Abstract
PURPOSE
To determine whether volumetric changes of enhancement as seen on contrast-enhanced magnetic resonance (MR) imaging can help assess early tumor response and predict survival in patients with metastatic uveal melanoma after one session of transarterial chemoembolization (TACE).
MATERIALS AND METHODS
Fifteen patients with 59 lesions who underwent MR imaging before and 3 to 4 weeks after the first TACE were retrospectively included. MR analysis evaluated signal intensities, World Health Organization (WHO), Response Evaluation Criteria in Solid Tumors (RECIST), European Association for the Study of the Liver (EASL), modified RECIST (mRECIST), tumor volume [volumetric RECIST (vRECIST)], and volumetric tumor enhancement [quantitative EASL (qEASL)]. qEASL was expressed in cubic centimeters [qEASL (cm3)] and as a percentage of the tumor volume [qEASL (%)]. Paired t test with its exact permutation distribution was used to compare measurements before and after TACE. The Kaplan-Meier method with the log-rank test was used to calculate overall survival for responders and non-responders.
RESULTS
In target lesions, mean qEASL (%) decreased from 63.9% to 42.6% (P = .016). No significant changes were observed using the other response criteria. In non-target lesions, mean WHO, RECIST, EASL, mRECIST, vRECIST, and qEASL (cm3) were significantly increased compared to baseline. qEASL (%) remained stable (P = .214). Median overall survival was 5.6 months. qEASL (cm3) was the only parameter that could predict survival based on target lesions (3.6 vs 40.5 months, P < .001) or overall (target and non-target lesions) response (4.4 vs 40.9 months, P = .001).
CONCLUSION
Volumetric tumor enhancement may be used as a surrogate biomarker for survival prediction in patients with uveal melanoma after the first TACE.
Introduction
Uveal melanoma is the most common primary intraocular malignant tumor in adults [1]. It is a rare disease with an incidence of 5.1 per million in the United States [2]. The 5-year survival rate ranges from 77% to 84% [2], [3]. Unlike cutaneous melanoma that has a widespread metastatic pattern [4], uveal melanoma has a significant predilection for metastasis to the liver [5]. Approximately 50% of the patients will develop metastatic liver disease. Although there are effective local therapies to eliminate and prevent recurrence within the eye (radioactive plaque, proton beam, enucleation), there are no effective systemic therapies for metastatic uveal melanoma [6]. As the liver is the first and, in many cases, the only site for metastatic disease, new modalities of therapy, including the use of regional therapies such transarterial chemoembolization (TACE), have been used.
The clinical course is highly dependent on disease progression within the liver. Once diagnosed with liver metastasis, the prognosis is dismal with a median survival of 2 months for patient receiving no treatment and 5 to 7 months for patients who received therapy [7], [8]. Thus, determining the response to TACE early after the locoregional treatment is crucial to guide the course of therapy.
Overall survival is the ultimate end point in clinical cancer research. However, most clinical trials rely on imaging criteria as a surrogate for survival [9]. For the purpose of radiologic response evaluation, the World Health Organization (WHO) response criteria were introduced in 1979. The WHO criteria are based on the sum of the product of bidimensional diameters of the lesions [10]. To address some limitations of the WHO criteria, the Response Evaluation Criteria in Solid Tumors (RECIST) was introduced in 2000 [11] and was revised to version 1.1 in 2009 [12]. RECIST is based on the sum of the unidimensional longest diameters. Both WHO criteria and RECIST were designed to evaluate systemic chemotherapy in which all tumors are equally exposed to systemic agents and address shrinkage of tumor size. In the case of locoregional therapy such as TACE, clinical benefit is not always correlated with tumor shrinkage but could be paralleled with necrosis of a viable tumor. Because the WHO criteria and RECIST are based on tumor size measurements, they do not address antitumor activity such as necrosis. Therefore, in response to these concerns, the European Association for the Study of the Liver (EASL) recommended measuring changes in the area of tumor enhancement [13]. More recently, the American Association for the Study of Liver Disease proposed an amendment of RECIST [modified RECIST (mRECIST)] to take into consideration changes in tumor enhancement as a biomarker of tumor viability [14].
It has been acknowledged that assessing treatment response using volumetric measurements should be a priority [14]. Prior studies showed the value of volumetric assessment in determining tumor response after intraarterial therapies [15], [16], [17].
The objective of this study was to determine whether quantitative volumetric changes as seen on contrast-enhanced magnetic resonance (MR) imaging can help assess early tumor response and predict survival in patients with metastatic uveal melanoma after one session of TACE.
Materials and Methods
This was a single-institution retrospective study. The study was compliant with the Health Insurance Portability and Accountability Act and was approved by the Institutional Review Board. Informed consent was waived.
Study Design
A review of the database of prospectively enrolled patients with uveal melanoma who underwent TACE at our institution from 2004 to 2014 was performed. A total of 21 patients were identified.
Inclusion criteria were given as follows: 1) diagnosis of liver metastasis confirmed by means of biopsy; 2) absence of previous systemic chemotherapy and/or liver directed therapies that might influence tumor response; 3) patients who underwent dynamic contrast-enhanced MR imaging before and approximately 3 to 4 weeks after TACE; 4) an Eastern Cooperative Oncology Group performance status of up to 2; 5) additional criteria included Child-Pugh class; unifocal or multifocal hepatic malignancy; absent or limited extrahepatic malignancy; absent or trace ascites; albumin level of more than 2.5 g/dl; alanine aminotransferase and aspartate aminotransferase levels of less than five times the upper normal limit; total serum bilirubin level of less than 3.0 mg/dl; serum creatinine level of less than 2.0 mg/dl; platelet count of at least 50,000/mm3; international normalized ratio of up to 1.5; at least partial patency of the portal venous system. Six patients were excluded for the following reasons: previous systemic and/or locoregional therapies (n = 1) and absence of follow-up MR imaging after TACE (n = 5). On the basis of these criteria, the final study population included 15 patients. Baseline characteristics are summarized in Table 1.
