Abstract
Background
Ethylene is one of the most used chemical monomers derived from non-renewable sources and we are investigating the possibility of producing it in yeast via the ethylene forming enzyme (EFE) from Pseudomonas syringae. To enable engineering strategies to improve the enzyme, it is necessary to identify the regions and amino acid residues involved in ethylene formation.
Results
We identified the open reading frame for the EFE homolog in Penicillium digitatum and also showed its capability of mediating ethylene production in yeast. The sequence of the EFE homologs from P.digitatum and P. syringae was compared to that of the non-functional EFE-homolog from Penicillium chrysogenum and ten amino acids were found to correlate with ethylene production. Several of these amino acid residues were found to be important for ethylene production via point mutations in P. syringae EFE. The EFE homolog from P. chrysogenum was engineered at 10 amino acid residues to mimic the P. syringae EFE, but this did not confer ethylene producing capability.
Furthermore, we predicted the structure of EFE by homology to known structures of 2-oxoglutarate/Fe(II) dependent dioxygenases. Three of the amino acids correlating with ethylene production are located in the predicted 2-oxoglutarate binding domain. A protein domain specific for the EFE-class was shown to be essential for activity. Based on the structure and alanine substitutions, it is likely that amino acids (H189, D191 and H268) are responsible for binding the Fe(II) ligand.
Conclusion
We provide further insight into the structure and function of the ethylene forming (EFE) - subclass of 2-oxoglutarate/Fe(II) dependent dioxygenases. We conclude that residues in addition to the 10 identified positions implicated in ethylene production by sequence comparison, are important for determining ethylene formation. We also demonstrate the use of an alternative EFE gene. The data from this study will provide the basis for directed protein engineering to enhance the ethylene production capability and properties of EFE.
Background
Ethylene (C2H4) is a compound of considerable interest: First, it is an important plant hormone with strong effects on plant physiology and secondly, it is produced in high amounts for use in chemical synthesis (e.g. plastics). Understanding the enzymatic production of ethylene therefore has a dual biotechnological interest, both from the aspect of understanding and possibly using the effect on plants by microorganisms producing it, or by creating microorganisms which can convert biomass into ethylene for use in industry. In nature, ethylene is synthesized by three pathways, (1) the path from S-adenosylmethionine (ACC-pathway) is used in plants, (2) the KMBA pathway is used by several microorganisms and (3) a 2-oxoglutarate dependent pathway is used by several Pseudomonas syringae species and by Penicillium digitatum[1,2]. Pathway 3 has been shown to mediate the highest ethylene production, both naturally [2] and when expressed in heterologous hosts such as yeast [3,4]. This route is therefore the most promising for biotechnological production of ethylene [4], especially since expression of the plant system yielded very low ethylene production [5].
The biosynthesis of ethylene via the 2-oxoglutarate dependent ethylene forming enzyme (EFE) mainly from Pseudomonas syringae species but also from Penicillium digitatum, has been intensively studied, resulting in a proposed “dual-circuit” enzyme mechanism [6]. The overall stoichiometry of this reaction is that 2-oxoglutarate, oxygen and arginine are converted to carbon dioxide, ethylene, succinate, 1-pyrroline-5-carboxylic acid (P5C) and guanidine. However, this stoichiometry is the sum of two separate reactions: One (reaction 1) in which 2-oxoglutarate + oxygen produces ethylene and carbon dioxide, and another reaction (reaction 2) in which 2-oxoglutarate, arginine and oxygen is converted to succinate, P5C, carbon dioxide and guanidine.
Due to this duality in function the classification of the EFE is somewhat complex, it belongs to the 2-oxoglutarate/L-arginine monooxygenase/decarboxylase (succinate-forming) family (EC 1.14.11.34) which is also termed the Fe(II)/α-ketoglutarate-dependent hydroxylase family [7]. The hydroxylases are a large and rather heterogenous group of enzymes which in general employ 2-oxoglutarate and oxygen to hydroxylate a co-substrate. This co-substrate can be a wide range of molecules, e.g. proteins, nucleotides, lipids or other small chemicals, for review see [7], in the case of EFE the co-substrate is arginine. However, due to the ethylene forming reaction in which arginine is merely a co-factor rather than co-substrate, the EFE can also be classified as belonging to 2-oxoglutarate/Fe(II) dependent dioxygenases (EC 1.13.12.19) [6].
The aim of this study was to identify regions and amino acid residues of importance for ethylene production of EFE. We have achieved this through comparison of two ethylene producing homologs and one close homolog which does not produce ethylene. Protein structure was predicted and will, in combination with the sequence comparison, serve as a foundation for directed protein engineering to enhance the ethylene production capability of EFE.
