Abstract
Various cytokines activated by the inhalation of coal dust may mediate inflammation and lead to tissue damage. Objective of this study was to examine the relationships between coal workers’ pneumoconiosis (CWP) progression over a 3 yr period and the serum levels of cytokines in 85 retired coal workers. To investigate the relevance of serum cytokines in CWP, serum levels of interleukin-8 (IL-8), tumor necrosis factor-alpha (TNF-α), transforming growth factor–beta1 (TGF-β1), and monocyte chemotactic protein-1 (MCP-1) as progressive CWP biomarkers were studied in relation to the progression of pneumoconiosis over a 3 yr period in 85 patients with CWP. CWP progression was evaluated through paired comparisons of chest radiographs. Median levels of TGF-β1 and MCP-1 were significantly higher in subjects with progressive CWP than in those without CWP progression. The area under the ROC curve for TGF-β1 (0.693) and MCP-1 (0.653) indicated that these cytokines could serve as biomarkers for the progression of CWP. Serum TGF-β1 levels were related to the progression of CWP (β=0.247, p=0.016). The results suggest that high serum levels of TGF-β1 and MCP-1 are associated with the progression of CWP.
Keywords: Interleukin-8, Monocyte chemotactic factor-1, Progressive coal workers’ pneumoconiosis, Transforming growth factor-β1, Tumor necrosis factor-α
Introduction
Of the various occupational lung diseases, those induced by the inhalation of dusts such as asbestos, crystalline silica, and coal are most prevalent. Dust inhalation can cause a variety of lung diseases such as coal workers’ pneumoconiosis (CWP), progressive massive fibrosis, chronic alveolitis, and emphysema1). Notably, crystalline silica has been classified as a class I carcinogen by the International Agency for Research on Cancer2). Once a silica threshold has been exceeded, silica-induced pulmonary disease may progress even without further exposure to silica. CWP is a lung disease caused by the inhalation of coal dust. The clinical detection of CWP, however, is currently dependent on radiological and lung function abnormalities, both of which are detectable only in the late stages of the disease. The identification of accurate and reliable biomarkers would enable earlier detection before irreversible radiological changes occur in the lung3, 4).
Cytokines influence various biological events such as inflammation, metabolism, cell growth and proliferation, morphogenesis, fibrosis, and homeostasis. The major sources of cytokines in the lung are epithelial cells, endothelial cells, fibroblasts, and inflammatory cells5). Serum cytokines such as tumor necrosis factor-alpha (TNF-α)6, 7), Interleukin-8 (IL-8)6, 8), transforming growth factor-beta 1 (TGF-β1)9, 10), and monocyte chemotactic protein-1 (MCP-1)11) have been considered as biomarkers for development or progression of CWP. Although evidence obtained from a various studies on the increased production of these cytokines following exposure crystalline silica or coal mine dust in macrophage and fibroblasts, only a limited number of human validation studies were reported in the literature1, 6). These reports suggest the importance of serum cytokines in the CWP, however there are not lots of studied reports about the in vivo relevance of predictive discrimination between the levels of cytokines and progression of CWP. To determine the significance of serum cytokines regarding the progression of CWP, a longitudinal design is necessary.
In the present study, we examined the relationship between serum levels of IL-8, TNF-α, TGF-β1, and MCP-1 and CWP findings in retired coal workers with previous cross sectional findings, and examined the relationship between initial levels of same cytokines and 3 yr progressive changes of CWP.
Subjects and Methods
Study design
A group of 110 male subjects were recruited retired coal workers who were examined for pneumoconiosis for two months at an affiliated hospital of Korea Workers’ Compensation & Welfare Service (KCOMWEL) in 2007. We excluded 25 subjects who showed greater than reference serum levels of indices of liver or kidney dysfunction, including aspartate aminotransferase, alanine aminotransferase, or gamma-glutamyl transpeptidase, blood urea nitrogen or creatinine. All of excluded subjects were pneumoconiosis patients (20 cases of 1/0, 4 cases of 1/2, and 1 case of 2/2) and a case showed progressive change (1/0 to 1/1). The progression of CWP was evaluated through paired comparison of chest radiographs made in 2007 and 2010. Ultimately, chest radiographs in 2010 were measured only in 85 subjects, and 85 subjects previously screened for serum cytokines participated in our follow-up. These subjects were divided into two groups according to the progressive changes identified on chest radiographs (no progression in 67 subjects, progression in 18 subjects).
