Abstract
BACKGROUND: The study aimed to compare the tolerability and efficacy of gefitinib combined with chemotherapy agents versus chemotherapy alone for the treatment of epidermal growth factor receptor (EGFR)–mutated lung adenocarcinoma in heavily pretreated patients. METHODS: The study was designed as a matched-pair case-control investigation to minimize intergroup heterogeneity. Patients were stratified into gefitinib plus chemotherapy and chemotherapy alone groups with matching for sex, age, ECOG performance status, progress-free survival (PFS) from previous EGFR tyrosine kinase inhibitor treatment, EGFR mutation types, and tumor metastasis status. RESULTS: Sixty-six patients were selected from our database using the matched-pair method. The median age was 61 years (95% confidence interval, 57-65 years). During a follow-up period of 14.5 months on average, the overall response rates of the gefitinib-integrated and chemotherapy alone groups were 9.1% and 6.5%, respectively (P > .05), whereas the corresponding disease-control rates were 39.4% and 30.3%, respectively (P > .05). No statistically significant differences in PFS (median, 4.2 vs 3.3 months; P = .06) and overall survival (median, 10.4 vs 7.9 months; P = .44) were observed between two groups. The 6-month survival rates of the gefitinib-integrated and chemotherapy alone groups were 21.2% and 12.1%, respectively (P < .05). Side effects were mild, and all treatments were well tolerated. CONCLUSIONS: Our results indicated that gefitinib-integrated therapy offered a trend to better PFS and an improved 6-month survival rate in heavily pretreated patients with metastatic EGFR-mutated lung adenocarcinoma. All treatments were well tolerated. Future prospective studies are warranted to confirm our findings.
Introduction
Platinum-based chemotherapy is the standard first-line therapeutic regimen for advanced non–small cell lung cancer (NSCLC) [1], [2], [3], [4]. In cases of disease progression, single-agent regimens such as docetaxel or pemetrexed are often provided as second-line chemotherapy [5], [6], [7]. Since its development approximately 10 years ago, epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) treatment has been another milestone in the management of NSCLC. For patients with EGFR-mutated lung adenocarcinoma, EGFR-TKIs, such as gefitinib, erlotinib, and icotinib, have demonstrated promising therapeutic efficacy. These agents have been used as first- or second-line therapy in patients with EGFR-mutated lung adenocarcinoma instead of chemotherapy [8], [9], [10], [11], [12], [13], [14], [15], [16], [17]. However, almost all patients with EGFR-mutated advanced lung adenocarcinoma with initial response to chemotherapy or subsequent EGFR-TKI eventually developed disease progression. As the mechanisms of such acquired resistance such as T790M and D761Y mutations are under investigation and remain poorly understood [18], additional treatment options for these patients whose general conditions are adequate remain necessary. Because limited data are available on the issue, such additional treatments are controversial. Although current treatment of TKI-resistant NSCLC is chemotherapy, many novel strategies are under investigation, including the continuation beyond progression of EGFR-TKIs or the usage of a different TKI [19], [20], [21]. Chaft et al. [22] reported incidences of disease flare after discontinuation of TKI in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib. The data available strengthen the hypothesis that at least two cell populations co-exist in EGFR-mutated NSCLC: one remains sensitive to TKIs, whereas the other one is resistant to TKIs [23]. Moreover, the 2014 National Comprehensive Cancer Network guidelines suggest the continuation beyond progression of EGFR-TKI combined with chemotherapy. Therefore, treatment options for NSCLC patients who have failed previous chemotherapy and the order of EGFR-TKI treatment remain under discussion. Thus, the present study aimed to compare the clinical outcomes of gefitinib plus chemotherapy and chemotherapy alone in heavily pretreated patients with EGFR-mutated lung adenocarcinoma.
Materials and Methods
Patient Selection
The study was designed as a matched-pair case-control investigation to minimize intergroup heterogeneity. All patients selected from our database had pathologically confirmed lung adenocarcinoma with the following inclusion criteria: 1) EGFR-19/21 activation mutations, 2) previously receiving sequential use of chemotherapy and TKI, TKI between two chemotherapy regimens, or chemotherapy between TKI treatments followed by the reintroduction of TKI in heavily pretreated patients, and 3) disease progression after previous treatment, entered gefitinib-integrated regimen versus chemotherapy alone. All patients provided informed consents previously to allow their clinical data for research or publication purposes, which was approved by our Institutional Ethics Committee. Clinical parameters examined at the time of gefitinib-integrated or chemotherapy alone treatments included age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS), EGFR mutation, prior systemic chemotherapy, progression-free survival (PFS) from previous EGFR-TKI treatment, and metastasis status.
