Abstract
Transarterial radioembolization (TARE) with 90Y microspheres delivers low dose rate radiation (LDR) to intrahepatic tumors. In the current study, we examined clonogenic survival, DNA damage, and cell cycle distribution in hepatocellular carcinoma (HCC) cell lines treated with LDR in combination with varying doses and schedules of 5-fluorouracil (5-FU), gemcitabine, and sorafenib. Radiosensitization was seen with 1 to 3 μM 5-FU (enhancement ratio 2.2–13.9) and 30 to 100 nM gemcitabine (enhancement ratio 1.9–2.9) administered 24 hours before LDR (0.26 Gy/h to 4.2 Gy). Sorafenib radiosensitized only at high concentrations (3–10 μM) when administered after LDR. For a given radiation dose, greater enhancement was seen with LDR compared to standard dose rate therapy. Summarizing our clinical experience with low dose rate radiosensitization, 13 patients (5 with HCC, 8 with liver metastases) were treated a total of 16 times with TARE and concurrent gemcitabine. Six partial responses and one complete response were observed with a median time to local failure of 7.1 months for all patients and 9.9 months for patients with HCC. In summary, HCC is sensitized to LDR with clinically achievable concentrations of gemcitabine and 5-FU in vitro. Encouraging responses were seen in a small cohort of patients treated with TARE and concurrent gemcitabine. Future studies are needed to validate the safety and efficacy of this approach.
Keywords: hepatocellular carcinoma, yttrium-90 microspheres, transarterial radioembolization, radiosensitization
Introduction
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related deaths worldwide [1]. Transarterial radioembolization (TARE) with yttrium-90 (90Y) microspheres is one of the many treatment options available for patients with unresectable HCC. Because tumors in the liver derive most of their blood supply from the hepatic artery versus the portal vein [2], this therapy preferentially targets the tumor and spares uninvolved liver parenchyma. Prior reports have shown that TARE with 90Y microspheres is associated with a 42% partial response rate [3], [4] and longer progression-free survival than chemoembolization [5].
Concurrent chemoradiotherapy has proven to be more efficacious than radiation alone in the majority of gastrointestinal malignancies. A drug which preferentially sensitizes HCC to the cytotoxic effects of low dose rate radiation (LDR) produced by 90Y microspheres would potentially improve the efficacy of this therapy. Candidate drugs for radiosensitization include gemcitabine and 5-fluorouracil (5-FU) in addition to agents with known efficacy in HCC such as sorafenib. Gemcitabine and 5-FU are used routinely in combination with external beam radiation therapy for several intra-abdominal malignancies including pancreatic and gastric cancer [6], [7], [8]. Sorafenib was shown in a preclinical study to be an effective radiosensitizer in HCC when given after radiation therapy but not when given before treatment [9].
In the current study, we evaluated the potential of gemcitabine, 5-FU, and sorafenib to radiosensitize HCC to 90Y microspheres. Because the mean dose rate achieved during an administration of 90Y microspheres is 0.05 to 0.5 Gy per hour, we used a novel in vitro LDR model system that could deliver a dose rate in this range. We assessed clonogenic survival, DNA damage repair, and cell cycle distribution in HCC cells in vitro. Additionally, we report our early clinical experience of combining TARE with gemcitabine in patients with primary liver cancer and liver metastases.
Materials and Methods
Cell Culture and Drug Treatment
Human HCC cell lines (Hep3B, HepG2) were maintained in F-12 or RPMI media supplemented with 10% fetal bovine serum and penicillin/streptomycin. Experiments involving 5-FU were carried out in dialyzed serum with leucovorin. Gemcitabine (Eli Lilly, Indianapolis, IN), 5-FU/leucovorin (Sigma-Aldrich, St. Louis, MO), and sorafenib (University of Michigan Pharmacy, Ann Arbor, MI) were tested in combination with LDR. Drugs were diluted in PBS to appropriate concentrations which were selected to correspond to clinically achievable levels.
Radiation Techniques
LDR was delivered using a custom-built LDR device consisting of an array of cesium-137 sources. This array is shielded by interlocking 6-cm–thick pieces of Cerrobend and resides inside a cell culture incubator at 37°C. Dose homogeneity determined by film was within ± 5%. Cells were irradiated at a dose rate of 0.07, 0.10, or 0.26 Gy/h for 16 hours to a total dose of 1.1, 1.6, or 4.2 Gy. Standard dose rate radiation (SDR) was delivered using a Philips RT250 orthovoltage unit (Kimtron Medical, Oxford, CT) at a dose rate of approximately 2 Gy per minute to a total dose of 2 to 4 Gy. Dosimetry was carried out using an ionization chamber connected to an electrometer system directly traceable to a National Institute of Standards and Technology calibration.