Pulmonary function was measured in 2007 by using spirometry. The data collected included the forced vital capacity (FVC) and the forced expiratory volume in one second (FEV1). The pulmonary function test (PFT) results and personal information, including age, body mass index (BMI), and demographic information (e.g., job and smoking status), were obtained using a questionnaire. All subjects provided their informed consent. The study was approved by the Research Ethics Committee at our institute.
Serum cytokine level measurement
Serum was separated from whole blood samples and stored at −80 °C until the assay. Serum cytokine levels were measured using a bead laser analyzer (Bio-Plex 200, Bio-Rad Laboratories, USA) and sandwich enzyme immunoassays.
PFT
PFT was performed in accordance with the guidelines recommended by the ATS/ERS Task Force12) by using a Vmax22 spirometer (SensorMedics, San Diego, CA, USA). The parameters measured included FVC (the volume delivered during an expiration made as forcefully and completely as possible starting from full inspiration), FEV1 (the volume delivered during the first second of an FVC maneuver), and the FEV1/FVC (%FEV1/FVC) ratio. The predicted FVC and FEV1 volumes were calculated using a previously reported equation13). Tests of pulmonary function were performed with the patient in the sitting position via closed-circuit method, measuring inhaled and exhaled air during the same test cycle. Tests were carried out until 3 adequate data points had been obtained. The threshold levels of %FVC predicted, %FEV1 predicted, and the %FEV1/FVC ratio were 80%, 80%, and 70%, respectively.
Chest radiography
Chest radiographs were obtained and scored according to the classification rules used by the International Labor Office (ILO 2002)14). Classifications of radiographs were decided by the guidelines of the pneumoconiosis review committee of KCOMWEL in Korea. Briefly, radiographs were identified after consensus of classification was acquired between two experienced radiologists.
Statistical analyses
Levels of serum IL-8, TNF-α and MCP-1 were log-normally distributed. The values were log-transformed for parametric statistical tests such as regression analysis. The Mann-Whitney U test was used to identify differences between the study groups. A logistic regression model was constructed to compare non-progressive CWP patients with progression cases, then adjusted for age, BMI, exposure period, ex-exposure period, smoking status, and pulmonary function. Receiver operating characteristic (ROC) curve analysis was used to assess the biomarker potential of each cytokine for the discrimination of CWP progression. Statistical “cutoff” values were calculated by minimizing the distance between the point with specificity=1 and sensitivity=1 and various points on the ROC curve. For ROC analysis, an area under the curve (AUC) of 1.0 indicates perfect discrimination, whereas an area of 0.5 indicates that the test discriminates no better than chance. Values of p<0.05 were considered statistically significant. All statistical evaluations were performed using SPSS 17.0 software (SPSS, Chicago, IL, USA).
Results
Study subjects
The general characteristics of the study subjects are shown in Table 1. Among the CWP patients, 67 and 18 subjects were classified as having non-progressive and progressive CWP, respectively. General characteristics, including median age, BMI, exposure period, pulmonary function (%FVC predicted, %FEV1 predicted, %FEV1/FVC ratio), and smoking status did not show difference between the two study groups.
Table 1. Patient characteristics.