Therapeutic Regimens
Patients were stratified into gefitinib plus chemotherapy and chemotherapy alone groups. In the gefitinib-integrated group, oral gefitinib was provided at a daily dose of 250 mg, except in chemotherapy administration days. Treatment was continued until disease progression, development of unacceptable toxicity, or patient's refusal of therapy. Therapeutic regimens for patients in the chemotherapy alone group were decided on the basis of their prior treatments. Pemetrexed at 500 mg/m2 was administrated every 21 days if patients had previously received docetaxel or paclitaxel. Otherwise, docetaxel at 75 mg/m2 was administered every 21 days.
Response Evaluation
Response evaluation was conducted according to the Response Evaluation Criteria in Solid Tumors version 1.0 guidelines [24] using chest computed tomography scans. Because this study was not a clinical trial, the evaluation timeline was not strictly predetermined. Instead, a follow-up was conducted every 6 to 8 weeks on average.
Statistical Analysis
Treatment outcomes were evaluated as response rate, disease-control rate, 6-month survival rate, PFS, and overall survival (OS). PFS was defined as the time from the date of gefitinib-integrated or chemotherapy treatment to that of disease progression or death of any cause. OS was defined as the time from the date of treatment to that of death. The Pearson chi-square test, Fisher exact test, and Kaplan-Meier method were employed in this study [25]. A P value of <.05 was considered statistical significant. Stata 10.0 software was used for all analyses.
Results
Baseline Patient Characteristics
A search in our database yielded 115 patients meeting all inclusion criteria. Of these, 70 patients were treated with gefitinib and 45 with gefitinib-integrated chemotherapy between January 2006 and June 2011. The matched-pair case-control method selected 66 patients (33 pairs) for this study. The baseline characteristics of all included patients are shown in Table 1. All variables (age, sex, ECOG PS, PFS from previous EGFR-TKI treatment, EGFR mutation types, and metastatic status) were well matched between the gefitinib-integrated and chemotherapy alone groups with no statistically significant differences observed.
Table 1.
Baseline Patient Characteristics
| Characteristics | Integrated | Chemotherapy | P Value |
|---|---|---|---|
| Age, years | .72 | ||
| Median | 62.09 | 61.06 | |
| 95% CI | 58.24-65.94 | 56.73-65.39 | |
| Sex | |||
| Male | 12 | 12 | 1.00 |
| Female | 21 | 21 | |
| ECOG PS | |||
| 0 | 20 | 27 | .141 |
| 1 | 12 | 5 | |
| 2 | 1 | 1 | |
| EGFR mutation | |||
| Exon 19 deletion | 27 | 26 | .914 |
| Exon 21 replacement | 6 | 7 | |
| Metastasis | |||
| Limited | 13 | 13 | |
| Multiple | 20 | 20 | |
| PFSp EGFR-TKI (months) | |||
| 1-6 | 2 | 3 | 1.00 |
| >6 | 31 | 30 | |
| Current chemotherapy | |||
| Docetaxel | 26 | 26 | 1.00 |
| Pemetrexed | 7 | 7 |
PFSp, progress-free survival from previous TKI treatment.
Response and Toxicity
The response rates and observed toxicity are shown in Table 2. The proportion of no disease progression at 6 months was more favorable in the gefitinib-integrated group than in the chemotherapy alone group. As patients had previously received EGFR-TKI, skin rash and diarrhea were expected at the beginning of treatment due to prolonged and chronic EGFR-TKI usage. However, no grade 3 skin rash and diarrhea were recorded. Grade 2 skin reaction and diarrhea might have been underestimated by both patients and physicians as patients might have ignored such common toxicity-related events and only records of mild diarrhea, dry skin, and itches were noted in patients' medical history. We therefore concluded that toxicity was mild, and both treatments were well tolerated.
Table 2.
