Summary
Percutaneous transluminal angioplasty (PTA) has recently become a noteworthy treatment option for significant stenosis involving the vertebral artery (VA) in selected patients. We conducted a prospective study to evaluate the efficacy, safety and mid-term follow up results of 206 cases received PTA with or without stent implant to treat their symptomatic atherosclerotic VA stenosis in all segments (V1-V4). In a prospective mono-arm trial from October 2008 to July 2012 in a single center, 239 lesions affecting the intra or extracranial VA (171 in V1, 17 in V2, 14 in V3, 21 in V4 and 16 in combined segments) were treated by PTA with or without stent implant. Non-disabling stroke patients who had failed conservative medical treatment and had angiographic evidence of >50% stenosis in the dominant VA with clinical signs and symptoms of VB stenosis were included in this study. They were mean followed for 13.15±5.24 months after treatment. Overall, 206 patients underwent the procedure. A stent was implemented in 199 patients (96.6%). The periprocedural complication rate was 7.2%. The procedural (technical) success rate was 97.6%. Of the total 239 lesions, 223 were treated with stent implant. Clinical success was achieved in all 206 symptomatic patients after the procedure. Restenosis occurred in 15.9% after a mean 10.8 (6-24) months. Of those, 63.1% and 34.2% had mild and moderate stenosis that was treated medically, whereas one case (2.6%) with severe restenosis underwent balloon angioplasty. No deaths occurred during the follow-up period. The follow-up complication rate was 6.3%. TIA occurred in 4.4%, a minor stroke in 1.4% and a major stroke in one patient. The overall patient event-free survival was 92.4%. These results demonstrate the safety and feasibility of PTA with or without stent implant, with a high technical success rate, a low complication rate, a low restenosis rate and durable clinical success in patients with symptomatic VA stenosis. This approach seems to improve patients' immediate and mid-term clinical results. Randomized controlled trials are necessary to further validate this treatment option.
Keywords: symptomatic vertebral artery stenosis, percutaneous transluminal angioplasty, stent implant, procedural success rate, clinical success rate, clinical follow-up, angiographic follow-up
Introduction
Strokes are 80% ischemic in origin, of which 20-25% involve the posterior circulation of the brain, the part supplied by the vertebrobasilar (VB) circulation 1-3. Data from a systematic review on the prognosis of ischemic events have shown that patients with posterior circulation events have a higher risk for subsequent stroke or death in the acute phase compared with patients who present with anterior circulation symptoms 4. Nevertheless, much less is known about the incidence of vertebral artery (VA) stenosis (VAS) in the general population. Patients with symptomatic VB circulation ischemia have a 25-40% incidence of VAS 5 and the incidence in patients with atherosclerotic peripheral arterial disease (PAD) is 40% 6. The prognosis for patients with atherosclerotic occlusion or thrombosis of the VB system is poor, with 80-100% mortality 7. Three approaches for symptomatic VAS have been described comprising medical therapy, surgical therapy and endovascular methods. Surgery for VAS is technically difficult, potentially hazardous, and is not considered in most centers. Therefore, VAS has traditionally been treated conservatively with medical care alone 8-10. However, traditional treatments for VAS are usually unlikely to achieve long-lasting effectiveness, and a VAS prognosis is often poor 11,12. Hence, selection of a proper therapeutic strategy for VAS has been challenging for clinicians and management has shifted to endovascular techniques with the evolution of endovascular device technologies. Endovascular procedures, such as percutaneous transluminal angioplasty (PTA), have been increasingly advocated when established treatments such as surgical therapy with mortality rates of 10-20% 10,13 fail to relieve the effects of stenosis. A nonrandomized case series suggested that VAS may be treated using an endovascular method such as PTA and/or stenting and is associated with low morbidity and mortality 14-16. However, results of PTA performed to relieve stenosis in the posterior intracranial circulation have been conflicting 17-19. Thus, we conducted a prospective study to evaluate the efficacy, safety, and long-term follow-up of 206 patients who received PTA with a stent implant to treat their symptomatic atherosclerotic VAS.