| NP (n=67) † | P (n=18)c | p-values | |
|---|---|---|---|
| Age, yr | 64.8 (41.9–84.0) | 63.5 (44.0–78.0) | 0.767 a |
| BMI, kg/m2 | 22.7 (17.2–31.6) | 23.7 (16.1–26.9) | 0.519 a |
| Exposure period, yr | 18.2 (7.0–37.0) | 16.5 (5.0–35.0) | 0.198 a |
| Ex-exposure period, yr | 18.0 (0.5–42.0) | 18.8 (2.0–31.0) | 0.214 a |
| %FVC predicted | 91.5 (61.3–125.6) | 89.7 (62.3–112.2) | 0.250 a |
| %FEV1 predicted | 86.8 (41.9–129.2) | 83.6 (50.6–110.4) | 0.268 a |
| %FEV1/FVC ratio | 71.3 (65.2–76.1) | 66.7 (43.0–81.8) | 0.464 a |
| Smoking, N (%) | |||
| Never | 7 (10.4) | 4 (22.2) | 0.405 b |
| Past | 32 (47.8) | 8 (44.4) | |
| Current | 28 (41.8) | 6 (33.3) |
† NP, CWP subjects without progression; P, CWP subjects with progression. a Calculated by Mann-Whitney U test, median (range). b Calculated by χ2-test, number of cases (%). c Three-year progression was evaluated by paired comparison of chest radiographs. Category 0 (3 subjects): 0/0 (2) to 1/0, 0/1 (1) to 1/1. Category I (8 subjects): 1/0 (2) to 1/1, 1/1 (3) to 1/2, 1/1 (2) to 2/1, 1/2 (1) to 2/1. Category II (7 subjects): 2/1 (3) to 2/2, 2/2 (3) to 4A, 2/3 (1) to 3/2.
Serum cytokine levels
Serum cytokine levels as related to CWP progression are shown in Table 2. Median level of TGF-β1 was significantly higher in subjects with progressive CWP (90.7 ng/ml vs. 74.4 ng/ml) (p=0.012). This effect held true in the adjusted logistic regression model (p=0.006 for all comparisons). Although median level of MCP-1 was significantly higher in subjects with progressive CWP (37.2 pg/ml vs. 30.6 pg/ml) (p=0.048), this effect disappeared upon analysis with the adjusted logistic regression model (p=0.842). Although median levels of IL-8 and TNF-α were similar in subjects with and without progressive CWP, the median IL-8 level tended to increase in association with the progression of CWP (14.7 pg/ml vs. 16.8 pg/ml, p=0.056).
Table 2. Median levels of serum cytokines as related to CWP progression.
| NP (n=67) † | P (n=18)c |
p-values |
||
|---|---|---|---|---|
| Univariate a | Multivariate b | |||
| IL-8, pg/ml | 14.7 (6.8–38.4) | 16.8 (10.5–26.4) | 0.056 | 0.054 |
| TNF-α, pg/ml | 2.03 (0.47–18.98) | 1.91 (0.73–9.19) | 0.817 | 0.926 |
| TGF-β1, ng/ml | 74.4 (30.4–119.8) | 90.7 (49.3–122.9) | 0.012 | 0.006 |
| MCP-1, pg/ml | 30.6 (10.5–238.7) | 37.2 (16.3–64.0) | 0.048 | 0.354 |
a Calculated by Mann-Whitney U test, median (range). b Calculated using a logistic regression model to adjust for age, BMI, exposure period, smoking status (never/past/current), and pulmonary function (%FVC predicted, %FEV1 predicted, and %FEV1/FVC ratio).
ROC analysis of serum cytokine levels
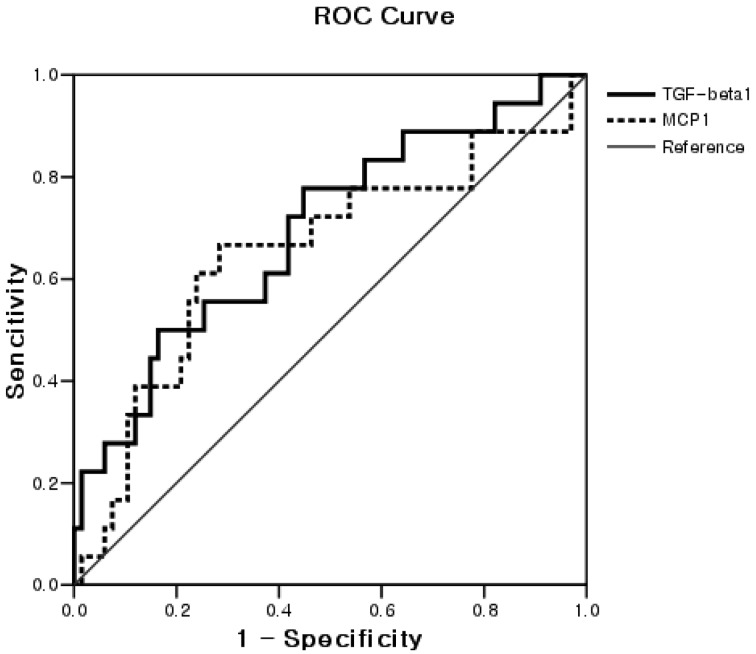
The area under the ROC curve for serum cytokine levels as related to the progression of CWP is shown in Table 3 and Fig. 1. The AUC of the ROC curve for serum TGF-β1 level was 0.69 (95% CI, 0.55–0.84). A serum TGF-β1 level of 79.2 ng/ml was determined as the optimal cutoff value with resulting sensitivity and specificity of 61.1% and 61.2%, respectively. The AUC of the ROC curve for serum MCP-1 levels was 0.65 (95% CI, 0.50–0.81). A serum MCP-1 level of 34.3 pg/ml was determined as the optimal cutoff value with resulting sensitivity and specificity of 66.7% and 67.2%, respectively.
