Abstract
CD8+ cytotoxic T lymphocytes recognize infected cells in which MHC class I molecules present pathogen-derived peptides that have been processed mainly by proteasomes. Many infections induce a set of proteases, the caspases involved in apoptosis or inflammation. In this study, we report that processing and presentation of a short vaccinia virus-encoded Ag can take place also by a nonproteasomal pathway, which was blocked in infected cells with chemical inhibitors of caspases. By cleaving at noncanonical sites, at least two caspases generated antigenic peptides recognized by T lymphocytes. The sites and the peptidic products were partially overlapping but different to those used and produced by proteasomes in vitro. Antigenic natural peptides produced in infected cells by either pathway were quantitatively and qualitatively similar. Finally, coexpression of the natural vaccinia virus protein B13, which is an inhibitor of caspases and apoptosis, impaired Ag presentation by the caspase pathway in infected cells. These data support the hypothesis that numerous cellular proteolytic systems, including those induced during infection, such as caspases involved in apoptosis or in inflammation, contribute to the repertoire of presented peptides, thereby facilitating immunosurveillance.
The initial event for Ag presentation of pathogen-derived epitopes is the proteolytic processing of newly synthesized pathogen proteins. The proteasome is the primary and major proteolytic complex involved in the cytosolic generation of peptides (1). This multicatalytic endoprotease is not dedicated to Ag processing and produces a broad diversity of peptides. Among them, a small fraction of the correct epitope or NH2-terminally extended precursors can be used for MHC class I Ag presentation. These epitope precursor peptides may be N-terminally trimmed by aminopeptidases in the cytosol (2). The peptides are then transported by TAP to the endoplasmic reticulum lumen and usually require further trimming to attain the final size epitope. This is performed mainly by endoplasmic reticulum aminopeptidases (3). Finally, binding to nascent MHC class I molecules generates the antigenic complex recognized by antiviral CD8+ CTLs at the infected cell surface.
In addition to proteasome-independent and TAP-independent Ag-processing secretory pathways (4), tripeptidyl peptidase II is the only endoproteolytic activity that can generate pathogen-derived peptides in the cytosol when proteasomes are inhibited (5, 6). The potential role of other cytosolic endoproteases in the processing of MHC class I viral ligands remains to be elucidated. Although it is not expected that these proteases are dedicated to Ag processing, their peptidic products could be useful for Ag presentation to CD8+ T lymphocytes. These proteases could thus expand the number of tissues and physiological and pathological situations compatible with Ag presentation, as well as the range of pathogen-derived sequences available for recognition by CD8+ T lymphocytes.
Infection by pathogens and the subsequent variety of immune responses may affect the cellular proteolytic machinery in contrasting ways. Induction of apoptosis after infection by many pathogens is a widespread phenomenon (7, 8), which always induces activation of a set of endoproteases, the caspases. Caspases are a family of cytoplasmic cysteine proteases, some of which are essential for programmed cell death in a variety of species, whereas others are involved in inflammatory responses (9). They can be detected in many different types of cells undergoing apoptosis, regardless of their origin or the death stimulus. Initiator caspases are the first to be activated, followed by effector caspases, which act on cellular target molecules. In recent years, over 14 different caspases have been identified in mammals (9). They are synthesized as zymogens with no or very low intrinsic enzymatic activity. Direct aggregation of initiator caspases after the triggering stimulus is sufficient to promote self-processing to generate active heterotetramers. Downstream caspases are then activated proteolytically in a cascade fashion.
In a previous study (10), we investigated Ag processing in cells infected with recombinant vaccinia viruses (rVACVs) encoding two closely related miniproteins, encompassing murine CMV pp89 immunodominant epitope (11). In that study, we defined the redundant role of proteasomes in endogenous Ag processing of short Ags. In the current study, we have characterized an additional proteasome-independent pathway for Ag processing of one of these miniproteins, which operates when apoptosis is induced by vaccinia virus infection, and demonstrate that caspases can generate viral antigenic peptides recognized by CTLs.
Materials and Methods
Mice
BALB/c mice (H-2d haplotype) were bred in our colony in accordance with national regulations. Animal studies were approved by the review board of Instituto de Salud Carlos III (Madrid, Spain).
Reagents
Lactacystin (LC) was purchased from Dr. E. J. Corey (Harvard University, Boston, MA). Benzyloxycarbonyl-VAD-fluoromethyl ketone (z-VAD-fmk) and z-FA-fmk inhibitors were from Bachem (Weil am Rhein, Germany). All three were used at 50 μM both for in vitro digestions and for cell-based experiments. Ac-WEHD-CHO, Ac-YVAD-CHO, and Ac-DEVD-CHO caspase inhibitors used in control digestions were purchased from Peptide Institute (Osaka, Japan). 9pp89 (YPHFMPTNL), m19 (MDIGAYPHFMPTNLAGDPY), and G9I (GPGRAFVTI) peptides were synthesized in an Applera (Applied Biosystems, Foster City, CA) peptide synthesizer model 433A, purified, and confirmed to be homogeneous by HPLC analysis.
Cell lines
Transfected L/Ld and L/Dd cells were obtained from Dr. U. H. Koszinowski (Max-von-Pettenkofer Institute, München, Germany) (11). The classical IL-2–dependent murine T cell line CTL-L2 was also used. All cell lines were cultured in IMDM supplemented with 10% FBS and 5 × 10−5 M 2-ME.
rVACVs
The rVACVs encoding the minigenes rVACV-m17 and rVACV-m19 have been described previously (12). The rVACV-m17 codes for a 17-mer peptide representing the immunodominant murine CMV 9pp89 epitope and the local flanking amino acids of hepatitis B virus e Ag (HBe) carrier protein (MDIGYPHFMPTNLGDPY). The rVACV-m19 codes for a 19-mer peptide (MDIGAYPHFMPTNLAGDPY) that differs only from the previous minigene by a biterminal alanine spacer between 9pp89 core and HBe flanking residues. The recombinant rVACV-HBe was used as a negative control (13). To generate specific CTL lines in vivo, the murine CMV 9pp89 immunodominant epitope flanked biterminally by alanines, inserted at the N terminus of HBe, and encoded by rVACV-HBe/N/A59pp89A5 was used (14). rVACV-sA encodes a secreted, glycosylated 86-aa protein (15) containing the Ld-restricted murine hepatitis virus nucleocapsid pN epitope linked to the HIV IIIB gp160 V3 loop comprising the p18 epitope presented by Dd. rVACV-sI encodes a recombinant 22-aa secretory miniprotein comprising both epitopes. Both rVACV-sA and rVACV-sI are similar to the published A and I recombinants (16), except that they are preceded by the adenovirus E19 signal sequence. The vaccinia virus strain Western Reserve (WR) mutant lacking the B13R gene, vΔB13R, and its revertant control, vB13R+rev, were reported previously (17) (Fig. 1). The recombinant viruses rVACV-m19, rVACV-HBe, and rVACV-HBe/N/A59pp89A5 are based on vaccinia virus strain Copenhagen, which naturally lacks the B13R gene. rVACV-sA, rVACV-sI, vΔB13R, and vB13R+rev were derived from vaccinia virus strain WR.