Results and discussion
Cloning EFE from Penicillium digitatum
The ethylene forming activity of the 2-oxoglutarate dependent route is mediated by a single enzyme termed EFE, which has been biochemically characterized from isolates of P. syringae and from P. digitatum[8]. The EFE from P. syringae pv phaseolicola is especially well studied and has been shown to mediate ethylene formation when expressed in S. cerevisiae, E. coli, T. reesii and P. putida[9-12]. Unfortunately, the gene sequence of the enzyme from P. digitatum was not known, apart from the N-terminal 25 amino acids, characterized from a purified enzyme [8]. The recently published genome sequence of P. digitatum[13] allowed us to find a protein sequence with high homology to the P. syringae EFE. However, the previously published N-terminal was only partially found in this sequence and we therefore inspected the upstream codons in the DNA-sequence. This revealed that the predicted start codon is most likely not correct. When using the first alternative start codon, which is located 147 basepairs upstream, a similar sequence to the published N-terminal was found, preceded by a stretch of amino acids predicted by the MitoProtII-software (http://ihg.gsf.de/ihg/mitoprot.html) [14] to encode a mitochondrial signal peptide (Figure 1A). The probability for mitochondrial localization was 0.9491, with a predicted cleavage site after amino acid 33 (Bold/Italic in Figure 1A). This fits well, but not perfectly, with the N-terminal identified previously [8], indicating either that the protein is further processed after cleavage or that the predicted site of cleavage is inaccurate. In further support of our change in start codon choice for the P. digitatum EFE, a highly similar protein to the protein from P. digitatum is found in Penicillium chrysogenum (accession number: XP_002562422). The P. chrysogenum protein is predicted to use a start codon at a homologous position to that which we predict in P. digitatum. It is therefore likely that the P. digitatum EFE is a mitochondrially localized protein. This is somewhat surprising in view of the fact that P. syringae EFE was found to be inactive when expressed in mitochondria of S. cerevisiae[10]. Furthermore, there was a discrepancy in the first five amino acids of the P. digitatum EFE homolog and the sequence of the purified enzyme (Figure 1B). We do not know if this reflects a technical issue of the amino acid sequencing (in which the determination of arginine, proline and threonine can be difficult) or whether it reflects the fact that the sequences are derived from two different isolates of P. digitatum. This issue, together with the fact that sequence homology does not necessarily imply equal function, made it necessary to investigate whether the EFE-homologs from Penicillium species can mediate production of ethylene. To this end we cloned and expressed the EFE - homologs from P. syringae, P. digitatum and P. chrysogenum in S. cerevisiae. The EFE-genes from Penicillium were expressed without the putative mitochondrial targeting signal. When grown in batch cultures, both the P. syringae and P. digitatum enzymes mediated ethylene production, although the P. digitatum enzyme mediated only 68% of the ethylene production seen for the P. syringae enzyme (75 ± 14 vs 111 ± 12 μg/gDW and hr, respectively; n = 2 for P. digitatum and n = 3 for P. syringae). The enzyme from P. chrysogenum produced no ethylene, despite its strong sequence similarity to the P. digitatum enzyme (data not shown).
Figure 1.

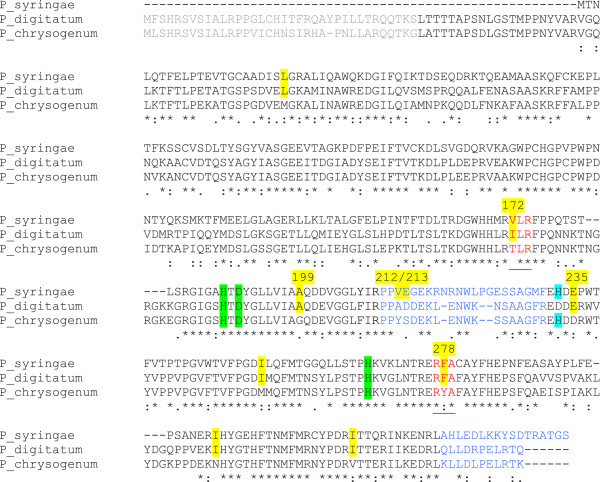
Sequence of the EFE-protein from P. digitatum. (A) Suggested revision of protein sequence of EFE from P. digitatum, isolate PHI26 (accession: EKV19239). In yellow shading is the present start codon of the protein EKV19239. Underlined sequence is translated from the 147 bp preceding the start codon. The sequence in red was not found in the purified EFE from P.digitatum. (B) Alignment of the sequences of P. digitatum and P. chrysogenum with the published N-terminal sequence of purified EFE from P. digitatum (indicated as: Nagahana et al.) [8]. The red X in the N-terminal sequencing is an R by homology, which fits well with the trouble of this amino acid in the chemistry of this method.
