Abstract
Accumulating evidence has shown a strong relationship between Alzheimer’s disease (AD), cerebral amyloid angiopathy (CAA), and cerebrovascular disease. Cognitive impairment in AD patients can result from cortical microinfarcts associated with CAA, as well as the synaptic and neuronal disturbances caused by cerebral accumulations of β-amyloid (Aβ) and tau proteins. The pathophysiology of AD may lead to a toxic chain of events consisting of Aβ overproduction, impaired Aβ clearance, and brain ischemia. Insufficient removal of Aβ leads to development of CAA and plays a crucial role in sporadic AD cases, implicating promotion of Aβ clearance as an important therapeutic strategy. Aβ is mainly eliminated by three mechanisms: (1) enzymatic/glial degradation, (2) transcytotic delivery, and (3) perivascular drainage (3-“d” mechanisms). Enzymatic degradation may be facilitated by activation of Aβ-degrading enzymes such as neprilysin, angiotensin-converting enzyme, and insulin-degrading enzyme. Transcytotic delivery can be promoted by inhibition of the receptor for advanced glycation end products (RAGE), which mediates transcytotic influx of circulating Aβ into brain. Successful use of the RAGE inhibitor TTP488 in Phase II testing has led to a Phase III clinical trial for AD patients. The perivascular drainage system seems to be driven by motive force generated by cerebral arterial pulsations, suggesting that vasoactive drugs can facilitate Aβ clearance. One of the drugs promoting this system is cilostazol, a selective inhibitor of type 3 phosphodiesterase. The clearance of fluorescent soluble Aβ tracers was significantly enhanced in cilostazol-treated CAA model mice. Given that the balance between Aβ synthesis and clearance determines brain Aβ accumulation, and that Aβ is cleared by several pathways stated above, multi-drugs combination therapy could provide a mainstream cure for sporadic AD.
Keywords: Alzheimer’s disease, cerebral amyloid angiopathy, treatment, perivascular drainage, cilostazol
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia in the elderly. AD is pathologically characterized by β-amyloid (Aβ) plaques within the brain parenchyma and Aβ accumulation in blood vessels (cerebral amyloid angiopathy; CAA), as well as by the formation of neurofibrillary tangles and neurodegeneration (Duyckaerts et al., 2009). AD was not previously thought to be closely linked to cerebrovascular disease (CVD), but accumulating lines of evidence have shown a strong relationship between AD and vascular dementia (VaD) (Fotuhi et al., 2009; Kalaria and Ihara, 2013). AD and CVD share common risk factors (Viswanathan et al., 2009; Kalaria et al., 2012), and treatment of vascular risk factors is associated with slower decline in cognitive impairments of AD patients (Deschaintre et al., 2009). The Nun study revealed that CVD plays an important role in determining the presence and severity of the clinical symptoms of AD (Snowdon et al., 1997). Aβ accumulation and other AD changes are also recognized in elderly patients without apparent dementia (Funato et al., 1998; Schneider et al., 2007), which implies a strong relationship between AD neuropathology and the aging processes. Many reports have described that a majority of sporadic dementia patients have a mixture of AD and CVD pathology (Neuropathology Group of Medical Research Council Cognitive Function and Aging Study (MRC CFAS), 2001; Toledo et al., 2013). Hemorrhage, infarctions, and vascular changes are not specific indicators for VaD.
Cerebral amyloid angiopathy often induces lobar hemorrhage and cortical microhemorrhage, which mainly affects the occipital lobe (Charidimou et al., 2012). In addition, imaging technology advances, including 7 T MRI, have identified numerous cortical microinfarcts (CMI), which have been attributed to CAA (Suter et al., 2002; van Veluw et al., 2013; Westover et al., 2013). Cognitive impairment in AD patients may result from hypoperfusion/ischemia and CMIs, as well as synaptic disturbance and neuronal loss caused by Aβ and tau accumulation (Okamoto et al., 2009; Launer et al., 2011; Smith et al., 2012). Small vessel injury is frequent in both AD and VaD. CAA was previously thought to be pathologically different from Binswanger disease, one of the common forms of VaD characterized by arteriolosclerosis and white matter change. However, Binswanger disease and CAA are now often regarded as part of the same spectrum disease; the former labeled type 1 and the latter type 2 small vessel disease (Pantoni, 2010). Both types of arteriopathies make dementia patients vulnerable to hemodynamic fluctuation through impairments in cerebral autoregulation and vascular reactivity (Tanoi et al., 2000; Pimentel-Coelho and Rivest, 2012). Consequently, hypoperfusion induces Aβ overproduction and elimination failure (Zlokovic, 2011; Carare et al., 2013; Elali et al., 2013). Brain ischemia and hypoxia modulates amyloid precursor protein (APP) cleavage enzymes such as β-secretase and γ-secretase, thereby resulting in increased Aβ production (Sun et al., 2006; Guglielmotto et al., 2009; Kitaguchi et al., 2009; Li et al., 2009). Excess Aβ contributes to the impairment of Aβ clearance and CAA (Joachim et al., 1989; Rovelet-Lecrux et al., 2006; Han et al., 2008). Aβ elimination failure could also result from arteriolosclerosis (Weller et al., 2009). Thus, dementia patients with a single simple etiology are scarcely seen, except for juvenile familial AD cases caused by mutations in the APP or presenilin genes, comprising <1% of AD cases (Campion et al., 1999).
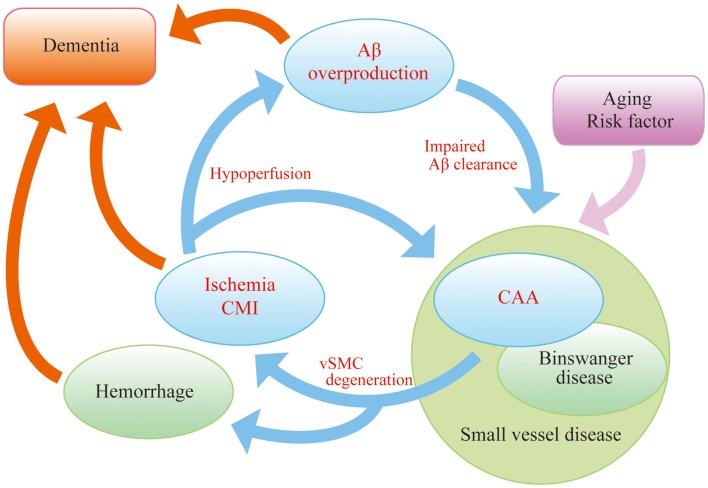
In order to explore novel therapies in AD, we must consider the “AD malignant cycle” (Figure 1). In this scheme, cessation of Aβ overproduction is not sufficient to treat patients with sporadic AD, and important components of the cycle, brain ischemia, and CAA should also be noted. Insufficient Aβ clearance seems to be more crucial than Aβ overproduction in sporadic AD patients (Mawuenyega et al., 2010). Even in familial AD cases, the onset of dementia is often delayed until the fifth or sixth decade, suggesting that the aging-associated failure in clearance also plays a part in the pathogenesis of inherited types of the disease (Weller et al., 2008). Therefore, recent work has focused on the failure of Aβ elimination as the most important therapeutic targets and adopted a “neurovascular” approach as a strategy to tackle AD (Vardy et al., 2005; Deane et al., 2008; Carare et al., 2013).
Figure 1.
“AD malignant cycle”. Aβ overproduction impairs Aβ elimination leading to vascular smooth muscle cells (vSMC) degeneration, cerebral ischemia, and microinfarcts. Ischemia also induces Aβ overproduction. Such vicious circle consists of core pathology in sporadic AD. Note that cessation of Aβ overproduction is not sufficient to sever the cycle.
This review mainly focuses on the mechanisms of Aβ elimination and the drug development to facilitate Aβ clearance. The perivascular lymphatic drainage system, one of the Aβ clearance mechanisms, is closely associated with AD and CAA (Carare et al., 2013). In addition, the possibility of drugs enhancing perivascular drainage as well as future strategies for AD and CAA treatment will be reviewed.
Aβ Clearance: 3-d Mechanism
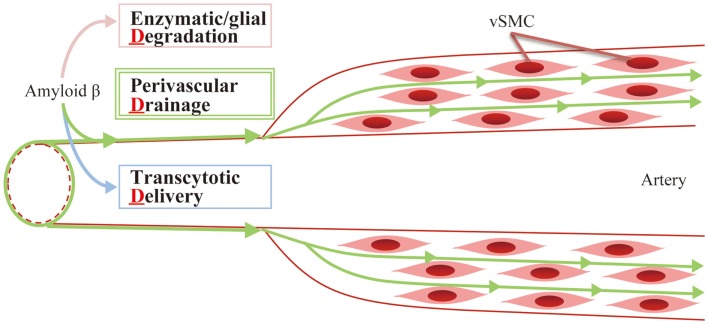
So far, several mechanisms of eliminating Aβ proteins have been identified, which fall into three main categories (3-“d,” Figure 2):
Enzymatic/glial degradation
Transcytotic delivery
Perivascular drainage
Figure 2.
Aβ clearance: 3-d mechanism. Aβ is mainly eliminated by the following mechanisms: (1) enzymatic/glial degradation, (2) transcytotic delivery, and (3) perivascular drainage.
