Abstract
Nanosilica is one of the most widely used nanomaterials across the world. However, their assessment data on the occupational exposure to nanoparticles is insufficient. The present study performed an exposure monitoring in workplace environments where synthetic powders are prepared using fumed nanosilica. Furthermore, after it was observed during exposure monitoring that nanoparticles were emitted through leakage in a vacuum cleaner (even with a HEPA-filter installed in it), the properties of the leaked nanoparticles were also investigated. Workers were exposed to high-concentration nanosilica emitted into the air while pouring it into a container or transferring the container. The use of a vacuum cleaner with a leak (caused by an inadequate sealing) was found to be the origin of nanosilica dispersion in the indoor air. While the particle size of the nanosilica that emitted into the air (during the handling of nanosilica by a worker) was mostly over 100 nm or several microns (µm) due to the coagulation of particles, the size of nanosilica that leaked out of vacuum cleaner was almost similar to the primary size (mode diameter 11.5 nm). Analysis of area samples resulted in 20% (60% in terms of peak concentration) less than the analysis of the personals sample.
Keywords: Exposure assessment, Nanomaterials, Silica nanoparticle, Aerosol characteristic, Vacuum cleaner leakage
Introduction
Nanosilica is one of the most widely used nanomaterials across the world. In South Korea, 34,136 tons of 93 types of nanomaterials are manufactured or imported annually, and silica occupies the second place with 9,408 tons1).
Silica exists in two phases: crystalline, and amorphous. Crystalline silica is especially notorious for inducing lung cancer and is categorized into Group 1 by International Agency for Research on Cancer. Nanosilica is mainly produced through pyrolysis or polymerization processes in the amorphous phase. The adverse health effect of amorphous nanosilica has not been clearly demonstrated to date, nor has any occupational exposure limit (OEL) been attributed to it yet2).
Besides toxicity evaluation, exposure monitoring plays a very important role in risk assessment for nanosilica. Therefore, it is necessary to establish a scale to systematically quantify the measurement results of exposure monitoring in various environments3).
On the other hand, the present study determined that a vacuum cleaner emits nanoparticles even with a high efficiency particulate absorption (HEPA)-filter installed in it. Studies that performed indoor environment assessments confirmed vacuum cleaners as the origin of nanoparticles that are found in indoor air4). However, HEPA-filtered vacuum cleaners are recommended as an appropriate cleaning method in workplace environments where nanomaterials are handled5). Therefore, the objectives of this study are first, to perform monitoring of the nanoparticle exposure during the manufacturing process of synthetic powders, and second, to deliver a case study of vacuum cleaners causing nanoparticle dispersion in the workplace indoor air, and third, to assess the properties of nanoparticles during the vacuum cleaner operation.
Subjects and Methods
Nano-specific and non-nano specific information of the workplace
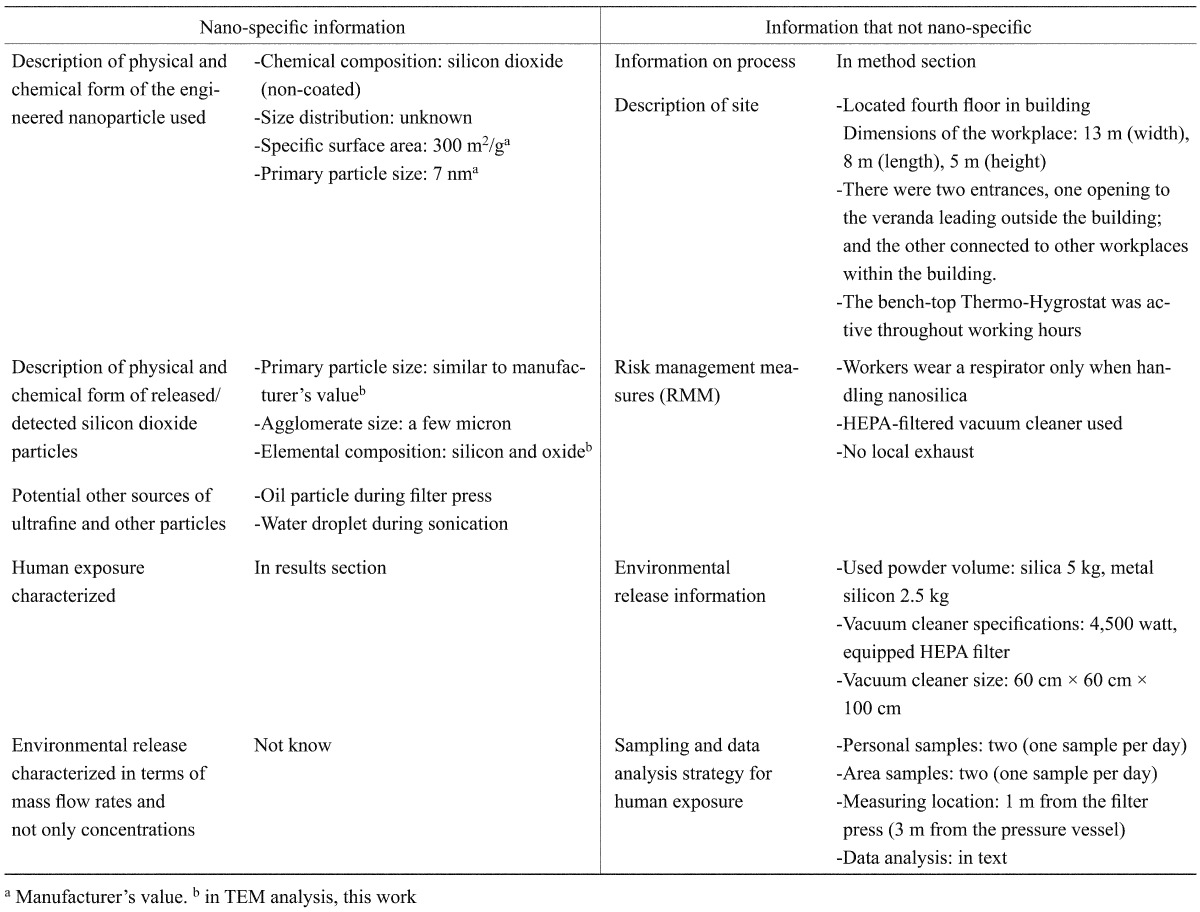
As there are numerous factors that affect the results of assessment of nanoparticle exposure, efforts were made to establish a precise mapping of information associated with such factors. To this end, nano specific information and non nano-specific information presented by Clark et al. (2012)6) was investigated in this study, and the results are shown in Table 1 .