FIGURE 1. Diagram showing nature of the rVACV.
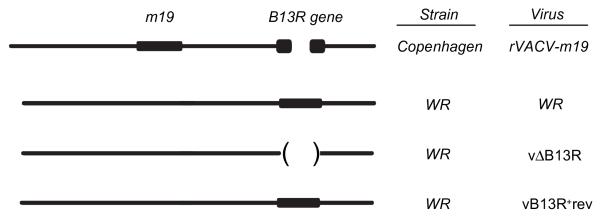
The rVACV-m19 and all viruses derived from vaccinia virus strain Copenhagen have the B13R gene disrupted. In contrast, this gene is functional in vaccinia virus strain WR.
T cell lines and cytotoxicity assays
Polyclonal 9pp89-monospecific CTLs were generated by immunization of mice with rVACV-HBe/N/A59pp89A5 as described previously (11).
Polyclonal ENV p18-specific CTLs were generated similarly (15). Either 9pp89- or ENV p18-specific CTLs were used as effector cells in standard 4–6-h cytotoxicity assays (11). Transfected cells were used as target cells after overnight infection with rVACVs as described (11). The data are expressed as mean values of at least two experiments. Recombinant human IL-2 was used for the long-term propagation of CTL lines and was provided by Hoffmann-La Roche (Basel, Switzerland). The percentage of specific inhibition was calculated as 100 × (A − Ai)/(A − N), where A is the percent specific lysis with target cells infected with rVACV-m19, Ai is the value with rVACV-m19 plus inhibitor, and N is the value with the negative control.
Proteasome and caspase digestion assays
Caspases that were selectively expressed in bacteria and 20S proteasomes were purified as described (18, 19). m19 digestions by caspases and proteasome were performed as follows (18, 19): the m19 peptide, dissolved in DMSO, was incubated overnight at room temperature with micromolar concentrations of different proteases (the proteasome and 10 human caspases) in 0.2 ml. Digestion time was chosen to mimic time of exposure of m19 to proteases in infected cells. The final concentration of m19 was 0.5 mM, and the incubation buffer consisted of 0.1 M HEPES (pH 7.5), 10% sucrose, 0.1% CHAPS, and 10 mM DTT. Postincubation, the samples were frozen at −70°C until further analysis. Similar experiments in presence of 0.2 mM WEHD-CHO or 2 mM YVAD-CHO (to inhibit caspase-5) or 0.2 mM DEVD-CHO (to block caspase-10 activity) were used as caspase-specificity controls.
Mass spectrometry
Aliquots of total digestions containing peptides were dried and dissolved in 10 μl 0.5% acetic acid in water and sequenced by quadrupole ion trap micro-HPLC electrospray mass spectrometry (MS)/MS in a Deca XP LCQ mass spectrometer (Thermo Electron, San Jose, CA). We used the MS/MS mode and concentrated the analysis on each theoretical parental peptide (20), using an isolation width (m/z) of 1.5 Da. The charge and mass of the ionic species were determined by high-resolution sampling of the mass/charge rank. Collision energy and ion-precursor resolution were improved to optimize the fragmentation spectrum. All peptides were sequenced by MS/MS fragmentation.
Isolation of naturally processed peptides
L/Ld cells (3 × 108) were infected with rVACV with 10 PFU/cell, and 16 h later, the naturally processed peptides were extracted from whole cells with trifluoroacetic acid, purified (21, 22) by reversed phase (RP)-HPLC, and tested in cytotoxicity assays (10) with 9pp89-specific CTLs. As an internal standard, a 9pp89-unrelated peptide was included in all HPLC runs. To ensure comparability of different runs, the actual conductivity gradient was monitored. Contamination of the HPLC columns was excluded by testing preceding HPLC runs with CTLs. For standardization, serial dilutions of synthetic peptides were always tested in parallel.
Flow cytometric analysis
Cells (4 × 105) were infected and/or treated with inhibitors or staurosporine (Sigma-Aldrich, St. Louis, MO). Four hours later, they were washed and stained with CaspACE FITC-VAD-fmk in situ marker (Promega, Madison, WI), followed by flow cytometry in a FACScan (BD Biosciences, Franklin Lakes, NJ).
Results
Caspases inhibitor specifically blocks m19 Ag presentation
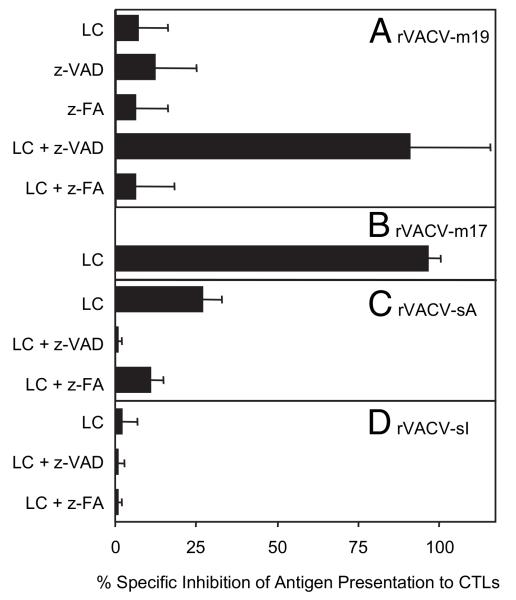
The 19-aa–long m19 miniprotein (MDIGAYPHFMPTNLAGDPY) encompasses the murine CMV pp89 immunodominant and protective nonamer epitope that is presented by the MHC class I H-2Ld molecule in BALB/c mice (11) and requires proteolytic processing for TAP-dependent presentation to CD8+ T lymphocytes (10). To study processing of this miniprotein, the effects of protease inhibitors were investigated. First, processing and presentation of m19 by H-2Ld-positive target cells following infection with rVACV-m19 (Fig. 1) was confirmed by their lysis by CTL lines specific for the core nonameric epitope 9pp89 (45% specific lysis versus 5% with control rVACV at an E:T ratio of 5:1). Some reagents, such as LC or z-VAD-fmk, had no effect on the specific recognition by CTLs of target cells infected with rVACV-m19 (Fig. 2A). LC is a powerful proteasome-specific inhibitor (23) whose inhibitory effect on Ag presentation was revealed with the m19-related, 2-aa–shorter m17 protein (Fig. 2B) (10). z-VAD-fmk blocks the activity of most caspases (24). In contrast to the lack of effect of each inhibitor on m19, the combination of both reagents blocked the presentation of rVACV-m19 completely (Fig. 2A). Because some nonspecific effects have been ascribed to the fluoro-methyl-ketone moiety of z-VAD-fmk, we also included the peptide z-FA-fmk as a control. This control compound inhibits lysosomal cathepsins B and L, but has little effect on caspase activity; only some effector caspases are inhibited marginally and 10–100-fold less effectively by z-FA-fmk than by z-VAD-fmk (25). In our studies, m19 Ag presentation was unaffected by this reagent, regardless of whether it was tested alone or in combination with LC (Fig. 2A). To exclude the possibility that the inhibitory effects of LC plus z-VAD-fmk were due to toxic effects on target cells or on rVACV replication, rather than to a specific block of the respective proteases, similar experiments were performed in parallel using either rVACV-sA or rVACV-sI viruses, which express secretory proteins that are presented in the presence of LC following uncharacterized Ag-processing pathways (15). Target cells infected with these rVACVs and incubated with the mix of LC and z-VAD-fmk were recognized efficiently by specific CTLs, and no inhibition was detected of either rVACV-sA or rVACV-sI (Fig. 2C, 2D). This excluded any general nonspecific effect on endogenous Ag presentation. These data thus suggest that inhibition of m19 miniprotein presentation by addition of LC plus z-VAD-fmk is due to specific blockage of the respective proteases and suggest that the 19-mer product can be processed in parallel and independently either by proteasomes or by caspases.