Enzymatic/Glial Degradation
Aβ catabolism is regulated by a series of degrading enzymes as well as glial cells, such as astrocytes and microglia, in the brain parenchyma (Vardy et al., 2005). Among them, neprilysin has received much attention (Iwata et al., 2000). Previous reports have described impaired Aβ degradation in neprilysin-deficient mice (Iwata et al., 2001) and amelioration of Aβ pathology in APP-transgenic mice, when injected with viral vector expressing human neprilysin gene (Marr et al., 2003; Iwata et al., 2004, 2013). Levels of neprilysin mRNA were found to be significantly lower in the hippocampus and middle temporal gyrus of AD brains compared with normal control patients (Yasojima et al., 2001). Decreased neprilysin activity was also associated with CAA (Miners et al., 2006). Thus, the up-regulation of cerebral neprilysin activity could potentially be targeted in the treatment of AD. Indeed, a somatostatin receptor agonist has recently been shown to increase neprilysin activity and decrease Aβ levels in senescence-accelerated mice (Sandoval et al., 2012). However, Meilandt et al. reported that an 11-fold greater neprilysin overexpression failed to reduce pathogenic Aβ oligomers and improve deficits in spatial learning and memory in AD model mice (Meilandt et al., 2009). It was also reported that cerebral Aβ concentration was too low to be degraded by neprilysin (Shibata et al., 2000). The affinity of neprilysin for its physiological substrates (e.g., enkephalins, tachykinins, atrial natriuretic peptide) is within the millimolar range (Hersh and Morihara, 1986), while the levels of Aβ in the brain are normally in the nanomolar range and up to 1 μM/kg even in APP-transgenic mice (Hsiao et al., 1996). Thus, only small concentrations of Aβ will likely bind to neprilysin under physiological and pathological conditions. Many issues should be solved to proceed to drug development of neprilysin activators.
Angiotensin-converting enzyme (ACE) is another Aβ-degrading agent. Captopril, a blood–brain barrier (BBB) penetrating ACE inhibitor, increases Aβ accumulation (Zou et al., 2007), and ACE overexpression in myelomonocytes reduces Aβ deposition in AD model mice (Bernstein et al., 2014). However, brain ACE deficient mice showed no significant alteration in endogenous Aβ levels (Eckman et al., 2006). In addition, two small studies assessing the clinical use of ACE inhibitors, found that they did not deteriorate dementia in AD and amnestic mild cognitive impairment (MCI) patients (Ohrui et al., 2004; Rozzini et al., 2006). Because of such conflicting findings, contributions of ACE to Aβ degradation in the brain per se remain ambiguous.
Insulin-degrading enzyme (IDE) is also known to have Aβ-degrading properties, and hyperinsulinemia in diabetes mellitus competitively inhibits Aβ degradation (Craft and Watson, 2004; Qiu and Folstein, 2006). Indeed, IDE deficient mice demonstrate increased cerebral accumulation of endogenous Aβ with hyperinsulinemia and glucose intolerance (Farris et al., 2003), and IDE overexpression ameliorates Aβ pathology (Leissring et al., 2003), suggesting a link between insulin metabolism and Aβ degradation. However, clinical evidence is still lacking and further studies on the association of IDE with AD pathogenesis may uncover potential treatment targets in AD. Some researchers have labeled AD “type 3 diabetes” (de la Monte and Wands, 2008). If hyperinsulinemia is related to resistance of neuronal cells to insulin, impaired insulin signaling in neurons is thought to lead to neuronal disturbances. A clinical trial assessing intranasal insulin therapy in the treatment of AD and amnestic MCI is anticipated to further elaborate on the relationship between AD and insulin signaling (Craft et al., 2012).
Transcytotic Delivery
The cerebral vasculature originates from large arteries, such as middle cerebral artery and the circle of Willis. These arteries branch into the leptomeningeal arteries, which travel on the surface of the brain in the subarachnoid space. Leptomeningeal arteries further branch into smaller arteries and arterioles consisting of three layers: tunica intima (endothelium), tunica media (smooth muscle cells), and tunica adventitia (mainly collagen fibers). Finally, the terminals of arterioles become capillaries. Capillary lumen and brain parenchyma are separated by the BBB, which prevents the passive exchange of solutes between blood and brain (Iadecola, 2004).
Lipoprotein receptor-related protein-1 (LRP-1), a multifunctional scavenger and signaling receptor, is expressed in neural cells and cerebral microvessels including capillaries, small venules, and arterioles (Wolf et al., 1992; Tooyama et al., 1995; Shibata et al., 2000). LRP-1 has received increasing attention as it mediates transport of Aβ out of the brain across the BBB (Bell and Zlokovic, 2009). Many reports have described the genetic linkage of LRP-1 with AD (Kang et al., 1997; Lambert et al., 1998; Wavrant-DeVrièze et al., 1999) and CAA (Christoforidis et al., 2005). Colocalization of LRP-1 with Aβ was pathologically recognized in senile plaques (Rebeck et al., 1993; Donahue et al., 2006), strengthening the linkage. The relationship is further supported by reduced LRP-1 staining in vessels both in AD patients (Shibata et al., 2000; Donahue et al., 2006) and CAA model mice carrying a vasculotropic Dutch/Iowa mutant form of APP gene (Deane et al., 2004).
Animal experiments have confirmed the importance of transcytosis in the regulation of cerebral Aβ levels. Five hours after microinjection of 125I-labeled Aβ1–40 into the caudate nucleus, 73.8% of labeled tracer had been found in blood across the BBB in young wild-type mice, while 125I-labeled Aβ1–40 in cerebrospinal fluid (CSF) measured 10.7%, and only 15.6% of the dose remained in the brain parenchyma (Shibata et al., 2000). These findings suggest that endothelial transcytosis by LRP-1 and others is probably one of the most prominent pathways in cerebral Aβ clearance, although this study might underestimate other clearance pathways as all the Aβ peptides found in blood are considered to derive from transcytotic delivery.
LRP-1 binds to Aβ directly (Deane et al., 2004), but also binds indirectly via its ligands including α2-macroglobulin, receptor-associated protein, and apolipoprotein E (ApoE) (Narita et al., 1997; Bu, 2009; Kanekiyo and Bu, 2009). ApoE is the main chaperone of Aβ in central nervous system (Holtzman et al., 2012; Zolezzi et al., 2014). To date, three isoforms of ApoE have been described (ε2, ε3, and ε4), and the ApoE ε4 variant is considered to be one of the most relevant risk factors for AD and CAA (Premkumar et al., 1996; Zolezzi et al., 2014). ApoE immunoreactivity is common in amyloid plaques, suggesting that ApoE interacts with Aβ directly in AD brains and could strongly influence the rate of Aβ removal (Namba et al., 1991; Holtzman et al., 2012). Several authors have proposed ApoE as therapeutic target for Aβ clearance (Cramer et al., 2012; Zolezzi and Inestrosa, 2014). Cramer et al. reported that bexarotene, a retinoid X receptor agonist, stimulated the ApoE-dependent Aβ clearance through the actions of liver X receptors and peroxisome proliferator-activated nuclear receptor gamma in AD model mice (Cramer et al., 2012). As a result, cognitive deficits improved with reduced burden of Aβ plaque. However, some conflicting reports have been also documented (Fitz et al., 2013; Price et al., 2013; Tesseur et al., 2013; Veeraraghavalu et al., 2013). Further analysis and experimentation should be performed.
Receptor for advanced glycation end products (RAGE), an immunoglobulin supergene family member, is also known to be a key molecule in Aβ transcytosis (Yan et al., 2012). Strong staining for RAGE has been reported in the vessels of AD patients (Yan et al., 1996; Donahue et al., 2006) and has been shown to mediate influx of circulating Aβ into brain across the BBB (Deane et al., 2003). In addition, RAGE contributes to Aβ-related synaptic dysfunction and microglial activation (Yan et al., 1996; Origlia et al., 2008, 2010). These findings suggest that RAGE could be a therapeutic target in AD and CAA. Indeed, a RAGE inhibitor ameliorated cerebral Aβ burden and normalized cognitive performance in APP-transgenic mice (Deane et al., 2012). The phase III 18 month clinical trial of the RAGE inhibitor TTP488 is being planned for mild to moderate AD patients (The U.S. National Institutes of Health, 2014); positive results in phase II testing have been reported (Burstein et al., 2014).
Perivascular Drainage
The central nervous system is devoid of conventional lymphatic vessels, unlike other organs containing networks of lymphatic vessels, which process various substances, such as wastes, fluid, proteins, and cells from tissues to lymph nodes. However, the lymphatic perivascular drainage system in the brain performs the main function assigned to systemic lymphatic vessels. Analysis of the lymphatic perivascular drainage system dates back as far as the nineteenth century, where it was shown that Indian ink injected into cisterna magna drained to the cervical lymph nodes (Schwalbe, 1869; Weller et al., 2010).
The detail of perivascular drainage system has been examined mainly by intracranial injection of various tracers, including 125I-labeled albumin (Bradbury et al., 1981; Szentistványi et al., 1984; Yamada et al., 1991), Indian ink (Zhang et al., 1992), and various fluorescent tracers (Carare et al., 2008). Recently, this drainage pathway was also confirmed by multi-photon imaging (Arbel-Ornath et al., 2013).