Table 1.
Baseline Patient Characteristics
| Characteristic | Value (%) |
|---|---|
| No. of Patients | 15 (100) |
| Sex | |
| Female | 8 (53.3) |
| Male | 7 (46.7) |
| Age* | |
| All patients | 63 (11.2) [range, 46-83] |
| Female | 59 (10.8) [range, 46-82] |
| Male | 69 (9.8) [range, 57-83] |
| Ethnicity | |
| White | 15 (100) |
| Eye treatment | |
| Enucleation | 6 (40) |
| Radioactive plaque | 5 (33.3) |
| Radioactive plaque + enucleation | 3 (20) |
| Radiotherapy | 1 (6.7) |
| Biopsy-proven liver metastasis | 15 (100) |
| Tumor number liver lesions | |
| 10-20 | 3 (20) |
| >20 | 12 (80) |
| Extrahepatic disease | 11 (73.3) |
| Eastern Cooperative Oncology Group performance status | |
| Grade 0 | 9 (60) |
| Grade 1 | 6 (40) |
| Child-Pugh class | |
| A | 15 (100) |
| Patent portal vein | 15 (100) |
Note: Except where indicated, data represent number of patients and numbers in parentheses are percentages.
*Data are represented as means ± standard deviation.
TACE Protocol
All patients considered for TACE were discussed at our multidisciplinary liver tumor board. All TACE procedures were performed by one experienced interventional radiologist with 16 years of experience by using a consistent approach as reported previously [18]. Briefly, an 18-gauge single-wall needle was used with the Seldinger technique to access the right common femoral artery. A 5-F vascular sheath was placed over a 0.035-inch Bentson guidewire (Cook, Bloomington, IN). With fluoroscopic guidance, a 5-F Simmons-1 catheter (Cordis, Miami Lakes, FL) was advanced over the wire and reformed into the aortic arch and used to select the celiac axis. Then, a Renegade HI-FLO microcatheter was advanced over a Fathom-16 wire (Boston Scientific, Natick, MA) into the desired hepatic artery branch, depending on the tumor location. Selective catheterization was performed to achieve lobar or sub-/segmental embolization based on the targeted lesions. A solution containing 50 mg of doxorubicin and 10 mg of mitomycin C in a 1:1 mixture with iodized oil (Lipiodol; Laboratoire Guerbet, Aulnay-sous-Bois, France) was infused and followed by 100- to 300-μm diameter microspheres (Embospheres; Merit Medical Systems, South Jordan, UT). Substantial arterial flow reduction to the tumor was defined as the technical end point of embolization; complete occlusion of the tumor-feeding blood vessels was avoided to maintain the arterial pathway for potential retreatment.
MR Imaging Protocol
MR imaging was performed at baseline and 3 to 4 weeks after the initial TACE by using a 1.5-T superconducting MR system (GE Signa; GE Medical Systems, Milwaukee, WI) and a phased-array torso coil for signal reception. The protocol included 1) axial T2-weighted fast spin-echo images (repetition time/echo time, 5000/100 milliseconds; matrix size, 256 × 256; section thickness, 8 mm; intersection gap, 2 mm; receiver bandwidth, 32 kHz), 2) axial T1-weighted dual fast gradient-recalled echo sequence, and 3) axial breath-hold unenhanced and contrast-enhanced [0.1 mmol per kilogram of body weight of intravenous gadodiamide (Omniscan; GE Healthcare, Princeton, NJ)] T1-weighted three-dimensional fat-suppressed spoiled gradient-recalled echo images (5.1/1.2; field of view, 320-400 mm; matrix size, 192 × 160; section thickness, 4-6 mm; receiver bandwidth, 64 kHz; flip angle, 15°) in the arterial, portal venous, and equilibrium phases (20 seconds, 60-70 seconds, and 180-200 seconds after intravenous contrast material injection, respectively).
MR Image Analysis
Quantitative volumetric image analysis was performed by a radiologist (with 7 years of experience). Tumor response assessment was conducted by two radiologists (with 7 and 9 years of experience) during the same reading session to ensure careful comparison of pretreatment and posttreatment findings. Any discrepancy was resolved by consensus.
For each patient, 2 lesions in the treated lobe of the liver (target lesions) and 2 lesions in the untreated lobe (non-target lesions) were evaluated [30 target and 29 non-target lesions (one patient had only one non-target lesion); a total of 59 lesions]. Lesions had a minimum diameter of 1 cm. To ensure independent sampling, the two largest lesions were evaluated in each lobe of the liver.
The signal intensity of all the target and non-target lesions was graded on T2-weighted and T1-weighted images as isointense, hypointense, or hyperintense in relation to normal liver tissue. High signal intensity lesions on T2-weighted images were also compared to the spleen. In heterogeneous lesions on T2- and T1-weighted images (e.g., with areas of hypointensity and hyperintensity), the lesions were deemed isointense, hypointense, or hyperintense depending on the most prevalent signal in each respective lesion. In cases of lesions that had hyperintense signal intensities in relation to the liver tissue on unenhanced T1-weighted images, subtraction was performed to assess tumor enhancement.