FIGURE 2. Ag-presentation assays with proteasome and caspases inhibitors.
L/Ld cells were infected overnight with 10 PFU/cell of rVACV-m19 or rVACV-m17 in the presence of LC, z-VAD-fmk (z-VAD), or z-FA-fmk (z-FA) and tested with 9pp89-specific CTLs (A, B). Similarly, L/Dd cells were infected with rVACV-sA (C) or rVACV-sI (D) and tested with HIV ENV-specific CTLs. Inhibitors were present throughout infection and during [51Cr] labeling until addition of specific CTLs to target cells. The percentage specific lysis in the absence of LC was 45%, 50%, 45%, and 39% for m19, m17, sA, and sI, respectively, and was used as a reference to calculate percentage of specific inhibition, which is expressed as the mean ± SD (n = 6).
Specific cleavage of m19 synthetic peptide by both caspase-5 and caspase-10
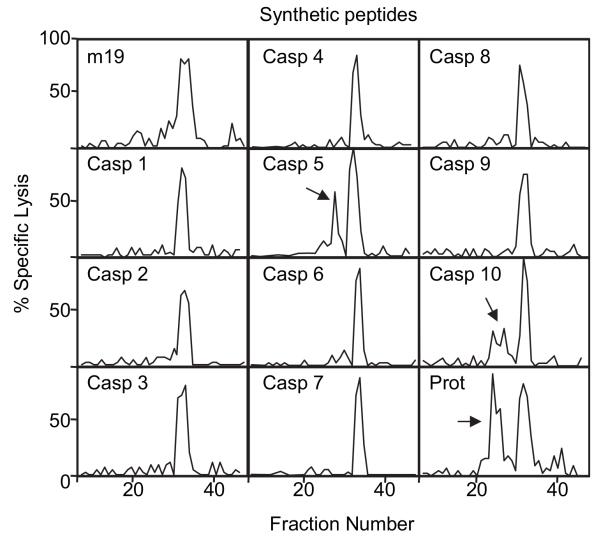
In recent years, the aspartate-specific cysteinyl proteases known as caspases have been studied intensively both in inflammation and apoptosis (9). Caspases cleave after selected Asp residues in selected target proteins. Over 14 caspases have been identified, and 10 of the human enzymes have been selectively expressed in bacteria, purified, and characterized thoroughly (18). A similar set of purified murine caspases is not available. Purified human caspase-1 through caspase-10 were incubated with m19 synthetic peptide, exactly the same substrate as expressed in living rVACV-m19–infected cells. The potential peptides generated after enzymatic digestion were separated by RP-HPLC and analyzed in cytotoxicity assays with 9pp89-specific CTLs (Fig. 3). In all cases, an antigenic activity that coelutes with the unprocessed m19 substrate peptide was observed. This 19-aa–long synthetic peptide is recognized by CTLs specific for the 9pp89 nonamer, but it is 103 times less antigenic (data not shown) than the 9pp89 peptide that is bound naturally to the MHC class I molecule Ld in infected cells (14). Notably, caspase-5 and caspase-10 generated additional antigenic fractions (arrows), indicating a partial cleavage of m19 peptide to generate smaller antigenic peptidic products. Control digestions in the presence of caspase-5 or caspase-10 inhibitors did not reveal any antigenic peaks other than the m19 substrate (data not shown; see also later controls in Fig. 4B). Caspase-5 is a caspase-1–like initiator proinflammatory caspase, whereas caspase-10 is an initiator caspase that triggers apoptosis. Similar experiments were also performed with the proteasome. This multicatalytic complex is also able to partially cleave the m19 peptide and produce smaller antigenic peptides (Fig. 3, last panel). The apparent yield of antigenically active products was lower after caspase-10 digestion, and specially after caspase-5 digestion, than after proteasome digestion (see approximate quantitation after MS experiments below). In conclusion, the proteasome as well as caspase-5 and caspase-10 are able to process m19 peptide independently and generate products that are recognized by 9pp89-specific CTLs.
FIGURE 3. In vitro digestions of m19 synthetic peptide with different caspases (Casp) or proteasomes (Prot).
The m19 synthetic peptide was digested with the indicated purified proteases. Peptides generated in each digestion were separated by RP-HPLC. Fractions were tested with 9pp89-specific CTLs to analyze the presence of antigenic peptides. The results obtained with each protease in the cytotoxicity assay are depicted. The arrows indicate antigenic peaks other than the m19 substrate peptide.
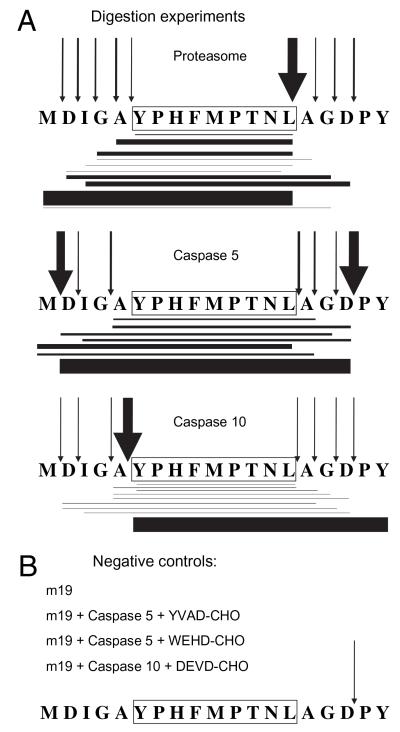
FIGURE 4. Digestion pattern of m19 synthetic peptide with purified proteasomes or different caspases analyzed by MS.