Fluorescent tracers, injected to the striatum, spread diffusely through the extracellular spaces of the brain parenchyma and enter the walls of blood vessels almost immediately. Confocal microscopy showed tracers colocalize with laminin in the basement membranes of capillary walls. Injected tracers were cleared from the basement membranes in the walls of capillaries and arteries, while some tracers were taken up by smooth muscle cells and perivascular macrophages (Zhang et al., 1992; Carare et al., 2008). Studies using radiolabeled tracers showed that drainage of interstitial fluid (ISF) and solutes continues along tunica media and the tunica adventitia of leptomeningeal and major cerebral arteries, through the base of the skull to the deep cervical lymph nodes (Szentistványi et al., 1984; Weller et al., 2010). Tissue soluble Aβ was detected by enzyme immunoassay in meningeal arteries and intracranial arteries but not in extracranial vessels (Shinkai et al., 1995). The clearance system leading to cervical lymph nodes was confirmed by subsequent injection into the inferior colliculus (Ball et al., 2010). Theoretical models have indicated that arterial pulsations could be the motive force behind ISF and solutes being driven centrifugally from the brain by reflection waves that follow each cardiac pulse wave (Schley et al., 2006).
This drainage route closely corresponds with the distribution of Aβ in the basement membranes of capillary and artery walls in CAA (Weller et al., 1998), which implies that the congestion of drainage pathway is associated with the pathogenesis of CAA. The fact that CAA was accelerated in the brains of immunized AD patients and that the CSF Aβ concentration was decreased both in AD and CAA patients may result from an impaired perivascular drainage pathway (Nicoll et al., 2004; Patton et al., 2006; Verbeek et al., 2009). Consistent with this, perivascular drainage of solutes is impaired in the aging mouse brain and in the presence of CAA (Hawkes et al., 2011). The fact that cerebral Aβ clearance was delayed after photothrombosis within individual vessels or middle cerebral artery occlusion (Garcia-Alloza et al., 2011), and after bilateral common carotid artery stenosis (Okamoto et al., 2012), further supports the notion that brain ischemia and impaired arterial pulsation could be an exacerbation factor of CAA. Consistent with the experimental data is the clinical finding that arterial stiffness, indicated by pulse wave velocity, has been associated with Aβ deposition in the brains of non-demented elderly adults (Hughes et al., 2013). Therefore, vasoactive drugs could have potential in the improvement of lymphatic congestion and facilitation of Aβ clearance in the brain.
Convincing Effects of Phosphodiesterase Inhibitor
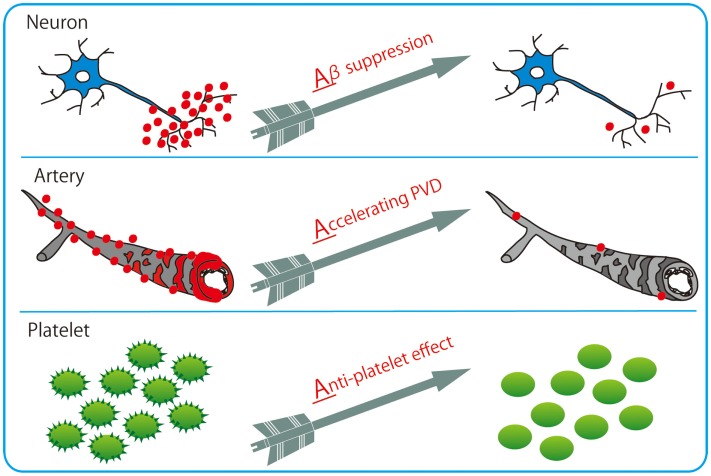
Among varieties of vasoactive drugs, cilostazol, a selective inhibitor of type 3 phosphodiesterase (PDE), is likely to be a promising agent for AD and CAA (Figure 3). PDE3 can hydrolyze both cAMP and cGMP, while increasing cAMP level is a major pharmacological effect of cilostazol (Ikeda, 1999). PDE3 is widely expressed in central nervous system and up-regulated in Aβ-positive vessels, especially in vascular smooth muscle cells (vSMC) (Maki et al., 2014), suggesting the possibility that PDE3 inhibition could be therapeutic for CAA. Cilostazol possesses multiple effects, such as increasing pulse rate (Shinohara et al., 2010) and arterial elasticity (Han et al., 2013), prolonging pulse duration time (Aruna and Naidu, 2007), and dilating cerebral vessels (Tanaka et al., 1989; Birk et al., 2004a,b); such vasoactive actions may promote efficiency of perivascular drainage. In support of this, clearance of fluorescent soluble Aβ tracers is significantly enhanced in cilostazol-treated CAA model mice, thereby resulting in maintenance of vascular integrity, amelioration of Aβ deposits (Figure 4), and prevention of cognitive decline (Maki et al., 2014). Memory-preserving activity of cilostazol has been demonstrated in aged wild-type mice (Yanai et al., 2014) and a rat model of chronic cerebral hypoperfusion (Watanabe et al., 2006), suggesting that cilostazol could be a potential disease modifying therapy of AD and other dementing disorders.
Figure 3.
Cilostazol with 3 Arrows: triple effects toward potential resolution of dementia. Cilostazol, a selective inhibitor of PDE3, has pleiotropic capabilities of suppressing Aβ production in neurons, enhancing Aβ clearance through perivascular drainage system, and inhibiting platelet aggregation (anti-platelet effects).
Figure 4.
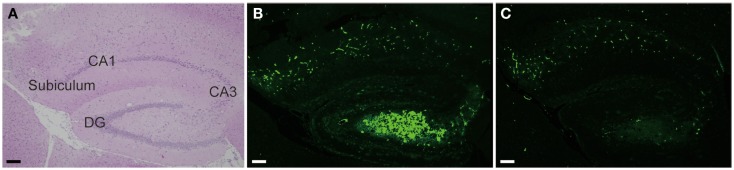
Cilostazol reduced Aβ deposition. Hippocampal images obtained from 17-month-old homozygous Tg-SwDI mice, a model of CAA, treated with vehicle (A,B) or cilostazol (C) for 15 months show that cilostazol treatment reduced levels of Aβ deposits in the hippocampus compared with vehicle treatment. Scale bars indicate 100 μm. (A) HE staining. (B,C) Thioflavin-S staining.
Recently, Nedergaad et al. suggested the “glymphatic pathway,” consisting of para-arterial CSF influx route, para-venous ISF efflux route, and convective bulk fluid flux (Iliff and Nedergaard, 2013; Nedergaard, 2013), as another clearance system in central nervous system. Aβ proteins may be cleared through this perivascular pathway, as well as the perivascular drainage system (Iliff et al., 2012), although the relationship to CAA pathogenesis remains to be clarified as Aβ does not accumulate in the venous system. Cerebral arterial pulsation with a vasoactive agent dobutamine drives perivascular CSF–ISF exchange (Iliff et al., 2013). Further investigation is required to determine whether other vasoactive drugs such as cilostazol could have a potential to facilitate paravascular clearance.
Many inhibitors of other PDE subtypes have been reported to produce cognitive enhancement (Reneerkens et al., 2009) and have been associated with neuronal cAMP signaling activation. Rolipram, a PDE4 inhibitor, reverses the decrease in cAMP regulatory element-binding protein (CREB) phosphorylation, which results in persistent improvement in synaptic function in AD model mice (Gong et al., 2004). Sildenafil, a PDE5 inhibitor, decreases Aβ levels in extracts of cerebral cortex and improves associative and spatial memory in AD model mice (Puzzo et al., 2009). Caffeine is a non-specific PDE inhibitor (Yoshimura, 2005), and its beneficial effects have been clarified in many clinical AD studies (Eskelinen et al., 2009; Eskelinen and Kivipelto, 2010). Caffeine stimulates cAMP-dependent protein kinase A signaling and increases CREB phosphorylation in AD model mice (Arendash et al., 2006; Zeitlin et al., 2011). Protein kinase A activation then suppresses the expression of Aβ-synthesizing enzymes such as β- and γ-secretase, leading to reduced Aβ production (Arendash et al., 2009). Cilostazol also reduces Aβ production in vitro (Lee et al., 2012, 2014; Maki et al., 2014), and suppresses Aβ-induced tauopathy and tau phosphorylation in vitro (Lee et al., 2012, 2014). However, as only a minor fraction of cilostazol passes through BBB (Akiyama et al., 1985), it remains to be elucidated whether these positive effects of cilostazol do occur in AD patients.
Cilostazol has a wide variety of pleiotropic effects capable of inducing neurogenesis (Lee et al., 2009; Tanaka et al., 2010), promoting oligodendrocyte precursor cell differentiation (Miyamoto et al., 2013), preventing lipid peroxidation (Hiramatsu et al., 2010; Kurtoglu et al., 2014), enhancing cholesterol efflux from macrophages (Nakaya et al., 2010), ameliorating insulin resistance (Wada et al., 2013), reducing inflammatory burden (Otsuki et al., 2001; Tsai et al., 2008; Hattori et al., 2009), and improving systemic lymphatic function by inducing proliferation and stabilization of lymphatic endothelial cells (Kimura et al., 2014). In a clinical setting, cilostazol is currently used as an anti-platelet drug (Gotoh et al., 2000; Shinohara et al., 2010), and may be used to prevent ischemic events in patients with CAA. Major manifestations of CAA include lobar hemorrhage and cortical microhemorrhage, as well as CMI. As most CAA patients are elderly (Zhang-Nunes et al., 2006), this necessitates the use of anti-platelet drugs with little risk of hemorrhage (Charidimou et al., 2012). The second Cilostazol Stroke Prevention Study (CSPS2) for patients with cerebral infarction showed that the hemorrhagic stroke was significantly less frequent in cilostazol treatment than with aspirin (Shinohara et al., 2010; Uchiyama et al., 2014). The prevention of cerebral hemorrhage may be explained by reproducible experimental evidence showing that cilostazol inhibits expression of matrix metalloproteinase-9 and protects vascular endothelial cells (Ishiguro et al., 2010; Hase et al., 2012; Kasahara et al., 2012). Endothelial protection with cilostazol mediates increase in nitric oxide, which dilates blood vessels (Oyama et al., 2011), leading to increased cerebral blood flow (Mochizuki et al., 2001; Matsumoto et al., 2011; Sakurai et al., 2013). These results suggest that cilostazol could be suitable for patients with both AD and CVD, the most common type of dementia in the elderly.