Table 1. Nano-specific and not nano-specific information in this study.
Operation Processes (in order of performance)
1) Mixing and stirring: First, the nanosilica is poured (10 kg paper bag) into a container on the floor. Then, the bag is weighed using a balance. Finally, the nanosilica is poured into a stirrer (includes walking for 5 m to the stirrer with the container). In order to prevent the nanosilica from being emitted to the air while pouring, a vacuum cleaner is switched on and placed close to the inlets of the container and the stirrer. Subsequently, micro-sized metal silicon and liquid is poured into the stirrer, which is then put into operation. The nanosilica that was scattered on the floor was suctioned into a vacuum cleaner. (1 h)
2) Drainage: The thoroughly stirred sludge is automatically transferred to the Filter-Press process through a delivery hose, and the pressing begins to enable liquid drainage. The press is powered by a hydraulic motor. (Automatic operation, 80 min)
3) Sonication: The filter is separated after the Filter-Press process and undergoes sonication in the water bath for reuse. (1 h)
4) Drying: In order to collect the cakes, the surface of the filter is scratched and a stainless steel tray is used for collection. The cakes are then stored in a drying machine in a different workplace. (20 m from the entrance)
5) Storage: On the next day, the tray is carried to the workplace and the dry powder is spooned into a plastic bag. (20 min) (Fig. 1).
Fig. 1.
A. Suctioning of nanosilica while pouring nanosilica into the container. B. Suctioning of nanosilica while pouring nanosilica into the stirrer. C. Collect the cakes from the filter press.
Sampling strategy
1) Area sample measurement using SMPS and NSAM
We used a scanning mobility particle sizer (SMPS; Model 3910; TSI Inc.), which measures the particle number concentration of 13 sets of particles per unit volume of ambient air (10−300 nm), and a nanoparticle surface area monitor (NSAM; Model 9000; TSI Inc.), which measures the surface area concentration of particles ranging from 10 to 1,000 nm. The measurements were made continuously in one-minute intervals for 24 h (from 10:00 a.m. on the 1st day, to 10:25 a.m. on the 2nd day). The measuring location was about 1 m from the filter press (about 3 m from the pressure vessel).
2) Personal sampling and area sampling using DiSCmini
DiSCmini (DM) (Matter Aerosol, Wohlen, Switzerland; Fierz et al., 2011)7), an instrument recently developed for nanoparticle monitoring and personal sampling, was used to measure the number concentration, surface area concentration, and mean particle size of the airborne particles ranging from 10 to 700 nm at one-minute intervals. The area sampling was also conducted using another DM. The battery of the DM used for personal sampling was charged during a lunch break. The personal sampling was measured for two days in keeping with the working schedule, and the area sampling was continuously measured until the next day.
3) Assessment of the background concentration
As nanoscale particles are found both in workplace environments and in ambient air, it is necessary to assess the background concentration to identify the concentration of nanoparticles generated in the process of handling nanomaterials7,8,9,10,11). In this study, the concentration measured before the handling of nanomaterials was used as the background concentration, since the particle concentration is at its lowest level of the day at this time. Additionally, air in other workplaces within the building and outside the building was also analyzed considering the fact that outside air infiltrates through two doors.
4) Filter-based sampling and FTIR analysis
Gravimetric analysis was performed by means of a polycarbonate filter (37 mm, pore 0.4 µm; SKC Inc., USA). Particulate sampling was performed at a rate of 5 LPM under a lowered detection limit in order to facilitate the gravimetric analysis. Similar to the DM analysis case, area samples were analyzed and measured for two days as per the working schedule. Because gravimetric analysis is used to determine the weights of all particles, FTIR analysis was performed to determine the weight of silica particles. After the gravimetric analysis, a fourier-transform infrared-spectroscopy (Nicolet 6700, Thermo fisher scientific Inc., USA) analysis was performed on the filter via low-temperature pyrolysis and KBr-pellet preparation in accordance with the NIOSH method 760212). The FTIR spectra confirmed an amorphous silica-specific peak of 800 cm−1. The limit of detection (LOD) of silica was 0.010 mg/sample.
5) TEM analysis
The shape and chemical composition of airborne nanoparticles was investigated using a transmission electron microscopy (TEM, H-7650, Hitachi Inc., Japan) analysis whereby the airborne nanoparticles were trapped in a TEM grid with a portable electric dust collector, ESPnano (model 100, Dash connector technology Inc., USA). For each task (pouring nanosilica into the container, operating vacuum cleaner, operating filter press, ultrasonic cleaning, spoon-transferring synthetic powder), samples was collected for 20 to 50 s at the operator’s breathing zone. The chemical composition was verified by an energy-dispersive X-ray spectroscopy (EDS).