Sequence based identification of amino acids important for ethylene formation
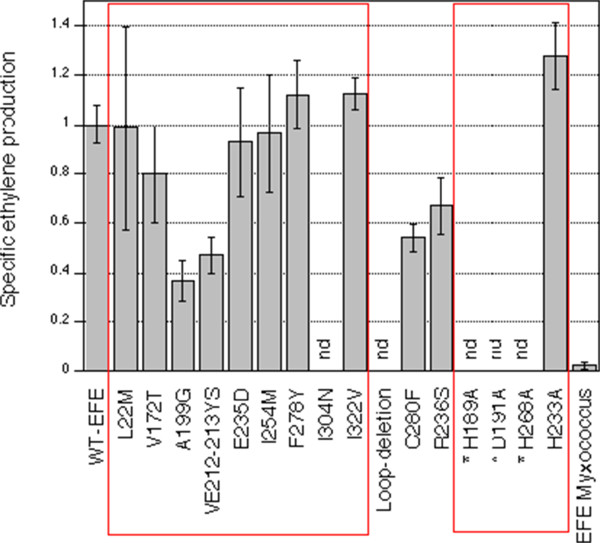
Aligning of the sequences from the two ethylene producing homologs of EFE and the ethylene production negative homolog from P. chrysogenum, using ClustalOmega [15], subsequently allowed us to assign amino acids correlating with ethylene forming ability of the enzyme. Ten residues came out as promising targets, i.e. being conserved in P. syringae and P. digitatum but non-conserved in P. chrysogenum (Figure 2, yellow shading). In order to test the hypothesis that amino acids at these ten positions would be important for ethylene production, we made point mutations on all ten positions, i.e. L22M, V172T, A199G, V212Y/E213S, E235D, I254M, F278Y, I304N and I322V, thereby changing the amino acid to those found in the ethylene production negative EFE-homolog from P. chrysogenum. The A199G, I304N and V212Y/E213S mutants all showed a clear reduction of ethylene production, while L22M, V172T, E235D, I254M, F278Y and I322V did not cause a significant change in ethylene production (Figure 3).
Figure 2.
ClustalOmega-alignment of the amino acid sequences of EFE-homologs from P. syringae, P. digitatum and P. chrysogenum. Amino acids in yellow shading correlate with ethylene production. Amino acids in blue are not included in the predicted structural model. Green shaded amino acids are predicted to bind the Fe(II), the blue shaded histidines were previously suggested to bind Fe(II). Amino acids in red are predicted to bind 2-oxoglutarate and correspond to the EFE-site I [6]. Putative mitochondrial targeting sequences are marked in grey.
Figure 3.
Specific ethylene production of strains with plasmids containing mutations in EFE from P. syringae. The production of the WT-EFE was set to 1. Standard deviation from at least 3 independent cultures. "nd" stands for not detectable. The large red rectangle indicates mutants in the 10 amino-acids correlating with ethylene production while the small red rectangle indicates mutations in amino acids putatively involved in iron binding.
C280 in P. syringae is close to the predicted 2-oxoglutarate binding motif (see below) and it is unusual in being a cystein. In most EFE homologs, including both the Penicillium sequences and the Myxococcus enzyme, a phenylalanine is found at the corresponding position, a non conservative substitution. Despite the fact that this amino acid was not implicated as crucial for ethylene production, we tested the effect of a C280F mutant for ethylene production. As can be seen in Figure 3, the mutation led to a reduction of ethylene production.
We also constructed a mutated version of the P. chrysogenum EFE-homolog, in which the 10 amino acids were exchanged for the amino acid found in P. syringae, since we expected that this would restore ethylene production. However, this hypothesis could be discarded since expression of this enzyme did not confer ethylene production (Data not shown). From these experiments we conclude that several of the 10 amino acids are indeed important for ethylene production, but that they are not sufficient in explaining why the two Penicillium enzymes differ in activity.