Favorable effects have already been described in observational clinical studies, which demonstrated the efficacy of cilostazol in patients with MCI (Taguchi et al., 2013), donepezil-treated patients with clinically probable AD (Arai and Takahashi, 2009; Ihara et al., 2014), and AD with CVD (Sakurai et al., 2013). Randomized placebo-controlled clinical trials are being planned for patients with MCI.
Future Strategy for AD and CAA Treatment
Aging inevitably increases the amount of Aβ burden in the brain, implying a strong relationship between impaired Aβ metabolism and age (Funato et al., 1998). Since heterogeneity and multimorbidity are common in the elderly (Barnett et al., 2012), dementia likely originates from a combination of different pathological substrates. As the population ages, the distribution of AD shifts to older ages in developed countries (Hebert et al., 2013), resulting in an increasing number of demented patients with numerous complicated etiologies. Given that the balance between Aβ synthesis and clearance determines brain Aβ accumulation, and that Aβ is cleared by several pathways stated above, multi-drugs combination therapy would likely be necessary for sporadic AD with complicated etiologies. Combination therapy has already been applied to various diseases, such as hypertension, diabetes mellitus, and malignant tumors. The ultimate goal will be to develop a sovereign remedy of AD, and we hope that the recent rapid advances in drug development will enable us to delay the onset or modify the progression of cognitive impairment with multi-targeting therapies. Further investigation from various viewpoints will thus be essential for the development of novel treatment for AD and CAA.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Akiyama H., Kudo S., Shimizu T. (1985). The absorption, distribution and excretion of a new antithrombotic and vasodilating agent, cilostazol, in rat, rabbit, dog and man. Arzneimittelforschung 35, 1124–1132. [PubMed] [Google Scholar]
- Arai H., Takahashi T. (2009). A combination therapy of donepezil and cilostazol for patients with moderate Alzheimer disease: pilot follow-up study. Am. J. Geriatr. Psychiatry 17, 353–354. 10.1097/JGP.0b013e31819431ea [DOI] [PubMed] [Google Scholar]
- Arbel-Ornath M., Hudry E., Eikermann-Haerter K., Hou S., Gregory J. L., Zhao L., et al. (2013). Interstitial fluid drainage is impaired in ischemic stroke and Alzheimer’s disease mouse models. Acta Neuropathol. 126, 353–364. 10.1007/s00401-013-1145-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendash G. W., Mori T., Cao C., Mamcarz M., Runfeldt M., Dickson A., et al. (2009). Caffeine reverses cognitive impairment and decreases brain amyloid-beta levels in aged Alzheimer’s disease mice. J. Alzheimers Dis. 17, 661–680. 10.3233/JAD-2009-1087 [DOI] [PubMed] [Google Scholar]
- Arendash G. W., Schleif W., Rezai-Zadeh K., Jackson E. K., Zacharia L. C., Cracchiolo J. R., et al. (2006). Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience 142, 941–952. 10.1016/j.neuroscience.2006.07.021 [DOI] [PubMed] [Google Scholar]
- Aruna D., Naidu M. U. (2007). Pharmacodynamic interaction studies of Ginkgo biloba with cilostazol and clopidogrel in healthy human subjects. Br. J. Clin. Pharmacol. 63, 333–338. 10.1111/j.1365-2125.2006.02759.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K. K., Cruz N. F., Mrak R. E., Dienel G. A. (2010). Trafficking of glucose, lactate, and amyloid-beta from the inferior colliculus through perivascular routes. J. Cereb. Blood Flow Metab. 30, 162–176. 10.1038/jcbfm.2009.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett K., Mercer S. W., Norbury M., Watt G., Wyke S., Guthrie B. (2012). Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 380, 37–43. 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- Bell R. D., Zlokovic B. V. (2009). Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 118, 103–113. 10.1007/s00401-009-0522-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein K. E., Koronyo Y., Salumbides B. C., Sheyn J., Pelissier L., Lopes D. H., et al. (2014). Angiotensin-converting enzyme overexpression in myelomonocytes prevents Alzheimer’s-like cognitive decline. J. Clin. Invest. 124, 1000–1012. 10.1172/JCI66541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk S., Edvinsson L., Olesen J., Kruuse C. (2004a). Analysis of the effects of phosphodiesterase type 3 and 4 inhibitors in cerebral arteries. Eur. J. Pharmacol. 489, 93–100. 10.1016/j.ejphar.2004.02.038 [DOI] [PubMed] [Google Scholar]
- Birk S., Kruuse C., Petersen K. A., Jonassen O., Tfelt-Hansen P., Olesen J. (2004b). The phosphodiesterase 3 inhibitor cilostazol dilates large cerebral arteries in humans without affecting regional cerebral blood flow. J. Cereb. Blood Flow Metab. 24, 1352–1358. 10.1097/01.WCB.0000143536.22131.D7 [DOI] [PubMed] [Google Scholar]
- Bradbury M. W., Cserr H. F., Westrop R. J. (1981). Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am. J. Physiol. 240, F329–F336. [DOI] [PubMed] [Google Scholar]
- Bu G. (2009). Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat. Rev. Neurosci. 10, 333–344. 10.1038/nrn2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein A. H., Grimes I., Galasko D. R., Aisen P. S., Sabbagh M., Mjalli A. M. (2014). Effect of TTP488 in patients with mild to moderate Alzheimer’s disease. BMC Neurol. 14:12. 10.1186/1471-2377-14-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campion D., Dumanchin C., Hannequin D., Dubois B., Belliard S., Puel M., et al. (1999). Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. Am. J. Hum. Genet. 65, 664–670. 10.1086/302553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carare R. O., Bernardes-Silva M., Newman T. A., Page A. M., Nicoll J. A., Perry V. H., et al. (2008). Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol. Appl. Neurobiol. 34, 131–144. 10.1111/j.1365-2990.2007.00926.x [DOI] [PubMed] [Google Scholar]
- Carare R. O., Hawkes C. A., Jeffrey M., Kalaria R. N., Weller R. O. (2013). Review: cerebral amyloid angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy. Neuropathol. Appl. Neurobiol. 39, 593–611. 10.1111/nan.12042 [DOI] [PubMed] [Google Scholar]
- Charidimou A., Gang Q., Werring D. J. (2012). Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J. Neurol. Neurosurg. Psychiatr. 83, 124–137. 10.1136/jnnp-2011-301308 [DOI] [PubMed] [Google Scholar]
- Christoforidis M., Schober R., Krohn K. (2005). Genetic-morphologic association study: association between the low density lipoprotein-receptor related protein (LRP) and cerebral amyloid angiopathy. Neuropathol. Appl. Neurobiol. 31, 11–19. 10.1111/j.1365-2990.2004.00614.x [DOI] [PubMed] [Google Scholar]
- Craft S., Baker L. D., Montine T. J., Minoshima S., Watson G. S., Claxton A., et al. (2012). Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch. Neurol. 69, 29–38. 10.1001/archneurol.2011.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S., Watson G. S. (2004). Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 3, 169–178. 10.1016/S1474-4422(04)00681-7 [DOI] [PubMed] [Google Scholar]
- Cramer P. E., Cirrito J. R., Wesson D. W., Lee C. Y., Karlo J. C., Zinn A. E., et al. (2012). ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science 335, 1503–1506. 10.1126/science.1217697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte S. M., Wands J. R. (2008). Alzheimer’s disease is type 3 diabetes-evidence reviewed. J. Diabetes Sci. Technol. 2, 1101–1113. 10.1177/193229680800200619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R., Du Yan S., Submamaryan R. K., Larue B., Jovanovic S., Hogg E., et al. (2003). RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 9, 907–913. 10.1038/nm890 [DOI] [PubMed] [Google Scholar]
- Deane R., Sagare A., Zlokovic B. V. (2008). The role of the cell surface LRP and soluble LRP in blood-brain barrier Abeta clearance in Alzheimer’s disease. Curr. Pharm. Des. 14, 1601–1605. 10.2174/138161208784705487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R., Singh I., Sagare A. P., Bell R. D., Ross N. T., Larue B., et al. (2012). A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Invest. 122, 1377–1392. 10.1172/JCI58642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R., Wu Z., Sagare A., Davis J., Du Yan S., Hamm K., et al. (2004). LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron 43, 333–344. 10.1016/j.neuron.2004.07.017 [DOI] [PubMed] [Google Scholar]
- Deschaintre Y., Richard F., Leys D., Pasquier F. (2009). Treatment of vascular risk factors is associated with slower decline in Alzheimer disease. Neurology 73, 674–680. 10.1212/WNL.0b013e3181b59bf3 [DOI] [PubMed] [Google Scholar]