Quantitative Volumetric Image Analysis
Quantitative volumetric image analysis was performed using a semiautomatic three-dimensional (3D) software prototype (Medisys; Philips Research, Suresnes, France), as described in detail previously [19], [20]. Briefly, a semiautomatic 3D segmentation mask was generated on the 20-second contrast-enhanced MR images (arterial phase) obtained before and after TACE (Figure 1, A and B). The arterial phase was chosen because all the lesions of the study population demonstrated much better enhancement than in the portal-venous phase. The overall tumor volume – defined as volumetric RECIST (vRECIST) – was obtained on the basis of this segmentation (Figure 1, C and D). The MR imaging scan obtained before contrast material administration (Figure 1, E and F) was subtracted from the 20-second scan to remove any background signal. This step is particularly important for the assessment of lesions that may exhibit high signal intensity on precontrast T1-weighted images due to hemorrhage with the presence of methemoglobin and/or due to melanin as seen in some metastasis of uveal melanoma [21], [22]. The 3D segmentation mask was then transposed onto this subtracted MR imaging scan. The average enhancement values from the subtracted MR imaging scan used for the quantitative volumetric tumor enhancement – defined as quantitative EASL (qEASL) – calculation were obtained as follows: a region of interest (ROI) formed by 1 cm3 was placed in the normal appearing liver parenchyma as a reference for normalization to calculate the relative enhancement within the tumor (Figure 1, G and H). The ROI was placed in the ipsilateral lobe of the evaluated lesion at a level of section on which the lesion had its largest diameter and on the extratumoral liver parenchyma identified as non-enhancing after image subtraction. ROI placement was carefully performed to avoid any adjacent main branch blood vessels, the gallbladder, liver periphery, and motion artifacts. Viable (i.e., enhancing) tumor [13] was defined as voxels within the 3D segmentation mask where the enhancement was higher (defined as >2 standard deviation value of the reference ROI) than that of the normal liver parenchyma. The volume of viable tumor expressed in cubic centimeters (cm3) was measured for each lesion [qEASL (cm3)] and also defined as a percentage of the total tumor volume [qEASL (%)]. Subsequently, to visually demonstrate viable tumor regions within the 3D segmentation mask based on the previous ROI calculations, a color map with a blue-red scale was automatically generated by the software (blue representing non-enhancing necrotic tissue and red representing viable enhancing tumor tissue; Figure 1, G and H). Further details of the technique are presented in the Supplementary Materials.
Figure 1.
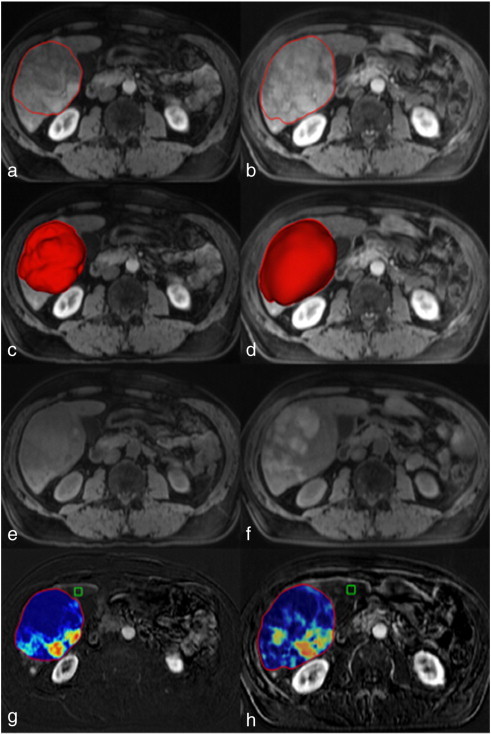
Quantitative volumetric contrast-enhanced MR imaging assessment technique (qEASL). The left column represents baseline MR imaging, and the right column represents the follow-up MR imaging after TACE. (a and b) The semiautomated tumor segmentation on a representative contrast-enhanced T1-weighted MR sequence at the arterial phase. (c and d) The corresponding volume of the segmented tumor in a 3D rendering. (e and f) The T1-weighted MR sequence before contrast administration to demonstrate baseline background signal intensity of the tumors. (g and h) The qEASL color maps of the tumor on the subtracted MR imaging scan [the scan before contrast material (E and F) was subtracted from the arterial phase scan (a and b) to remove any background signal intensity]. Color maps: red represents maximum enhancement and blue represents no enhancement, normalized by the ROI. Green box: 3D ROI used as the reference background of image intensity.
Tumor Response Assessment and Overall Survival
Each patient was classified as a responder or non-responder according to WHO, RECIST, EASL, mRECIST, vRECIST, and qEASL criteria on the basis of pretreatment and 3 to 4 weeks posttreatment MR imaging results of the target and non-target lesions. Response categorization for EASL, mRECIST, qEASL (cm3), and qEASL (%) was based on arterial phase images. Because no guidelines exist for volumetric tumor response criteria, we deliberately selected the same cutoff values that are currently used in RECIST and mRECIST for vRECIST and qEASL to unify and simplify response assessment in a clinical setting. Thus, by using the formula: Volume = 4/3πr3, where r is the radius and π is the mathematical constant representing the ratio of a circle's circumference to its diameter, a decrease of 30% defining partial response (PR) using the unidimensional RECIST and mRECIST corresponds to a decrease of 65% of tumor volume. Table 2 summarizes tumor response criteria.
Table 2.
Response Criteria
| WHO | RECIST | EASL | mRECIST | vRECIST | qEASL (cm3) | qEASL (%) | |
|---|---|---|---|---|---|---|---|
| CR | Disappearance of all target lesions | Disappearance of all target lesions | Disappearance of all enhancing tissue in all target lesions | Disappearance of all enhancing tissue in all target lesions | Disappearance of all target lesions | Disappearance of all enhancing tissue in all target lesions | Disappearance of all enhancing tissue in all target lesions |
| PR | ≥50% decrease in the sum of the product of bidimensional diameter of the target lesions | ≥30% decrease in the sum of the longest diameter of the target lesions | ≥50% decrease in the sum of the product of bidimensional diameter of enhancing tissue of the lesions | ≥30% decrease in the sum of the longest enhancing diameter of the target lesions | ≥65% decrease in the sum of the volume of the target lesions | ≥65% decrease in the sum of enhancing tissue volume of the lesions | ≥65% decrease in the sum of percentage of enhancing tissue of the lesions |
| PD | ≥25% increase in the sum of the product of bidimensional diameter of the target lesions | ≥20% increase in the sum of the longest diameter of the target lesions | ≥25% increase in the sum of the product of bidimensional diameter of the lesions | ≥20% increase in the sum of the longest enhancing diameter of the target lesions | ≥73% increase in the sum of the volume of the target lesions | ≥73% increase in the sum of enhancing tissue volume of the lesions | ≥73% increase in the sum of percentage of enhancing tissue of the lesions |
| SD | Any case that does not qualify for either CR, PR, or PD | Any case that does not qualify for either CR, PR, or PD | Any case that does not qualify for either CR, PR, or PD | Any case that does not qualify for either CR, PR, or PD | Any case that does not qualify for either CR, PR, or PD | Any case that does not qualify for either CR, PR, or PD | Any case that does not qualify for either CR, PR, or PD |
Note: WHO is calculated by measuring the longest diameter of the tumor in the axial plane and by drawing a line perpendicular to it. EASL is calculated by measuring the longest diameter of the enhancing tumor in the axial plane and by drawing a line perpendicular to it. mRECIST is calculated by measuring the longest diameter of the enhancing tumor in the axial plane. qEASL (cm3) is calculated by measuring the volume of enhancing tumor. qEASL (%) is calculated by measuring the percentage of enhancing tumor in the lesion volume.