Materials and Methods
Patients
In a continuation of the first published report of this prospective non-controlled study 20, 211 patients who presented with a wide variety of posterior circulation ischemia symptoms despite receiving adequate medical treatment and were referred to our institute (a single center) were evaluated prospectively from October 2008 to July 2012. An episode of posterior circulation ischemia was defined as the sudden onset of two or more symptoms attributable to the posterior circulation which were presumed to be of non-traumatic vascular origin after adequate investigation. Symptoms commonly included vertigo, visual changes, Wallenberg syndrome, ataxia, numbness, paresis, and other symptoms (headache or confusion). Patients were diagnosed with VAS by clinical assessment coupled with transcranial Doppler ultrasound or magnetic resonance angiography (MRA) and confirmed by an independent neurological evaluation conducted by a member of the stroke team not participating in the intervention, or intra-arterial catheter angiography before any endovascular intervention.
Stenosis severity was calculated according to the carotid stenosis method proposed in the North American Symptomatic Carotid Endarterectomy Trial (NASCET) and Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS) and was classified as mild (0% to 49%), moderate (50% to 69%), severe (70% to 99%), or occluded 21-23. Aortic arch, renal artery angiography and four-vessel digital subtraction angiography (DSA) were performed to evaluate extra and intracranial arteries in all patients. Special attention was paid to the subclavian, VAS, and the collateral blood flow to the posterior cerebral circulation. A duplex study of the carotid, vertebral and renal arteries, and transesophageal echocardiography was performed in each patient.
Arterial stenosis was considered to be angiographically significant when the stenosis was >50%, but only if the other VA was equally diseased or absent. The intensity of neurological deficit at admission was assessed by neurologists using the National Institutes of Health Stroke Scal 24.
Cases with angiographic evidence of >50% stenosis in the dominant VA with clinical signs and symptoms of VB stenosis confirmed by an experienced stroke neurologist were included in this study. The exclusion criteria were: tandem intracranial stenosis lesions (lesions in other arteries spatially in the basilar artery except in the involved VA and several lesions in a single VA not considered tandem lesions), other potential cause of stroke, such as a cardiac source embolism, VA dissection, vasculitis, terminally ill patients, patients unable to give their informed consent or unwilling to undergo intervention, and those with a disabling stroke (NIHSS≤10).
A standard protocol was used to evaluate all patients. Demographic features and vascular risk factors were recorded by an expert neurologist including hypertension (defined as receiving medication for hypertension or blood pressure >140/90 mmHg on repeated measurements), diabetes mellitus (defined as receiving medication for diabetes mellitus, fasting blood glucose level ≥126 mg/dl), ischemic heart disease, dyslipidemia (defined as receiving lipid lowering agents or having an overnight fasting cholesterol level >200 mg/dl and low-density lipoprotein >100 mg/dl), current cigarette smoking (current smokers or those who quit smoking <6 months) and previous transient ischemia attack (TIA) (neurologic deficit lasting <24 hours) (Table 1). All patients' paraclinical data were evaluated, including red and white blood cell and platelet counts, blood glucose and glycosylated hemoglobin, lipid profile, serum electrolytes, liver transaminase, blood urea and creatinine levels, measurement of prothrombin and activated partial thromboplastin time, antinuclear antibodies, antiphospholipid antibodies, anticardiolipin antibodies, lupus anticoagulant, troponin, creatine kinase, antithrombin III, protein C, protein S, and factor V Leiden. They also underwent brain magnetic resonance imaging (1.5 Tesla) after the initial brain computed tomography (data not shown).
Table 1.
Patient characteristics*.
| Characteristics | 206 (100%) |
| Gender Male Female |
121 (58.7%) 85 (41.3%) |
| Age (years) | 67.88±8.42 |
| Vascular risk factors: Diabetes Hypertension Dyslipidemia Cigarette smoking |
89 (43.2%) 153 (74.3%) 24 (11.7%) 49 (23.8%) |
| Presenting symptoms: Ataxia Visual disturbance Vertigo Wallenberg syndrome Numbness Hemiparesia Others |
95 (46.1%) 19 (9.2%) 32 (15.5%) 10 (4.8%) 24 (11.6%) 16 (7.7%) 10 (4.8%) |
| Functional status (initial mRS† score) 1 2 3 4 |
3 (1.5%) 85 (41.3%) 111 (53.9%) 7 (3.4%) |
| Initial National Institutes of Health Stroke Scale (NIHSS) | 7.94±2.61 |
| Concomitant arterial lesion: Unilateral internal carotid artery (ICA) stenosis† Bilateral ICA stenosis Unilateral ICA occlusion Unilateral renal artery stenosis Unilateral subclavian stenosis Unilateral middle cerebral artery (MCA) stenosis Unilateral anterior cerebral artery (ACA) stenosis |
146 (100%) 57 (39.0%) 15 (10.2%) 4 (2.7%) 41 (28.0%) 24 (16.4%) 4 (2.73%) 1 (0.6%) |
| Degree of vertebral arteries stenosis | 70.93±8.645 |
* Data are presented as mean ± SD or n (%).