Vacuum cleaner analysis
It was confirmed that nanoparticles are generated in the air path of the vacuum cleaner (age: manufactured 2010; type: dry vacuums; model: CLEON, made in Korea; power: 4.5HP), and a follow-up assessment of the vacuum cleaner was performed on a later date after the exposure monitoring.
This paper reports the experiments conducted to measure the particle mode size and number concentration of nanoparticles emitted from the vacuum cleaner using SMPS. The experiments were conducted in the same situation and the working conditions (silica amount, work method, work time, etc.). The measurement was conducted at a distance of 1 m from the exhaust vent of the vacuum cleaner.
The measurement was conducted sequentially by measuring the size and concentration of nanoparticles:
-
(1)
Present in the workplace environment (background concentration).
-
(2)
When the vacuum cleaner is ON (i.e., the air in the workspace is inhaled) in order to see whether the nanoparticles are generated by the vacuum cleaners’ motor.
-
(3)
Scattered in air when silica powder is poured into a container when the vacuum cleaner is OFF.
-
(4)
Scattered in air when silica powder is poured into a container when the vacuum cleaner is ON.
During the process of exposure monitoring, it was found that the concentration of nanoparticles fell abruptly when the bench-top HEPA-filtered thermo-hygrostat was turned on. Therefore, the vacuum cleaner analysis was performed with the Thermo-Hygrostat off.
Analysis of other factors
Temperature and humidity were measured during working hours (except during the night), and the wind speeds at the inlet and outlet of vacuum cleaner was measured using a hot-wire anemometer (model AS-202A, Graywolf sensing solutions Inc.).
Data analysis
A distribution of the DM measurement results on a log probability plot resulted in linearity. Thus, the geometric mean concentration and the GSD were calculated after the logarithmic conversion.
Results
1) Aerosol characterization by task
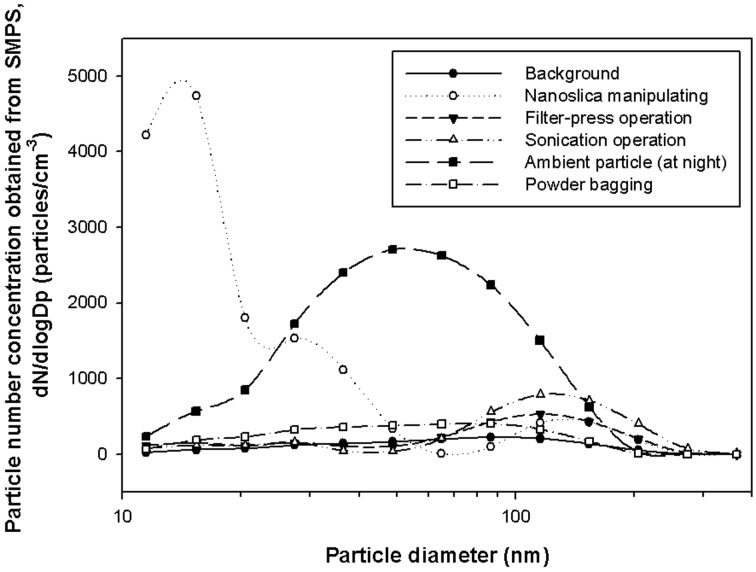
Particle number concentration increased compared to the background number concentration during the handling of nanosilica, operation of the filter press, ultrasonic cleaning of the filter, and during the night, when the Thermo-Hygrostat was off. The particle size by SMPS measurement, 62 nm, observed in the background concentration estimation prior to operation was abruptly reduced to 24 nm when simultaneously handling nanosilica and operating the vacuum cleaner, and abruptly increased to 76, 93 nm during the operation of the filter press and ultrasonic cleaner, respectively (Fig. 2, Table 2). While the DM measurements showed similar particle sizes to those of the SMPS measurements in each work process, the results of particle number concentration measurements were slightly different, with DM results showing somewhat higher general values than SMPS values (range: −12%–+81, average: +33%) and even as much as 270% higher, when the number concentration of nanoparticles rose to the peak value during the handling of silica (Table 2). Figure 3 depicts the SMPS-measured particle size distribution in each work process, wherein a multi-modal distribution (15, 30, and 150 nm) is shown during the handling of silica. This is attributable to the agglomeration of silica particles in air whereas primary silica particles measure 7 nm.
Fig. 2.
Real-time particle measurement in nanosilica handling workplace: particle number concentration and geometric mean diameter measured by the SMPS. Evants were nanosilica handling (silica nanoparticle), filter press operation (oil particles), cleaning sonication (water particles), infiltration of outside air (ambient particles).
Table 2. Summary statistics by tasks in workplace.