Table 3. Area under the ROC curve for serum cytokines according to CWP progression.
| Cytokines | AUROC | SE | 95% CI | p-values | Cut off | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|
| IL-8 | 0.65 | 0.07 | 0.52−0.78 | 0.056 | - | - | - |
| TNF-α | 0.48 | 0.09 | 0.31−0.65 | 0.817 | - | - | - |
| TGF-β1 | 0.69 | 0.07 | 0.55−0.84 | 0.012 | 79.2 ng/ml | 61.1 | 61.2 |
| MCP-1 | 0.65 | 0.08 | 0.50−0.81 | 0.048 | 34.3 pg/ml | 66.7 | 67.2 |
AUROC, area under the ROC curve; SE, standard error; CWP, coal workers’ pneumoconiosis; 95% CI, 95% confidence interval a In all subjects (n=85).
Fig. 1.
ROC curve for TGF-β1 and MCP-1 according to progression of CWP (n=85). The areas under the ROC curve for TGF-β1 and MCP-1 were 0.69 (95% CI, 0.55–0.84, p=0.012) and 0.65 (95% CI, 0.50–0.81, p=0.048), respectively. The optimal cutoff values of TGFβ1 and MCP1 for the progression of pneumoconiosis were 79.2 ng/ml (sensitivity and specificity of 61.1% and 61.2%) and 34.3 pg/ml (sensitivity and specificity of 66.7% and 67.2%), respectively.
Relationships between serum cytokine levels and associated variables
Stepwise multiple regression analysis (Table 4) showed that serum TGF-β1 levels were related to the progression of CWP (β=0.247, p=0.016), %FEV1/FVC ratio (β=0.229, p=0.023) and log MCP-1 levels (β=0.252, p=0.014) (adjusted R2=0.178, p<0.001). Serum levels of IL-8, TNF-α, and MCP-1 were not related to the progression of CWP.
Table 4. Stepwise multiple regression analysis for serum cytokines.
| Variables |
B† | SE | β | p-values | |
|---|---|---|---|---|---|
| Dependents | Independents | ||||
| Log TNF-α (pg/ml) | Constant | −0.276 | 0.205 | 0.183 | |
| Log IL-8 | 0.485 | 0.173 | 0.295 | 0.006 | |
| adjusted R2 | 0.076 | ||||
| F | 7.887 (p = 0.006) | ||||
| Log IL-8 (pg/ml) | Constant | 1.105 | 0.128 | 0.000 | |
| Log TNF-α | 0.179 | 0.058 | 0.295 | 0.003 | |
| TGF-β1 | 0.002 | 0.001 | 0.339 | 0.001 | |
| BMI | −0.011 | 0.005 | −0.240 | 0.014 | |
| ILO category | 0.048 | 0.024 | 0.191 | 0.050 | |
| adjusted R2 | 0.235 | ||||
| F | 7.455 (p<0.001) | ||||
| TGF-β1 (ng/ml) | Constant | −129.544 | 70.618 | 0.000 | |
| Progression of CWP | 12.264 | 4.961 | 0.247 | 0.016 | |
| Log MCP-1 | 30.060 | 11.920 | 0.252 | 0.014 | |
| %FEV1/FVC ratio | 2.238 | 0.969 | 0.229 | 0.023 | |
| adjusted R2 | 0.178 | ||||
| F | 7.069 (p<0.001) | ||||
| Log MCP-1 (pg/ml) | Constant | 1.300 | 0.069 | 0.000 | |
| TGF-β1 | 0.002 | 0.001 | 0.259 | 0.014 | |
| Smoking status | 0.084 | 0.036 | 0.242 | 0.022 | |
| adjusted R2 | 0.124 | ||||
| F | 6.955 (p = 0.002) | ||||
† Subjects: CWP patients with or without progression (n=85). B, regression coefficients; SE, standard error; β, standardized B. Independent variables: age, BMI, exposure period, smoking status (yes/no), ILO category of CWP, progression of CWP (yes/no), %FVC predicted, %FEV1 predicted, %FEV1/FVC ratio, and measured serum cytokines.
