Autoimmune encephalitides have been recognized in association with antibodies targeting neuronal surface antigen antibodies [NSAbs].1 Glycine receptors (GlyRs) are fundamental in motor neuron excitability, and antibodies against GlyRα1 (GlyR-Abs) were identified in a case of progressive encephalomyelitis with rigidity and myoclonus (PERM)2 and subsequently in other cases related to the stiff person syndrome spectrum.3
GlyRs are expressed mainly in the spine and brainstem but also in the hippocampus.4 Here we report 2 patients with subacute onset of refractory temporal lobe seizures associated with behavioral changes and memory deficits whose sera were positive for GlyR-Abs.
Case reports.
Patient 1.
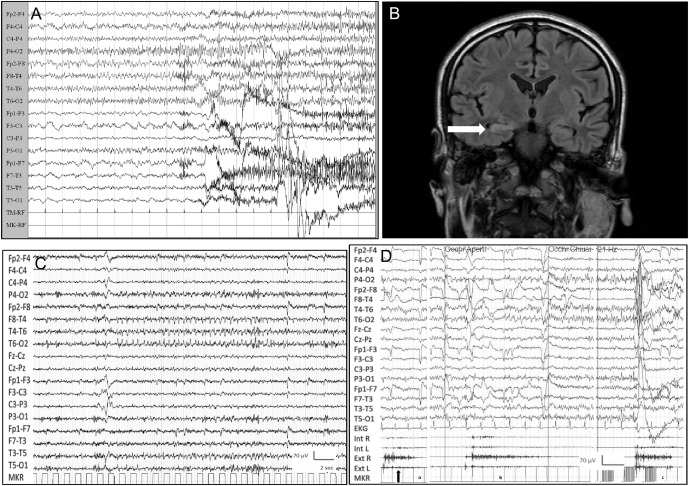
A 25-year-old man presented with a generalized convulsive status epilepticus (GCSE). Apathy and anxiety were noted over a few days. Status epilepticus was controlled with propofol. Vital signs, neurologic examination, routine blood tests, CSF analysis, and brain MRI were normal. Postictal EEG disclosed diffuse slow abnormalities. On day 2, GCSE recurred and he was transferred to the intensive care unit (ICU). Over the following days, when sedation was withdrawn, seizures continued despite IV phenytoin and levetiracetam. An EEG performed on day 7 showed an ictal discharge over right temporal leads, clinically associated with a convulsive generalized seizure (figure, A). Screening for pathogenic microorganisms, systemic autoimmunity, serum “onconeural,” GAD65, NMDAR, AMPAR, GABAbR, and voltage-gated potassium channel (VGKC)–complex autoantibodies was negative. Brain MRI was uninformative. On day 17, IV steroid (methylprednisolone 1 g/d for 7 days) was started, and 3 days later seizure frequency declined. Off sedation, the patient had only sporadic focal seizures. However, when steroids were tapered, status epilepticus reappeared. IV immunoglobulin (IVIg) (0.4 g/kg/d for 5 days) was started with marked improvement, and on day 40 he was discharged from the ICU. Seizures were controlled with a combination of phenobarbital, phenytoin, and zonisamide. EEG showed diffuse slow waves while brain MRI revealed a T2-weighted hyperintensity with mild swelling of right hippocampus, likely due to local edema induced by status epilepticus (figure, B). A wider autoantibody screening with a live cell-based assay subsequently showed GlyR-Ab positivity in serum; CSF was not available for further testing. The patient was transferred to the rehabilitation department and discharged after 8 weeks. By telephone interview 5 months after discharge, he was seizure-free on the same antiepileptic drug (AED) combination.
Figure. Patient 1's ictal EEG and brain MRI and patient 2's ictal EEG and polygraphic recording.
(A) Patient 1's EEG showing an ictal discharge over right temporal leads followed by diffuse polyspikes. (B) Patient 1's fluid-attenuated inversion recovery brain MRI coronal section showing hyperintensity with mild swelling of right hippocampus, likely due to local edema induced by the status epilepticus. (C) Patient 2's ictal EEG showing rhythmic sharp theta activity over the right temporal derivations lasting approximately 24 seconds, associated with above-mentioned symptoms. (D) Patient 2's polygraphic recording showing spasms involving upper limb muscles (arrows), more prominent proximally: Spontaneous (a), provoked by eye-opening (b), and provoked by intermittent photic stimulation (c). Note absence of EEG correlates. Ext R and L = extensor carpi muscle right and left; Int R and L = interosseous muscle right and left.
Patient 2.
A 41-year-old woman presented with a 3-month history of daily short-lasting (seconds) episodes characterized by abdominal pain followed by lightheadedness, nausea, goose bumps, and sensation of uncomfortable breathing. In the same period she started complaining of short-term memory difficulty. EEG revealed ictal discharges over right temporal leads (figure, C). 3T brain MRI showed a mild asymmetry of hippocampal volume (right 5.80 mL; left 5.24 mL; normal value 4.48–6.41 mL) with no different signal intensities on fluid-attenuated inversion recovery and T2-weighted images. She was given different AEDs (oxcarbazepine, lacosamide, valproate) without benefit. Four months later she was admitted because of persisting seizures with new onset of sudden spasms involving the whole body. Neurologic examination revealed mild cerebellar ataxia. Neuropsychological examination showed severe impairment of attention as well as short- and long-term verbal memory, moderate anxiety, and depression. Polygraphy disclosed sudden diffuse spasms, both spontaneous and induced by eye-opening or intermittent photic stimulation, with no EEG correlate (figure, D). CSF analysis was normal; oligoclonal bands were absent. Neoplastic markers, systemic autoimmunity, serum “onconeural”and GAD65, and serum and CSF NMDAR, AMPAR, GABAbR, and VGKC-complex autoantibodies were negative. GlyR-Abs were positive in serum but not detected in CSF. She was given IVIg 0.4 g/kg/d for 5 days with disappearance of the spasms and discharged home on oxcarbazepine and valproate. Over the following year, seizure frequency decreased but memory complaints persisted. Brain MRI showed no change. IV methylprednisolone 0.5 g/d for 5 days followed by oral prednisone was given. Due to inefficacy of steroid treatment (persistence of weekly seizures as well as severe short- and long-term verbal memory impairment), prednisone was gradually tapered off over a period of 3 months. The patient is currently receiving only the above-mentioned antiepileptic treatment and refuses further trial with IVIg.