| Task/Activity | SMPS |
DiSCmini |
NSAM |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GM | size | Peak | GM | Peak | size | GSD | SA | Peak | |||
| Before the start of operations (inside workplace) | 1.3×103 | 62 | 1.6×103 | 2.4×103 | 5.3×104 | 64 | 1.71 | 5.2 | 6.1 | ||
| Manipulating nanosilica | 9.6×103 | 24 | 1.9×103 | 2.5×104 | 3.0×105 | 22 | 1.83 | 14.1 | 23.8 | ||
| Filter press operation (oil particle) | 2.6×103 | 76 | 5.2×103 | 3.4×103 | 7.8×105 | 53 | 1.46 | 8.3 | 28.2 | ||
| Sonication (water drop) | 3.2×103 | 93 | 6.2×103 | 4.7×103 | 2.9×104 | 51 | 1.11 | 20.5 | 42.2 | ||
| At night (ambient particle) | 1.4×104 | 49 | 2.5×104 | 1.6×104 | 3.8×104 | 41 | 1.07 | 35.2 | 67.8 | ||
| Powder bagging (dry cake) | 2.8×103 | 50 | 2.9×103 | 2.5×103 | 1.8×104 | 55 | 1.45 | 1.6 | 2.7 | ||
| Outside laboratory (semi-ambient particle) | 1.2×104 | 53 | 1.4×104 | 48.0 | 50.6 | ||||||
| Outside building (ambient particle) | 1.1×104 | 40 | 1.5×104 | 43.6 | 52.4 | ||||||
SMPS, scanning mobility particle sizer; DiSCmini, miniature diffusion size classifier; NSAM, nanoparticle surface area monitor; GM, geometric mean of number concentration; size, particle diameter; Peak, peak concentration; GSD, geometric standard deviation; SA, alveolar-deposited surface area; Unit, GM: particles/cc, size: nm, SA: µm2/cc, Peak: particles/cc
Fig. 3.
Real-time particle measurement by the SMPS in each work process.
2) Comparison of results of the personal samples and the area samples
The particle number concentration of the personal samples (except during the night) had an arithmetic mean (AM) of 7,054 particles/cc, a geometric mean (GM) of 3,131 particles/cc. The average particle diameter had an AM of 45 nm and a GM of 42 nm; while their surface area has an AM of 12 µm2/cc, and a GM of 7 µm2/cc. The particle number concentration observed in the personal samples was higher than that of the area sample by 1.6 times (GM 1.2 times), and the peak was also 1.6 times higher (Fig 4, Table 3).
Fig. 4.
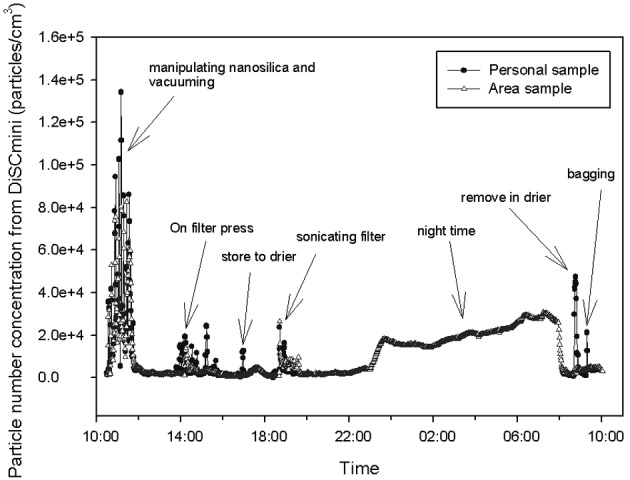
Comparison of particle number concnentration in personal sample and area sample measured by two sets of DISCmini in nanosilica handling workplace.
Table 3. Summary of results in personal sample and area sample measured by two sets of DiSCmini.
| Number concentration | Mode diameter | Surface area | ||||||
|---|---|---|---|---|---|---|---|---|
| Personal | Area | Personal | Area | Personal | Area | |||
| GM | 3,131 | 2,692 | 42 | 49 | 7 | 7 | ||
| Min | 108 | 568 | 10 | 13 | 1.4 | 1.7 | ||
| Max | 134,154 | 82,736 | 300 | 79 | 120.6 | 58.1 | ||
GM, geometric mean of number concentration; GM, geometric mean; Unit, number concentration: particles/cc, mode diameter: nm, surface area: µm2/cc
In the case of the personal samples, the worker’s assigned activity was shown to greatly affect the exposure to nanoparticles. In area sampling, it is difficult to consider operations that are performed sporadically, such as opening and closing of the drying machine and bagging, or sampling that is performed at a long distance from the measurement instrument.
3) Assessment of background concentration
The background concentration inside the workplace was about 10 times lower compared to that outside the workplace. This is explained by the use of a HEPA-filtered Thermo-Hygrostat in the workplace, which provides air circulation, which enhances the filtering of nanoparticles through the HEPA filter. The background concentration at other workplaces and outside the building showed similar levels.
4) Results of filter sample assessment
The particle concentration on the filter was 0.047 mg/m3 on the first day and 0.045 mg/m3 on the second day. The FTIR analysis showed, however, that amorphous silica was detected in the sample only on the first day (Table 4). The mass concentrations in the filter on the 1st and 2nd days are similar: 0.047 and 0.045, respectively. However, on the 2nd day, silica powders were not treated, and consequently, the FTIR analysis detected silica only on the 1st day (Table 4). This result suggests that the exposure assessment of amorphous silica nanoparticles can be performed using assessment methods for crystalline silica such as NIOSH 7500 and 7602.
Table 4. Results of mass concentration on filter and FTIR analysis.
| Sampling day | Sampling duration | Total dust concentration | Detection of amorphous silica in FTIR analysis |
|---|---|---|---|
| 1st | 570 | 0.047 | YES |
| 2nd | 73 | 0.045 | NO |
FTIR, fourier-transform infrared-spectroscopy; Unit, sampling duration: min; total dust concentration, mg/m−3.
5) Results of TEM analysis
Measurements collected during pouring of nanosilica into the container exhibit a wide variation in silica particle sizes, ranging from several nm to several µm. However, particles extracted near the air outlet of the vacuum cleaner were mostly small, ranging from 20 nm to 200 nm (Fig. 5). No nanosilica particles were detected during the operation of filter press, ultrasonic cleaning, and bagging.
Fig. 5.