The m19 synthetic peptide was digested with the indicated purified proteases (A) or negative controls (B) and analyzed by MS/MS. The sequence of the CMV epitope is boxed. Horizontal lines show peptide products identified by MS/MS analysis. Line thickness indicates approximate amount detected. The arrows indicate cleavages. Their thickness indicates frequency of each cleavage, deduced from the approximate amounts of identified peptide products. Amounts are depicted relative to the most abundant product within each digestion.
Identification by MS fragmentation of peptides generated by different proteases
Proteasomes are not dedicated to Ag processing and seldom produce the final antigenic peptide. Rather, a collection of N-terminally extended precursors is frequently generated, which, after further trimming by additional aminopeptidases, can give rise to the final antigenic products. The same could apply to caspases. Fig. 3 revealed only antigenic products. Therefore, our next aim was to further reveal potential relevant precursor peptides generated by caspases. To this end, the peptides generated by the different proteases involved in m19 processing were studied by micro-HPLC electrospray MS. Total digestions with proteasome, caspase-5, or caspase-10 were analyzed extensively by MS/MS to detect all relevant products (i.e., potential epitope precursors as well as the final epitope). Micro-liquid chromatography coupled to MS/MS was used, and the analysis was restricted to each theoretical parent ion and followed by manual interpretation (20). This technique was employed because it increases peptide detection and optimizes the identification of generated peptides, because it can resolve isomeric products generated from this miniprotein. Fig. 4A summarizes the results. The proteasome generates mainly N-terminally extended peptides with the correct C-terminal leucine residue, as reported previously (12, 20). Both caspase-5 and caspase-10 produced a broad spectrum of processed peptides. This was unexpected given their canonical specificity. The nature and amount of these products differed from those produced by proteasomes. Caspase-5 caused a major cleavage at a canonical site after the three C-extended residues Ala-Gly-Asp, and caspase-10 induced a major cleavage upstream of the exact N-terminal Tyr of 9pp89 epitope. Similar to the proteasome digestion, some peptides that can be relevant for Ag presentation were produced by caspase-5, such as 1MDIGAYPHFMPTNL14, or by caspase-10, such as 5AYPHFMPTNL14, and the exact optimally antigenic 6YPHFMPTNL14 9pp89 nonamer. The global yield of products generated by caspase-5 or caspase-10 was around 1/25 or 1/3 of the proteasome yield, respectively (data not shown). Peptide 1-17, having a possible cleavage site on the carboxyl side of an aspartate residue, which would fit canonical caspase activity, was found at low levels after electrospray ionization-MS in all cleavage experiments, including the negative control experiments (m19 synthetic peptide with no proteases and digestions in the presence of caspase inhibitors; Fig. 4B). Thus, no conclusions could be drawn when low levels of this peptide were detected. This finding is in agreement with a report that indicates that Asp-Pro bonds of synthetic peptides can undergo spontaneous cleavage under acidic conditions, preferentially under those generally used in solubilization and purification of peptides prior to electrospray ionization-MS analysis (26). Of note, the lack of other peptidic products in these control digestions, as well as the diversity of products generated by digestion with each enzyme, speaks for the purity of the caspase preparations and supports their noncanonical specificities.
In summary, these results show that caspases can cleave at sites other than the specific consensus sites reported previously, albeit with lower efficiency than the proteasome. Most importantly, the products of these noncanonical cleavages include peptides that can be recognized by CTLs, as well as potential N-extended precursor peptides for Ag presentation in infected cells.
Proteasomes and caspases generate similar natural antigenic peptides in living infected cells
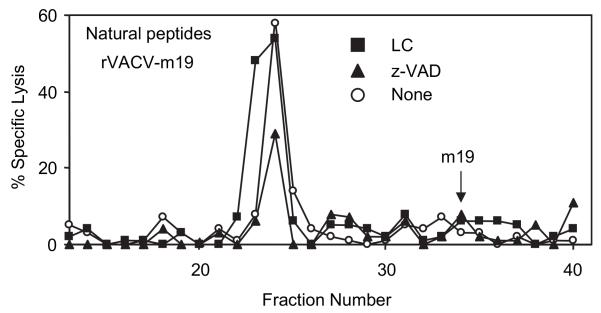
Following the study of the products of in vitro processing by caspases, the natural endogenously processed peptides derived from the m19 miniprotein and generated by both independent pathways in infected cells were analyzed. To this end, we performed acid extractions of the peptides generated after infection of L/Ld cells with rVACV-m19 in the presence or absence of protease inhibitors. The peptides were separated by RP-HPLC and their antigenicity was analyzed in cytotoxicity assays. Cells infected with m19 without protease inhibitors showed one peak of antigenic activity (Fig. 5, empty circles). This biological activity coelutes with the synthetic nonapeptide YPHFMPTNL, 9pp89, (data not shown) reported previously as the natural CTL epitope in rVACV and murine CMV infection (14, 27). The low levels of expression of unprocessed m19 did not allow detection of its antigenicity in these whole-cell extracts (Fig. 5, arrow). 9pp89-specific CTLs did not recognize control rVACV-infected target cells, indicating the specificity of the antigenic peak (data not shown). Results in Fig. 2 had suggested that m19 can be processed in infected cells either by proteasomes or caspases. Therefore, natural peptides were also extracted from cells infected in the presence of proteasome inhibitor LC to reveal the products derived mainly from the caspases pathway (Fig. 5, triangles) or from cells infected in the presence of caspase inhibitor z-VAD-fmk to reveal the products derived mainly from the proteasomal pathway (Fig. 5, squares). Both cell extracts showed similar antigenic activities, suggesting that either pathway generates similar antigenic peptides in sufficient amount to allow effective Ag presentation to CTLs.
FIGURE 5. Natural endogenous peptides generated after processing of m19 in infected cells.
Naturally processed peptides were acid-extracted from L/Ld cells infected overnight with 10 PFU/cell of rVACV-m19 in the presence of LC (■), z-VAD-fmk (▲), or nothing (○) and separated by RP-HPLC. Fractions were tested with 9pp89-specific CTLs to analyze the presence of antigenic peptides.
Activation of caspases in rVACV-m19–infected cells
Caspases are activated in different cells and also after vaccinia virus infection (7–9). Therefore, caspase activation in L/Ld cells infected with rVACV-m19 was studied. As shown in Fig. 6A, the same levels of activation were attained in cells infected with rVACV-m19 as those observed following classical staurosporine-induced cell killing. This cellular death was mediated by caspase activity because the presence of z-VAD-fmk specifically impaired it (Fig. 6B).
FIGURE 6. Activation of caspases after vaccinia virus infection.