- Donahue J. E., Flaherty S. L., Johanson C. E., Duncan J. A., Silverberg G. D., Miller M. C., et al. (2006). RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 112, 405–415. 10.1007/s00401-006-0115-3 [DOI] [PubMed] [Google Scholar]
- Duyckaerts C., Delatour B., Potier M. C. (2009). Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 118, 5–36. 10.1007/s00401-009-0532-1 [DOI] [PubMed] [Google Scholar]
- Eckman E. A., Adams S. K., Troendle F. J., Stodola B. A., Kahn M. A., Fauq A. H., et al. (2006). Regulation of steady-state beta-amyloid levels in the brain by neprilysin and endothelin-converting enzyme but not angiotensin-converting enzyme. J. Biol. Chem. 281, 30471–30478. 10.1074/jbc.M605827200 [DOI] [PubMed] [Google Scholar]
- Elali A., Thériault P., Préfontaine P., Rivest S. (2013). Mild chronic cerebral hypoperfusion induces neurovascular dysfunction, triggering peripheral beta-amyloid brain entry and aggregation. Acta Neuropathol Commun 1, 75. 10.1186/2051-5960-1-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen M. H., Kivipelto M. (2010). Caffeine as a protective factor in dementia and Alzheimer’s disease. J. Alzheimers Dis. 20(Suppl. 1), S167–S174. 10.3233/JAD-2010-1404 [DOI] [PubMed] [Google Scholar]
- Eskelinen M. H., Ngandu T., Tuomilehto J., Soininen H., Kivipelto M. (2009). Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J. Alzheimers Dis. 16, 85–91. 10.3233/JAD-2009-0920 [DOI] [PubMed] [Google Scholar]
- Farris W., Mansourian S., Chang Y., Lindsley L., Eckman E. A., Frosch M. P., et al. (2003). Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A 100, 4162–4167. 10.1073/pnas.0230450100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz N. F., Cronican A. A., Lefterov I., Koldamova R. (2013). Comment on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models”. Science 340, 924–c. 10.1126/science.1235809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotuhi M., Hachinski V., Whitehouse P. J. (2009). Changing perspectives regarding late-life dementia. Nat. Rev. Neurol. 5, 649–658. 10.1038/nrneurol.2009.175 [DOI] [PubMed] [Google Scholar]
- Funato H., Yoshimura M., Kusui K., Tamaoka A., Ishikawa K., Ohkoshi N., et al. (1998). Quantitation of amyloid beta-protein (A beta) in the cortex during aging and in Alzheimer’s disease. Am. J. Pathol. 152, 1633–1640. [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alloza M., Gregory J., Kuchibhotla K. V., Fine S., Wei Y., Ayata C., et al. (2011). Cerebrovascular lesions induce transient β-amyloid deposition. Brain 134, 3697–3707. 10.1093/brain/awr300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B., Vitolo O. V., Trinchese F., Liu S., Shelanski M., Arancio O. (2004). Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J. Clin. Invest. 114, 1624–1634. 10.1172/JCI22831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh F., Tohgi H., Hirai S., Terashi A., Fukuuchi Y., Otomo E., et al. (2000). Cilostazol stroke prevention study: a placebo-controlled double-blind trial for secondary prevention of cerebral infarction. J. Stroke Cerebrovasc. Dis. 9, 147–157. 10.1053/jscd.2000.7216 [DOI] [PubMed] [Google Scholar]
- Guglielmotto M., Aragno M., Autelli R., Giliberto L., Novo E., Colombatto S., et al. (2009). The up-regulation of BACE1 mediated by hypoxia and ischemic injury: role of oxidative stress and HIF1alpha. J. Neurochem. 108, 1045–1056. 10.1111/j.1471-4159.2008.05858.x [DOI] [PubMed] [Google Scholar]
- Han B. H., Zhou M. L., Abousaleh F., Brendza R. P., Dietrich H. H., Koenigsknecht-Talboo J., et al. (2008). Cerebrovascular dysfunction in amyloid precursor protein transgenic mice: contribution of soluble and insoluble amyloid-beta peptide, partial restoration via gamma-secretase inhibition. J. Neurosci. 28, 13542–13550. 10.1523/JNEUROSCI.4686-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S. W., Lee S. S., Kim S. H., Lee J. H., Kim G. S., Kim O. J., et al. (2013). Effect of cilostazol in acute lacunar infarction based on pulsatility index of transcranial Doppler (ECLIPse): a multicenter, randomized, double-blind, placebo-controlled trial. Eur. Neurol. 69, 33–40. 10.1159/000338247 [DOI] [PubMed] [Google Scholar]
- Hase Y., Okamoto Y., Fujita Y., Kitamura A., Nakabayashi H., Ito H., et al. (2012). Cilostazol, a phosphodiesterase inhibitor, prevents no-reflow and hemorrhage in mice with focal cerebral ischemia. Exp. Neurol. 233, 523–533. 10.1016/j.expneurol.2011.11.038 [DOI] [PubMed] [Google Scholar]
- Hattori Y., Suzuki K., Tomizawa A., Hirama N., Okayasu T., Hattori S., et al. (2009). Cilostazol inhibits cytokine-induced nuclear factor-kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Cardiovasc. Res. 81, 133–139. 10.1093/cvr/cvn226 [DOI] [PubMed] [Google Scholar]
- Hawkes C. A., Hartig W., Kacza J., Schliebs R., Weller R. O., Nicoll J. A., et al. (2011). Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol. 121, 431–443. 10.1007/s00401-011-0801-7 [DOI] [PubMed] [Google Scholar]
- Hebert L. E., Weuve J., Scherr P. A., Evans D. A. (2013). Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 80, 1778–1783. 10.1212/WNL.0b013e31828726f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh L. B., Morihara K. (1986). Comparison of the subsite specificity of the mammalian neutral endopeptidase 24.11 (enkephalinase) to the bacterial neutral endopeptidase thermolysin. J. Biol. Chem. 261, 6433–6437. [PubMed] [Google Scholar]
- Hiramatsu M., Takiguchi O., Nishiyama A., Mori H. (2010). Cilostazol prevents amyloid beta peptide(25-35)-induced memory impairment and oxidative stress in mice. Br. J. Pharmacol. 161, 1899–1912. 10.1111/j.1476-5381.2010.01014.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman D. M., Herz J., Bu G. (2012). Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med 2, a006312. 10.1101/cshperspect.a006312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., et al. (1996). Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274, 99–102. 10.1126/science.274.5284.99 [DOI] [PubMed] [Google Scholar]
- Hughes T. M., Kuller L. H., Barinas-Mitchell E. J., Mackey R. H., Mcdade E. M., Klunk W. E., et al. (2013). Pulse wave velocity is associated with β-amyloid deposition in the brains of very elderly adults. Neurology 81, 1711–1718. 10.1212/01.wnl.0000435301.64776.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. (2004). Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 5, 347–360. 10.1038/nrn1387 [DOI] [PubMed] [Google Scholar]
- Ihara M., Nishino M., Taguchi A., Yamamoto Y., Hattori Y., Saito S., et al. (2014). Cilostazol add-on therapy in patients with mild dementia receiving donepezil: a retrospective study. PLoS ONE 9:e89516. 10.1371/journal.pone.0089516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y. (1999). Antiplatelet therapy using cilostazol, a specific PDE3 inhibitor. Thromb. Haemost. 82, 435–438 [PubMed] [Google Scholar]
- Iliff J. J., Nedergaard M. (2013). Is there a cerebral lymphatic system? Stroke 44, S93–S95. 10.1161/STROKEAHA.112.678698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff J. J., Wang M., Liao Y., Plogg B. A., Peng W., Gundersen G. A., et al. (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4, 147ra111. 10.1126/scitranslmed.3003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff J. J., Wang M., Zeppenfeld D. M., Venkataraman A., Plog B. A., Liao Y., et al. (2013). Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 33, 18190–18199. 10.1523/JNEUROSCI.1592-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro M., Mishiro K., Fujiwara Y., Chen H., Izuta H., Tsuruma K., et al. (2010). Phosphodiesterase-III inhibitor prevents hemorrhagic transformation induced by focal cerebral ischemia in mice treated with tPA. PLoS ONE 5:e15178. 10.1371/journal.pone.0015178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata N., Mizukami H., Shirotani K., Takaki Y., Muramatsu S., Lu B., et al. (2004). Presynaptic localization of neprilysin contributes to efficient clearance of amyloid-beta peptide in mouse brain. J. Neurosci. 24, 991–998. 10.1523/JNEUROSCI.4792-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata N., Sekiguchi M., Hattori Y., Takahashi A., Asai M., Ji B., et al. (2013). Global brain delivery of neprilysin gene by intravascular administration of AAV vector in mice. Sci. Rep. 3, 1472. 10.1038/srep01472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata N., Tsubuki S., Takaki Y., Shirotani K., Lu B., Gerard N. P., et al. (2001). Metabolic regulation of brain Abeta by neprilysin. Science 292, 1550–1552. 10.1126/science.1059946 [DOI] [PubMed] [Google Scholar]