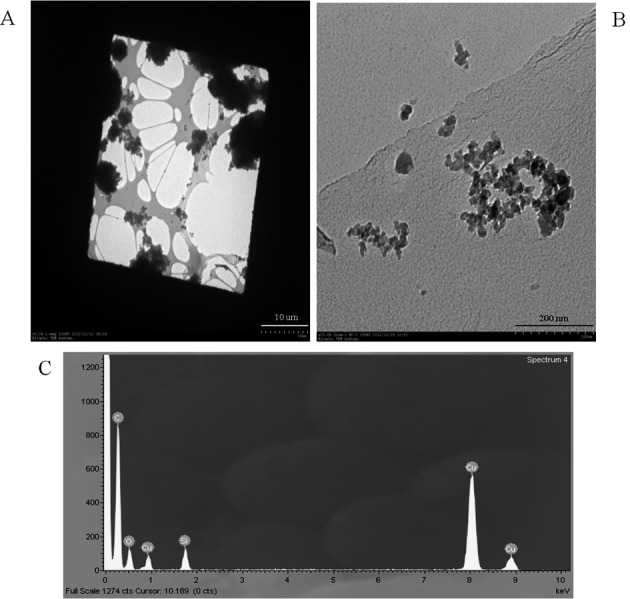
Transmission electron micrograph of airborne nanosilica.
A. Silica particles observed in the air while pouring nanosilica into the container.
B. Silica particles in the air while the vacuum cleaner was on (i.e., with suctioning of nanosilica).
C. Energy dispersive X-ray profile (silicone dioxide nanoparticles on carbon-coated copper grid).
Results of assessment with the vacuum cleaner
Experimental conditions (1) and (2) confirmed that nanoparticles are not produced by the vacuum cleaner itself (the concentrations before and after the usage of vacuum cleaner were the same). When nanosilica is poured into the container, the size of the entrained particles was 154 nm and the concentration was 523,879 particle/cc, which is a 25-fold increase in the concentration compared to the background concentration. On the other hand, when the nanosilica particles were suctioned, the size of the particles measured at the exhaust vent of the vacuum cleaner was 11.5 nm and their concentration was 67,437 particle/cc, three times more than the background concentration (Fig. 6).
Fig. 6.

Particle size distribution comparison (weighted by number) according to the four experimental conditions. Measurements were conducted by the SMPS.
The nanoparticles were confirmed to be produced only one of the several exhaust vent. Thus, the nanoparticles that entered the chamber through the inlet at high speed were observed to be discharged between the back cover/back housing (where leakage occurs), without going through the HEPA filter.
Temperature, humidity, and wind speed
The average temperature was 27.6°C (SD 4.0°C), and the humidity, 39.9% (SD 12.9%). The wind speed was 10 m/s at the vacuum cleaner inlet (The flow volume was 0.0158 m3/s), 3 m/s at the outlet, and less than 0.5 m/s at the table (3 m from the vacuum cleaner).
Discussion
Compact type instruments recently developed
The recently developed SMPS 3910 and DM are compact type portable devices suitable for workplace assessment. In particular, DM can also be used for collecting personal samples.
However, not enough assessment cases exist that has used these devices in workplaces, and concerns may be raised regarding their performance level in comparison with conventional devices. In our study, SMPS 3910 clearly displayed multi-modal distribution during the silica treatment process in which number concentration of nanoparticles was high (>105 particles/cc) and thus proved its suitability for workplace application. Nevertheless, because the size channel of SMPS 3910 is only 13, its accuracy of measuring the particle size distribution and particle size may be occasionally lower than the conventional multi-channel SMPS, despite its advantage of shorter measuring time. Moreover, according to our experience, it is necessary to regularly clean and check the device to prevent occasional flow and pulse errors, especially after the use in a high-concentration workplace.
In this study, the mean particle number concentration measured with DM showed an approximately 30% higher average value than that measured with SMPS, presumably on account of the different measurement ranges of the two devices. However, DM measures were 270% higher than SMPS measures during the handling of silica. The exact reason for this result is not clear, but Mills et al. (2013) reported that DM’s accuracy decreases in the presence of particles exceeding 300 nm in diameter, yielding too high measures with an error range up to +101%, and during the handling of nanosilica in the workplace of this study, the particle agglomerates showed sub-micron sizes exceeding 300 nm and even several microns (Fig. 5).
On the contrary, while Fierz et al. (2011) reported that DM clearly tended to overestimate particle diameters in broader particle size distributions (GSD>2), the GSD in our study was less than 2 in all work processes. Table 2 shows that the particles sizes measured by SMPS and DM in our workplace are similar.
Personal sampling and area sampling using real-time instruments
Because of the lack of appropriate real-time measurement tools for the assessment of nanoparticles in personal samples, however, most assessments were performed on the area samples. Collection and analysis of personal samples are required in order to precisely measure the degree of worker exposure to nanoparticles13).
Our study performed a simultaneous assessment and comparison of personal samples and area samples by using two DM instruments. The results revealed that the particle number concentration was 1.6 times higher and that some tasks were not covered in the area sampling. This is due to the limited mobility of the area samples and the consequent impossibility to reflect all varying concentration depending on worker activities, given the distance between the measurement spot and working area. The workplace where we conducted the study was of a scale (104 m2) small enough such that the distance between the measurement spot and each operation was within 1–3 m (except for the drying machine). The possibility of underestimating the particle concentration in area sampling increases in proportion to the increase in the size of the workplace, the distance to the measurement spot, and the distance of movement to carry out the activities.