Patients with confirmed acute symptomatic VB insufficiency underwent a standard protocol by the stroke team and were usually discharged one week after primary admission. Stent eligible patients were recalled four to six weeks after the acute event and a neuroradiologist informed the patients about the benefits and potential risks of endovascular stent implant therapy. Patients who gave informed consent received aspirin (80 mg/d) and clopidogrel (75 mg/d) three days before the procedure and were transferred to the angiography room. All these patients were required to have no contraindications for endovascular procedures (renal failure, coagulopathy, or contrast allergy). PTA and stent implantations were performed using a transfemoral artery approach (all stenting procedures were performed under local anesthesia) by the same interventional neuroradiologists who implemented the angiography. Diagnostic four-vessel angiography and a general anesthetic were administered if required. All collateral flow to the infarcted area was systematically assessed. The artery that was clinically assumed to be obturated was investigated last and endovascular therapy was performed directly after diagnostic angiography.
Procedure
PTA was planned to decrease the risk of new or recurrent cerebral infarction in patients with significant atherosclerotic stenosis of the dominant vertebral artery when 1) symptoms of stenosis recurred or progressed despite antiplatelet pharmacotherapy, and 2) previous infarctions had occurred and the collateral circulation could not establish adequate blood flow to that region. PTA and stent implants were applied to those who had VA involvement at the v1, v2, or v4 segments, and those who had v3 segment stenosis underwent PTA alone. Balloon-mounted stents were used for v1 and v2 stenotic segments and self-expandable stents were applied for v4 segment involvement. All patients were fully heparinized during the procedure (100 IU/kg plus 1,000-2,000 IU/h) and this was continued for 24 hours following the procedure. Blood pressure and heart rate were continuously monitored and controlled pre and post procedurally. Balloon angioplasty was performed in all cases by neuroradiologists experienced with interventional techniques. Dilatation was achieved by inflating the balloon to about 8-10 atmospheres for 10-20 seconds to deploy the stent into the inner arterial wall. For those who underwent PTA only, the balloon was deflated, the result verified and eventually inflation was repeated if necessary. The length of the balloon and stent used was determined by the length and the anatomic site of the stenosis by quantitative angiography before balloon angioplasty and stent placement, but the balloon was always longer than the stenosis itself. The balloon was inflated to a diameter equal to or less than the diameter of the normal vessel. In five cases (four patients with v1 stenosis and one with v4 stenosis) we could not perform any endovascular procedure because of difficult accessibility and severe stenosis. Although these patients fulfilled the inclusion criteria, we excluded them because of our procedural failure and performed our procedure on 206 cases.
DSA was performed to assess the treated vessel immediately after the procedure. All patients underwent a complete neurologic examination after which their neurological status was monitored closely for 24 hours. Careful attention was paid to the intracranial circulation to rule out any sign of distal embolization. NIHSS and mRS scores were determined before and after angioplasty. Technical success was defined as reduction of stenosis to <20% of the completely enveloped lesion after the procedure without in-hospital stroke or death. Clinical success was defined as technical success with resolution of VBS symptoms.