- Iwata N., Tsubuki S., Takaki Y., Watanabe K., Sekiguchi M., Hosoki E., et al. (2000). Identification of the major Abeta1-42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat. Med. 6, 143–150. 10.1038/72237 [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Mori H., Selkoe D. J. (1989). Amyloid beta-protein deposition in tissues other than brain in Alzheimer’s disease. Nature 341, 226–230. 10.1038/341226a0 [DOI] [PubMed] [Google Scholar]
- Kalaria R. N., Akinyemi R., Ihara M. (2012). Does vascular pathology contribute to Alzheimer changes? J. Neurol. Sci. 322, 141–147. 10.1016/j.jns.2012.07.032 [DOI] [PubMed] [Google Scholar]
- Kalaria R. N., Ihara M. (2013). Dementia: vascular and neurodegenerative pathways-will they meet? Nat. Rev. Neurol. 9, 487–488. 10.1038/nrneurol.2013.164 [DOI] [PubMed] [Google Scholar]
- Kanekiyo T., Bu G. (2009). Receptor-associated protein interacts with amyloid-beta peptide and promotes its cellular uptake. J. Biol. Chem. 284, 33352–33359. 10.1074/jbc.M109.015032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D. E., Saitoh T., Chen X., Xia Y., Masliah E., Hansen L. A., et al. (1997). Genetic association of the low-density lipoprotein receptor-related protein gene (LRP), an apolipoprotein E receptor, with late-onset Alzheimer’s disease. Neurology 49, 56–61. 10.1212/WNL.49.1.56 [DOI] [PubMed] [Google Scholar]
- Kasahara Y., Nakagomi T., Matsuyama T., Stern D., Taguchi A. (2012). Cilostazol reduces the risk of hemorrhagic infarction after administration of tissue-type plasminogen activator in a murine stroke model. Stroke 43, 499–506. 10.1161/STROKEAHA.111.635417 [DOI] [PubMed] [Google Scholar]
- Kimura T., Hamazaki T. S., Sugaya M., Fukuda S., Chan T., Tamura-Nakano M., et al. (2014). Cilostazol improves lymphatic function by inducing proliferation and stabilization of lymphatic endothelial cells. J. Dermatol. Sci. 74, 150–158. 10.1016/j.jdermsci.2014.01.001 [DOI] [PubMed] [Google Scholar]
- Kitaguchi H., Tomimoto H., Ihara M., Shibata M., Uemura K., Kalaria R. N., et al. (2009). Chronic cerebral hypoperfusion accelerates amyloid beta deposition in APPSwInd transgenic mice. Brain Res. 1294, 202–210. 10.1016/j.brainres.2009.07.078 [DOI] [PubMed] [Google Scholar]
- Kurtoglu T., Basoglu H., Ozkisacik E. A., Cetin N. K., Tataroglu C., Yenisey C., et al. (2014). Effects of cilostazol on oxidative stress, systemic cytokine release, and spinal cord injury in a rat model of transient aortic occlusion. Ann. Vasc. Surg. 28, 479–488. 10.1016/j.avsg.2013.08.005 [DOI] [PubMed] [Google Scholar]
- Lambert J. C., Wavrant-De Vrièze F., Amouyel P., Chartier-Harlin M. C. (1998). Association at LRP gene locus with sporadic late-onset Alzheimer’s disease. Lancet 351, 1787–1788. 10.1016/S0140-6736(05)78749-3 [DOI] [PubMed] [Google Scholar]
- Launer L. J., Hughes T. M., White L. R. (2011). Microinfarcts, brain atrophy, and cognitive function: the Honolulu Asia aging study autopsy study. Ann. Neurol. 70, 774–780. 10.1002/ana.22520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. R., Park S. Y., Kim H. Y., Shin H. K., Lee W. S., Rhim B. Y., et al. (2012). Protection by cilostazol against amyloid-β(1-40)-induced suppression of viability and neurite elongation through activation of CK2α in HT22 mouse hippocampal cells. J. Neurosci. Res. 90, 1566–1576. 10.1002/jnr.23037 [DOI] [PubMed] [Google Scholar]
- Lee H. R., Shin H. K., Park S. Y., Kim H. Y., Lee W. S., Rhim B. Y., et al. (2014). Attenuation of β-amyloid-induced tauopathy via activation of CK2α/SIRT1: targeting for cilostazol. J. Neurosci. Res. 92, 206–217. 10.1002/jnr.23310 [DOI] [PubMed] [Google Scholar]
- Lee J. H., Shin H. K., Park S. Y., Kim C. D., Lee W. S., Hong K. W. (2009). Cilostazol preserves CA1 hippocampus and enhances generation of immature neuroblasts in dentate gyrus after transient forebrain ischemia in rats. Exp. Neurol. 215, 87–94. 10.1016/j.expneurol.2008.09.013 [DOI] [PubMed] [Google Scholar]
- Leissring M. A., Farris W., Chang A. Y., Walsh D. M., Wu X., Sun X., et al. (2003). Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 40, 1087–1093. 10.1016/S0896-6273(03)00787-6 [DOI] [PubMed] [Google Scholar]
- Li L., Zhang X., Yang D., Luo G., Chen S., Le W. (2009). Hypoxia increases Abeta generation by altering beta- and gamma-cleavage of APP. Neurobiol. Aging 30, 1091–1098. 10.1016/j.neurobiolaging.2007.10.011 [DOI] [PubMed] [Google Scholar]
- Maki T., Okamoto Y., Carare R., Hase Y., Hattori Y., Hawkes C., et al. (2014). Phosphodiesterase III inhibitor promotes drainage of cerebrovascular β-amyloid. Ann Clin Transl Neurol. 1, 519–533. 10.1002/acn3.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr R. A., Rockenstein E., Mukherjee A., Kindy M. S., Hersh L. B., Gage F. H., et al. (2003). Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J. Neurosci. 23, 1992–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S., Shimodozono M., Miyata R., Kawahira K. (2011). Effect of cilostazol administration on cerebral hemodynamics and rehabilitation outcomes in poststroke patients. Int. J. Neurosci. 121, 271–278. 10.3109/00207454.2010.551431 [DOI] [PubMed] [Google Scholar]
- Mawuenyega K. G., Sigurdson W., Ovod V., Munsell L., Kasten T., Morris J. C., et al. (2010). Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 330, 1774. 10.1126/science.1197623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilandt W. J., Cisse M., Ho K., Wu T., Esposito L. A., Scearce-Levie K., et al. (2009). Neprilysin overexpression inhibits plaque formation but fails to reduce pathogenic Abeta oligomers and associated cognitive deficits in human amyloid precursor protein transgenic mice. J. Neurosci. 29, 1977–1986. 10.1523/JNEUROSCI.2984-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miners J. S., Van Helmond Z., Chalmers K., Wilcock G., Love S., Kehoe P. G. (2006). Decreased expression and activity of neprilysin in Alzheimer disease are associated with cerebral amyloid angiopathy. J. Neuropathol. Exp. Neurol. 65, 1012–1021. 10.1097/01.jnen.0000240463.87886.9a [DOI] [PubMed] [Google Scholar]
- Miyamoto N., Pham L. D., Hayakawa K., Matsuzaki T., Seo J. H., Magnain C., et al. (2013). Age-related decline in oligodendrogenesis retards white matter repair in mice. Stroke 44, 2573–2578. 10.1161/STROKEAHA.113.001530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki Y., Oishi M., Mizutani T. (2001). Effects of cilostazol on cerebral blood flow, P300, and serum lipid levels in the chronic stage of cerebral infarction. J. Stroke Cerebrovasc. Dis. 10, 63–69. 10.1053/jscd.2001.24657 [DOI] [PubMed] [Google Scholar]
- Nakaya K., Ayaori M., Uto-Kondo H., Hisada T., Ogura M., Yakushiji E., et al. (2010). Cilostazol enhances macrophage reverse cholesterol transport in vitro and in vivo. Atherosclerosis 213, 135–141. 10.1016/j.atherosclerosis.2010.07.024 [DOI] [PubMed] [Google Scholar]
- Namba Y., Tomonaga M., Kawasaki H., Otomo E., Ikeda K. (1991). Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 541, 163–166. 10.1016/0006-8993(91)91092-F [DOI] [PubMed] [Google Scholar]
- Narita M., Holtzman D. M., Schwartz A. L., Bu G. (1997). Alpha2-macroglobulin complexes with and mediates the endocytosis of beta-amyloid peptide via cell surface low-density lipoprotein receptor-related protein. J. Neurochem. 69, 1904–1911. 10.1046/j.1471-4159.1997.69051904.x [DOI] [PubMed] [Google Scholar]
- Nedergaard M. (2013). Neuroscience. Garbage truck of the brain. Science 340, 1529–1530. 10.1126/science.1240514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuropathology Group of Medical Research Council Cognitive Function and Aging Study (MRC CFAS). (2001). Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet 357, 169–175. 10.1016/S0140-6736(00)03589-3 [DOI] [PubMed] [Google Scholar]
- Nicoll J. A., Yamada M., Frackowiak J., Mazur-Kolecka B., Weller R. O. (2004). Cerebral amyloid angiopathy plays a direct role in the pathogenesis of Alzheimer’s disease. Pro-CAA position statement. Neurobiol. Aging 25, 589–597. 10.1016/j.neurobiolaging.2004.02.003 [DOI] [PubMed] [Google Scholar]