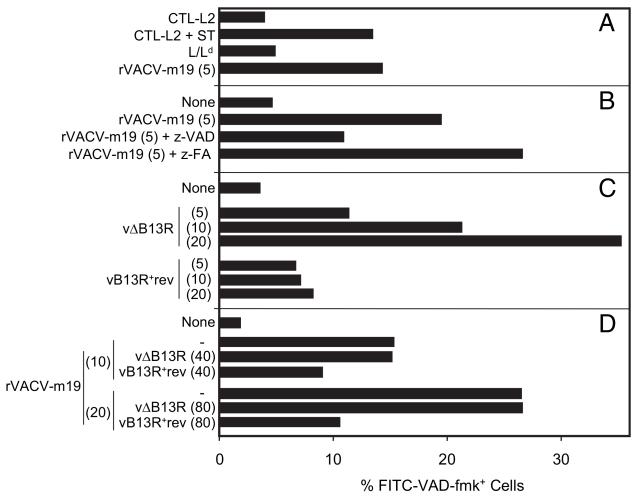
Inhibition by the vaccinia virus protein B13. L/Ld cells were infected for 4 h with the PFU/cell shown in brackets with the indicated vaccinia viruses or combinations of two viruses (A–D). Refer to Fig. 1 for presence of B13R in each virus. The percentage of apoptotic cells was determined by flow cytometry after staining with CaspACE FITC-VAD-fmk. CTL-L2 cells in A were incubated for 4 h with 1 μM staurosporine (ST).
Vaccinia virus wild-type strain WR expresses the B13 protein, which inhibits the proinflammatory caspase-1, the IL-1–converting enzyme, in human (17) and murine cells (28). B13 is closely related to crmA from cowpox virus. Like crmA, it blocks apoptosis (17, 28, 29) and thus is likely to also inhibit proapoptotic caspases. We reasoned that B13 might target the z-VAD-fmk–sensitive enzymes involved in m19 presentation to CTLs. Analysis with a WR strain of vaccinia virus lacking the B13R gene, vΔB13R, and with the revertant control virus, vB13R+rev, shows, first, that induction of apoptosis by vΔB13R is infection- and dose-dependent and, second, that apoptosis is inhibited by the B13 protein in L/Ld cells infected with the revertant virus vB13R+rev (Fig. 6C). The B13R gene is naturally disrupted in vaccinia virus strain Copenhagen, the parental virus for rVACV-m19 (Fig. 1). This explains why rVACV-m19 infection induced apoptosis in Fig. 6A, 6B. To express the viral apoptosis inhibitor B13 protein in cells expressing m19, cells were coinfected with rVACV-m19 and with either one of the two WR-based vaccinia viruses. Fig. 6D shows that apoptosis induced by rVACV-m19 decreased after coinfection with vB13R+rev but not with control vΔB13R. Thus, in addition to inhibiting the proinflammatory caspase-1, vaccinia virus B13 protein also prevented apoptosis in rVACV-m19–infected cells.
Blockade of the endogenous processing of 9pp89 epitope by LC combined with vaccinia virus protein B13
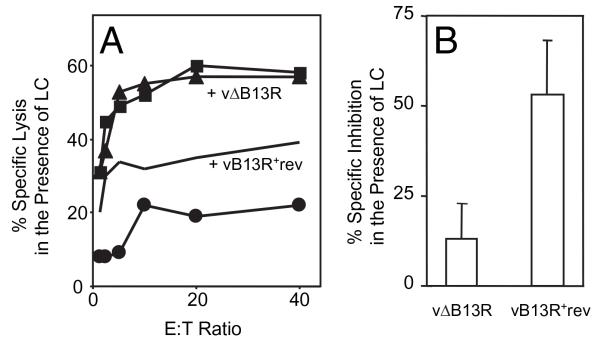
Next, cytotoxic assays were performed with rVACV-m19–infected cells treated with the proteasome inhibitor LC and coinfected with vaccinia viruses that do or do not encode the gene B13R for the viral caspase inhibitor. These experiments were designed to mimic the combined treatment with LC and the pan-caspase inhibitor z-VAD-fmk that blocks m19 Ag presentation (Fig. 2). The results are shown in Fig. 7. When only the proteasome pathway was blocked, by using LC only (Fig. 7A) or control vΔB13R and LC (Fig. 7B, left bar), Ag presentation remained unaffected, as expected. In contrast, when both pathways were blocked (by LC plus vaccinia virus expressing protein B13), Ag presentation was considerably decreased (Fig. 7B, right bar). Therefore, because caspase inhibition by either a chemical or a viral protein impairs recognition of infected cells by antiviral MHC class I-restricted CTLs, these data substantiate that caspases are involved in m19 Ag processing.
FIGURE 7. Inhibition of Ag presentation to CTLs by a combination of LC and the vaccinia virus protein B13.
L/Ld cells were singly infected overnight in the presence of LC with 10 PFU/cell of rVACV-m19 (■) or a negative control (●) or doubly infected with 10 PFU/cell of either vΔB13R (▲) or vB13R+rev (lines). LC was kept throughout infection and [51Cr] labeling until the addition of CTLs to target cells. The percentage specific lysis at each E:T ratio is shown on the vertical axis (A). The percentage specific inhibition caused by the coinfection is plotted as the mean ± SD (n = 4) of all E:T ratios (B).
Discussion
We have investigated the role of cytoplasmic nonproteasomal proteases in Ag presentation of short viral Ags by MHC class I molecules in infected cells. By employing a chemical inhibitor of caspases as well as a viral protein that inhibits caspases and apoptosis in infected cells, we find that caspases are involved in Ag presentation to CD8+ T lymphocytes of a vaccinia virus-expressed viral epitope.
Each caspase has different substrate specificities determined by tetrapeptide motifs upstream of the cleavage site, but all cleave mainly on the carboxyl side of an aspartate residue. Our findings show that at least two human initiator caspases can process the m19 peptide in vitro and that they generate a variety of peptidic products, some of which are recognized by specific antiviral CD8+ T lymphocytes. The protease activity of these caspases was unexpected in view of their reported cleavage specificity, and so these data establish an additional minor new activity for these caspases. Extending recent results (30) in which a model epitope was positioned at the end of a protein and had an engineered N-terminal canonical cleavage site for caspase-3 and -7, we now show that, for efficient viral Ag processing in infected cells, caspases do not need an optimal consensus cleavage site. This ability broadens the repertoire of potential MHC ligands or precursors generated by caspases. Precursor peptides are especially relevant for Ags like 9pp89 and for MHC class I molecules like Ld and HLA-B7 supertype that preferentially bind peptides with a proline in position 2, which is poorly transported by TAP (31). Furthermore, in addition to the generation of potential precursor peptides, we show that caspases can generate with limited efficiency both N and C ends of the viral peptide in vitro.
Our results also show that in vivo the 19-mer substrate peptide is processed naturally in infected cells by caspases as well as by proteasomes. In our system, the production of a similar final antigenic activity by either pathway in living cells may reflect the potential ability of either protease to generate correct C-terminals aided by the likely contribution to both pathways of downstream trimming by auxiliary enzymes (2, 3), followed by selection by the MHC class I molecule Ld of the optimal binding peptide.