Clonogenic Survival Assay
After radiation was complete, cells were suspended and counted then plated at set densities based on the dose of radiation received. Cells were incubated until visible colonies were present. Colonies were fixed with methanol/acetic acid (7:1) and stained with crystal violet. The number of colonies containing ≥ 50 cells was determined. Enhancement ratios were calculated by dividing the surviving fraction without drug by the surviving fraction with drug for each dose of radiation with an adjustment for plating efficiency. Experiments were performed in at least triplicate, and the mean and standard error were calculated.
Cell Cycle Distribution
Cell cycle distribution was determined using propidium iodide (PI, 0.018 mg/ml) staining and flow cytometry. Cells were fixed in 70% ethanol at the appropriate time points then incubated with PI before quantification using flow cytometry. Trout erythrocytes were used as the internal standard. Data were analyzed using FlowJo (Tree Star, Ashland, OR). Single-cell populations were gated, and histograms were modeled using the Watson method. A fixed distance between the G1 and G2 peaks was used for each cell line based on untreated controls.
γH2AX Detection
Cells were fixed with 70% ethanol after treatment at the appropriate time points. Fixed cells were incubated with anti-γH2AX mouse antibody (Millipore, Billerica, MA) at a concentration of 1:500 overnight followed by fluorescein isothiocyanate–labeled anti-mouse secondary antibody (Sigma-Aldrich) for 2 hours. Cells were then counted with flow cytometry. Trout erythrocytes were used as the internal standard. FlowJo software was used to quantify the percentage of cells staining positive for γH2AX.
Transarterial Radioembolization and Gemcitabine in Patients
Thirteen patients with primary liver cancer or liver metastases were treated with a single dose of gemcitabine (200–400 mg/m2) 1 day before TARE with TheraSpheres (Nordion, Ottawa, Canada). Radioembolization dose was defined as the dose to the entire lobar volume. Response was determined based on the Response Evaluation Criteria in Solid Tumors (RECIST). Survival endpoints were calculated from the start of treatment. Local failure was defined as progression in the region of the liver targeted with TARE. Patient were typically seen 1, 3, and 6 months after treatment with follow-up imaging obtained 2 to 3 months after treatment then every 4 to 6 months or as clinically indicated. Data were retrospectively collected and analyzed under an Institutional Review Board–approved protocol.
Statistical Analysis
The mean and standard error were calculated using Microsoft Excel Software (Seattle, WA). For in vitro studies, a Student’s t test was used to compare treatment groups. A P value of ≤ .05 was considered statistically significant. Experiments were performed in at least triplicate to ensure reproducibility. The Kaplan-Meier method was used to determine overall survival, local progression-free survival, and time to local failure for all patients treated. Median survival was calculated with JMP software (version 10; SAS, Cary, NC).
Results
Low Dose Rate Radiosensitization
To test our hypothesis that systemic therapy enhances the cytotoxic effect of LDR, we first determined the optimal schedule and concentration of each agent. Clonogenic survival assays with HCC cell lines were performed using gemcitabine, 5-FU/leucovorin, and sorafenib at different dosing schedules. Schedules were chosen based on our experience using these agents with external beam radiation therapy. For gemcitabine, cells were treated for 2 hours either 1 day before or just before LDR. Both schedules resulted in effective radiosensitization at a cytotoxic concentration of gemcitabine (100 nM); however, at noncytotoxic concentrations (10–30 nM), treatment 24 hours before LDR was required for optimal radiosensitization (Figure 1A). Similar to our findings with gemcitabine, treatment with 5-FU resulted in greater radiosensitization if started 24 hours before LDR compared to treatment just before LDR (Figure 1B). This schedule provided greater enhancement ratios at cytotoxic (3–10 μM) and noncytotoxic concentrations (1 μM) of 5-FU.
Figure 1.