Discussion
CWP results from exposure to coal mine dust and is characterized by a progressive fibrotic reaction in the lung that can cause functional damage and irreversible changes1). Pulmonary fibrosis is an irreversible accumulation of connective tissue in the interstitium of the lung. Research on animal models and studies of human lung disease suggest that the initiating events may be a combination of pulmonary injury and the recruitment of inflammatory cells15).
The identification of biomarkers that are accurate and reliable in the prediction and early detection of CWP is imperative for the implementation of timely intervention strategies3). The present study was performed using prospective findings collected over a 3 yr period (2007–2010) from retired coal workers who were examined for pneumoconiosis. Serum levels of inflammatory cytokines may be elevated in these status16, 17). In the present study, we excluded subjects who showed greater than reference serum levels of indices of liver-kidney dysfunction.
IL-8 is a chemokine secreted by a variety of cells, including fibroblasts, in response to IL-1 and TNF-α stimulation and is an important activator and chemoattractant for neutrophils. The accumulation of inflammatory leukocytes in the lung is hallmark of either acute or chronic pulmonary inflammation18). IL-8 is one of the important mediators in the lung inflammation induced by crystalline silica19, 20). In present study, we found that levels of IL-8 levels were related to those of serum TNF-α (β=0.295, p=0.003). Levels of IL-8 were reportedly elevated in the supernatants of spontaneous or dust-stimulated monocytes isolated from peripheral blood and sera from non-progressing CWP patients8). We previously found that serum levels of IL-8 were related to the presence of CWP, but were not related to the progression of CWP6). In the present study, although no difference between study groups was identified, serum IL-8 levels tended to increase in association with CWP progression (p=0.056) and ILO category (β=0.191, p=0.050). Future studies will be required to ascertain the role of IL-8 for the progression of CWP using more longitudinal follow-up. TNF-α is a proinflammatory cytokine, important in the early onset of inflammation, development, and progression of several diseases, including pulmonary fibrosis1, 3). Workers who showed abnormally high dust-stimulated levels of TNF-α had an increased risk of CWP progression. Serum TNF-α levels were reported to be a powerful tool for the estimation of disease progression in patients with coal dust-induced pneumoconiosis, even after any occupational exposure had ended19). In our previous study, high levels of serum TNF-α are associated with the progression of CWP in 1 yr follow-up. In the present study, however, serum TNF-α levels were not related to progression of CWP. Future studies will be required to ascertain the role of TNF-α for the progression of CWP using more longitudinal follow-up.