It is possible that the sequence signatures could be used to predict which EFE-homologs would produce ethylene and which would not. To test this we performed a BLAST search using the P. syringae EFE as a query. 102 hits with an E-value ≤1e-22 were scored. From this list of hits we selected enzymes which we believe will make ethylene, based on the conservation of motifs containing the 10 amino acids correlating with ethylene production. The top of the list consisted of homologs from different species of Pseudomonas, which was not surprising. A protein from Myxococcus stipitatus (YP 007362884.1) was found to contain conserved residues at all ten positions, with a BLAST E-value of 3e-164 and a 63% identity and a 98% query cover. (A ClustalOmega alignement of the M. stipitatus and P. syringae enzymes is provided as a supplement file (Additional file 1)). In comparison, the protein from P. digitatum had an E-value of 1e-122, an identity of 55% and a query cover of 95%. Since Myxococcus is a group of organisms evolutionarily far removed from Pseudomonas, and since no evidence of ethylene production from this class of bacteria exists, we decided to express the enzyme from M. stipitatus in yeast, from identical construct as the ones used for the other EFE-homologs. As can be seen from Figure 3, the M. stipitatus enzyme did indeed produce ethylene, albeit at low amounts. This is to our knowledge the first demonstration of a 2-oxoglutarate dependent EFE from a non-plant-pathogenic organism.
EFE-homologs from different isolates of P. syringae have been examined [16,17]. The most active EFE enzyme was that from the pathovar (pv) phaseolicola. The pathovars cannabina, sesami and glycinea displayed a roughly 50% reduced ethylene production while the enzyme from P. syringae pv pisi showed a 20-fold lower ethylene production. The pathovars cannabina, sesami and glycinea differ in sequence of EFE from that found in pv phaseolicola only in that R236 in phaseolicola is exchanged for an S, which is thus likely to explain the reduced ethylene production of these strains [17]. Also in our hands, an R236S mutation mediated a clear reduction of the ethylene production upon expression in yeast (Figure 3), further corroborating the importance of this site. From examination of the amino acid sequences it was found that the P. syringae pv pisi sequence lacks 13 amino acids on the C-terminal and differs in 29 amino acids over the aligned sequence [17]. 9 out of the 10 amino acid substitutions found to correlate with ethylene production in the comparison of two Penicillium EFE's and the EFE from P. syringae pv phaseolicola were conserved also in the P. syringae pv pisi-enzyme. However, the E235 position was exchanged for a D in the EFE from P. syringae pv pisi, which is the same as that found in the EFE from P. chrysogenum. However, although it is tempting to speculate that the strongly reduced ethylene production from the P. syringae pv pisi enzyme is due to the E235D-mutation, since the E235D mutant of the normal EFE did not show a major effect on ethylene production, it is instead likely that the reason for the low ethylene production is due to a combined effect of the other 29 amino acid substitutions and perhaps lack of the C-terminal.
EFE-structure prediction
The ten amino acids correlating with ethylene production are distributed across the entire protein sequence of EFE. It was therefore of interest to investigate whether they would occur close to each other in the 3D-structure of the protein. However, the structure of EFE has not been determined for any of the three homologs, despite the fact that the protein can be produced at high levels in E.coli[18]. We have succesfully over-expressed EFE in E. coli and have attempted to determine the structure by both NMR and crystallography. However, for NMR it was found that labelling with deuterium causes protein precipitation (data not shown). A batch of highly purified EFE was also subjected to high throughput screening (SARomics, Lund, Sweden), but formation of crystals was not observed under any of the tested conditions. Therefore, to obtain indications of the EFE-structure, we submitted the amino acid sequences of P. syringae, P. digitatum and P. chrysogenum for structure prediction using the Phyre2-server (http://www.sbg.bio.ic.ac.uk/phyre2) [19]. All three sequences gave the same top hit, namely Leucoanthocyanidin dioxygenase from Arabidopsis thaliana (pdb-ID: 1gp6; http://www.uniprot.org/uniprot/Q96-323), henceforth termed LDOX. The structure prediction homology model for the EFE from P. syringae (and P. digitatum and P. chrysogenum) was downloaded and visualized using the Chimera software [20]. All three predicted models are found in the supplementary material with this paper (Additional files 2,3 and 4).