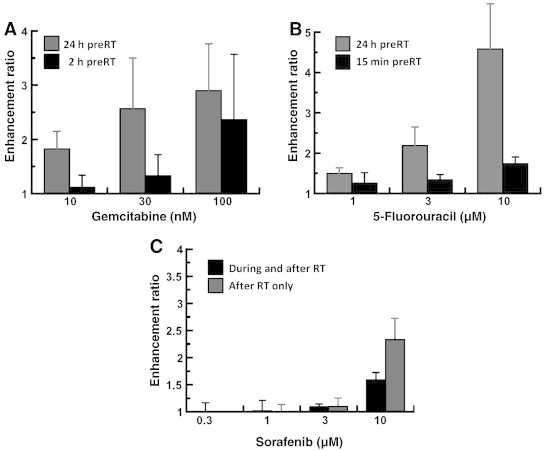
Effects of dose and schedule of systemic therapy on LDR radiosensitization. HepG2 cells were treated with gemcitabine (A), 5-FU (B), or sorafenib (C) at the indicated doses and schedules in combination with LDR (0.26 Gy/h to 4.2 Gy). Cells were assayed for clonogenic survival 24 hours after LDR. Shown are the mean enhancement ratios with standard error (n ≥ 3 for each condition).
Prior reports demonstrate that sorafenib radiosensitizes if administered after radiation but has protective effects if given before [9]. Using this information, we treated cells with sorafenib at the start of or immediately after LDR. Sorafenib was not an effective radiosensitizer at noncytotoxic concentrations (0.3–1 μM) with either dosing schedule. However, at a cytotoxic concentration (10 μM), radiosensitization was observed with both schedules (Figure 1C).
Using the optimal dosing schedules determined from the prior experiment, we next tested the effect of changing the radiation dose rate on radiosensitization with gemcitabine and 5-FU. Increasing the dose rate over the LDR range (from 0.07 to 0.10 to 0.26 Gy/h) resulted in increasing levels of radiosensitization with gemcitabine and 5-FU in both HCC cell lines (Table 1). Radiation delivered at a standard dose rate (2 Gy/min or 120 Gy/h) was associated with less radiosensitization compared to LDR for gemcitabine and 5-FU at most concentrations tested (Table 1). Overall, these data suggest that combining gemcitabine or 5-FU with LDR produced by 90Y microspheres is potentially an efficacious strategy in HCC.
Table 1.
Radiosensitization in Hepatocellular Carcinoma
| Dose rate Total dose |
Gem 30 nM |
Gem 100 nM |
5-FU 1 μM |
5-FU 3 μM |
Sorafenib 3 μM |
Sorafenib 10 μM |
|---|---|---|---|---|---|---|
| A. Enhancement Ratios for Hep3B Cells with Low and Standard Dose Rate Radiation | ||||||
| LDR 0.07 Gy/h 1.1 Gy |
0.99 | 1.14 | 2.26 | 1.81 | 1.61 | 1.19 |
| LDR 0.10 Gy/h 1.6 Gy |
1.62 | 1.55 | 1.02 | 3.51 | 1.22 | 2.36 |
| LDR 0.26 Gy/h 4.2 Gy |
1.90 | 2.31 | 7.92 | 13.85 | 1.58 | 5.55 |
| SDR 2 Gy/min 2 Gy |
1.27 | 1.73 | 1.48 | 1.18 | 1.14 | 1.58 |
| SDR 2 Gy/min 4 Gy |
1.03 | 1.41 | 1.87 | 2.84 | 1.56 | 2.09 |
| B. Enhancement Ratios for HepG2 Cells with Low and Standard Dose Rate Radiation | ||||||
| LDR 0.07 Gy/h 1.1 Gy |
2.06 | 2.69 | 1.89 | 2.09 | 0.91 | 4.19 |
| LDR 0.10 Gy/h 1.6 Gy |
2.42 | 2.83 | 2.03 | 1.95 | 1.24 | 4.50 |
| LDR 0.26 Gy/h 4.2 Gy |
2.16 | 2.87 | 2.16 | 3.30 | 1.05 | 2.61 |
| SDR 2 Gy/min 2 Gy |
1.63 | 0.94 | 0.92 | 1.72 | 1.10 | 1.48 |
| SDR 2 Gy/min 4 Gy |
1.53 | 1.19 | 2.45 | 1.79 | 1.46 | 1.11 |
Effect of Gemcitabine and 5-FU on LDR-Induced DNA Damage
Given the promising findings from the clonogenic survival assays, we next studied the formation and resolution of DNA double-strand breaks using γH2AX immunostaining and flow cytometry. Cells were treated with LDR (0.26 Gy/h for 16 hours) and gemcitabine or 5-FU as described above. Compared to LDR alone, treatment with 30 nM gemcitabine and LDR resulted in more unresolved DNA double-strand breaks in the HepG2 cell line immediately after radiation was complete (16 hours from the start of LDR). Flow cytometry analysis showed that 35% of HepG2 cells treated with gemcitabine and LDR were positive for γH2AX compared to 12% of cells treated with gemcitabine alone (P = .03) and 17% of cells treated with radiation alone (P = .07). These differences persisted at 6 and 24 hours after LDR (Figure 2).