Efficacy and Toxicity in Each Group
| Response | Integrated, % (n) | Chemotherapy, % (n) | P Value |
|---|---|---|---|
| Response rate | 9.10% (3/33) | 6.45% (2/33) | .70 |
| Disease control rate | 39.39% (13/33) | 30.30% (10/33) | .60 |
| No progression rate at 6 months | 21.2% (7/33) | 12.1% (4/33) | .04 |
| Toxicity | |||
| Grade 3 myelosuppression | 12.1% (4/33) | 12.1% (4/33) | 1.00 |
| Grade 2 skin rash | 9% (3/33) | 0 | .238 |
| Diarrhea ≤ 2 | 36.4% (10/33) | 6.0% (2/33) | .02 |
PFS and OS
The median PFS of the gefitinib-integrated group was 4.15 months [95% confidence interval (CI), 2.89–6.01], whereas that of the chemotherapy alone group was 3.25 months (95% CI, 1.69–4.73; hazard ratio, 0.806; P = .061; Figure 1A). The corresponding median OS of the two groups was 10.36 months (95% CI, 9.15–12.24) and 7.9 months (95% CI, 6.00–11.35), respectively (hazard ratio, 0.872; P = .44; Figure 1B). No significant differences in PFS and OS were observed between the two groups.
Figure 1.
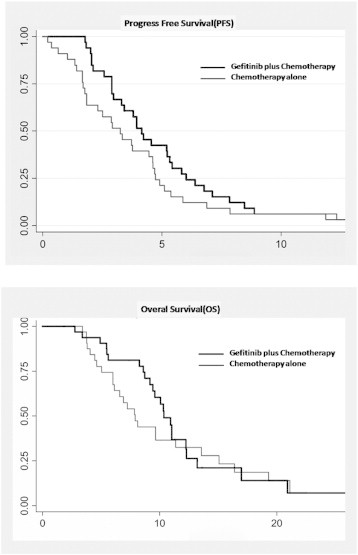
Kaplan-Meier curves for the OS (A) and PFS (B) of patients treated with gefitinib-integrated regimen and chemotherapy alone.
Discussion
The role of EGFR-TKI when used in combination with chemotherapy for NSCLC patients who are likely to respond to treatment in first- or second-line setting is uncertain. Both gefitinib and erlotinib have been extensively evaluated in phase III trials in combination with standard chemotherapy for previously untreated NSCLC patients who were not selected on the basis of EGFR mutation status [26], [27], [28]. EGFR-TKI combined with platinum-based therapy did not offer a clinical benefit in response rate, time to progression, or survival. However, despite no observable increase in survival, it remains possible that clinical benefits in some patients were obscured in a molecularly heterogeneous population. This was suggested by a subset analysis of 274 patients to evaluate the survival impact of mutations in EGFR and k-ras genes [29], [30]. Patients with EGFR-mutated tumors showed a trend toward improved PFS when erlotinib was added to chemotherapy compared to chemotherapy alone. In contrast, those with EGFR wild-type tumors tended to favor chemotherapy alone. Wu et al. [31] reported that intercalated combination of chemotherapy and erlotinib significantly prolonged PFS in patient with advanced NSCLC. In a randomized phase II trial conducted by Cancer and Leukemia Group B (CALGB 30406) [32], 181 patients with advanced lung adenocarcinoma were randomly assigned to receive erlotinib alone or erlotinib plus chemotherapy with carboplatin and paclitaxel. Tissue samples were analyzed for EGFR mutation status in 164 patients (91%). The presence of an EGFR mutation was associated with a statistically significant increase in PFS compared to wild-type EGFR in both arms of the study (16 vs 3 months with erlotinib alone and 17 vs 5 months with erlotinib plus chemotherapy). Similar differences were also observed in the OS (31 vs 18 months for erlotinib alone and 39 vs 14 months for erlotinib plus chemotherapy). The addition of chemotherapy to an EGFR-TKI did not result in an improved survival in patients whose tumors expressed EGFR mutations.