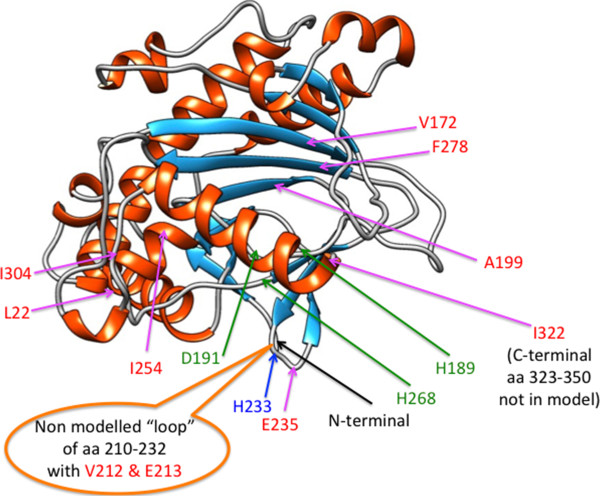
Studies of the LDOX enzyme have indicated that the regions corresponding to positions 172-174 and 277-279 in the P. syringae EFE are involved in binding of the 2-oxoglutarate [21], these regions are shown in red in Figure 2. The involvement of an arginine (at position corresponding to R277 in P. syringae EFE) in binding the C-5 of 2-oxoglutarate has previously been suggested [7]. Interestingly, both of these regions contain one residue correlating with ethylene formation, V172 is isoleucine (I) in P. digitatum and threonine (T) in P. chrysogenum, the former being a conservative replacement and the latter constituting a shift from hydrophobic to hydrophilic group. Similarly, the phenylalanine (F) at position 278 is substituted for a more hydrophilic tyrosine (Y) residue in the ethylene-negative EFE from P. chrysogenum. The predicted structure of P. syringae EFE from Phyre2 results (Figure 4) show that in addition to the previously mentioned V172 and F278, the A199 which also correlates with ethylene production, is located close to the others, in the beta-sheet region thought to be involved in 2-oxoglutarate binding. The A199G mutation is somewhat conservative, but is likely to influence structure. The amino acids 172-174 and 277-279 are predicted to comprise at least part of the 2-oxoglutarate binding site (Site I) as previously proposed in the dual circuit reaction model of EFE [6]. Since the spatial alignement of this site I has been proposed to determine whether the ethylene formation (reaction 1) or the arginine/2-oxoglutarate breakdown (reaction 2) occurs, it was interesting that 3 of the 10 amino acids (V172, F278 and A199) occurred in this domain. However, only the A199G mutant led to a reduction of ethylene production (Figure 3). The I304N is a non-conservative mutation and was also found to display the greatest effect on ethylene production (Figure 3). The strong effect of a I304N-mutation could be due to alteration of the position of the predicted C-terminal alpha-helix which we find it likely will change the structure of the essential iron-binding site (Figure 4).
Figure 4.
Predicted structure of EFE from P. syringae pv phaseolicola, based on the structure of Leucoanthocyanidin dioxygenase from Arabidopsis thaliana generated by the Phyre2 server. Amino acids marked in red correlate with ethylene production, amino acids in green are involved in binding Fe(II), Histidine 233 is marked in blue and the orange callout-bubble corresponds to the non-modelled "loop".
From the structure modelling and sequence alignment it was evident that EFE from Pseudomnoas syringae and from the Penicillium EFE-homologs have an additional stretch of amino acids (corresponding to amino acids 210-232 in P. syringae EFE) which is not present in the non-ethylene forming structural homolog from Arabidopsis (Figures 2 and 4). This region is predicted to be unstructured. However, 2 positions in this non-modelled "loop" correlate with ethylene production, i.e. position 212 is a small hydrophobic residue (valine (V) or an alanine (A)) and position 213 is an acidic amino acid (aspartate (D) or glutamate (E)) in the EFE homologs mediating ethylene production. In contrast, the EFE from P. chrysogenum has a tyrosine (Y) followed by a serine (S), both polar residues, at the corresponding positions. The function and structure of this "loop" is at present not known, but deletion of the corresponding amino acids from the P. syringae enzyme leads to loss of function ("loop deletion", Figure 3). Furthermore, a VE212-213YS mutation displayed a reduced ethylene production (Figure 3).
From the predicted structure it is easy to envision that the non-conservative mutations in the 2-oxoglutarate binding site would affect ethylene production. The other substitutions would be predicted to mediate a more subtle change in the enzyme structure, possibly leading to a "stretching" of the active site previously suggested to favour the ethylene forming reaction [6]. However, the Phyre2-based structure prediction of the normal syringae EFE and a sequence with 10 substitutions to the amino acids found in the chrysogenum EFE showed no major difference.