Discussion.
The clinical picture of our 2 patients is in keeping with limbic encephalitis (LE). Patient 2 also displayed spontaneous and stimulus-sensitive spasms suggesting rhomboencephalitis and the diagnosis of PERM. It is thought that early diagnosis of LE should be considered in patients with multiple mesiotemporal seizures even in the absence of typical MRI abnormalities.5 The possibility of an NSAb-associated autoimmune encephalitis1 justified a trial of immunomodulatory treatment in both patients and was further supported by the finding of serum GlyR-Abs. Immunomodulation was effective in controlling symptoms in patient 1. In patient 2 IVIg provoked disappearance of spasms but only a mild reduction of seizure frequency and had no effect on attention and memory impairment; steroids were ineffective.
To our knowledge, seizures were reported in 1 patient with GlyR-Ab/PERM and another had seizures with hippocampal inflammatory involvement, but NMDAR-Ab coexisted.6 Of course, we cannot exclude the possibility that other antibodies were present and could explain hippocampal involvement in our patients. The antibody assay employed measured Ab to the α1 subunit, but these antibodies often cross-react with hippocampal GlyRs α2 and 3 subunits.6 Of note, GlyR expression is altered in patients with temporal lobe epilepsy4 and GlyR-Abs were identified in 3% of a large cohort of patients with epilepsy.7
We suggest that screening for NSAb could include GlyR-Ab in patients with autoimmune LE with seizures.
Footnotes
Author contributions: Luigi Zuliani, Edoardo Ferlazzo, Umberto Aguglia, Angela Vincent: interpretation of the data, drafting and revision of the manuscript. Cinzia Andrigo, Alessandro Casano, Vittoria Cianci, Marco Zoccarato, Maria Isabel Leite, Patrick Waters, Mark Woodhall, Ernesto Della Mora, Michele Morra, Bruno Giometto: interpretation of the data, revision of the manuscript.
Study funding: No targeted funding reported.
Disclosure: L. Zuliani, C. Andrigo, A. Casano, V. Cianci, M. Zoccarato, M. Woodhall, E. Della Mora, M. Morra, B. Giometto, and U. Aguglia report no disclosures. E. Ferlazzo has received honoraria for travel and speaking from UCB. M.I. Leite is supported by NHS National Specialised Commissioning Group for Neuromyelitis optica, UK, and by NIHR Oxford Biomedical Research Centre and has received speaking honoraria from Biogen Idec and travel and educational grants from Biogen Idec and UK. P.Waters has received speaker honoraria from Biogen Idec and Euroimmun AG; is a review editor for Frontiers in Molecular Innate Immunity; and holds a patent for assays for the detection of antibodies to lgi1, Caspr2, and tag-1. A. Vincent has received honoraria for travel and speaking from Baxter International Inc and Biogen Inc; is on the advisory board and is an associate editor for Brain; holds a patent with Oxford University for VGKC complex antibodies licensed to Euroimmun AG; receives royalties from Athena Diagnostics, Euroimmun AG, Blackwell Publishing, and Mac Keith Press; has consulted with Athena Diagnostics for assessing antibody assays; and has received research support from NIHR. Go to Neurology.org/nn for full disclosures. The Article Processing Charge was paid by the authors.
References
- 1.Zuliani L, Graus F, Giometto B, Bien C, Vincent A. Central nervous system neuronal surface antibody associated syndromes: review and guidelines for recognition. J Neurol Neurosurg Psychiatry 2012;83:638–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutchinson M, Waters P, McHugh J, et al. Progressive encephalomyelitis, rigidity, and myoclonus: a novel glycine receptor antibody. Neurology 2008;71:1291–1292 [DOI] [PubMed] [Google Scholar]
- 3.McKeon A, Martinez-Hernandez E, Lancaster E, et al. Glycine receptor autoimmune spectrum with stiff-man syndrome phenotype. JAMA Neurol 2013;70:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eichler SA, Kirischuk S, Jüttner R, et al. Glycinergic tonic inhibition of hippocampal neurons with depolarizing GABAergic transmission elicits histopathological signs of temporal lobe epilepsy. J Cell Mol Med 2008;12:2848–2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cianci V, Labate A, Lanza P, et al. Non-paraneoplastic limbic encephalitis characterized by mesio-temporal seizures and extratemporal lesions: a case report. Seizure 2010;19:446–449 [DOI] [PubMed] [Google Scholar]
- 6.Carvajal-González A, Leite MI, Waters P, et al. Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain Epub ahead of print 2014 June 20. pii: awu142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner T, Sills GJ, Hart Y, et al. Prevalence of neurologic autoantibodies in cohorts of patients with new and established epilepsy. Epilepsia 2013;54:1028–1035 [DOI] [PubMed] [Google Scholar]