Objective response was defined as complete response (CR) and PR. The patients with objective response were classified as responders, and the other patients [with stable disease (SD) and progressive disease (PD)] were classified as non-responders. As no data exist for the response assessment in uveal melanoma metastatic to the liver with regard to the inclusion of target and non-target lesions, the final response assessment was based on the target lesions alone and also determined by incorporating the target and non-target lesion responses (overall response) [10], [11], [12], [14].
Statistical Analysis
Data were summarized using descriptive statistics (count and frequency for categorical variables and mean and range for continuous variables). Significance levels and confidence intervals (CIs) were calculated, when possible, with exact methods that do not rely on normal approximations, to increase validity of the findings. A paired Student's t test with its exact permutation distribution was used to compare size, tumor volume, and tumor enhancement before and after TACE to evaluate tumor response to treatment. To evaluate the change in signal intensities on T2- and T1-weighted images before versus after TACE, the ratio of the sample proportion of “T2 = 2” and “T1 = 2” in all target and non-target lesions before versus after treatment was calculated. The significance level of this ratio was obtained as twice its tail probability from its exact permutation distribution [23], where permutations were performed, for each patient independently, between the pretreatment and the posttreatment vector of the T2 and T1 signal values of the target and non-target lesions. The overall survival was calculated from the date of the first TACE until death. The median overall survival of the entire cohort was estimated from the 50% point of the Kaplan-Meier curve, and its standard error and 95% CI were obtained using the jackknife technique. The predictive value of each response criterion was evaluated on its own (univariate) and then in a multivariate analysis. The univariate predictive value was evaluated by 1) the survival curves for responders and non-responders using Kaplan-Meier; 2) Cox proportional hazard ratios (HRs) between the curves of responders and non-responders, whose significance level was calculated using the log-rank test and its exact permutation distribution; and 3) the percent variance (R2) in survival that is explained by the “response”/”non-response” categorization. The 95% CIs for the HR between responders and non-responders were calculated for every method using the exact inference procedure for HRs [24], implemented with the algorithm for computing exact CIs for odds ratios in conditional logistic regression (Georg Heinze and Tobias Ladner (2013). logistiX: Exact logistic regression including Firth correction. R package version 1.0-1). To minimize bias, R2 was estimated by cross-validation. A multivariate analysis was explored by a rule that selects the first predictor as the one that has the highest predictive value of survival based on R2 and then including the next predictor if the inclusion increases the predictive value. A difference with a two-tailed P value of less than .05 was considered statistically significant. Statistical analysis was performed with a software package (R: A Language and Environment for Statistical Computing, R Core Team, R Foundation for Statistical Computing, Vienna, Austria, 2013).
Results
Patient Data
Mean time from uveal melanoma diagnosis and liver metastasis was 103.4 ± 110.6 months (range, 3-424). Mean time from pretreatment MR imaging to the first TACE was 2.2 ± 1.8 weeks (range, 0-7). Mean time from the TACE to posttreatment MR imaging was 4 ± 1.3 weeks (range, 3-7). Mean follow-up period was 13.5 ± 18.2 months (range, .7-58.7). A mean of 2.9 ± 1.7 TACE (range, 1-6) was performed per patient, for a total of 43 procedures. Four patients (26.7%) underwent only one TACE session. After the first TACE, the number of patients who underwent second, third, fourth, fifth, and sixth session of TACE was 4 (26.7%), 1 (6.7%), 3 (20%), 2 (13.3%), and 1 (6.7%), respectively. Thirteen TACE (86.7%) were performed on the right lobe of the liver and 2 (13.3%) on the left. A total of 114 MR imaging studies were reviewed in this cohort (mean MR imaging exam per patient, 7.6 ± 7.5; range, 2-27).
MR Imaging Data
Signal intensities
Signal intensities before and after TACE are summarized in Table 3. On fat-suppressed T2-weighted fast spin-echo sequences, there were no statistically significant differences in signal intensity in target and non-target lesions before and after TACE (P = .367 and P = .25, respectively). Similar results were obtained on single-shot T2-weighted sequences with no significant change in signal intensity in target and non-target lesions before and after TACE (P = .504 and P = .761, respectively). However, on T1-weighted images, target lesions depicted significantly more hyperintense signals relative to the liver after TACE compared to the baseline MR imaging (P = .002), whereas this was not the case for non-target lesions (P = .124).
Table 3.