Clinical and angiographic follow-up
Patients were discharged from the neurological ward two to seven days (mean, four days) after the procedure, when their neurological condition was stable. Clopidogrel (75 mg/d) was continued for six months after the procedure in all patients and then they were maintained on daily aspirin indefinitely. All patients received a low-dose statin (atrovastatin, 20 mg/daily) for at least six months and it was continued in patients with hypercholesterolemia. All cases were managed similarly and followed up clinically every month for six months and then every two months until the end of follow-up time (this will occur when the last patient has been followed for six months). The follow-up was performed by independent neurologists (not involved in the interventional procedure) via an outpatient visit to assess any changes in previous symptoms, appearance of new symptoms, and to manage vascular risk factors. If new neurological signs occurred during these visits and were suspected to be posterior circulation ischemia, the neurologist examined the patient for a future study by magnetic resonance imaging or brain computed tomography. TCD and color duplex sonography were used during the follow-up period to assess stent patency. Control angiography was performed in all patients after six months and repeated every six months. The stented artery and other major intra and extracranial arteries were reexamined in the control angiography but the main focus was to find any restenosis or occlusion in the target artery. High blood pressure (preferred systolic blood pressure was <140 mm Hg and <130 mm Hg) was treated aggressively and patients who smoked were asked to stop cigarette smoking and become involved in a lifestyle modification program including weight reduction, exercise and nutritional diet. Target vessel revascularization was defined as repeat intervention of the target vessel driven by clinical symptoms with ≥70% stenosis. In-stent restenosis was described as recurrent artery stenosis with >30% luminal narrowing after excluding post stent implant residual stenosis. In these cases, severe restenosis was treated with balloon angioplasty and if there was moderate and mild stenosis or no symptoms the patients were treated medically based on NASCET and CAVATAS classification. The occurrence of clinical adverse events (AEs) for all patients was evaluated until the follow-up ended. The AEs were classified as procedure-related and follow-up adverse events (FAEs). The FAEs arose 30 days after enrollment during the follow-up period and included ischemic stroke (stroke in territory of qualifying artery), TIA, and mortality related to these events. The patients were informed that whenever symptoms suggestive of AEs occurred that they should visit their physicians. All AEs were arbitrated by expert neurologists during outpatient visits. mRS scores were calculated at the end of the follow-up period. The results of AEs and follow-up are summarized in Table 3.
Table 2.
Procedural data.
| Characteristics | N (%) |
| Treated Artery: left vertebral Right vertebral Bilateral |
206 110 (53.4%) 79 (38.3%) 17 (8.3%) |
| Lesion locations: V1 V2 V3 V4 Total combined ipsilateral lesions: V1+v2 V1+v3 V1+v4 V3+v1 V4+v1 V4+v2 |
239 171 (71.5%) 17 (7.1%) 14 (5.8%) 21 (8.8%) 16 (6.6%) 5 (2.0%) 2 (0.8%) 3 (1.2%) 2 (0.8%) 2 (0.8%) 2 (0.8%) |
| Procedure: Percutaneous transluminal angioplasty (PTA) PTA and stent implant |
206 7 (3.4%) 199 (96.6%) |
| Stent number | 223 |
| Stent type: Balloon expandable bare-metal Self expandable stent |
223 199 (89.2%) 24 (10.8%) |
Table 3.
Complications and follow-up results of 206 patients who underwent endovascular procedures*.
| Periprocedural complication | 15/206 (7.2%) |
| Hematoma | 12/206 (5.8%) |
| Bradycardia | 2/206 (1%) |
| Dissection (without the need for transfusion) | 1/206 (0.5%) |
| Follow up results | |
| Restenosis: V1 V2 V3 V4 |
38/239 (15.9%) 26/38 (68.4%) (14 mild, 11 moderate, 1 severe) 4/38 (10.5%) (4 mild) 3/38 (7.9%) (3 mild) 5/38 (13.1%) (3 mild, 2 moderate) |
| Complications: Transient ischemic attack (TIA) Non disabling stroke Disabling stroke Follow-up (months) |
13/206 (6.3%) 9 (4.4%) 3 (1.4%) 1 (0.5%) 13.15±5.24 |
| * Data are presented as mean ± SD or n (%). | |
Statistical method
Numerical variables are presented as mean ± standard deviation, whereas categorical variables are summarized as absolute frequencies and percentages. Observation time was from study enrollment to the last patient's six-month follow-up ended. Survival rates were calculated with the Kaplan-Meier product limit method. SPSS version 13.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for the analysis. All p-values were two-tailed, and p ≤0.05 was considered significant.