When processing of endogenously synthesized Ags proceeds independently of the classical proteasome pathway, the involvement of three proteases of the secretory pathway, signal peptidase (32, 33), furin (34–36), and cathepsins (36), has been reported. There are some indications that cytosolic nonproteasomal endoproteases are involved in Ag processing (15, 37, 38), but their identification remains elusive. The exception is cytosolic tripeptidyl peptidase II, which, in addition to acting as an aminopeptidase, exhibits an endoproteolytic activity and contributes to the generation of at least some selected epitopes also when proteasomes are inhibited (5, 6, 39). Caspases generate endoproteolytic cleavages, and the current study shows that, as for tripeptidyl peptidase II, Ag processing of endogenous Ags by these proteases can be detected when proteasomes are impaired. Caspases may thus also be important under infection conditions under which proteasome activities cannot generate given peptides or even directly destroy some epitopes (5, 6, 40). For this particular Ag, we show that it can be processed by proteasomes and by at least two human caspases. There are no exact murine counterpart genes for these caspases (41, 42). It is thus expected that in murine cells, the viral Ag is processed by a set of zVAD-cmk- and B13-sensitive caspases with canonical or noncanonical specificities related to those of human caspase-5 and -10. We cannot anticipate whether this redundancy can be extended to other Ags. When the sole effect of B13R deletion on selected epitopes was assayed in the absence of proteasome blockade, no differences were found (43, 44). Our data thus demonstrate the existence of supplemental activities in the cytosolic Ag-processing pathway for presentation by MHC class I molecules and identify the enzyme family involved.
A recent report showed that caspases can partially cleave cellular proteins in apoptotic cells, suggesting that after endocytosis of apoptotic cells by dendritic cells, these partially cleaved self-proteins would serve as substrates for the proteasome during cross-presentation to autoreactive T lymphocytes (45). Our results show that caspases can fully process the viral Ag already in the apoptotic infected cell, rendering it a target for elimination by antiviral CD8+ T lymphocytes.
On the other hand, processing of Ags that are exogenously added to cells can also proceed via a variety of proteases, including the proteasome and cathepsin S in professional APCs and signal peptidase and furin in other cell types (46). Of note, these are the same proteases as those involved in processing of endogenously synthesized viral proteins. We show in this study that caspases in infected cells can process endogenous viral Ags. A relevant issue that deserves further study is whether caspases in dendritic cells can also process endocytosed Ags for cross-presentation, an event which contributes to initiating the antiviral CD8+ T lymphocyte responses within hours of host infection (47).
Apoptosis by caspase activation is a universal cellular defense mechanism against viruses and other intracellular pathogens, including vaccinia virus (7, 8), which interferes with virus production and transmission to neighboring cells. As we show in this paper, as a spin-off benefit, caspases process Ag. Therefore, these proteases are good candidates to increase the diversity of the pool of viral peptides available for MHC binding, thereby complementing the products of the proteasome and of the IFN-γ-induced immunoproteasomes. Like these complexes, some caspases, including caspase-1 and caspase-5, are induced by IFN-γ expressed during inflammation (48). It is plausible that the increase in caspase activation that we observe postinfection may compensate for the lower efficiency of peptide production in vitro by caspases as compared with the proteasome. In this context, it is relevant that we study naturally induced apoptosis by vaccinia virus infection. Close to physiological blockade of apoptosis and Ag presentation via caspases was also achieved with the vaccinia virus protein B13 that was naturally expressed during infection. Our results thus extend previous findings with an engineered model of induction of apoptosis (30). Because caspases are selectively activated under infection or physiological stress, their supplementary role in Ag processing may facilitate the production of MHC class I ligands for Ag presentation in a critical moment postinfection. This regulation would decrease the likelihood of immune escape by viruses or other pathogens. Not surprisingly, several pathogens including vaccinia virus attenuate caspase activity by encoding inhibitors of apoptosis and of caspases (7, 17). The inhibitory effects of the vaccinia virus apoptosis inhibitor B13 both on apoptosis and Ag presentation are predicted to add up to favoring virus replication in the infected host. Indeed, a virus deletion mutant lacking the B13R gene is mildly attenuated after i.p. infection in vivo (49), although not if applied intranasally (50). Apoptotic cells show endogenous adjuvant properties to initiate an immune response (51). Our results support the notion that viral Ag presentation by caspases induced during apoptosis also contributes to immunogenicity of apoptotic infected cells.
Acknowledgments
We thank U.H. Koszinowski for kindly providing the rVACV encoding the m19 miniprotein and L/Ld and L/Dd cells and C.C. Bergmann for providing rVACV-sA and rVACV-sI. We also thank A.F. Kisselev for proteasome isolation and purification. Recombinant human IL-2 was a gift from Hoffmann-La Roche. The excellent technical assistance of F. Vélez, C. Mir, O. Calero, and M. Jiménez is gratefully acknowledged.
This work was supported by grants provided by the European Union, Ministerio de Ciencia e Innovación, Comunidad de Madrid, and Instituto de Salud Carlos III (to M.D.V.) and Programa Ramón y Cajal, Comunidad de Madrid, and Instituto de Salud Carlos III (to D.L.). G.L.S. is a Wellcome Principal Research Fellow.
Abbreviations used in this paper
- HBe
hepatitis B virus e Ag
- LC
lactacystin
- MS
mass spectrometry
- RP-HPLC
reversed phase-HPLC
- rVACV
recombinant vaccinia virus
- WR
Western Reserve
Footnotes
Disclosures: The authors have no financial conflicts of interest.