The major sources of TGF-β proteins are blood platelets, alveolar macrophages, monocytes, and neutrophils21). TGF-β is expressed in injured lung tissue and produced by inflammatory cells removed from the lung. TGF-β production is increased prior to collagen synthesis. The cytokine is mainly produced by alveolar macrophages and can be deposited in epithelial cells in areas of lung regeneration and remodeling. Borm and Schins22) proposed that TGF-β1 release from silica-stimulated monocytes was significantly lower than baseline levels of release. These previous studies have demonstrated that TGF-β has anti-fibrotic properties and that its pro/anti-fibrotic action may be concentration dependent7). Vanhee et al.10) reported that TGF-β levels were significantly higher in the BAL fluid of patients with simple pneumoconiosis in comparison with those in control subjects and patients with progressive massive fibrosis. However, TGF-β1 continues to be regarded as the most important of the growth factors involved in pulmonary fibrogenesis9). Notably, aberrant tissue repair may lead directly to progressive fibrosis15). Gosset et al.23) reported that coal and silica dust-stimulated TGF release was significantly higher in workers than in non-exposed individuals. Jagirdar et al.24) reported that scar tissue in the progressive massive fibrosis (PMF) lesions contained TGF-β. These data suggest a major role for TGF-β in silicosis, particularly in the development of PMF. In fetal monkey study25), over expression of TGF-β1 within lung results in severe and progressive fibrosis in lung parenchyma and pleural membrane. In the present study, serum TGF-β1 level in subjects with progressive CWP were higher than those in patients without progressive CWP (90.7 ng/ml vs. 74.4 ng/ml, p=0.012). The ROC curve analyses identified TGF-β1 (AUC=0.69) levels as predictive of CWP progression. The associated threshold was 79.2 ng/ml (sensitivity, 61.1%; specificity, 61.2%). Applying stepwise multiple regression analysis, serum TGF-β1 levels were correlated with the progression of CWP (β=0.247, p=0.016) and the %FEV1/FVC ratio (β=0.229, p=0.023). These results suggest that levels of serum TGF-β1 might serve as biomarkers for the progression of CWP and the decreasing of lung function.
MCP-1 is a potent chemotactic factor for blood monocytes26) and produced by various inflammatory cells27). MCP-1 may play an important role in the development of pulmonary fibrosis, but not in the initiation of acute lung inflammation28, 29). MCP-1 levels are reported to increase in the bronchoalveolar lavage fluid of CWP patients11). MCP-1 may also be involved in the development of chronic macrophagic inflammation in CWP patients. This process stems from the pulmonary overproduction of MCP-1. Fibroblasts and type II cells might also mediate the enhanced production of MCP-1 in CWP patients. However, no human study has been performed in patients with progressive CWP. In the present study, the median serum MCP-1 level in subjects with progressive CWP was higher than that in patients without progressive CWP (37.2 pg/ml vs. 30.6 pg/ml, p=0.048). The AUC values for the predictive value of MCP-1 (0.65) identified the cytokine as a reasonable biomarker. The cutoff MCP-1 value for the progression of pneumoconiosis was 34.3 pg/ml (sensitivity, 66.7%; specificity, 67.2%). Stepwise multiple regression analysis showed that serum MCP-1 levels correlated with serum TGF-β1 levels (β=0.259, p=0.014). These results suggest that levels of serum MCP-1 might serve as a biomarker for the progression of CWP.
The study has several limitations. One is that the follow-up period of 3 yr was insufficient to draw conclusions about the long-term progression of CWP. Another limitation concerns the lack of data on co-factors such as serum leptin (for TNF-α)30, 31) and neurotrophic factor (for IL-8)32). Serum cytokines levels can be measured by various techniques, the enzyme-linked immunosorbent assay, flow cytometry, and a biochip array33). The optimal cutoff values may, therefore, differ depending on the method of analysis. In the current study, serum levels of TGF-β1 and MCP-1 tended to increase in association with CWP progression. The ROC analysis identified TGF-β1 and MCP-1 levels as potential biomarkers.
In conclusion, high serum levels of TGF-β1 and MCP-1 are associated with the progression of CWP. Further longitudinal follow-up studies are needed to investigate the potential of serum cytokines for use as CWP biomarkers. Future studies will be required to ascertain the cytokine profiles of bronchoalveolar lavage fluid, exhaled breath condensate, and lung tissue.
Acknowledgments
The authors thank all of the retired CWP patients who participated in this study. This study was supported by the Korea Workers’ Compensation and Welfare Service research grant.