Results
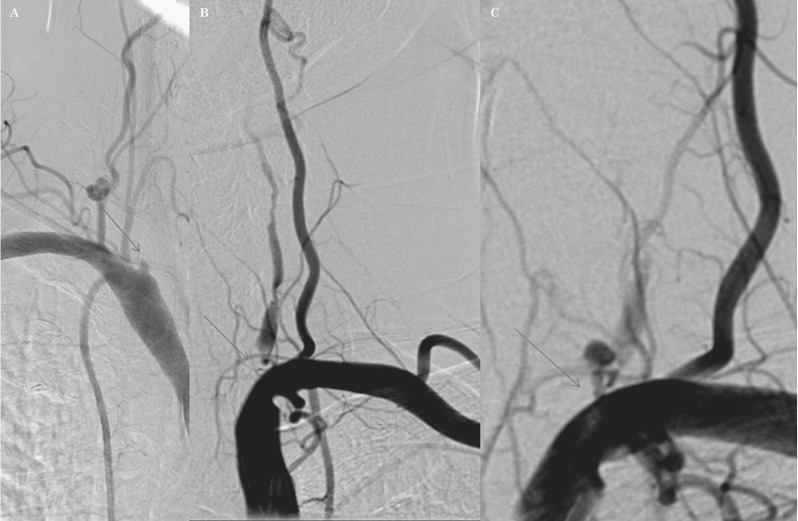
Overall, 206 patients with confirmed symptomatic VA stenosis underwent the procedure at our institute over a 46 month period. The mean age of all patients was 64.73 ± 9.85 years (range, 40-86 years). Male gender was predominant (58.7%). Stents were implemented in 199 (96.6%) patients, and the remaining patients underwent balloon angioplasty only because of difficult anatomical access but achieved significant dilatation and symptom relief. The patient characteristics are summarized in Table 1. The prevalence of vascular risk factors was 74.3%, 43.2%, 23.8%, and 11.7% for hypertension, diabetes mellitus, hyperlipidemia, and cigarette smoking, respectively. Concomitant PAD involvement was found in 5.8% of cases for subclavian artery stenosis and in 10.1% of patients for renal artery stenosis. Ataxia was the most frequent (46.1%) presenting symptom in our cases. The baseline mRS and NIHSS scores were 2.59 ± 0.58 (range, 1-4) and 7.94 ± 2.6 (range, 3-17), respectively. Procedural data are presented in Table 2. The left VA was the most frequent (53.4%) treated site, whereas the pure v1 segment was the most affected part (71.5%) overall. The frequency of combined lesions ipsilateral to the main lesion is listed in Table 2. Internal carotid artery involvement occurred in 52% of concomitant arterial lesions. Balloon-mounted stents were utilized in most of our cases (89.2%). Procedural data and follow-up outcomes are summarized in Table 3. The periprocedural complication rate was 7.2%. An access site local hematoma was the most frequent (5.8%) periprocedural complication. We had only one case of artery dissection during the wiring procedure in a 75-year-old woman with a history of diabetes and hypertension who presented with homonymous hemianopia and underwent PTA and stent implanting in the left v1 segment. The patient complained of neck pain after the dissection occurred and a balloon-mounted stent was implanted immediately at the dissection site to successfully control the dissection (Figure 1). She was followed up for 11 months without complications. No other patients developed any serious changes in consciousness, electrocardiographic waves, or arterial oxygen saturation rates during the procedure.
Figure 1.
A 75-year-old woman with a history of diabetes and hypertension who presented with homonymous hemianopia. DSA revealed occlusion of the right vertebral artery (A), and severe stenosis in the orifice of the left side (B). There was evidence of dissection after wire manipulation (C).