References
- 1.Kloetzel PM, Ossendorp F. Proteasome and peptidase function in MHC-class-I-mediated antigen presentation. Curr. Opin. Immunol. 2004;16:76–81. doi: 10.1016/j.coi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Rock KL, York IA, Goldberg AL. Post-proteasomal antigen processing for major histocompatibility complex class I presentation. Nat. Immunol. 2004;5:670–677. doi: 10.1038/ni1089. [DOI] [PubMed] [Google Scholar]
- 3.Hammer GE, Kanaseki T, Shastri N. The final touches make perfect the peptide-MHC class I repertoire. Immunity. 2007;26:397–406. doi: 10.1016/j.immuni.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Del Val M, López D. Multiple proteases process viral antigens for presentation by MHC class I molecules to CD8 T lymphocytes. Mol. Immunol. 2002;39:235–247. doi: 10.1016/s0161-5890(02)00104-9. [DOI] [PubMed] [Google Scholar]
- 5.Seifert U, Marañón C, Shmueli A, Desoutter JF, Wesoloski L, Janek K, Henklein P, Diescher S, Andrieu M, de la Salle H, et al. An essential role for tripeptidyl peptidase in the generation of an MHC class I epitope. Nat. Immunol. 2003;4:375–379. doi: 10.1038/ni905. [DOI] [PubMed] [Google Scholar]
- 6.Guil S, Rodríguez-Castro M, Aguilar F, Villasevil EM, Antón LC, Del Val M. Need for tripeptidyl-peptidase II in major histocompatibility complex class I viral antigen processing when proteasomes are detrimental. J. Biol. Chem. 2006;281:39925–39934. doi: 10.1074/jbc.M608522200. [DOI] [PubMed] [Google Scholar]
- 7.Pogo BG, Melana SM, Blaho J. Poxvirus infection and apoptosis. Int. Rev. Immunol. 2004;23:61–74. doi: 10.1080/08830180490265547. [DOI] [PubMed] [Google Scholar]
- 8.Clarke P, Tyler KL. Apoptosis in animal models of virus-induced disease. Nat. Rev. Microbiol. 2009;7:144–155. doi: 10.1038/nrmicro2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel RM. Caspases at the crossroads of immune-cell life and death. Nat. Rev. Immunol. 2006;6:308–317. doi: 10.1038/nri1809. [DOI] [PubMed] [Google Scholar]
- 10.López D, Del Val M. Selective involvement of proteasomes and cysteine proteases in MHC class I antigen presentation. J. Immunol. 1997;159:5769–5772. [PubMed] [Google Scholar]
- 11.Del Val M, Volkmer H, Rothbard JB, Jonjić S, Messerle M, Schickedanz J, Reddehase MJ, Koszinowski UH. Molecular basis for cytolytic T-lymphocyte recognition of the murine cytomegalovirus immediate-early protein pp89. J. Virol. 1988;62:3965–3972. doi: 10.1128/jvi.62.11.3965-3972.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eggers M, Boes-Fabian B, Ruppert T, Kloetzel PM, Koszinowski UH. The cleavage preference of the proteasome governs the yield of antigenic peptides. J. Exp. Med. 1995;182:1865–1870. doi: 10.1084/jem.182.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlicht HJ, Schaller H. The secretory core protein of human hepatitis B virus is expressed on the cell surface. J. Virol. 1989;63:5399–5404. doi: 10.1128/jvi.63.12.5399-5404.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Val M, Schlicht HJ, Ruppert T, Reddehase MJ, Koszinowski UH. Efficient processing of an antigenic sequence for presentation by MHC class I molecules depends on its neighboring residues in the protein. Cell. 1991;66:1145–1153. doi: 10.1016/0092-8674(91)90037-y. [DOI] [PubMed] [Google Scholar]
- 15.López D, Gil-Torregrosa BC, Bergmann C, Del Val M. Sequential cleavage by metallopeptidases and proteasomes is involved in processing HIV-1 ENV epitope for endogenous MHC class I antigen presentation. J. Immunol. 2000;164:5070–5077. doi: 10.4049/jimmunol.164.10.5070. [DOI] [PubMed] [Google Scholar]
- 16.Bergmann CC, Tong L, Cua R, Sensintaffar J, Stohlman S. Differential effects of flanking residues on presentation of epitopes from chimeric peptides. J. Virol. 1994;68:5306–5310. doi: 10.1128/jvi.68.8.5306-5310.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kettle S, Alcamí A, Khanna A, Ehret R, Jassoy C, Smith GL. Vaccinia virus serpin B13R (SPI-2) inhibits interleukin-1β-converting enzyme and protects virus-infected cells from TNF- and Fas-mediated apoptosis, but does not prevent IL-1β-induced fever. J. Gen. Virol. 1997;78:677–685. doi: 10.1099/0022-1317-78-3-677. [DOI] [PubMed] [Google Scholar]
- 18.García-Calvo M, Peterson EP, Rasper DM, Vaillancourt JP, Zamboni R, Nicholson DW, Thornberry NA. Purification and catalytic properties of human caspase family members. Cell Death Differ. 1999;6:362–369. doi: 10.1038/sj.cdd.4400497. [DOI] [PubMed] [Google Scholar]
- 19.Kisselev AF, Akopian TN, Woo KM, Goldberg AL. The sizes of peptides generated from protein by mammalian 26 and 20 S proteasomes. Implications for understanding the degradative mechanism and antigen presentation. J. Biol. Chem. 1999;274:3363–3371. doi: 10.1074/jbc.274.6.3363. [DOI] [PubMed] [Google Scholar]
- 20.López D, Calero O, Jiménez M, García-Calvo M, Del Val M. Antigen processing of a short viral antigen by proteasomes. J. Biol. Chem. 2006;281:30315–30318. doi: 10.1074/jbc.M605973200. [DOI] [PubMed] [Google Scholar]
- 21.Samino Y, López D, Guil S, de León P, Del Val M. An endogenous HIV envelope-derived peptide without the terminal NH +3 group anchor is physiologically presented by major histocompatibility complex class I molecules. J. Biol. Chem. 2004;279:1151–1160. doi: 10.1074/jbc.M305343200. [DOI] [PubMed] [Google Scholar]
- 22.Samino Y, López D, Guil S, Saveanu L, van Endert PM, Del Val M. A long N-terminal-extended nested set of abundant and antigenic major histocompatibility complex class I natural ligands from HIV envelope protein. J. Biol. Chem. 2006;281:6358–6365. doi: 10.1074/jbc.M512263200. [DOI] [PubMed] [Google Scholar]
- 23.Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 24.Zhu H, Fearnhead HO, Cohen GM. An ICE-like protease is a common mediator of apoptosis induced by diverse stimuli in human monocytic THP.1 cells. FEBS Lett. 1995;374:303–308. doi: 10.1016/0014-5793(95)01116-v. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Hernandez FJ, Ortiz MA, Bayon Y, Piedrafita FJ. Z-FA-fmk inhibits effector caspases but not initiator caspases 8 and 10, and demonstrates that novel anticancer retinoid-related molecules induce apoptosis via the intrinsic pathway. Mol. Cancer Ther. 2003;2:255–263. [PubMed] [Google Scholar]
- 26.Skribanek Z, Mezo G, Mák M, Hudecz F. Mass spectrometric and chemical stability of the Asp-Pro bond in herpes simplex virus epitope peptides compared with X-Pro bonds of related sequences. J. Pept. Sci. 2002;8:398–406. doi: 10.1002/psc.395. [DOI] [PubMed] [Google Scholar]