- Ohrui T., Tomita N., Sato-Nakagawa T., Matsui T., Maruyama M., Niwa K., et al. (2004). Effects of brain-penetrating ACE inhibitors on Alzheimer disease progression. Neurology 63, 1324–1325. 10.1212/01.WNL.0000140705.23869.E9 [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Ihara M., Fujita Y., Ito H., Takahashi R., Tomimoto H. (2009). Cortical microinfarcts in Alzheimer’s disease and subcortical vascular dementia. Neuroreport 20, 990–996. 10.1097/WNR.0b013e32832d2e6a [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Yamamoto T., Kalaria R. N., Senzaki H., Maki T., Hase Y., et al. (2012). Cerebral hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol. 123, 381–394. 10.1007/s00401-011-0925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Origlia N., Bonadonna C., Rosellini A., Leznik E., Arancio O., Yan S. S., et al. (2010). Microglial receptor for advanced glycation end product-dependent signal pathway drives beta-amyloid-induced synaptic depression and long-term depression impairment in entorhinal cortex. J. Neurosci. 30, 11414–11425. 10.1523/JNEUROSCI.2127-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Origlia N., Righi M., Capsoni S., Cattaneo A., Fang F., Stern D. M., et al. (2008). Receptor for advanced glycation end product-dependent activation of p38 mitogen-activated protein kinase contributes to amyloid-beta-mediated cortical synaptic dysfunction. J. Neurosci. 28, 3521–3530. 10.1523/JNEUROSCI.0204-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki M., Saito H., Xu X., Sumitani S., Kouhara H., Kurabayashi M., et al. (2001). Cilostazol represses vascular cell adhesion molecule-1 gene transcription via inhibiting NF-kappaB binding to its recognition sequence. Atherosclerosis 158, 121–128. 10.1016/S0021-9150(01)00431-2 [DOI] [PubMed] [Google Scholar]
- Oyama N., Yagita Y., Kawamura M., Sugiyama Y., Terasaki Y., Omura-Matsuoka E., et al. (2011). Cilostazol, not aspirin, reduces ischemic brain injury via endothelial protection in spontaneously hypertensive rats. Stroke 42, 2571–2577. 10.1161/STROKEAHA.110.609834 [DOI] [PubMed] [Google Scholar]
- Pantoni L. (2010). Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 9, 689–701. 10.1016/S1474-4422(10)70104-6 [DOI] [PubMed] [Google Scholar]
- Patton R. L., Kalback W. M., Esh C. L., Kokjohn T. A., Van Vickle G. D., Luehrs D. C., et al. (2006). Amyloid-beta peptide remnants in an-1792-immunized Alzheimer’s disease patients: a biochemical analysis. Am. J. Pathol. 169, 1048–1063. 10.2353/ajpath.2006.060269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel-Coelho P. M., Rivest S. (2012). The early contribution of cerebrovascular factors to the pathogenesis of Alzheimer’s disease. Eur. J. Neurosci. 35, 1917–1937. 10.1111/j.1460-9568.2012.08126.x [DOI] [PubMed] [Google Scholar]
- Premkumar D. R., Cohen D. L., Hedera P., Friedland R. P., Kalaria R. N. (1996). Apolipoprotein E-epsilon4 alleles in cerebral amyloid angiopathy and cerebrovascular pathology associated with Alzheimer’s disease. Am. J. Pathol. 148, 2083–2095. [PMC free article] [PubMed] [Google Scholar]
- Price A. R., Xu G., Siemienski Z. B., Smithson L. A., Borchelt D. R., Golde T. E., et al. (2013). Comment on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models”. Science 340, 924–d. 10.1126/science.1234089 [DOI] [PubMed] [Google Scholar]
- Puzzo D., Staniszewski A., Deng S. X., Privitera L., Leznik E., Liu S., et al. (2009). Phosphodiesterase 5 inhibition improves synaptic function, memory, and amyloid-beta load in an Alzheimer’s disease mouse model. J. Neurosci. 29, 8075–8086. 10.1523/JNEUROSCI.0864-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W. Q., Folstein M. F. (2006). Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer’s disease: review and hypothesis. Neurobiol. Aging 27, 190–198. 10.1016/j.neurobiolaging.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Rebeck G. W., Reiter J. S., Strickland D. K., Hyman B. T. (1993). Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron 11, 575–580. 10.1016/0896-6273(93)90070-8 [DOI] [PubMed] [Google Scholar]
- Reneerkens O. A., Rutten K., Steinbusch H. W., Blokland A., Prickaerts J. (2009). Selective phosphodiesterase inhibitors: a promising target for cognition enhancement. Psychopharmacology (Berl.) 202, 419–443. 10.1007/s00213-008-1273-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovelet-Lecrux A., Hannequin D., Raux G., Le Meur N., Laquerrière A., Vital A., et al. (2006). APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat. Genet. 38, 24–26. 10.1038/ng1718 [DOI] [PubMed] [Google Scholar]
- Rozzini L., Chilovi B. V., Bertoletti E., Conti M., Del Rio I., Trabucchi M., et al. (2006). Angiotensin converting enzyme (ACE) inhibitors modulate the rate of progression of amnestic mild cognitive impairment. Int. J. Geriatr. Psychiatry 21, 550–555. 10.1002/gps.1523 [DOI] [PubMed] [Google Scholar]
- Sakurai H., Hanyu H., Sato T., Kume K., Hirao K., Kanetaka H., et al. (2013). Effects of cilostazol on cognition and regional cerebral blood flow in patients with Alzheimer’s disease and cerebrovascular disease: a pilot study. Geriatr. Gerontol. Int. 13, 90–97. 10.1111/j.1447-0594.2012.00866.x [DOI] [PubMed] [Google Scholar]
- Sandoval K. E., Farr S. A., Banks W. A., Crider A. M., Morley J. E., Witt K. A. (2012). Somatostatin receptor subtype-4 agonist NNC 26-9100 decreases extracellular and intracellular Abeta(1-42) trimers. Eur. J. Pharmacol. 683, 116–124. 10.1016/j.ejphar.2012.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schley D., Carare-Nnadi R., Please C. P., Perry V. H., Weller R. O. (2006). Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J. Theor. Biol. 238, 962–974. 10.1016/j.jtbi.2005.07.005 [DOI] [PubMed] [Google Scholar]
- Schneider J. A., Arvanitakis Z., Bang W., Bennett D. A. (2007). Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69, 2197–2204. 10.1212/01.wnl.0000271090.28148.24 [DOI] [PubMed] [Google Scholar]
- Schwalbe G. (1869). Der arachnoidalraum ein lymphraum und sein zusammenhang mit dem perichoroidalraum. Zentralb Med Wiss 7, 465–467 [Google Scholar]
- Shibata M., Yamada S., Kumar S. R., Calero M., Bading J., Frangione B., et al. (2000). Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J. Clin. Invest. 106, 1489–1499. 10.1172/JCI10498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y., Yoshimura M., Ito Y., Odaka A., Suzuki N., Yanagisawa K., et al. (1995). Amyloid beta-proteins 1-40 and 1-42(43) in the soluble fraction of extra- and intracranial blood vessels. Ann. Neurol. 38, 421–428. 10.1002/ana.410380312 [DOI] [PubMed] [Google Scholar]
- Shinohara Y., Katayama Y., Uchiyama S., Yamaguchi T., Handa S., Matsuoka K., et al. (2010). Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol. 9, 959–968. 10.1016/S1474-4422(10)70198-8 [DOI] [PubMed] [Google Scholar]
- Smith E. E., Schneider J. A., Wardlaw J. M., Greenberg S. M. (2012). Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 11, 272–282. 10.1016/S1474-4422(11)70307-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon D. A., Greiner L. H., Mortimer J. A., Riley K. P., Greiner P. A., Markesbery W. R. (1997). Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA 277, 813–817. [PubMed] [Google Scholar]
- Sun X., He G., Qing H., Zhou W., Dobie F., Cai F., et al. (2006). Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci U S A 103, 18727–18732. 10.1073/pnas.0606298103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter O. C., Sunthorn T., Kraftsik R., Straubel J., Darekar P., Khalili K., et al. (2002). Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke 33, 1986–1992. 10.1161/01.STR.0000024523.82311.77 [DOI] [PubMed] [Google Scholar]
- Szentistványi I., Patlak C. S., Ellis R. A., Cserr H. F. (1984). Drainage of interstitial fluid from different regions of rat brain. Am. J. Physiol. 246, F835–F844. [DOI] [PubMed] [Google Scholar]
- Taguchi A., Takata Y., Ihara M., Kasahara Y., Tsuji M., Nishino M., et al. (2013). Cilostazol improves cognitive function in patients with mild cognitive impairment: a retrospective analysis. Psychogeriatrics 13, 164–169. 10.1111/psyg.12021 [DOI] [PubMed] [Google Scholar]
- Tanaka K., Gotoh F., Fukuuchi Y., Amano T., Uematsu D., Kawamura J., et al. (1989). Effects of a selective inhibitor of cyclic AMP phosphodiesterase on the pial microcirculation in feline cerebral ischemia. Stroke 20, 668–673. 10.1161/01.STR.20.5.668 [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Tanaka R., Liu M., Hattori N., Urabe T. (2010). Cilostazol attenuates ischemic brain injury and enhances neurogenesis in the subventricular zone of adult mice after transient focal cerebral ischemia. Neuroscience 171, 1367–1376. 10.1016/j.neuroscience.2010.10.008 [DOI] [PubMed] [Google Scholar]
- Tanoi Y., Okeda R., Budka H. (2000). Binswanger’s encephalopathy: serial sections and morphometry of the cerebral arteries. Acta Neuropathol. 100, 347–355. 10.1007/s004010000203 [DOI] [PubMed] [Google Scholar]