References
- 1.Schins RPF, Borm PJA. (1999) Mechanisms and mediators in coal dust induced toxicity: a review. Ann Occup Hyg 43, 7–33. [DOI] [PubMed] [Google Scholar]
- 2.IARC (1997) IARC Monograph on the evaluation of the carcinogenic risk of chemicals to humans. In: Silica, some silicates, coal dust and para-aramid fibrils. Vol. 68. IARC Press, Geneva. [PMC free article] [PubMed] [Google Scholar]
- 3.Gulumian M, Borm PJA, Vallyathan V, Castranova V, Donaldson K, Nelson G, Murray J. (2006) Mechanistically identified suitable biomarkers of exposure, effect, and susceptibility for silicosis and coal-worker’s pneumoconiosis: a comprehensive review. J Toxicol Environ Health B Crit Rev 9, 357–95. [DOI] [PubMed] [Google Scholar]
- 4.Porter DW, Hubbs AF, Mercer R, Robinson VA, Ramsey D, McLaurin J, Khan A, Battelli L, Brumbaugh K, Teass A, Castranova V. (2004) Progression of lung inflammation and damage in rats after cessation of silica inhalation. Toxicol Sci 79, 370–80. [DOI] [PubMed] [Google Scholar]
- 5.Elias JA, Zitnik RJ. (1992) Cytokine-cytokine interactions in the context of cytokine networking. Am J Respir Cell Mol Biol 7, 365–7. [DOI] [PubMed] [Google Scholar]
- 6.Lee JS, Shin JH, Lee JO, Lee KM, Kim JH, Choi BS. (2010) Serum Levels of Interleukin-8 and Tumor Necrosis Factor-alpha in Coal Workers’ Pneumoconiosis: one-year Follow-up Study. Saf Health Work 1, 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schins RPF, Borm PJA. (1995) Epidemiological evaluation of release of monocyte TNF-α as an exposure and effect marker in pneumoconiosis: a five year follow up study of coal workers. Occup Environ Med 52, 441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim KA, Lim Y, Kim JH, Kim EK, Chang HS, Park YM, Ahn BY. (1999) Potential biomarker of coal workers’ pneumoconiosis. Toxicol Lett 108, 297–302. [DOI] [PubMed] [Google Scholar]
- 9.Kelly M, Kolb M, Bonniaud P, Gauldie J. (2003) Re-evaluation of fibrogenic cytokines in lung fibrosis. Curr Pharm Des 9, 39–49. [DOI] [PubMed] [Google Scholar]
- 10.Vanhee D, Gosset P, Wallaert B, Voisin C, Tonnel AB. (1994) Mechanisms of fibrosis in coal workers’ pneumoconiosis. Increased production of platelet-derived growth factor, insulin-like growth factor type I, and transforming growth factor beta and relationship to disease severity. Am J Respir Crit Care Med 150, 1049–55. [DOI] [PubMed] [Google Scholar]
- 11.Boitelle A, Gosset P, Copin MC, Vanhee D, Marquette CH, Wallaert B, Gosselin B, Tonnel AB. (1997) MCP-1 secretion in lung from nonsmoking patients with coal worker’s pneumoconiosis. Eur Respir J 10, 557–62. [PubMed] [Google Scholar]
- 12.Brusasco V, Crapo R, Viegi G. (2005) Series “ATS/ERS Task Force: standardisation of lung function testing”. Eur Respir J 26, 318–38. [DOI] [PubMed] [Google Scholar]
- 13.Morris JF, Koski A, Johnson LC. (1971) Spirometric standards for healthy nonsmoking adults. Am Rev Respir Dis 103, 57–67. [DOI] [PubMed] [Google Scholar]
- 14.International Labour Office (ILO) (2002) Guidelines for the use of the ILO international classification of radiographs of pneumoconiosis. Revised Edition 2000. (Occupational Safety and Health Series, No. 22.) International Labor Organization, Geneva. [Google Scholar]
- 15.Khalil N, O’Connor RN, Unruh HW, Warren PW, Flanders KC, Kemp A, Bereznay OH, Greenberg AH. (1991) Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 5, 155–62. [DOI] [PubMed] [Google Scholar]
- 16.Laso FJ, Vaquero JM, Almeida J, Marcos M, Orfao A. (2007) Production of inflammatory cytokines by peripheral blood monocytes in chronic alcoholism: relationship with ethanol intake and liver disease. Cytometry B Clin Cytom 72, 408–15. [DOI] [PubMed] [Google Scholar]