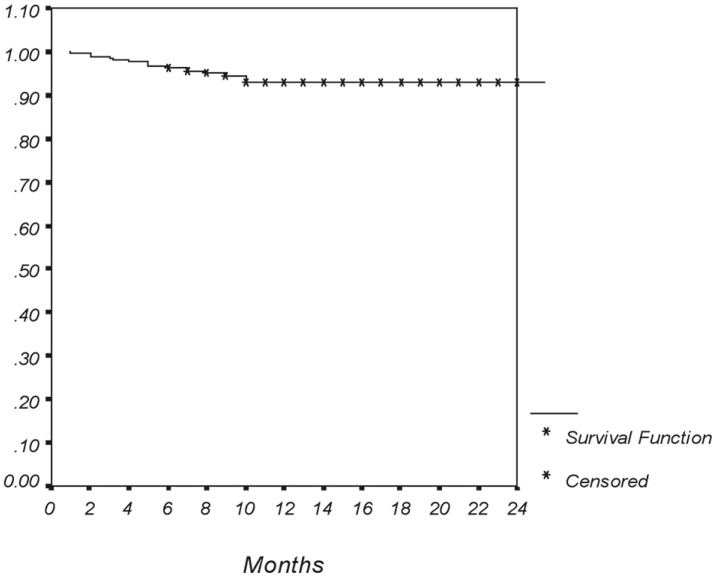
The mean post procedure NIHSS score was 4.52 ± 2.23 (range, 1-10). Mean follow-up time was 13.15 ± 5.24 months (range, 6-30 months). The procedural (technical) success rate was 97.6% (206/211). The total number of lesions was 239 and 223 were treated with stent implants (the lesions in the v3 segment [16 cases] were treated only by angioplasty). Clinical success was achieved in all 206 symptomatic patients after the procedure. Restenosis occurred in 15.9% (38/239) after a mean of 10.89 ± 5.0 months (range, 6-24 months), and 63.1% and 34.2% of them had mild and moderate stenosis that were treated medically. Only one PTA-treated case (2.6%) had severe restenosis. Of all 206 patients, clinical follow-up was obtained in 100% with a mean duration of 13.1 ± 5.24 months (range, 6-30 months). Of these, 56.3% has at least a one-year follow-up. The mean final mRS score was 0.36 ± 0.63 (range, 0-5). No mortalities were observed during the follow-up. The follow-up complication rate was 6.3%. TIAs occurred in 4.4% (nine cases) at a mean duration of 4.68 ± 2.90 months. A minor stroke occurred at 1.4% (three cases) of patients at a mean duration of 8 ± 2.64 months. One case experienced a major stroke. The overall patient event-free survival was 92.4% (Figure 2).
Figure 2.
Kaplan-Meier curve of cumulative events-free survival exclude restenosis. Note that all adverse events occurred within 1 to 10 months after the procedure.
Discussion
The current trial showed the feasibility of stent-assisted angioplasty for VAS in 206 cases treated with PTA with or without stents placed at different sites of the VA, with low peri-procedural complication rates. Our mean follow-up time of 13.15 months showed the durability of treatment outcome and long-term clinical outcomes. Clinical success and procedural (technical) success rates were 100% and 97.6%, respectively. Restenosis occurred in 15.9% of cases (63.1% mild, 34.2% moderate and 2.6% severe) after 10.8 months. TIA occurred in 4.4% (nine cases), minor stroke in 1.4% (three cases) and major stroke in 0.5% (one case) during the follow-up period. All of these patients were followed up for 14.92 ± 6.33 months, and they showed a significant improvement in their clinical outcomes. The overall patient event-free survival was 92.4%.
Pharmacotherapy and surgical intervention are traditional treatments for VAS. However, the risk for stroke is still relatively high in patients with VB artery stenosis receiving routine pharmacotherapy and their prognosis remains poor. A previous investigation demonstrated that the incidence of stroke in the region supplied by stenotic arteries remains high even if patients are treated with warfarin or aspirin 26.
The development of interventional techniques such as stent-assisted angioplasty has been an effective strategy for treating VB artery stenosis due to its minimal invasiveness and high efficiency 27,28. PTA with a stent implant for stenotic arteries recanalizes the arteries, improves blood supply, and attenuates symptoms. In addition, a stent can prevent the atherosclerotic plaque from tearing and reduce the risk for stroke due to atherosclerotic plaque.
In a review study on endovascular VA interventions, the 30-day major stroke and death rate was 3.2% and the 30-day TIA and nondisabling stroke rate was 3.2%. This meta-analysis suggested that VA stenting is safe and effective 29.
Kim et al. evaluated the feasibility and follow-up results of 17 consecutive patients treated with stent-assisted angioplasty for symptomatic intracranial VB artery stenosis with a mean follow-up duration of 17 months. Periprocedural complications occurred in 12%. Three patients (17.6%) had TIA and one (5.9%) developed a minor stroke after the procedure during the follow-up period. Restenosis occurred in 11.7% of cases and no mortalities were reported 30.