- Tesseur I., Lo A. C., Roberfroid A., Dietvorst S., Van Broeck B., Borgers M., et al. (2013). Comment on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models”. Science 340, 924–e. 10.1126/science.1233937 [DOI] [PubMed] [Google Scholar]
- The U.S. National Institutes of Health. (2014). Evaluation of the Efficacy and Safety of TTP488 in Patients with Mild Alzheimer’s Disease. Available at: http://clinicaltrial.gov/ct2/show/NCT02080364?term=TTP488&rank=2
- Toledo J. B., Arnold S. E., Raible K., Brettschneider J., Xie S. X., Grossman M., et al. (2013). Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the national Alzheimer’s coordinating centre. Brain 136, 2697–2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooyama I., Kawamata T., Akiyama H., Kimura H., Moestrup S. K., Gliemann J., et al. (1995). Subcellular localization of the low density lipoprotein receptor-related protein (alpha 2-macroglobulin receptor) in human brain. Brain Res. 691, 235–238. 10.1016/0006-8993(95)00735-9 [DOI] [PubMed] [Google Scholar]
- Tsai C. S., Lin F. Y., Chen Y. H., Yang T. L., Wang H. J., Huang G. S., et al. (2008). Cilostazol attenuates MCP-1 and MMP-9 expression in vivo in LPS-administrated balloon-injured rabbit aorta and in vitro in LPS-treated monocytic THP-1 cells. J. Cell. Biochem. 103, 54–66. 10.1002/jcb.21388 [DOI] [PubMed] [Google Scholar]
- Uchiyama S., Shinohara Y., Katayama Y., Yamaguchi T., Handa S., Matsuoka K., et al. (2014). Benefit of cilostazol in patients with high risk of bleeding: subanalysis of cilostazol stroke prevention study 2. Cerebrovasc. Dis. 37, 296–303. 10.1159/000360811 [DOI] [PubMed] [Google Scholar]
- van Veluw S. J., Zwanenburg J. J., Engelen-Lee J., Spliet W. G., Hendrikse J., Luijten P. R., et al. (2013). In vivo detection of cerebral cortical microinfarcts with high-resolution 7T MRI. J. Cereb. Blood Flow Metab. 33, 322–329. 10.1038/jcbfm.2012.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy E. R., Catto A. J., Hooper N. M. (2005). Proteolytic mechanisms in amyloid-beta metabolism: therapeutic implications for Alzheimer’s disease. Trends Mol. Med. 11, 464–472. 10.1016/j.molmed.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Veeraraghavalu K., Zhang C., Miller S., Hefendehl J. K., Rajapaksha T. W., Ulrich J., et al. (2013). Comment on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models”. Science 340, 924–f. 10.1126/science.1235505 [DOI] [PubMed] [Google Scholar]
- Verbeek M. M., Kremer B. P., Rikkert M. O., Van Domburg P. H., Skehan M. E., Greenberg S. M. (2009). Cerebrospinal fluid amyloid beta(40) is decreased in scerebral amyloid angiopathy. Ann. Neurol. 66, 245–249. 10.1002/ana.21694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan A., Rocca W. A., Tzourio C. (2009). Vascular risk factors and dementia: how to move forward? Neurology 72, 368–374. 10.1212/01.wnl.0000341271.90478.8e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T., Onogi Y., Kimura Y., Nakano T., Fusanobori H., Ishii Y., et al. (2013). Cilostazol ameliorates systemic insulin resistance in diabetic db/db mice by suppressing chronic inflammation in adipose tissue via modulation of both adipocyte and macrophage functions. Eur. J. Pharmacol. 707, 120–129. 10.1016/j.ejphar.2013.03.016 [DOI] [PubMed] [Google Scholar]
- Watanabe T., Zhang N., Liu M., Tanaka R., Mizuno Y., Urabe T. (2006). Cilostazol protects against brain white matter damage and cognitive impairment in a rat model of chronic cerebral hypoperfusion. Stroke 37, 1539–1545. 10.1161/01.STR.0000221783.08037.a9 [DOI] [PubMed] [Google Scholar]
- Wavrant-DeVrièze F., Lambert J. C., Stas L., Crook R., Cottel D., Pasquier F., et al. (1999). Association between coding variability in the LRP gene and the risk of late-onset Alzheimer’s disease. Hum. Genet. 104, 432–434. 10.1007/s004390050980 [DOI] [PubMed] [Google Scholar]
- Weller R. O., Djuanda E., Yow H. Y., Carare R. O. (2009). Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 117, 1–14. 10.1007/s00401-008-0457-0 [DOI] [PubMed] [Google Scholar]
- Weller R. O., Galea I., Carare R. O., Minagar A. (2010). Pathophysiology of the lymphatic drainage of the central nervous system: implications for pathogenesis and therapy of multiple sclerosis. Pathophysiology 17, 295–306. 10.1016/j.pathophys.2009.10.007 [DOI] [PubMed] [Google Scholar]
- Weller R. O., Massey A., Newman T. A., Hutchings M., Kuo Y. M., Roher A. E. (1998). Cerebral amyloid angiopathy: amyloid beta accumulates in putative interstitial fluid drainage pathways in Alzheimer’s disease. Am. J. Pathol. 153, 725–733. 10.1016/S0002-9440(10)65616-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller R. O., Subash M., Preston S. D., Mazanti I., Carare R. O. (2008). Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol. 18, 253–266. 10.1111/j.1750-3639.2008.00133.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westover M. B., Bianchi M. T., Yang C., Schneider J. A., Greenberg S. M. (2013). Estimating cerebral microinfarct burden from autopsy samples. Neurology 80, 1365–1369. 10.1212/WNL.0b013e31828c2f52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf B. B., Lopes M. B., Vandenberg S. R., Gonias S. L. (1992). Characterization and immunohistochemical localization of alpha 2-macroglobulin receptor (low-density lipoprotein receptor-related protein) in human brain. Am. J. Pathol. 141, 37–42. [PMC free article] [PubMed] [Google Scholar]
- Yamada S., Depasquale M., Patlak C. S., Cserr H. F. (1991). Albumin outflow into deep cervical lymph from different regions of rabbit brain. Am. J. Physiol. 261, H1197–H1204. [DOI] [PubMed] [Google Scholar]
- Yan S. D., Chen X., Fu J., Chen M., Zhu H., Roher A., et al. (1996). RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature 382, 685–691. 10.1038/382685a0 [DOI] [PubMed] [Google Scholar]
- Yan S. S., Chen D., Yan S., Guo L., Du H., Chen J. X. (2012). RAGE is a key cellular target for Abeta-induced perturbation in Alzheimer’s disease. Front Biosci (Schol Ed) 4:240–250. 10.2741/265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai S., Semba Y., Ito H., Endo S. (2014). Cilostazol improves hippocampus-dependent long-term memory in mice. Psychopharmacology (Berl.) 231, 2681–2693. 10.1007/s00213-014-3442-4 [DOI] [PubMed] [Google Scholar]
- Yasojima K., Akiyama H., Mcgeer E. G., Mcgeer P. L. (2001). Reduced neprilysin in high plaque areas of Alzheimer brain: a possible relationship to deficient degradation of beta-amyloid peptide. Neurosci. Lett. 297, 97–100. 10.1016/S0304-3940(00)01675-X [DOI] [PubMed] [Google Scholar]
- Yoshimura H. (2005). The potential of caffeine for functional modification from cortical synapses to neuron networks in the brain. Curr. Neuropharmacol. 3, 309–316. 10.2174/157015905774322543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin R., Patel S., Burgess S., Arendash G. W., Echeverria V. (2011). Caffeine induces beneficial changes in PKA signaling and JNK and ERK activities in the striatum and cortex of Alzheimer’s transgenic mice. Brain Res. 1417, 127–136. 10.1016/j.brainres.2011.08.036 [DOI] [PubMed] [Google Scholar]
- Zhang E. T., Richards H. K., Kida S., Weller R. O. (1992). Directional and compartmentalised drainage of interstitial fluid and cerebrospinal fluid from the rat brain. Acta Neuropathol. 83, 233–239. 10.1007/BF00296784 [DOI] [PubMed] [Google Scholar]
- Zhang-Nunes S. X., Maat-Schieman M. L., Van Duinen S. G., Roos R. A., Frosch M. P., Greenberg S. M. (2006). The cerebral beta-amyloid angiopathies: hereditary and sporadic. Brain Pathol. 16, 30–39. 10.1111/j.1750-3639.2006.tb00559.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic B. V. (2011). Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 12, 723–738. 10.1038/nrn3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolezzi J. M., Bastías-Candia S., Santos M. J., Inestrosa N. C. (2014). Alzheimer’s disease: relevant molecular and physiopathological events affecting amyloid-β brain balance and the putative role of PPARs. Front. Aging Neurosci. 6:176. 10.3389/fnagi.2014.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolezzi J. M., Inestrosa N. C. (2014). Brain metabolite clearance: impact on Alzheimer’s disease. Metab. Brain Dis. 29, 553–561. 10.1007/s11011-014-9527-2 [DOI] [PubMed] [Google Scholar]
- Zou K., Yamaguchi H., Akatsu H., Sakamoto T., Ko M., Mizoguchi K., et al. (2007). Angiotensin-converting enzyme converts amyloid beta-protein 1-42 (Abeta(1-42)) to Abeta(1-40), and its inhibition enhances brain Abeta deposition. J. Neurosci. 27, 8628–8635. 10.1523/JNEUROSCI.1549-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]