Particle size
It is very important to identify the aerosol characteristics in nanomaterial handing process, because particle size is a key metric affecting the risks of manufactured nanomaterials. The mode calculated while transferring nanosilica into the container was 154 nm in our study as well. The mode of the nanosilica emitted through the vacuum cleaner, however, was 11.5 nm, which is close to the primary size (7 nm). This may be because the rapid suctioning of nanoparticles into the vacuum cleaner reduced the coagulation between particles and increased the dustiness. Dustiness is a major determinant of worker exposure while handling powder14). The dustiness of fumed oxides, including nanosilica, is one of the highest among nanoparticles15). Despite their primary size of 100 nm or less, most nanoparticles grow to over 100 nm by the time they are emitted to the air due to the coagulation among particles. For example, a typical CNF measures 200–400 nm (SMPS mode size)16); 200 nm (ELPI mode size)17), CNTs, over 1 µm (microscopy size)18, 19); metal oxides, 0.1–1.0 µm (microscopy size)20); carbon black over 1 µm (SMPS mode size)21); and Silver (Ag), 150 nm (SMPS mode size)22). Various results from dustiness tests on carbon fiber and metallic nanoparticles also reported that all nanoparticles measured over 300 nm in mode15). The particle size distribution was reported to be 300–600 nm during a bag-emptying activity of somewhat bigger nanosilica (primary size 12 nm, surface area 200 g/m3) than that used in our study23).
Filter sampling and nanosilica analysis
In recent years, battery-powered small real-time instruments capable of measuring the particle number concentration of nanoscale particles have been developed, thus facilitating the assessment of nanoparticles in personal samples. Nevertheless, the limitation of particle number concentration measurement remains because it is impossible to differentiate between operation-generated nanoparticles and background nanoparticles. Therefore, many studies emphasize the mass concentration assessment through filter-based sampling20, 24, 25). Data available on occupational exposure of nanosilica is not sufficient: the total dust concentration in a factory that manufactures fumed silica was 0.61–6.5 mg/m3, and the respirable dust concentration 0.2–2.1 mg/m3. In a factory that manufactures nanosilica using a wet process, a total dust concentration of 1.0–8.8 mg/m3 and a respirable dust concentration of 0.5–2.1 mg/m3 were observed26). These results reveal that workers are exposed to a high concentration of nanosilica in a nanosilica manufacturing factory, but the difference in concentration relative to the manufacturing method cannot be ascertained clearly. However, those studies assessed only the mass concentration of micro particles, and there is a need for assessment of nanoparticles. In order to analyze the mass concentration of silica, which is a metal oxide; assessment can be performed by means of filter sampling, followed by XRD or FTIR. NIOSH 7501 is a representative XRD method for the assessment of amorphous silica12). The FTIR method has the disadvantage of sensitivity to interference materials, but unlike XRD, it analyzes amorphous silica directly. Care should be taken with respect to reference material selection, too, because XRD and FTIR show different reaction values depending on particle size. Uncontaminated nanosilica used in workplace is considered the most suitable reference material. As shown in the present study, the mass concentration on the filter on the first and second day was similar, but on the second day, no silica was detected in the FTIR analysis. Therefore, XRD or FTIR analysis lends itself well to the nanosilica exposure assessment. Another important observation is that the detection limit of XRD or FTIR method is 0.01 mg/m3(assuming a sampling of 8 h at flow volume of 1l of general SMPS). Moreover, after converting this concentration into a particle number concentration in accordance with the results reported by Broekhuizen et al. (2012)27), analysis can be performed only at concentration exceeding 954,930 particles/cc when the nanosilica size is 20 nm, and 7,639 particles/cc when the nanosilica size is 100 nm.
Vacuum cleaner as a source of particle emission
In the present study, the vacuum cleaner analysis was performed in 4 steps as described in the Methods section. The 1st step was the SMPS measurement of background concentration, and in the 2nd step, the measurement was made when only the vacuum cleaner was on. While no significant difference was observed in SMPS particle size distributions between the 1st and 2nd stages, a conspicuous difference was demonstrated in the number concentration/particle size distribution between the measurement at the vacuum cleaner outlet and that of the ambient air during the off-state of the vacuum cleaner, as shown in the 3rd and 4th step experiments. From this, it can be inferred that the leak occurred in the vacuum cleaner. In particular, since the SMPS measurement was performed in the immediate vicinity of the vacuum clear outlet with a very strong outflow of air, there was hardly any possibility of other particles being measured than those leaking from the vacuum cleaner.
Vacuum cleaners are known to be sources of fine and ultrafine particles. A HEPA filter refers to a filter capable of capturing more than 99.97% of 0.3 µm sized particles. In other words, HEPA-filtered vacuum cleaners can capture almost all particles bigger than 0.3 µm28). The most-penetrating particle size, MPPS, being 0.3 µm, particles smaller than this size will have an even higher rate of capture according to the single-fiber filtration theory. The result of the assessment on p100 filter mask under the same conditions as on the HEPA filter showed very low values of MPPS (30–60 nm) and a 0.009% penetration rate29).
The vacuum inlet speed measured in the present study was 10 m/s and the outlet speed was 3 m/s, which is very rapid. The rate of capture decreases as the speed increases30, 31). In particular, if the speed exceeds 0.5 m/s, particularly, particles hit the fiber and bounce, continuing to pass through the filter31). There are few objective data, if any, regarding this aspect of a vacuum cleaner.