Figure 2.
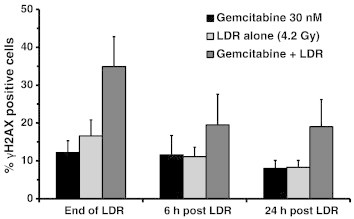
Formation and resolution of γH2AX foci. HepG2 cells were treated with 30 nM gemcitabine for 2 hours 1 day before LDR (0.26 Gy/h to 4.2 Gy). Cells were fixed and incubated with anti-γH2AX antibody and then analyzed by flow cytometry. Shown are the mean percent cells with DNA double-strand breaks after completion of LDR (n = 4).
For comparison, the above experiment with γH2AX was repeated using standard dose rate radiation (2 Gy/min) in place of LDR. We anticipated that there would be less DNA damage and/or impaired DNA repair in cells treated with SDR compared to LDR due to the lower levels of radiosensitization seen in the clonogenic survival study. Shortly after radiation (0–6 hours), HepG2 cells treated with radiation at either dose rate had a similar amount of DNA double-strand breaks with and without 30 nM gemcitabine. However, 24 hours after radiation, gemcitabine-treated HepG2 cells receiving LDR had impaired resolution of γH2AX (19% cells positive) compared to SDR (4% cells positive). These results suggest that DNA repair is impaired more in gemcitabine -treated cells receiving LDR compared to SDR.
The effect of 5-FU on the formation and resolution of LDR-induced DNA double-strand breaks was tested in a similar fashion as gemcitabine. Treatment with 5-FU and LDR was associated with more γH2AX-positive cells in the HepG2 cell line compared to 5-FU or LDR radiation alone. Treatment of HepG2 cells with 1 μM 5-FU and LDR resulted in 48% γH2AX-positive cells immediately after radiation was complete compared to 13% with 5-FU alone or RT alone, suggesting that 5-FU and LDR interact to induce DNA damage and/or impair DNA damage repair.
Effects of Gemcitabine or 5-FU and LDR Radiation on Cell Cycle Distribution
To further understand the mechanism behind LDR radiosensitization with gemcitabine and 5-FU, we next studied the effects of these treatments on cell cycle distribution. Treatment with 30 nM gemcitabine with LDR (0.26 Gy/h to 4.2 Gy) had significant cell cycle effects in the Hep3B cell line. Immediately after 16 hours of LDR, Hep3B cells treated with gemcitabine were more likely to be in G2/M phase (24%) than cells treated with RT alone (7%, P = .009) or gemcitabine alone (14%, P = .015) (Figure 3). This difference persisted at 2, 6, 12, and 24 hours after radiation (Figure 3C). Additionally, treatment with gemcitabine alone led to an increase in the number of Hep3B cells in S phase 24 hours later (corresponding to the start of LDR). In the HepG2 cell line, treatment with gemcitabine plus LDR resulted in a similar number of cells in G2/M as treatment with LDR alone, whereas treatment with gemcitabine alone was associated with a higher percentage of cells in S phase.
Figure 3.