Iron binding site of EFE
Previously, based on point mutations of histidines to glutamines in EFE from P. syringae[22], it was suggested that H189 and H233 would be the main iron binding residues. However, the histidine 233 is not conserved in P. digitatum, where it is an aspartate, making it unlikely that H233 would have this role. Furthermore, we find that a H233A mutation is fully active (Figure 3). This is in strong contrast to the previous finding that a H233Q mutant is inactive for ethylene production [22], but we cannot at present provide an explanation for this. Also, from the structural and sequence homology to LDOX and other members of this enzyme class, we believe that histidines 189 and 268 together with aspartate 191 are involved in iron binding, which has also been proposed previously [3,7]. It is also evident from the structure prediction (Figure 4) that the histidine 233 would be located on the outer side of the protein, further making its involvement in iron binding unlikely. The fact that alanine substitutions of H189, H268 and D191 all result in complete abolition of ethylene production (Figure 3) strongly supports their role in binding the Fe(II) co-factor.
Conclusions
We have corrected the predicted sequence and cloned the EFE from P. digitatum, demonstrating that it is somewhat less efficient than EFE from P. syringae in terms of productivity upon expression in yeast. An EFE-homolog from M. stipitatus was even less efficient for ethylene production while the EFE-homolog from P. chrysogenum was inactive.
By amino acid homology, we have furthermore identified amino acid residues and domains important for ethylene production, although it is clear that other residues are important for the EFE-activity. We also suggest a structure for the EFE class of 2-oxoglutarate/Fe(II) dependent dioxygenases. We anticipate that a more detailed comparison of several heterologously expressed EFE-enzymes with varying ethylene forming efficiency, in combination with the suggested protein structures, will enable improvement of EFE-function, and will thus be valuable for further development of ethylene production in microorganisms.
Why are homologs of EFE common, while comparatively few of the organisms carrying these homologs seem to make ethylene? It is possible that the role of EFE-homologs in most organisms is to make P5C from arginine by using 2-oxoglutarate as a co-substrate with formation of succinate. This would adhere to the more "normal" reaction mechanism for this enzyme class [7]. The formation of ethylene from the co-substrate 2-oxoglutarate could have been selected as beneficial for certain plant pathogens, such as P. digitatum and P. syringae. EFE seems to be important for the ability of P. syringae to proliferate in plants [23]. However, it is still unclear which role ethylene formation plays in the pathogenesis, e.g. by P. syringae and P. digitatum, since plants also produce ethylene as part of their stress response, including infection by pathogens [24,25]. A detailed understanding of the enzyme EFE itself could therefore contribute to elucidating the role of ethylene production in plant pathogenes.
Methods
Cloning of EFE-homologs
The coding regions for the EFE-homologs from P. digitatum, P. chrysogenum and M. stipitatus were ordered as synthetic, codon-optimized genes (GeneScript, Piscataway, NJ). The P. chrysogenum and M. stipitatus EFE homologs were expressed via integration of a TDH3-promotor regulated construct in the genome (1000 bp downstream of the DAK2 gene) as performed previously [26,27]. The P. digitatum and P. syringae EFE homologs were expressed in a multicopy plasmid (pYX212), for the P. digitatum EFE using the sequence following the predicted mitochondrial targetting sequence (red amino acids in Figure 1), preceded by a methionine-codon.
The plasmid constructs bearing homologs of EFE were transformed into S. cerevisiae strain CEN.PK 113_5D (MATa MAL2-8c SUC2 ura3-52) while the strain CEN.PK 113_7D (MATa MAL2-8cSUC2) was used for the genomic integrations.
Growth of yeast and detection of ethylene
Throughout this study a minimal mineral media [28], with 10 g glucose L−1 was employed. Double amounts of trace metals compared to the original version were used, as well as somewhat higher KH 2 PO 4 and MgSO4 levels, 3.5 g L−1 and 0.75 g L−1 respectively. The vitamin solution was prepared according to the original published version. 7,5 g L−1 of ammonium sulfate was used as nitrogen source throughout the study.
For the experiments in Figure 3, cells were grown in 250 ml Erlenmeyer shake flasks with 50 ml liquid cell culture. Minimal mineral media was inoculated with ON cultures to OD600 of 0.4. The cell cultures were grown at 30°C, 200 rpm until OD600 in-between 1-2 was reached. Custom made rubber caps with syringe needles were used to trap the gases produced inside the flask. The cultures were plugged with caps 30 minutes ahead of sampling. Gas samples were extracted from the shake flasks using 50 ml syringes. The gas samples were subsequently injected to the loop of a HP5890II GC-FID (Hewlett-Packard, USA) with a HayeSep Q 80/100 porous packed column (Grace, USA). The GC column was heated to a constant 65°C, injection temperature 70°C and detection temperature 125°C. The ethylene detected from the bioreactor was compared to an ethylene standard with a known concentration to determine the amount. The samples were analyzed using the ChromNav version (1.14A) software (JASCO, Japan).