Signal Intensities before and after TACE
| Target Lesions |
Non-Target Lesions |
|||||
|---|---|---|---|---|---|---|
| Before TACE | After TACE | P Value | Before TACE | After TACE | P Value | |
| FS T2-weighted images | ||||||
| Hypointense relative to liver | 0 (0) | 0 (0) | .367 | 1 (4) | 0 (0) | .250 |
| Hyperintense relative to liver | 21 (70) | 17 (57) | 21 (72) | 18 (62) | ||
| Hyperintense relative to liver and spleen | 9 (30) | 13 (43) | 7 (24) | 11 (38) | ||
| SS T2-weighted images | ||||||
| Hypointense relative to liver | 0 (0) | 1 (3) | .504 | 0 (0) | 0 (0) | .761 |
| Hyperintense relative to liver | 17 (57) | 14 (47) | 19 (66) | 17 (59) | ||
| Hyperintense relative to liver and spleen | 13 (43) | 15 (50) | 10 (34) | 12 (41) | ||
| T1-weighted images | ||||||
| Isointense relative to liver | 1 (3) | 0 (0) | .002 | 0 (0) | 0 (0) | .124 |
| Hypointense relative to liver | 23 (77) | 11 (37) | 25 (86) | 20 (69) | ||
| Hyperintense relative to liver | 6 (20) | 19 (63) | 4 (14) | 9 (31) | ||
Target and Non-Target Lesions
Table 4 summarizes the pretreatment and 3 to 4 weeks posttreatment changes in conventional tumor response criteria according to WHO, RECIST, EASL, and mRECIST, as well as volumetric changes according to vRECIST and qEASL in all target and non-target lesions. In target lesions, none of the conventional tumor response criteria showed significant changes after TACE. Likewise, the volume of enhancing tumor [qEASL (cm3)] did not show any statistically significant difference (P = .270), while the percentage of enhancing tumor [qEASL (%)] decreased significantly (P = .016), reflecting tumor necrosis induced by TACE. As opposed to the target lesions, non-target lesions showed statistically significant increase in all conventional criteria as well as in vRECIST and qEASL (cm3), while the percentage of enhancing tumor [qEASL (%)] remained stable.
Table 4.
Changes in Conventional and Volumetric Tumor Response Criteria in Target and Non-Target Lesions after TACE
| Target Lesions |
Non-Target Lesions |
|||||
|---|---|---|---|---|---|---|
| Before TACE | After TACE | P Value | Before TACE | After TACE | P Value | |
| Conventional response criteria | ||||||
| WHO | 55.4 cm2 (59.6) | 60.6 cm2 (79.1) | .526 | 8.7 cm2 (7.1) | 11.2 cm2 (9) | <.001 |
| RECIST | 7.5 cm (4.7) | 7.8 cm (5.7) | .594 | 3.2 cm (1.3) | 3.7 cm (1.6) | .006 |
| EASL | 33.4 cm2 (38.9) | 36.9 cm2 (64.2) | .701 | 5.1 cm2 (4.6) | 7.9 cm2 (7.8) | .003 |
| mRECIST | 6.4 cm (4.1) | 6.3 cm (6) | .878 | 2.6 cm (1.1) | 3.1 cm (1.6) | .008 |
| Volumetric response criteria | ||||||
| vRECIST | 297.2 cm3 (473.4) | 409.5 cm3 (847.2) | .244 | 15 cm3 (19.9) | 20.5 cm3 (25.2) | <.001 |
| qEASL[cm3] | 152.1 cm3 (240.6) | 268.3 cm3 (644.8) | .270 | 10.1 cm3 (15) | 14.2 cm3 (17.5) | .008 |
| qEASL[%] | 63.9 % (26.2) | 42.6 % (33.8) | .016 | 72.9 % (28.7) | 72.4 % (27.9) | .214 |
Tumor Response Criteria in Target and Non-Target Lesions
Table 5 summarizes the tumor response in all patients according to target and non-target lesions. No new lesion appeared in the study population between the pretreatment and 3 to 4 weeks posttreatment MR imaging.
Table 5.
Tumor Response Assessment after the First TACE in Target and Non-Target Lesions
| Target Lesion |
Non-Target Lesion |
|||||||
|---|---|---|---|---|---|---|---|---|
| CR | PR | SD | PD | CR | PR | SD | PD | |
| Conventional response criteria | ||||||||
| WHO | 0 | 0 | 12 | 3 | 0 | 0 | 7 | 8 |
| RECIST | 0 | 0 | 13 | 2 | 0 | 0 | 12 | 3 |
| EASL | 1 | 5 | 3 | 6 | 0 | 1 | 3 | 11 |
| mRECIST | 1 | 4 | 8 | 2 | 0 | 0 | 10 | 5 |
| Volumetric response criteria | ||||||||
| vRECIST | 0 | 0 | 13 | 2 | 0 | 0 | 9 | 6 |
| qEASL (cm3) | 0 | 5 | 8 | 2 | 0 | 0 | 8 | 7 |
| qEASL (%) | 0 | 5 | 9 | 1 | 0 | 0 | 15 | 0 |
Overall Response
Conventional Response Criteria
When using WHO measurements, six patients (40%) had SD and the remaining nine patients (60%) had PD. According to RECIST, eleven patients (73%) had SD and four patients (27%) had PD. Thus, the use of both anatomic conventional criteria did not classify any patients as responders after TACE and no comparative survival analysis between responders and non-responders could be performed. When stratifying according to the EASL guideline, one patient (7%) showed PR, one patient (7%) had SD, and thirteen patients (86%) had PD. According to mRECIST, four patients (27%) showed PR, five patients (33%) had SD, and six patients (40%) had PD. The overall rate of responders was higher for mRECIST as compared to EASL (27% and 7%, respectively).
Volumetric Response Criteria
When quantifying tumor response with vRECIST, nine patients (60%) showed SD and six patients (40%) showed PD. When using qEASL (cm3), four patients (26.7%) showed PR, four patients (26.7%) had SD, and seven patients (46.6%) had PD. As for qEASL (%), five patients (33.3%) showed PR, nine patients (60%) had SD, and one patient (6.7%) had PD.