Du et al. evaluated the safety, feasibility, and long-term results of stent-assisted angioplasty in 48 cases of atherosclerotic ostial stenosis of the VA. Technical success was achieved in 97.9% of the lesions, and restenosis occurred in 34.6%. The clinical follow-up outcomes showed that there were no lesion-related strokes and deaths, but that three patients appeared to have a TIA 31.
Zavala et al. evaluated the results of PTA and stent implants for symptomatic VB insufficiency unresponsive to medical therapy in 28 patients and reported a technical success rate of 96% and a clinical success rate of 88% with a follow-up of 14.2 months (range, 3.5-24.3 months). Their restenosis rate was 3.5% and mortality rate was 3.5% 32. Weber et al. demonstrated a technical success rate of 100%, morbidity rate of 14%, and a restenosis rate of 9.9% without clinical symptoms in their investigation of stent angioplasty for intracranial VB stenosis in 21 symptomatic patients 33.
Jenkins et al. reported a high procedural success rate with low morbidity in a series of patients undergoing endovascular stent placement for symptomatic VA stenosis at their institution over an 11-year period. Procedural and clinical success was achieved in 105 (100%) and 95 (90.5%) patients, respectively. The periprocedural complication rate was 4.8%. The one-year mortality rate was 5.7%, and the stroke rate was 5%. They concluded that stenting for symptomatic VA stenosis can be accomplished with a very high success rate (100%), with few periprocedural complications, and the procedures are associated with durable symptom resolution in the majority (approximately 80%) of patients 34.
Dabus et al. evaluated 25 cases of endovascular treatment for VA stenosis in patients presenting with symptomatic VB ischemia. Overall technical success was achieved in 92.8%. No procedure-related TIA, stroke, or death was noted 35. Edgell et al. assessed the safety and efficacy of VA origin angioplasty and stenting for stroke prevention in a multicenter clinical experience. A total of 148 patients were included with a mean age of 66.2 ± 11.5 years. One patient (0.8%) had a stroke at one month and 5.2% patients had TIAs at three months. No immediate procedural events or deaths were reported. The restenosis (≥50%) rate on follow-up angiography was 15.5%. They concluded that endovascular intervention for a VA orifice stenosis is associated with low periprocedural complication rates 36.
Taken together, the results of our case series offer further evidence in favor of this new treatment option in patients with symptomatic VA stenosis. In SSYLVIA the incidence of re-stenosis was as high as 32.4% within six months after the procedure 37. The restenosis rates in different series are difficult to compare due to the different techniques used to image the treated VA segment during follow-up, but the lower rate of restenosis in our investigation may result from super-selection of patients (NIHSS≤10; restricted and characterized lesion, exclusion of any diseases affecting this process, close observation and aggressive control of risk factors) and the use of appropriate techniques by an experienced team of neuro-interventionists. The overall periprocedural complication rate was 7.2%. However, stent-assisted PTA for VB artery stenosis has some advantages such as a high success rate, fewer complications, prevention of recurrent stroke, and better short-term clinical improvement 23,38, but still presents a risk for rupture, artery dissection, perforating artery occlusion, thrombosis, cerebral hyperperfusion, and restenosis 39,40. However the incidence of these complications is relatively low and some of these can be avoided with experience.
Some limitations of our study should be mentioned. It is known that morphological factors of vertebral artery lesions may affect the outcome of stent therapy. For example, lesion length affects the restenosis rate and excessive vessel tortuosity precludes successful stent delivery. We did not have a control group to compare other treatment options.
Conclusion
Our results demonstrate the safety and feasibility of PTA with or without stent implants with a high technical success rate, low complication rate, low restenosis rate, and durable clinical success in patients with symptomatic VA stenosis. This approach seems to improve patients' immediate and long-term clinical results. Randomized controlled trials are necessary to further validate this treatment option. We declare that we have not conflict of interest.