For the evaluation of ethylene production from the Penicillium strains, 2 L aerobic batch cultivations were performed in 3.5 L Belach (Belach Bioteknik AB, Skogås, Sweden) bioreactors in the above described minimal media. Overnight shake flask cultures were used to inoculate the bioreactor to a starting OD600 of 0.1. In the bioreactor temperature and air flow were held constant at 30°C and 1 L min−1 respectively. Agitation was maintained around 800 rpm and the pH was kept at 5.0 using 1M NaOH and 1M HCl. Off-gas and dissolved oxygen were monitored using the Bleach gas-analyzer module CP460 and regulatory module CP400 respectively. Ethylene was determined directly in the off gas by connecting the off-gas via a pump with over pressure release to the GC-FID (as described above).
Competing interests
The authors declare no competing interests.
Authors’ contributions
NJ and KP performed all experimental work and participated in the writing of the manuscript. CL and JN wrote the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Alignment of EFE from P. syringae with EFE from M. stipitatus. Showing position of the 10 amino acids correlating with ethylene production.
Protein model of EFE from P. syringae.
Protein model of EFE from P. digitatum.
Protein model of EFE from P. chrysogenum.
Contributor Information
Nina Johansson, Email: nina.johansson@chalmers.se.
Karl O Persson, Email: karperss@chalmers.se.
Christer Larsson, Email: christer.larsson@chalmers.se.
Joakim Norbeck, Email: norbeck@chalmers.se.
Acknowledgements
We would like to thank Anders Pedersen at the Swedish NMR centre for introducing us to the use of Phyre2. The research leading to these results has received funding from the European Community's Seventh Framework Programme (FP7/ 2007-2013) under the grant agreement n°FP7-241566 – BIOCORE.
References
- De Paepe A, Van Der Straeten D. Ethylene biosynthesis and signaling: an overview. Plant Hormone. 2005;72:399–430. doi: 10.1016/S0083-6729(05)72011-2. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Ogawa T, Tanase S. Ethylene production by micro-organisms. Adv Microb Physiol. 1993;35:275–306. doi: 10.1016/s0065-2911(08)60101-0. [DOI] [PubMed] [Google Scholar]
- Eckert C, Xu W, Xiong W, Lynch S, Ungerer J, Tao L, Gill R, Maness PC, Yu J. Ethylene-forming enzyme and bioethylene production. Biotechnol Biofuels. 2014;7(1):33. doi: 10.1186/1754-6834-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson C, Snoep JL, Norbeck J, Albers E. Flux balance analysis for ethylene formation in genetically engineered Saccharomyces cerevisiae. IET Syst Biol. 2011;5(4):245–251. doi: 10.1049/iet-syb.2010.0027. [DOI] [PubMed] [Google Scholar]
- Lu BW, Yu B, Li N. Expression of a novel ethylene-producing bifunctional fusion enzyme in yeast. Botanical Bull Acad Sinica. 1999;40:107–114. [Google Scholar]
- Fukuda H, Ogawa T, Tazaki M, Nagahama K, Fujii T, Tanase S, Morino Y. Two reactions are simultaneously catalyzed by a single enzyme: the arginine-dependent simultaneous formation of two products, ethylene and succinate, from 2-oxoglutarate by an enzyme from Pseudomonas syringae. Biochem Biophys Res Commun. 1992;188(2):483–489. doi: 10.1016/0006-291X(92)91081-Z. [DOI] [PubMed] [Google Scholar]
- Hausinger RP. FeII/alpha-ketoglutarate-dependent hydroxylases and related enzymes. Crit Rev Biochem Mol Biol. 2004;39(1):21–68. doi: 10.1080/10409230490440541. [DOI] [PubMed] [Google Scholar]
- Nagahama K, Ogawa T, Fujii T, Tazaki M, Tanase S, Morino Y, Fukuda H. Purification and properties of an ethylene-forming enzyme from Pseudomonas syringae pv. phaseolicola PK2. J Gen Microbiol. 1991;137(10):2281–2286. doi: 10.1099/00221287-137-10-2281. [DOI] [PubMed] [Google Scholar]