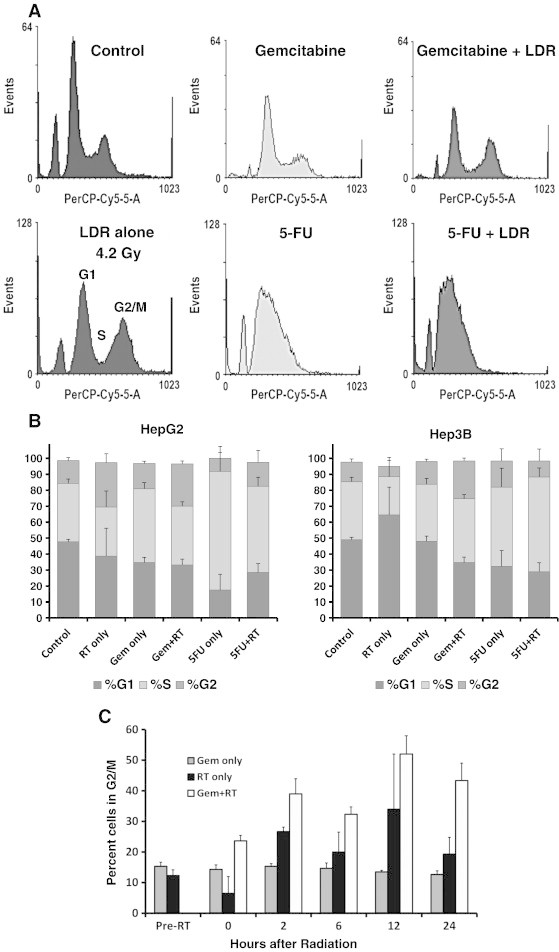
Effect of gemcitabine and 5-FU on LDR-induced cell cycle changes. HCC cell lines were treated with 30 nM gemcitabine or 3 μM 5-FU 24 hours before LDR (0.26 Gy/h for 16 hours). Cells were stained with PI and analyzed by flow cytometry. Shown are representative histograms at 24 hours after LDR (A), cell cycle distribution after LDR (B), and the percentage of Hep3B cells in G2/M at the indicated time points after LDR (C).
Similar to gemcitabine, we tested the effects of 5-FU and sorafenib on cell cycle in combination with LDR. Treatment with 3 μM 5-FU resulted in an increased number of cells in S phase compared to controls in both HepG2 (37% vs 57%, P < .001) and Hep3B (36% vs 54%, P = .06) cell lines (Figure 3). Additionally, adding 5-FU to radiation resulted in a higher percentage of cells in S phase in HepG2 (31% vs 54%, P = .01) and Hep3B (24% vs 59%, P = .01) cell lines compared to cells treated with LDR alone (Figure 3B). These data suggest that 5-FU induces S phase arrest in cells undergoing LDR. Of note, treatment with sorafenib after LDR did not significantly alter cell cycle distribution.
Clinical Experience with Liver Radioembolization and Concurrent Gemcitabine
Based on our preclinical results showing gemcitabine is an effective LDR radiosensitizer, we performed a review of our clinical experience with gemcitabine in combination with radioembolization. Thirteen patients with primary liver cancer or liver metastases were treated with 90Y microspheres and concurrent gemcitabine administered 24 hours before TARE. Three patients were treated to separate lobes of the liver at different times. Table 2 shows the characteristics of each patient with the doses of radiation and gemcitabine they received. Five patients were treated for liver-confined unresectable HCC, seven patients for metastatic melanoma, four patients for metastatic cholangioncarcinoma, and one patient for metastatic carcinoid. Three of the five patients with HCC had cirrhosis (all Child-Pugh score A), and three of the patients were HCV positive. A noncytotoxic gemcitabine dose of 200 mg/m2 (standard therapeutic dose is 1000 mg/m2) was used for 14 of the 16 treatments.
Table 2.
Patients Treated with 90Y Microspheres and Gemcitabine
| Radiation Dose Gy¶ | Gemcitabine Dose mg/m2 | Diagnosis | Number of Lesions | Vascular Involvement | Overall Survival Months | Time to Local Failure | Best Response (RECIST) | |
|---|---|---|---|---|---|---|---|---|
| 1 | 99.3 | 200 | Hepatocellular | 8 | No | 6.9 | 6.9‡ | CR |
| 2A§ | 80.2 | 200 | Hepatocellular | 4 | No | 32.9⁎ | 16.8 | PR |
| 2B§ | 155.0 | 200 | Hepatocellular | 4 | No | 17.9 | PR | |
| 3 | 85.3 | 200 | Hepatocellular | 2 | Yes | 12.3 | 9.9 | PR |
| 4 | 115.7 | 400 | Hepatocellular | 1 | No | 21.1⁎ | 3.8 | PD |
| 5 | 130.0 | 200 | Hepatocellular | 5 | No | 12.5 | 7.1 | SD |
| 6A§ | 97.4 | 200 | Melanoma | 8 | No | 27.7 | 6.4 | PR |
| 6B§ | 134.5 | 200 | Melanoma | > 10 | No | 7.8 | PR | |
| 7 | 102.4 | 200 | Melanoma | > 10 | No | 5.0 | 1.6 | PR |
| 8 | 89.0 | 200 | Melanoma | > 10 | No | 6.6† | 3.4 | PD |
| 9A§ | 103.7 | 200 | Melanoma | > 10 | No | 9.2 | 6.8 | SD |
| 9B§ | 109.8 | 200 | Melanoma | > 10 | No | 4.9 | PD | |
| 10 | 85.7 | 200 | Melanoma | > 10 | No | 15.9 | 9.3 | SD |
| 11 | 124.4 | 200 | Cholangio | > 10 | No | 2.2 | 2.2‡ | PD |
| 12 | 110.2 | 400 | Cholangio | > 10 | Yes | 5.5 | 6.4 | PD |
| 13 | 92.7 | 200 | Carcinoid | > 10 | No | 10.4 | 10.4‡ | SD |
Alive at time of analysis.