- 27.Del Val M, Hengel H, Häcker H, Hartlaub U, Ruppert T, Lucin P, Koszinowski UH. Cytomegalovirus prevents antigen presentation by blocking the transport of peptide-loaded major histocompatibility complex class I molecules into the medial-Golgi compartment. J. Exp. Med. 1992;176:729–738. doi: 10.1084/jem.176.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M, Beg AA. Induction of necrotic-like cell death by tumor necrosis factor alpha and caspase inhibitors: novel mechanism for killing virus-infected cells. J. Virol. 2000;74:7470–7477. doi: 10.1128/jvi.74.16.7470-7477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dobbelstein M, Shenk T. Protection against apoptosis by the vaccinia virus SPI-2 (B13R) gene product. J. Virol. 1996;70:6479–6485. doi: 10.1128/jvi.70.9.6479-6485.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pang B, Neijssen J, Qiao X, Janssen L, Janssen H, Lippuner C, Neefjes J. Direct antigen presentation and gap junction mediated cross-presentation during apoptosis. J. Immunol. 2009;183:1083–1090. doi: 10.4049/jimmunol.0900861. [DOI] [PubMed] [Google Scholar]
- 31.Knuehl C, Spee P, Ruppert T, Kuckelkorn U, Henklein P, Neefjes J, Kloetzel PM. The murine cytomegalovirus pp89 immunodominant H-2Ld epitope is generated and translocated into the endoplasmic reticulum as an 11-mer precursor peptide. J. Immunol. 2001;167:1515–1521. doi: 10.4049/jimmunol.167.3.1515. [DOI] [PubMed] [Google Scholar]
- 32.Wei ML, Cresswell P. HLA-A2 molecules in an antigen-processing mutant cell contain signal sequence-derived peptides. Nature. 1992;356:443–446. doi: 10.1038/356443a0. [DOI] [PubMed] [Google Scholar]
- 33.Henderson RA, Michel H, Sakaguchi K, Shabanowitz J, Appella E, Hunt DF, Engelhard VH. HLA-A2.1-associated peptides from a mutant cell line: a second pathway of antigen presentation. Science. 1992;255:1264–1266. doi: 10.1126/science.1546329. [DOI] [PubMed] [Google Scholar]
- 34.Gil-Torregrosa BC, Castaño AR, López D, Del Val M. Generation of MHC class I peptide antigens by protein processing in the secretory route by furin. Traffic. 2000;1:641–651. doi: 10.1034/j.1600-0854.2000.010808.x. [DOI] [PubMed] [Google Scholar]
- 35.Gil-Torregrosa BC, Castaño AR, Del Val M. Major histocompatibility complex class I viral antigen processing in the secretory pathway defined by the trans-Golgi network protease furin. J. Exp. Med. 1998;188:1105–1116. doi: 10.1084/jem.188.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tiwari N, Garbi N, Reinheckel T, Moldenhauer G, Hämmerling GJ, Momburg F. A transporter associated with antigen-processing independent vacuolar pathway for the MHC class I-mediated presentation of endogenous transmembrane proteins. J. Immunol. 2007;178:7932–7942. doi: 10.4049/jimmunol.178.12.7932. [DOI] [PubMed] [Google Scholar]
- 37.Hammond SA, Johnson RP, Kalams SA, Walker BD, Takiguchi M, Safrit JT, Koup RA, Siliciano RF. An epitope-selective, transporter associated with antigen presentation (TAP)-1/2-independent pathway and a more general TAP-1/2-dependent antigen-processing pathway allow recognition of the HIV-1 envelope glycoprotein by CD8+ CTL. J. Immunol. 1995;154:6140–6156. [PubMed] [Google Scholar]
- 38.Snyder HL, Bacík I, Bennink JR, Kearns G, Behrens TW, Bächi T, Orlowski M, Yewdell JW. Two novel routes of transporter associated with antigen processing (TAP)-independent major histocompatibility complex class I antigen processing. J. Exp. Med. 1997;186:1087–1098. doi: 10.1084/jem.186.7.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.York IA, Bhutani N, Zendzian S, Goldberg AL, Rock KL. Tripeptidyl peptidase II is the major peptidase needed to trim long antigenic precursors, but is not required for most MHC class I antigen presentation. J. Immunol. 2006;177:1434–1443. doi: 10.4049/jimmunol.177.3.1434. [DOI] [PubMed] [Google Scholar]
- 40.Antón LC, Snyder HL, Bennink JR, Vinitsky A, Orlowski M, Porgador A, Yewdell JW. Dissociation of proteasomal degradation of biosynthesized viral proteins from generation of MHC class I-associated antigenic peptides. J. Immunol. 1998;160:4859–4868. [PubMed] [Google Scholar]
- 41.Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Jänicke RU, Sohn D, Totzke G, Schulze-Osthoff K. Caspase-10 in mouse or not? Science. 2006;312:1874. doi: 10.1126/science.312.5782.1874a. [DOI] [PubMed] [Google Scholar]
- 43.Blake NW, Kettle S, Law KM, Gould K, Bastin J, Townsend ARM, Smith GL. Vaccinia virus serpins B13R and B22R do not inhibit antigen presentation to class I-restricted cytotoxic T lymphocytes. J. Gen. Virol. 1995;76:2393–2398. doi: 10.1099/0022-1317-76-9-2393. [DOI] [PubMed] [Google Scholar]
- 44.Müllbacher A, Wallich R, Moyer RW, Simon MM. Poxvirus-encoded serpins do not prevent cytolytic T cell-mediated recovery from primary infections. J. Immunol. 1999;162:7315–7321. [PubMed] [Google Scholar]
- 45.Rawson PM, Molette C, Videtta M, Altieri L, Franceschini D, Donato T, Finocchi L, Propato A, Paroli M, Meloni F, et al. Cross-presentation of caspase-cleaved apoptotic self antigens in HIV infection. Nat. Med. 2007;13:1431–1439. doi: 10.1038/nm1679. [DOI] [PubMed] [Google Scholar]
- 46.Johnstone C, Del Val M. Traffic of proteins and peptides across membranes for immunosurveillance by CD8 T lymphocytes: a topological challenge. Traffic. 2007;8:1486–1494. doi: 10.1111/j.1600-0854.2007.00635.x. [DOI] [PubMed] [Google Scholar]
- 47.Shen L, Rock KL. Priming of T cells by exogenous antigen cross-presented on MHC class I molecules. Curr. Opin. Immunol. 2006;18:85–91. doi: 10.1016/j.coi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Lin XY, Choi MS, Porter AG. Expression analysis of the human caspase-1 subfamily reveals specific regulation of the CASP5 gene by lipo-polysaccharide and interferon-γ. J. Biol. Chem. 2000;275:39920–39926. doi: 10.1074/jbc.M007255200. [DOI] [PubMed] [Google Scholar]
- 49.Legrand FA, Verardi PH, Jones LA, Chan KS, Peng Y, Yilma TD. Induction of potent humoral and cell-mediated immune responses by attenuated vaccinia virus vectors with deleted serpin genes. J. Virol. 2004;78:2770–2779. doi: 10.1128/JVI.78.6.2770-2779.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kettle S, Blake NW, Law KM, Smith GL. Vaccinia virus serpins B13R (SPI-2) and B22R (SPI-1) encode M(r) 38.5 and 40K, intracellular poly-peptides that do not affect virus virulence in a murine intranasal model. Virology. 1995;206:136–147. doi: 10.1016/s0042-6822(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 51.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 2009;9:353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]