The current treatment for TKI-resistant NSCLC is chemotherapy; however, many patients require further management after chemotherapy, even TKI reintroduction that eventually fails. An incidence of disease flare occurring after EGFR-TKI discontinuation might predict a poor survival [32], [33], which suggests that the continuation beyond progression of EGFR-TKIs is a reasonable strategy. In this matched-pair case-control study, the overall response rates in the gefitinib-integrated and chemotherapy alone groups were 9.1% and 6.45%, respectively (P > .05). The corresponding disease-control rates were 39.39% and 30.30%, respectively (P > .05). Such low response rates might be owing to the acquired resistance to EGFR-TKI and chemotherapy in heavily pretreated patients as they had all received prior EGFR-TKI and one or two lines of chemotherapy. Furthermore, the median OS (10.36 vs 7.9 months) and PFS (4.15 vs 3.25 months) did not significantly differ between the gefitinib-integrated and chemotherapy groups. In our study enrolling metastatic EGFR-mutated lung adenocarcinoma patients who had failed prior EGFR-TKI and platinum-based chemotherapy, no significant survival differences were observed between the gefitinib plus chemotherapy and chemotherapy alone groups either. Although this was a retrospective study rather than a clinical trial, the results were comparable since the matched-pair case-control design was employed, and selected patients were well matched between the two groups regarding age, sex, ECOG PS, EGFR mutation, PFS from previous EGFR-TKI treatment, and metastasis status. On the basis of those limited data, several clinical trials were designed, including the ongoing phase III randomized multicenter IMPRESS (A Study of IRESSA Treatment Beyond Progression in Addition to Chemotherapy Versus Chemotherapy Alone) trial to assess the safety and efficacy of continuing gefitinib at 250 mg in addition to chemotherapy versus chemotherapy alone in patients with EGFR-mutated NSCLC who have progressed on first-line gefitinib. The results of this study are being expected.
Nevertheless, the present retrospective study cannot replace a randomized clinical trial since selection bias might exist in other unmeasured clinical factors and the evaluation timeline was not strictly predetermined. Furthermore, the study cohort was limited, and other important issues such as dose intensity, toxicity profiles, and treatment compliance were not considered. In conclusion, to the best of our knowledge, this is the first matched-pair case-control study that evaluated and compared the outcomes between gefitinib-integrated regimens and chemotherapy alone in EGFR-mutated lung adenocarcinoma patients who had failed prior EGFR-TKI and chemotherapy treatments. Our analysis demonstrated that heavily pretreated patients tended to achieve improved PFS and OS if treated with chemotherapy plus gefitinib. Future prospective studies are warranted to elucidate any differences in the efficacy, toxicity, dose intensity, and quality of life between gefitinib-integrated treatment and chemotherapy alone.
Acknowledgement
We thank all patients and investigators for their participation during follow-ups and processing of medical records.
Footnotes
Y.W. received grant support from the Capital Development Foundation for Medical Research (2007-1049) and research fund from the Doctoral Program of Higher Education of China (RFDP20101106110045).
Conflict of interest: None declared.
References
- 1.Non-Small Cell Lung Cancer Collaborative Group Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 2.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH. Comparison of four chemotherapy regimens for advanced non–small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 3.Kim SY, Lee SA, Ryoo HM, Lee KH, Hyun MS, Bae SH. Efficacy of administration of weekly docetaxel combined with platinum as a first-line treatment for patients with advanced non-small cell lung cancer. Korean J Med. 2007;72:625–631. [Google Scholar]
- 4.Yuh YJ, Lee HR, Kim SR. Gemcitabine and carboplatin combination chemotherapy for elderly patients with advanced non-small cell lung cancer: a feasibility study. Cancer Res Treat. 2008;40:116–120. doi: 10.4143/crt.2008.40.3.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Burkes R. Prospective randomized trial of docetaxel versus best supportive care in patients with non–small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 6.Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non–small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 7.Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M, Muller T. Randomized phase III trial of pemetrexed versus docetaxel in patients with non–small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R. Erlotinib in previously treated non–small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 9.Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H, Sandler A. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non–small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 10.Cella D, Herbst RS, Lynch TJ, Prager D, Belani CP, Schiller JH, Heyes A, Ochs JS, Wolf MK, Kay AC. Clinically meaningful improvement in symptoms and quality of life for patients with non-small-cell lung cancer receiving gefitinib in a randomized controlled trial. J Clin Oncol. 2005;23:2946–2954. doi: 10.1200/JCO.2005.05.153. [DOI] [PubMed] [Google Scholar]