Lost to follow up.
No local progression at time of death.
Treated to separate regions at different times.
Calculated dose to lobe.
We reviewed the records of the 13 patients to determine treatment-related toxicity. The mean total bilirubin for the entire group did not change from baseline (0.68 mg/dl) to 1 month (0.68 mg/dl). However, at 3 and 6 months after TARE, the mean bilirubin of the group was higher at 0.95 mg/dl and 1.05 mg/dl, respectively. A clinically significant increase defined as a rise above 1.2 mg/dl was only seen in two patients at 3 and 6 months. In these patients, the rise in bilirubin was associated with an increased burden of disease. Absolute neutrophil or lymphocyte count did not substantially change from baseline to 1 month or 3 months after treatment. No patient developed neutropenia defined as a neutrophil count of less than 1500 per microliter. Clinically, two patients developed worsening ascites following treatment requiring hospitalization and/or intervention. It is unclear if their ascites were directly related to treatment or tumor progression. No variceal bleeding or encephalopathy was seen following treatment. One patient developed a duodenal ulcer months after TARE which was attributed to antiangiogenic therapy.
For all patients, median survival from the time of gemcitabine plus TARE was 12.3 months, and the time to local failure, defined as progression in the region targeted by TARE, was 7.1 months. In the five patients with liver-confined HCC there were one complete response, three partial responses, one patient with stable disease, and one patient with no response/progressive disease after treatment (Figure 4). Median time to local failure was 9.9 months and overall survival was 12.5 months for the patients with HCC. The eight patients treated for liver metastases had a median survival of 9.2 months and time to local failure of 6.4 months (Table 2). Overall, these findings suggest that radiosensitizing doses of gemcitabine can be combined with 90Y microspheres in patients with HCC and liver metastases.
Figure 4.
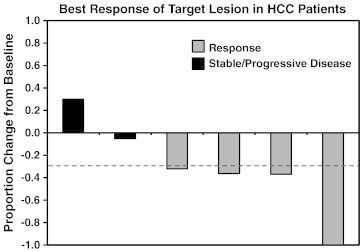
Response rate of patients treated with TARE and concurrent gemcitabine. Five patients with liver-confined HCC underwent treatment with 200 to 400 mg/m2 gemcitabine 1 day before TARE. One patient was treated to separate lobes of the liver at different times. Shown is the percent change in size of the target lesion based on RECIST.
Discussion
Despite the proven benefit of adding chemotherapy to radiation in most GI malignancies, combining chemotherapy with 90Y microspheres for HCC has not been previously studied. In the current study, we found that gemcitabine and 5-FU were effective radiosensitizing agents at noncytotoxic and clinically achievable concentrations in HCC cell lines treated with LDR (0.07–0.26 Gy/h). Interestingly, the level of radiosensitization with LDR was greater than what was observed in cells treated with SDR (2 Gy/min) under otherwise similar conditions. Sorafenib produced radiosensitization when administered after LDR; however, the doses required to radiosensitize were above a concentration which is achievable in patients. Given these results, gemcitabine and 5-FU are promising agents to combine with 90Y microspheres, whereas sorafenib may not produce more than an additive effect at clinically relevant concentrations.