- Chen X, Liang Y, Hua J, Tao L, Qin W, Chen S. Overexpression of bacterial ethylene-forming enzyme gene in Trichoderma reesei enhanced the production of ethylene. Int J Biol Sci. 2010;6(1):96–106. doi: 10.7150/ijbs.6.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkov I, Albers E, Norbeck J, Larsson C. Ethylene production by metabolic engineering of the yeast Saccharomyces cerevisiae. Metab Eng. 2008;10:276–280. doi: 10.1016/j.ymben.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Wang JP, Wu LX, Xu F, Lv J, Jin HJ, Chen SF. Metabolic engineering for ethylene production by inserting the ethylene-forming enzyme gene (efe) at the 16S rDNA sites of Pseudomonas putida KT2440. Bioresour Technol. 2010;101(16):6404–6409. doi: 10.1016/j.biortech.2010.03.030. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Ogawa T, Ishihara K, Fujii T, Nagahama K, Omata T, Inoue Y, Tanase S, Morino Y. Molecular cloning in Escherichia coli, expression, and nucleotide sequence of the gene for the ethylene-forming enzyme of Pseudomonas syringae pv. phaseolicola PK2. Biochem Biophys Res Commun. 1992;188(2):826–832. doi: 10.1016/0006-291X(92)91131-9. [DOI] [PubMed] [Google Scholar]
- Marcet Houben M, Ballester AR, De La Fuente B, Harries E, Marcos JF, Gonzalez Candelas L, Gabaldon T. Genome sequence of the necrotrophic fungus Penicillium digitatum, the main postharvest pathogen of citrus. BMC Genomics. 2012;13:646. doi: 10.1186/1471-2164-13-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claros MG, Vincens P. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem. 1996;241(3):779–786. doi: 10.1111/j.1432-1033.1996.00779.x. [DOI] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, Thompson JD, Higgins DG. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingart H, Volksch B. Ethylene production by pseudomonas syringae pathovars in vitro and in planta. Appl Environ Microbiol. 1997;63(1):156–161. doi: 10.1128/aem.63.1.156-161.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingart H, Volksch B, Ullrich MS. Comparison of ethylene production by pseudomonas syringae and ralstonia solanacearum. Phytopathology. 1999;89(5):360–365. doi: 10.1094/PHYTO.1999.89.5.360. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Matsuoka M, Inoue Y, Tanase S, Ogawa T, Fukuda H. Overexpression and In Vitro Reconstitution of the Ethylene-Forming Enzyme from Pseudomonas syringae. J Ferment Bioeng. 1995;79(3):205–211. doi: 10.1016/0922-338X(95)90604-X. [DOI] [Google Scholar]
- Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc. 2009;4(3):363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Wilmouth RC, Turnbull JJ, Welford RW, Clifton IJ, Prescott AG, Schofield CJ. Structure and mechanism of anthocyanidin synthase from Arabidopsis thaliana. Structure. 2002;10(1):93–103. doi: 10.1016/S0969-2126(01)00695-5. [DOI] [PubMed] [Google Scholar]
- Nagahama K, Yoshino K, Matsuoka M, Tanase S, Ogawa T, Fukuda H. Site-directed mutagenesis of histidine residues in the ethylene-forming enzyme from pseudomonas syringae. J Ferment Bioeng. 1998;85(3):255–258. doi: 10.1016/S0922-338X(97)85671-1. [DOI] [Google Scholar]
- Weingart H, Ullrich H, Geider K, Volksch B. The role of ethylene production in virulence of pseudomonas syringae pvs. Glycinea and phaseolicola. Phytopathology. 2001;91(5):511–518. doi: 10.1094/PHYTO.2001.91.5.511. [DOI] [PubMed] [Google Scholar]
- Abeles FB, Morgan PW, Saltweit ME. Ethylene in Plant Biology. 2. San Diego: Academic Press; 1992. [Google Scholar]
- Mattoo AK, Suttle JC. The Plant Hormone Ethylene. Boca Raton, FL: CRC Press; 1991. [Google Scholar]
- Ghiaci P, Norbeck J, Larsson C. Physiological adaptations of Saccharomyces cerevisiae evolved for improved butanol tolerance. Biotechnol Biofuels. 2013;6(1):101. doi: 10.1186/1754-6834-6-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson N, Quehl P, Norbeck J, Larsson C. Identification of factors for improved ethylene production via the ethylene forming enzyme in chemostat cultures of Saccharomyces cerevisiae. Microb Cell Factories. 2013;12:89. doi: 10.1186/1475-2859-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verduyn C, Postma E, Scheffers WA, Van Dijken JP. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast. 1992;8(7):501–517. doi: 10.1002/yea.320080703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignment of EFE from P. syringae with EFE from M. stipitatus. Showing position of the 10 amino acids correlating with ethylene production.
Protein model of EFE from P. syringae.
Protein model of EFE from P. digitatum.
Protein model of EFE from P. chrysogenum.