Survival Data
At the time of the redaction of the present study, all patients were dead. The median overall survival of the entire cohort was 5.6 months (95% CI = 2.6 months, 12.2 months). All patients were non-responders using the anatomic criteria WHO, RECIST, and vRECIST; thus, no stratification was possible and no survival data could be calculated. For the remaining criteria, Figure 2 illustrates the survival analysis according to the target lesion response and Figure 3 illustrates the survival analysis according to overall response (target and non-target lesions). Whether using the analysis based on target lesions or the overall response, there was no significant difference in responders and non-responders as assessed according to EASL and mRECIST (Table 6). However, quantitative volumetric assessment according to qEASL (cm3) was the only criteria that showed a significant difference in responders and non-responders according to response based on target lesions with a median survival of 3.6 versus 40.5 months (HR = 0.00; 95% CI = 0.00-0.34; P < .001), respectively, and according to overall response with a median survival of 4.4 versus 40.9 months (HR = 0.00; 95% CI = 0.00-0.4; P = .001), respectively. qEASL (%) had the same responders based on target lesions and on overall response assessment; it showed a trend but failed to reach statistical significance (P = .052; Table 6). Statistical analyses also showed that qEASL (cm3) had the highest value in predicting survival on its own (R2 = 79%). Among all the analyses that added a second predictor, the multivariate R2 was either lower than or equal to the one that had already been achieved by qEASL (cm3) alone (results not shown).
Figure 2.
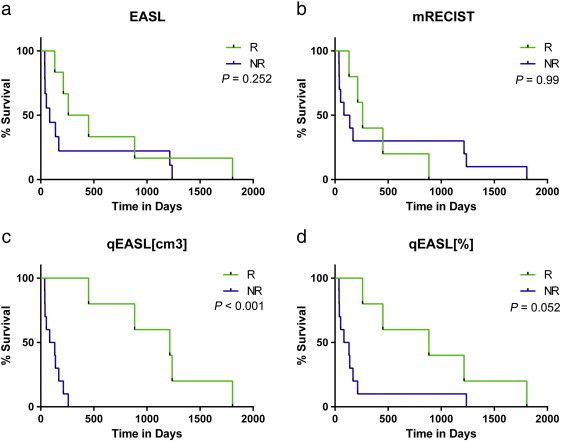
Survival analysis based on target lesion response. (a–d) Survival analysis according to tumor response criteria [EASL, mRECIST, qEASL (cm3), and qEASL (%)]. All patients were non-responders using WHO, RECIST, and vRECIST criteria; thus, no survival data could be calculated.
Figure 3.
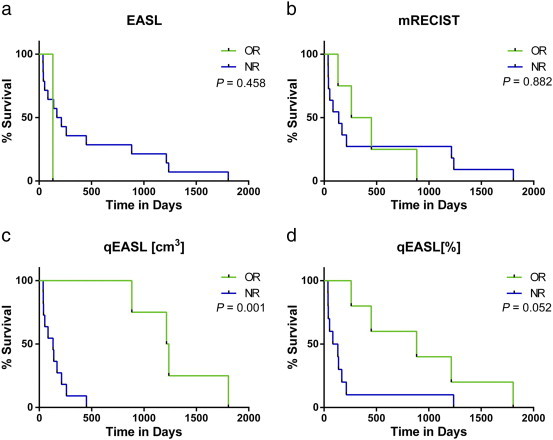
Survival analysis based on overall patient response. (a–d) Survival analysis according to tumor response criteria [EASL, mRECIST, qEASL (cm3), and qEASL (%)]. All patients were non-responders using WHO, RECIST, and vRECIST criteria; thus, no survival data could be calculated.
Table 6.
Survival Based on Target Lesions and Overall Patient Survival
| Response Criteria | Survival Based on Target Lesions |
Overall Patient Survival |
||||||
|---|---|---|---|---|---|---|---|---|
| Survival (Months) | HR (95% CI) | R2 (%) | P Value | Survival (months) | HR (95% CI) | R2 (%) | P Value | |
| WHO | ||||||||
| R | – | 1 | – | – | 1 | – | ||
| NR | 5.6 | 5.6 | ||||||
| RECIST | ||||||||
| R | – | 1 | – | – | 1 | – | ||
| NR | 5.6 | 5.6 | ||||||
| EASL | ||||||||
| R | 11.8 | 0.48 (0.12-1.63) | 0 | .252 | 4.4 | 2.26 (0.05-20.29) | 0 | .458 |
| NR | 2.8 | 6.4 | ||||||
| mRECIST | ||||||||
| R | 8.6 | 0.96 (0.24-3.60) | 0 | .99 | 11.8 | 0.91 (0.20-3.46) | 0 | .882 |
| NR | 3.7 | 4.6 | ||||||
| vRECIST | ||||||||
| R | – | 1 | – | – | 1 | – | ||
| NR | 5.6 | 5.6 | ||||||
| qEASL (cm3) | ||||||||
| R | 40.5 | 0.00 (0.00-0.34) | 66 | <.001 | 40.9 | 0.00 (0.00-0.40) | 79 | .001 |
| NR | 3.6 | 4.4 | ||||||
| qEASL (%) | ||||||||
| R | 29.5 | 0.27 (0.06-1.01) | 18 | .052 | 29.5 | 0.27 (0.06-1.01) | 18 | .052 |
| NR | 3.6 | 3.6 | ||||||
Note: R, responders; NR, non-responders; qEASL (%) had the same responders based on target lesions and on overall response assessments.
Discussion
The main finding of this study is that quantitative volumetric changes in tumor enhancement (qEASL) accurately predicted response to therapy and survival in patients with uveal melanoma after the first TACE.
Survival is the ultimate marker for treatment efficacy in solid tumors, and radiologic objective response has been widely used and accepted as a surrogate end point to the survival-based end points traditionally used in clinical trials [9]. Because the prognosis of uveal melanoma is highly dependent on disease progression in the liver, a local therapy holds promise in managing this otherwise highly chemoresistant disease. Hence, it is crucial to track the response to therapy early in the course of treatment to prevent a loss of chance for the patient.