Gemcitabine and 5-FU are antimetabolites with different mechanisms of action. Gemcitabine radiosensitizes by depleting phosphorylated deoxynucleotide pools, and maximum sensitization occurs under conditions producing S phase distribution and dATP depletion [10], [11], [12], [13]. We found that when noncytotoxic concentrations of gemcitabine were used, an incubation period of 24 hours was associated with increased radiosensitization compared to treatment just before LDR. This finding is consistent with prior reports showing that it takes several hours to deplete dNTP pools [13], [14]. Additionally, an increase in the number of cells in S phase was seen with our dosing schedule consistent with conditions needed for radiosensitization with gemcitabine [13], [14]. 5-FU’s main mechanism of action is through inhibition of thymidylate synthase [15]. In our study, 5-FU was associated with a pronounced S phase arrest in both HCC cell lines tested. Pretreatment for 24 hours was associated with improved radiosensitivity at noncytotoxic concentrations. Because high levels of enhancement were seen at noncytotoxic concentrations, the mechanism of radiosensitivity is not simply related to killing of radioresistant cells in S phase. More likely, treatment with 5-FU leads to inappropriate S phase progression during LDR [16], [17].
The findings from our study suggest that LDR and gemcitabine or 5-FU have complementary effects on cell cycle distribution leading to enhanced radiosensitivity. LDR alone was associated with G2 arrest which persisted for ≥ 24 hours after the 16-hour course of LDR was complete. LDR-induced G2 arrest is well established and is the basis of the inverse dose rate effect [18]. Treatment with gemcitabine plus LDR was associated with a higher percentage of cells in S phase compared to LDR alone in addition to G2 arrest. Additionally, treatment with 5-FU produced S phase arrest in both cell lines. Abnormal progression through S phase in conjunction with LDR-induced G2 arrest would be predicted to lead to increased radiosensitivity.
The formation and resolution of γH2AX foci provide insight into the induction of DNA damage and subsequent repair after LDR. As expected, in our study, we saw increased DNA damage and impaired DNA double-strand break repair when 5-FU or gemcitabine was added to LDR. A surprising finding was that DNA double-strand break repair was impaired for a longer period of time after LDR compared to cells treated with SDR at the same dose (4 Gy). Prior reports show that DNA damage is repaired during a course of LDR [19]. Therefore, one might predict less DNA damage after LDR compared to SDR therapy at the same dose. In this study, the percentage of γH2AX-positive cells was relatively similar for each dose rate when cells were examined shortly after radiation therapy; however, at 24 hours, treatment with gemcitabine and LDR was associated with a higher percentage of γH2AX-positive cells compared to treatment with SDR and gemcitabine. This finding could be explained by the long time (16 hours) required to deliver LDR resulting in greater depletion of dNTP pools by gemcitabine. Alternatively, prolonged exposure to LDR in combination with gemcitabine (or 5-FU) may cause permanent or prolonged cell cycle arrest affecting DNA damage response. A prior study found prolonged exposure to LDR leads to downregulation of critical DNA repair proteins including DNA-PKcs and Ku70, consistent with this hypothesis [20].
To our knowledge, this is the first report combining LDR with radiosensitizing chemotherapy to treat HCC. Treatment with TARE and concurrent gemcitabine was associated with an encouraging response in our small patient cohort. Prior reports of TARE have shown response rates of approximately 40% in HCC using similar response criteria as used in our study [4], [5]. In our experience, four of six primary liver tumors responded to gemcitabine followed by TARE including one complete response. Given the small number of patients and potential for selection bias in our cohort, the safety and efficacy of this approach cannot be determined.
In conclusion, gemcitabine and 5-FU are effective LDR radiosensitizers at clinically achievable concentrations. Given the preclinical findings, scientific rationale, and local control seen in our experience, the combination of radioembolization and chemotherapy should be prospectively studied in a larger patient cohort to determine if this treatment is safe and more efficacious than TARE alone.
Footnotes
Disclosure: The authors have no conflicts of interest to disclose.