Summing up the results of our study described above, the high concentration of nanoparticles caused by the vacuum cleaner is rather attributable to the leakage in the vacuum cleaner than to the nanoparticles that passed through the HEPA filter. Willeke et al. (2001)28) reported the occurrence of leakage, caused by an inadequate sealing between bag housing and bag cover (which was large with a long perimeter), of the particles that did not pass through the HEPA filter. Additionally, there are some related reports that found nanomaterial releases from a vacuum cleaner in carbon nanotube handling process32, 33).
On the other hand, the structure of the vacuum cleaner itself caused leakage of 109 ultrafine particles/min due to the abrasion of the commutator and the carbon brush of the motor34). In our study, however, no increase of nanoparticles was observed while the vacuum cleaner was on. The reason is that recent models of vacuum cleaners use brushless motors and hence cannot cause leakage of carbon ultrafine particles.
Limitation
We are aware that a comparative assessment of many different vacuum cleaners under various conditions, as performed in a study by Knibbs et al. (2012)4), can enhance the value of this study, but the focus of our study was on the vacuum cleaner analysis in a real job setting of a real-life workplace. Therefore, the results of this study cannot be generalized, because this study was conducted at one workplace and the size and concentration of the nanoparticles emitted from the vacuum cleaner depend on the type of nanoparticles and the state of the vacuum cleaner. Therefore, further study is required in better designed conditions. Nevertheless, this study is meaningful as the first experiment conducted in real job setting that generate nanoparticles.
Conclusion
Exposure monitoring was performed in a workplace handling nanosilica under simultaneous use of real-time measurement instruments and traditional filter measurement method.
Exposure to nanosilica emitted to the air occurred while pouring nanosilica into the container or transferring the container. The use of a vacuum cleaner with leakage also caused the emission of nanosilica within the workplace. The sizes of nanosilica particles emitted into the air in occupational environments where nanosilica is treated exceeded 100 nm, or were of micro-scale due to the coagulation of particles, but the nanosilica that leaked out of the vacuum cleaner had smaller sizes close to the primary size. Nanoparticles were generated also during the operation of the filter press and ultrasonic cleaning, but they were oil particles and water particles, respectively.
The area sample measurement resulted in 20% (60% in terms of peak concentration) less than the personal sample measurement. The area sample measurement was limited when the activities were carried out at a longer distance or were of short duration.
The use of HEPA-filtered Thermo-Hygrostat in the workplace reduced the concentration of nanoparticles and lowered the background concentration.
To conclude, high-concentration nanoparticles are emitted to the air while pouring and transferring nanosilica. Therefore, a respirator capable of capturing nanoparticles must be worn, and activities must be carried out within the HEPA-filtered hood. A regular check on the vacuum cleaner is necessary to prevent leakage of nanoparticles. Additionally, wet cleaning is safer in reducing exposure risk.
Acknowledgments
We thank two anonymous reviewers for their helpful comments that improved an earlier version of this article. and thank Dr. Sewan Oh (Ministry of Employment and Labor) for his support.
References
- 1.National Institute of Environmental Research (NIER) Report on the investigation for nanomaterial circulation in 2010. Incheon, Korea: 2012. (in Korean). [Google Scholar]
- 2.Napierska D, Thomassen LC, Lison D, Martens JA, Hoet PH. (2010) The nanosilica hazard: another variable entity. Part Fibre Toxicol 7, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murashov V, Schulte P, Geraci C, Howard J. (2011) Regulatory approaches to worker protection in nanotechnology industry in the USA and European union. Ind Health 49, 280–96. [DOI] [PubMed] [Google Scholar]
- 4.Knibbs LD, He C, Duchaine C, Morawska L. (2012) Vacuum cleaner emissions as a source of indoor exposure to airborne particles and bacteria. Environ Sci Technol 46, 534–42. [DOI] [PubMed] [Google Scholar]
- 5.NIOSH (2012) General safe practices for working with engineered nanomaterials in research laboratories. National Institute for Occupational Safety and Health, Washington. [Google Scholar]
- 6.Clark K, Tongeren M, Christensen F, Brouwer D, Nowack B, Gottschalk F, Micheletti C, Schmid K, Gerritsen R, Aitken R, Vaquero C, Gkanis V, Housiadas C, Ipiña J, Riediker M. (2012) Limitations and information needs for engineered nanomaterial-specific exposure estimation and scenarios: recommendations for improved reporting practices. J Nanopart Res 14, 970. [Google Scholar]
- 7.Fierz M, Houle C, Steigmeier P, Burtscher H. (2011) Design, calibration, and field performance of a miniature diffusion size classifier. Aerosol Sci Technol 45, 1–10. [Google Scholar]
- 8.Ono-Ogasawara M, Serita F, Takaya M. (2009) Distinguishing nanomaterial particles from background airborne particulate matter for quantitative exposure assessment. J Nanopart Res 11, 1651–9. [Google Scholar]