Our study showed that conventional response criteria assessing anatomic changes in the tumor (WHO, RECIST, and vRECIST) failed to stratify patients according to the tumor response and to predict survival. Moreover, while achieving stratification between responders and non-responders, EASL and mRECIST failed to predict survival, while qEASL was the only criteria predictive of overall survival. These results collectively show that quantitative volumetric tumor response assessing viable tumor is the optimal tumor response criteria in patients with metastatic uveal melanoma to the liver after the first session of TACE. This may be explained by the fact that conventional tumor response criteria that measure the tumor unidimensionally or bidimensionally wrongly assume that the tumor proportionally grows or shrinks in a spherical manner. Indeed, unidimensional and bidimensional tumor response criteria presume that lesion diameter (RECIST), enhancing diameter (mRECIST), and the product of diameters (WHO) or enhancing diameters (EASL) correlate with the tumor volume. However, most liver tumors exhibit asymmetrical and heterogeneous pattern of necrosis that challenge precise tumor response assessment after chemoembolization [9]. However, by the nature of quantitative volumetric measurement methods such as qEASL, these limitations may be overcome. Indeed, qEASL has several methodological strengths: this approach utilizes a semiautomatic tumor segmentation that evaluates the entire tumor volume, including the viable enhancing as well as necrotic parts of the tumor. Moreover, the semiautomatic approach of the software allows for time efficient tumor segmentation while allowing for adjustments by the radiologist [19]. This technique has been shown to be reproducible between radiologic readers and its precision was demonstrated with a strong correlation with tumor necrosis as measured on histopathology [20], [25].
In contrast to most tumors, uveal melanoma liver metastasis may be heterogeneous depicting high signal intensity on baseline precontrast T1-weighted images due to hemorrhage with the presence of methemoglobin and/or melanin [21], [22]. Furthermore, as shown by our results, uveal melanoma lesions treated with TACE exhibited more high signal intensities on precontrast T1-weighted images compared to baseline imaging, making oftentimes challenging the assessment of tumor enhancement, even when image subtraction is used. This might explain why a quantitative measurement may be more precise in assessing these lesions in comparison to a more subjective method such as EASL, in that the calculation of volume eliminates potential variability in the assessment based on slice selection.
The aggressiveness of the disease with potential changes in non-target lesions already seen in the short interval between the baseline and after TACE MR imaging provided the rationale to investigate the effect of the untreated lesions in the overall response. Our study demonstrated that the analysis based on the target lesions provided similar results as when including target and non-target lesions in the assessment of early tumor response. This may potentially lead to simplification of imaging assessment after one session of TACE.
There were several limitations to this study. First, the sample of the study was relatively small. However, uveal melanoma is a rare disease, and even in centers with high patient volume, it is unlikely to have a large sample from a single institution. Thus, a multi-institutional study with a larger cohort is needed to confirm our data. Moreover, a thorough statistical analysis was performed including exact permutation distribution in the calculations to overcome this limitation. Second, this study included only patients with pretreatment and posttreatment MR imaging, leading to a selection bias. However, accumulation of iodized oil (as used in TACE) into treated lesions limits the reliability of contrast enhancement on computed tomography scans; thus, only contrast-enhanced MR imaging is used in our institution in a post-TACE setting. Third, the quantitative volumetric measurements used in this study lack radiologic-pathologic validation [20]. However, this is unrealistic as patients with uveal melanoma metastatic to the liver were not considered appropriate candidates for any surgical treatment and were referred for TACE. Fourth, this study did not investigate the potential role of quantitative volumetric diffusion-weighted MR imaging. Diffusion-weighted MR imaging is increasingly used to evaluate tumor response to therapy [26]. Buijs et al. [27] showed an increase in conventional apparent diffusion coefficient values in liver metastasis of uveal melanoma in patients undergoing several cycles of TACE. 3D quantitative apparent diffusion coefficient has shown promising preliminary results [20]. Future work could investigate the role of this novel technique alone or in combination with enhancement-based methods in the response assessment of patients with uveal melanoma metastatic to the liver.
In conclusion, the current analysis indicates that quantitative volumetric tumor enhancement (qEASL) may be used as a surrogate biomarker for the prediction of survival in patients with uveal melanoma metastatic to the liver after one session of TACE.
Footnotes
Support for this work was provided by National Institutes of Health (NIH)/National Cancer Institute (NCI) R01 CA160771 and P30 CA006973 (Philips Research North America, Briarcliff Manor, NY). Disclosure: J.-F.G. is a consultant for Biocompatibles/BTG, Bayer HealthCare, Guerbet, BTG, Threshold, Guerbet/BTG, Philips Healthcare, and Jennerex and the founder and CEO of PreScience Labs, LLC. Grant support: Biocompatibles/BTG, Bayer HealthCare, Philips Medical, BTG, Threshold, Guerbet/BTG, Threshold, Guerbet, Department of Defense, NCI-Eastern Cooperative Oncology Group, and NIH-R01. M. L. is a Philips employee and supported by NIH-R01.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.tranon.2014.05.004.
Contributor Information
Rafael Duran, Email: jfg@jhmi.edu.
Julius Chapiro, Email: j.chapiro@googlemail.com.
Constantine Frangakis, Email: cfranga1@jhu.edu.
MingDe Lin, Email: ming.lin@philips.com.
Todd R. Schlachter, Email: tschlac1@jhmi.edu.
Rüdiger E. Schernthaner, Email: rschern1@jhmi.edu.
Zhijun Wang, Email: zwang58@jhmi.edu.
Lynn J. Savic, Email: lsavic1@jhmi.edu.
Vania Tacher, Email: vaniatacher@gmail.com.
Ihab R. Kamel, Email: ikamel@jhmi.edu.
Jean-François Geschwind, Email: jfg@jhmi.edu.
Appendix A. Supplementary data
Supplementary material.