References
- 1.Siegel R., Ward E., Brawley O., Jemal A. Cancer statistics 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Breedis C., Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969–977. [PMC free article] [PubMed] [Google Scholar]
- 3.Lewandowski R.J., Salem R. Yttrium-90 radioembolization of hepatocellular carcinoma and metastatic disease to the liver. Semin Intervent Radiol. 2006;23:64–72. doi: 10.1055/s-2006-939842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulik L.M., Carr B.I., Mulcahy M.F., Lewandowski R.J., Atassi B., Ryu R.K., Sato K.T., Benson A., Nemcek A.A., Gates V.L. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 5.Salem R., Lewandowski R.J., Kulik L., Wang E., Riaz A., Ryu R.K., Sato K.T., Gupta R., Nikolaidis P., Miller F.H. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497–507. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macdonald J.S., Smalley S.R., Benedetti J., Hundahl S.A., Estes N.C., Stemmermann G.N., Haller D.G., Ajani J.A., Gunderson L.L., Jessup J.M. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 7.McGinn C.J., Zalupski M.M., Shureiqi I., Robertson J.M., Eckhauser F.E., Smith D.C., Brown D., Hejna G., Strawderman M., Normolle D. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2001;19:4202–4208. doi: 10.1200/JCO.2001.19.22.4202. [DOI] [PubMed] [Google Scholar]
- 8.Moertel C.G., Frytak S., Hahn R.G., O'Connell M.J., Reitemeier R.J., Rubin J., Schutt A.J., Weiland L.H., Childs D.S., Holbrook M.A. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil, and high dose radiation + 5-fluorouracil: the Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705–1710. doi: 10.1002/1097-0142(19811015)48:8<1705::aid-cncr2820480803>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Plastaras J.P., Kim S.H., Liu Y.Y., Dicker D.T., Dorsey J.F., McDonough J., Cerniglia G., Rajendran R.R., Gupta A., Rustgi A.K. Cell cycle-dependent and schedule-dependent antitumor. Effects of sorafenib combined with radiation. Cancer Res. 2007;67:9443–9454. doi: 10.1158/0008-5472.CAN-07-1473. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence T.S., Blackstock A.W., McGinn C. The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Semin Radiat Oncol. 2003;13:13–21. doi: 10.1053/srao.2003.50002. [DOI] [PubMed] [Google Scholar]
- 11.Abbruzzese J.L., Grunewald R., Weeks E.A., Gravel D., Adams T., Nowak B., Mineishi S., Tarassoff P., Satterlee W., Raber M.N. A Phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol. 1991;9:491–498. doi: 10.1200/JCO.1991.9.3.491. [DOI] [PubMed] [Google Scholar]
- 12.Grunewald R., Abbruzzese J.L., Tarassoff P., Plunkett W. Saturation of 2’,2’-difluorodeoxycytidine 5’-triphosphate accumulation by mononuclear cells during a phase I trial of gemcitabine. Cancer Chemother Pharmacol. 1991;27:258–262. doi: 10.1007/BF00685109. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence T.S., Chang E.Y., Hahn T.M., Hertel L.W., Shewach D.S. Radiosensitization of pancreatic cancer cells by 2’,2’-difluoro-2’-deoxycytidine. Int J Radiat Oncol Biol Phys. 1996;34:867–872. doi: 10.1016/0360-3016(95)02134-5. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence T.S., Chang E.Y., Hahn T.M., Shewach D.S. Delayed radiosensitization of human colon carcinoma cells after a brief exposure to 2',2'-difluoro-2'-deoxycytidine (gemcitabine) Clin Cancer Res. 1997;3:777–782. [PubMed] [Google Scholar]
- 15.Lawrence T.S., Tepper J.E., Blockstock A.W. Fluropyrimidine-radiation interactions in cells and tumors. Semin Radiat Oncol. 1997;4:260–266. doi: 10.1053/SRAO00700260. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence T.S., Davis M.A., Loney T.L. Fluoropyrimidine-mediated radiosensitization depends on cyclin E–dependent kinase activation. Cancer Res. 1996;56:3203–3206. [PubMed] [Google Scholar]
- 17.Lawrence T.S., Davis M.A., Tang H.Y., Maybaum J. Flurodeoxyuridine-mediated cytotoxicity and radiosensitization require S phase progression. Int J Radiat Biol. 1996;70:273–280. doi: 10.1080/095530096145003. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell J.B., Bedofrd J.S., Bailey S.M. Dose rate effects on the cell cycle and survival of S3 HeLA and V79 cells. Radiat Res. 1979;79:520–536. [PubMed] [Google Scholar]
- 19.Steel G.G. The ESTRO Breur lecture. Cellular sensitivity to low dose-rate irradiation focuses the problem of tumour radioresistance. Radiother Oncol. 1991;20:71–83. doi: 10.1016/0167-8140(91)90140-c. [DOI] [PubMed] [Google Scholar]
- 20.Wang H., Qu A., Zhoa Y., Wang J. The different biological effects of single, fractionated, and continuous low dose rate irradiation on CL 187 colorectal cancer cells. Radiat Oncol. 2013;8:196. doi: 10.1186/1748-717X-8-196. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