- 9.Kuhlbusch TA, Asbach C, Fissan H, Göhler D, Stintz M. (2011) Nanoparticle exposure at nanotechnology workplaces: a review. Part Fibre Toxicol 8, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramachandran G, Ostraat M, Evans DE, Methner MM, O’Shaughnessy P, D’Arcy J, Geraci CL, Stevenson E, Maynard A, Rickabaugh K. (2011) A strategy for assessing workplace exposures to nanomaterials. J Occup Environ Hyg 8, 673–85. [DOI] [PubMed] [Google Scholar]
- 11.Brouwer D, Berges M, Virji MA, Fransman W, Bello D, Hodson L, Gabriel S, Tielemans E. (2012) Harmonization of measurement strategies for exposure to manufactured nano-objects; report of a workshop. Ann Occup Hyg 56, 1–9. [DOI] [PubMed] [Google Scholar]
- 12.NIOSH (2003) Manual of analytical methods (NMAM). 4th Ed., silica, amorphous. National Institute for Occupational Safety and Health, Cincinnati. [Google Scholar]
- 13.Kim B, Lee JS, Choi BS, Park SY, Yoon JH, Kim H. (2013) Ultrafine particle characteristics in a rubber manufacturing factory. Ann Occup Hyg 57, 728–39. [DOI] [PubMed] [Google Scholar]
- 14.Brouwer DH, Links IH, De Vreede SA, Christopher Y. (2006) Size selective dustiness and exposure; simulated workplace comparisons. Ann Occup Hyg 50, 445–52. [DOI] [PubMed] [Google Scholar]
- 15.Evans DE, Turkevich LA, Roettgers CT, Deye GJ, Baron PA. (2013) Dustiness of fine and nanoscale powders. Ann Occup Hyg 57, 261–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ku BK, Emery MS, Maynard AD, Stolzenburg MR, McMurry PH. (2006) In situ structure characterization of airborne carbon nanofibres by a tandem mobility-mass analysis. Nanotechnology 17, 3613–21. [DOI] [PubMed] [Google Scholar]
- 17.Evans DE, Ku BK, Birch ME, Dunn KH. (2010) Aerosol monitoring during carbon nanofiber production: mobile direct-reading sampling. Ann Occup Hyg 54, 514–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maynard AD, Baron PA, Foley M, Shvedova AA, Kisin ER, Castranova V. (2004) Exposure to carbon nanotube material: aerosol release during the handling of unrefined single-walled carbon nanotube material. J Toxicol Environ Health A 67, 87–107. [DOI] [PubMed] [Google Scholar]
- 19.Cena LG, Peters TM. (2011) Characterization and control of airborne particles emitted during production of epoxy/carbon nanotube nanocomposites. J Occup Environ Hyg 8, 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curwin B, Bertke S. (2011) Exposure characterization of metal oxide nanoparticles in the workplace. J Occup Environ Hyg 8, 580–7. [DOI] [PubMed] [Google Scholar]
- 21.Kuhlbusch TA, Neumann S, Fissan H. (2004) Number size distribution, mass concentration, and particle composition of PM1, PM2.5, and PM10 in bag filling areas of carbon black production. J Occup Environ Hyg 1, 660–71. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Kwon M, Ji JH, Kang CS, Ahn KH, Han JH, Yu IJ. (2011) Exposure assessment of workplaces manufacturing nanosized TiO2 and silver. Inhal Toxicol 23, 226–36. [DOI] [PubMed] [Google Scholar]
- 23.Brouwer D, Duuren-Stuurman B, Berges M, Jankowska E, Bard D, Mark D. (2009) From workplace air measurement results toward estimates of exposure? Development of a strategy to assess exposure to manufactured nano-objects. J Nanopart Res 11, 1867–81. [Google Scholar]
- 24.Dahm MM, Evans DE, Schubauer-Berigan MK, Birch ME, Fernback JE. (2012) Occupational exposure assessment in carbon nanotube and nanofiber primary and secondary manufacturers. Ann Occup Hyg 56, 542–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JH, Ahn KH, Kim SM, Jeon KS, Lee JS, Yu IJ. (2012) Continuous 3-day exposure assessment of workplace manufacturing silver nanoparticles. J Nanopart Res 14, 1134. [Google Scholar]
- 26.IARC (1997) Monographs on the evaluation of carcinogenic risk of chemicals to humans. Vol. 68. Silica Lyon, France: International Agency for Research on Cancer. [Google Scholar]
- 27.van Broekhuizen P, van Veelen W, Streekstra WH, Schulte P, Reijnders L. (2012) Exposure limits for nanoparticles: report of an international workshop on nano reference values. Ann Occup Hyg 56, 515–24. [DOI] [PubMed] [Google Scholar]
- 28.Willeke K, Trakumas S, Grinshpun SA, Reponen T, Trunov M, Friedman W. (2001) Test methods for evaluating the filtration and particulate emission characteristics of vacuum cleaners. AIHAJ 62, 313–21. [DOI] [PubMed] [Google Scholar]
- 29.Rengasamy S, Eimer BC, Shaffer RE. (2009) Comparison of nanoparticle filtration performance of NIOSH-approved and CE-marked particulate filtering facepiece respirators. Ann Occup Hyg 53, 117–28. [DOI] [PubMed] [Google Scholar]
- 30.Mostofi R, Wang B, Haghighat F, Bahloul A, Jaime L. (2010) Performance of mechanical filters and respirators for capturing nanoparticles—limitations and future direction. Ind Health 48, 296–304. [DOI] [PubMed] [Google Scholar]
- 31.Hinds W .(1999) Aerosol technology: properties, behavior, and measurement of airborne particles. 2nd Ed. John Wiley and Sons, New York. [Google Scholar]
- 32.Mills JB, Park JH, Peters TM. (2013) Comparison of the DiSCmini aerosol monitor to a handheld condensation particle counter and a scanning mobility particle sizer for submicrometer sodium chloride and metal aerosols. J Occup Environ Hyg 10, 250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogura I, Sakurai H, Mizuno K, Gamo M. (2011) Release potential of single-wall carbon nanotubes produced by super-growth method during manufacturing and handling. J Nanopart Res 13, 1265–80. [Google Scholar]
- 34.Lioy PJ, Wainman T, Zhang J, Goldsmith S. (1999) Typical household vacuum cleaners: the collection efficiency and emissions characteristics for fine particles. J Air Waste Manag Assoc 49, 200–6. [DOI] [PubMed] [Google Scholar]