References
- 1.Strickland D, Lee JA. Melanomas of eye: stability of rates. Am J Epidemiol. 1981;113:700–702. doi: 10.1093/oxfordjournals.aje.a113150. [DOI] [PubMed] [Google Scholar]
- 2.Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118:1881–1885. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 3.Singh AD, Topham A. Survival rates with uveal melanoma in the United States: 1973–1997. Ophthalmology. 2003;110:962–965. doi: 10.1016/S0161-6420(03)00077-0. [DOI] [PubMed] [Google Scholar]
- 4.Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807–810. doi: 10.1016/0002-9610(78)90171-x. [DOI] [PubMed] [Google Scholar]
- 5.Einhorn LH, Burgess MA, Gottlieb JA. Metastatic patterns of choroidal melanoma. Cancer. 1974;34:1001–1004. doi: 10.1002/1097-0142(197410)34:4<1001::aid-cncr2820340406>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Woodman SE. Metastatic uveal melanoma: biology and emerging treatments. Cancer J. 2012;18:148–152. doi: 10.1097/PPO.0b013e31824bd256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gragoudas ES, Egan KM, Seddon JM, Glynn RJ, Walsh SM, Finn SM, Munzenrider JE, Spar MD. Survival of patients with metastases from uveal melanoma. Ophthalmology. 1991;98:383–389. doi: 10.1016/s0161-6420(91)32285-1. [discussion 390] [DOI] [PubMed] [Google Scholar]
- 8.Kath R, Hayungs J, Bornfeld N, Sauerwein W, Hoffken K, Seeber S. Prognosis and treatment of disseminated uveal melanoma. Cancer. 1993;72:2219–2223. doi: 10.1002/1097-0142(19931001)72:7<2219::aid-cncr2820720725>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Guindalini FD, Botelho MP, Harmath CB, Sandrasegaran K, Miller FH, Salem R, Yaghmai V. Assessment of liver tumor response to therapy: role of quantitative imaging. Radiographics. 2013;33:1781–1800. doi: 10.1148/rg.336135511. [DOI] [PubMed] [Google Scholar]
- 10.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodes J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 14.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 15.Bonekamp S, Jolepalem P, Lazo M, Gulsun MA, Kiraly AP, Kamel IR. Hepatocellular carcinoma: response to TACE assessed with semiautomated volumetric and functional analysis of diffusion-weighted and contrast-enhanced MR imaging data. Radiology. 2011;260:752–761. doi: 10.1148/radiol.11102330. [DOI] [PubMed] [Google Scholar]
- 16.Halappa VG, Bonekamp S, Corona-Villalobos CP, Li Z, Mensa M, Reyes D, Eng J, Bhagat N, Pawlik TM, Geschwind JF. Intrahepatic cholangiocarcinoma treated with local-regional therapy: quantitative volumetric apparent diffusion coefficient maps for assessment of tumor response. Radiology. 2012;264:285–294. doi: 10.1148/radiol.12112142. [DOI] [PubMed] [Google Scholar]
- 17.Gowdra Halappa V, Corona-Villalobos CP, Bonekamp S, Li Z, Reyes D, Cosgrove D, Pawlik TM, Diaz LA, Bhagat N, Eng J. Neuroendocrine liver metastasis treated by using intraarterial therapy: volumetric functional imaging biomarkers of early tumor response and survival. Radiology. 2013;266:502–513. doi: 10.1148/radiol.12120495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liapi E, Geschwind JF. Transcatheter arterial chemoembolization for liver cancer: is it time to distinguish conventional from drug-eluting chemoembolization? Cardiovasc Intervent Radiol. 2011;34:37–49. doi: 10.1007/s00270-010-0012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin M, Pellerin O, Bhagat N, Rao PP, Loffroy R, Ardon R, Mory B, Reyes DK, Geschwind JF. Quantitative and volumetric European Association for the Study of the Liver and Response Evaluation Criteria in Solid Tumors measurements: feasibility of a semiautomated software method to assess tumor response after transcatheter arterial chemoembolization. J Vasc Interv Radiol. 2012;23:1629–1637. doi: 10.1016/j.jvir.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapiro J, Wood L, Lin M, Duran D, Cornish T, Lesage D, Charu V, Schernthaner R, Wang Z., Tacher V, Savic LJ. A radiological-pathological analysis of contrast-enhanced and diffusion-weighted MRI in patients with HCC after TACE – testing the diagnostic accuracy of 3D quantitative image analysis. Radiology. 2014 doi: 10.1148/radiol.14140033. Accepted in. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enochs WS, Petherick P, Bogdanova A, Mohr U, Weissleder R. Paramagnetic metal scavenging by melanin: MR imaging. Radiology. 1997;204:417–423. doi: 10.1148/radiology.204.2.9240529. [DOI] [PubMed] [Google Scholar]
- 22.Marx HF, Colletti PM, Raval JK, Boswell WD, Jr, Zee CS. Magnetic resonance imaging features in melanoma. Magn Reson Imaging. 1990;8:223–229. doi: 10.1016/0730-725x(90)90093-h. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbaum PR. Conditional permutation tests and the propensity score in observational studies. J Am Stat Assoc. 1984;79:565–574. [Google Scholar]
- 24.Samuelsen SO. Exact inference in the proportional hazard model: possibilities and limitations. Lifetime Data Anal. 2003;9:239–260. doi: 10.1023/a:1025880618819. [DOI] [PubMed] [Google Scholar]
- 25.Tacher V, Lin M, Chao M, Gjesteby L, Bhagat N, Mahammedi A, Ardon R, Mory B, Geschwind JF. Semiautomatic volumetric tumor segmentation for hepatocellular carcinoma: comparison between C-arm cone beam computed tomography and MRI. Acad Radiol. 2013;20:446–452. doi: 10.1016/j.acra.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology. 2010;254:47–66. doi: 10.1148/radiol.09090021. [DOI] [PubMed] [Google Scholar]
- 27.Buijs M, Vossen JA, Hong K, Georgiades CS, Geschwind JF, Kamel IR. Chemoembolization of hepatic metastases from ocular melanoma: assessment of response with contrast-enhanced and diffusion-weighted MRI. AJR Am J Roentgenol. 2008;191:285–289. doi: 10.2214/AJR.07.2467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.