- 11.Lee DH, Park K, Kim JH, Lee JS, Shin SW, Kang JH, Ahn MJ, Ahn JS, Suh C, Kim SW. Randomized Phase III trial of gefitinib versus docetaxel in non–small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16:1307–1314. doi: 10.1158/1078-0432.CCR-09-1903. [DOI] [PubMed] [Google Scholar]
- 12.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 13.Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu DT, Saijo N. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non–small-cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29:2866–2874. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 14.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 15.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 16.Rosell R., Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E., Palmero R., Garcia-Gomez R., Pallares C., Sanchez J.M. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 17.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 18.Balak MN, Gong Y, Riely GJ, Somwar R, Li AR, Zakowski MF, Chiang A, Yang G, Ouerfelli O, Kris MG. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor–mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12:6494–6501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- 19.Riely GJ, Kris MG, Zhao B, Akhurst T, Milton DT, Moore E, Tyson L, Pao W, Rizvi NA, Schwartz LH. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res. 2007;13:5150–5155. doi: 10.1158/1078-0432.CCR-07-0560. [DOI] [PubMed] [Google Scholar]
- 20.Costa DB, Nguyen KS, Cho BC, Sequist LV, Jackman DM, Riely GJ, Yeap BY, Halmos B, Kim JH, Jänne PA. Effects of erlotinib in EGFR mutated non-small cell lung cancers with resistance to gefitinib. Clin Cancer Res. 2008;14:7060–7067. doi: 10.1158/1078-0432.CCR-08-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choong NW, Dietrich S, Seiwert TY, Tretiakova MS, Nallasura V, Davies GC, Lipkowitz S, Husain AN, Salgia R, Ma PC. Gefitinib response of erlotinib-refractory lung cancer involving meninges—role of EGFR mutation. Nat Clin Pract Oncol. 2006;3:50–57. doi: 10.1038/ncponc0400. [DOI] [PubMed] [Google Scholar]
- 22.Chaft JE, Oxnard GR, Sima CS, Kris MG, Miller VA, Riely GJ. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res. 2011;17(19):6298–6303. doi: 10.1158/1078-0432.CCR-11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.La Monica S, Caffarra C, Saccani F, Galvani E, Galetti M, Fumarola C, Bonelli M, Cavazzoni A, Cretella D, Sirangelo R. Gefitinib inhibits invasive phenotype and epithelial-mesenchymal transition in drug-resistant NSCLC cells with MET amplification. PloS One. 2013;8(10):e0078656. doi: 10.1371/journal.pone.0078656. [electronic resource] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 26.Park K, Goto K. A review of the benefit-risk profile of gefitinib in Asian patients with advanced non-small-cell lung cancer. Curr Med Res Opin. 2006;22:561–573. doi: 10.1185/030079906X89847. [DOI] [PubMed] [Google Scholar]
- 27.Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, Milanowski J, Karnicka-Mlodkowski H, Pesek M, Serwatowski P. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non–small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 28.Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, Natale RB, Schiller JH, Von Pawel J, Pluzanska A. Gefitinib in combination with gemcitabine and cisplatin in advanced non–small-cell lung cancer: a phase III trial—INTACT 1. J Clin Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, Scagliotti G, Rosell R, Oliff I, Reeves JA. Gefitinib in combination with paclitaxel and carboplatin in advanced non–small-cell lung cancer: a phase III trial—INTACT 2. J Clin Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 30.Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, Ince WL, Jänne PA, Januario T, Johnson DH. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non–small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900-5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 31.Janne P.A., Wang X.F., Socinski M.A., Crawford J., Stinchcombe T.E., Gu L., Capelletti M., Edelman M.J., Villalona-Calero M.A., Kratzke R. Randomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 trial. J Clin Oncol. 2012;10:2063–2069. doi: 10.1200/JCO.2011.40.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu YL, Lee JS, Thongprasert S, Yu CJ, Zhang L, Ladrera G, Srimuninnimit V, Sriuranpong V, Sandoval-Tan J, Zhu Y. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol. 2013;14(8):777–786. doi: 10.1016/S1470-2045(13)70254-7. [DOI] [PubMed] [Google Scholar]
- 33.Chen HJ, Yan HH, Yang JJ, Chen ZH, Su J, Zhang XC, Wu YL. Disease flare after EGFR tyrosine kinase inhibitor cessation predicts poor survival in patients with non-small cell lung cancer. Pathol Oncol Res. 2013;19(4):833–838. doi: 10.1007/s12253-013-9651-z. [DOI] [PubMed] [Google Scholar]