- 17.Manco M, Marcellini M, Giannone G, Nobili V. (2007) Correlation of serum TNF-alpha levels and histologic liver injury scores in pediatric nonalcoholic fatty liver disease. Am J Clin Pathol 127, 954–60. [DOI] [PubMed] [Google Scholar]
- 18.Strieter RM, Standiford TJ, Rolfe MW, Kunkel SL. (1993) Cytokines of lung: Interleukin-8, Kelly J (Ed.), 281–305, Marcel Dekker, New York. [Google Scholar]
- 19.Strieter RM, Chensue SW, Basha MA, Standiford TJ, Lynch JP, Baggiolini M, Kunkel SL. (1990) Human alveolar macrophage gene expression of interleukin-8 by tumor necrosis factor-alpha, lipopolysaccharide, and interleukin-1 beta. Am J Respir Cell Mol Biol 2, 321–6. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Chen H. (2002) [The study on the interleukin-8 (IL-8)]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 19, 697–702 (in Chinese with English abstract). [PubMed] [Google Scholar]
- 21.Grotendorst GR, Smale G, Pencev D. (1989) Production of transforming growth factor beta by human peripheral blood monocytes and neutrophils. J Cell Physiol 140, 396–402. [DOI] [PubMed] [Google Scholar]
- 22.Borm PJA, Schins RPF. (2001) Genotype and phenotype in susceptibility to coal workers’ pneumoconiosis. the use of cytokines in perspective. Eur Respir J Suppl 32, 127s–33s. [PubMed] [Google Scholar]
- 23.Gosset P, Lassalle P, Vanhée D, Wallaert B, Aerts C, Voisin C, Tonnel AB. (1991) Production of tumor necrosis factor-α and interleukin-6 by human alveolar macrophages exposed in vitro to coal mine dust. Am J Respir Cell Mol Biol 5, 431–6. [DOI] [PubMed] [Google Scholar]
- 24.Jagirdar J, Begin R, Dufresne A, Goswami S, Lee TC, Rom WN. (1996) Transforming growth factor-beta (TGF-beta) in silicosis. Am J Respir Crit Care Med 154, 1076–81. [DOI] [PubMed] [Google Scholar]
- 25.Tarantal AF, Chen H, Shi TT, Lu CH, Fang AB, Buckley S, Kolb M, Gauldie J, Warburton D, Shi W. (2010) Overexpression of transforming growth factor-beta1 in fetal monkey lung results in prenatal pulmonary fibrosis. Eur Respir J 36, 907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leonard EJ, Yoshimura T. (1990) Human monocyte chemoattractant protein-1 (MCP-1). Immunol Today 11, 97–101. [DOI] [PubMed] [Google Scholar]
- 27.Rollins BJ, Yoshimura T, Leonard EJ, Pober JS. (1990) Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol 136, 1229–33. [PMC free article] [PubMed] [Google Scholar]
- 28.Inoshima I, Kuwano K, Hamada N, Hagimoto N, Yoshimi M, Maeyama T, Takeshita A, Kitamoto S, Egashira K, Hara N. (2004) Anti-monocyte chemoattractant protein-1 gene therapy attenuates pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 286, L1038–44. [DOI] [PubMed] [Google Scholar]
- 29.Zhang K, Gharaee-Kermani M, Jones ML, Warren JS, Phan SH. (1994) Lung monocyte chemoattractant protein-1 gene expression in bleomycin-induced pulmonary fibrosis. J Immunol 153, 4733–41. [PubMed] [Google Scholar]
- 30.Kim SJ. (2010) Leptin potentiates Prevotella intermedia lipopolysaccharide-induced production of TNF-alpha in monocyte-derived macrophages. J Periodont Implant Sci 40, 119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kythreotis P, Kokkini A, Avgeropoulou S, Hadjioannou A, Anastasakou E, Rasidakis A, Bakakos P. (2009) Plasma leptin and insulin-like growth factor I levels during acute exacerbations of chronic obstructive pulmonary disease. BMC Pulm Med 9, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langford D, Masliah E. (2002) Role of trophic factors on neuroimmunity in neurodegenerative infectious diseases. J Neurovirol 8, 625–38. [DOI] [PubMed] [Google Scholar]
- 33.Rostaing L, Peres C, Tkaczuk J, Charlet JP, Bories P, Durand D, Ohayon E, de Préval C, Abbal M. (2000) Ex vivo flow cytometry determination of intracytoplasmic expression of IL-2, IL-6, IFN-gamma, and TNF-alpha in monocytes and T lymphocytes, in chronic hemodialysis patients. Am J Nephrol 20, 18–26. [DOI] [PubMed] [Google Scholar]