References
- 1.Hornig RC, Büttner T, Hoffmann O, et al. Short-term prognosis of vertebrobasilar ischemic stroke. Cerebrovasc Dis. 1992;2:273–281. [Google Scholar]
- 2.Bamford J, Sandercock P, Dennis M, et al. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–1526. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 3.Caplan LR. The intracranial vertebral artery: a neglected species. The Johann Jacob Wepfer Award 2012. Cerebrovasc Dis. 2012;34:20–30. doi: 10.1159/000339629. [DOI] [PubMed] [Google Scholar]
- 4.Flossman E, Rothwell P. Prognosis of vertebrobasilar transient ischaemic attack and minor stroke. Brain. 2003;126:1940–1954. doi: 10.1093/brain/awg197. [DOI] [PubMed] [Google Scholar]
- 5.Wityk RJ, Chang HM, Rosengart A, et al. Proximal extracranial vertebral artery disease in the New England Medical Center Posterior Circulation Registry. Arch Neurol. 1998;55:470–478. doi: 10.1001/archneur.55.4.470. [DOI] [PubMed] [Google Scholar]
- 6.Phatouros CC, Higashida RT, Malek AM, et al. Endovascular treatment of noncarotid extracranial cerebrovascular disease. Neurosurg Clin N Am. 2000;11:331–350. [PubMed] [Google Scholar]
- 7.Levy EI, Horowitz MB, Koebbe CJ, et al. Transluminal stent-assisted angioplasty of the intracranial vertebrobasilar system for medically refractory, posterior circulation ischemia: early results. Neurosurgery. 2001;48:1215–1221. doi: 10.1097/00006123-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Berguer R, Morasch MD, Kline RA. A review of 100 consecutive reconstructions of the distal vertebral artery for embolic and hemodynamic disease. J Vasc Surg. 1998;27:852–859. doi: 10.1016/s0741-5214(98)70265-4. [DOI] [PubMed] [Google Scholar]
- 9.Hass WK, Easton JD, Adams HP Jr., et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. Ticlopidine Aspirin Stroke Study Group. N Engl J Med. 1989;321:501–507. doi: 10.1056/NEJM198908243210804. [DOI] [PubMed] [Google Scholar]
- 10.Thevenet A, Ruotolo C. Surgical repair of vertebral artery stenoses. J Cardiovasc Surg. 1984;25:101–110. [PubMed] [Google Scholar]
- 11.Levy EI, Turk AS, Albuquerque FC, et al. Wingspan in-stent restenosis and thrombosis: incidence, clinical presentation, and management. Neurosurgery. 2007;61(3):644–651. doi: 10.1227/01.NEU.0000290914.24976.83. [DOI] [PubMed] [Google Scholar]
- 12.Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Warfarin-Aspirin Symptomatic Intracranial Disease Trial Investigators. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl Med. 2005;352(13):1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 13.Koskas F, Kieffer E, Rancurel G, et al. Direct transposition of the distal cervical vertebral artery into the internal carotid artery. Ann Vasc Surg. 1995;9:515–524. doi: 10.1007/BF02018824. [DOI] [PubMed] [Google Scholar]
- 14.Cloud GC, Crawley F, Clifton A, et al. Vertebral artery origin angioplasty and primary stenting: safety and restenosis rates in a prospective series. J Neurol Neurosurg Psychiatry. 2003;74:586–590. doi: 10.1136/jnnp.74.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruigrok Y, Cox TC, Markus HS. Positional vertebrobasilar transient ischaemic attacks treated with vertebral angioplasty. Cerebrovasc Dis. 1999;9:171–174. doi: 10.1159/000015949. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins JS, White CJ, Ramee SR, et al. Vertebral artery stenting. Catheter Cardiovasc Interv. 2001;54:1–5. doi: 10.1002/ccd.1228. [DOI] [PubMed] [Google Scholar]
- 17.Clark WM, Barnwell SL, Nesbit G, et al. Safety and efficacy of percutaneous transluminal angioplasty for intracranial atherosclerotic stenosis. Stroke. 1995;26:1200–1204. doi: 10.1161/01.str.26.7.1200. [DOI] [PubMed] [Google Scholar]
- 18.Callahan ASI, Berger BL. Balloon angioplasty of intracranial arteries for stroke prevention. J Neuroimaging. 1997;7:232–235. doi: 10.1111/jon199774232. [DOI] [PubMed] [Google Scholar]
- 19.Yokote H, Terada T, Ryujin K, et al. Percutaneous transluminal angioplasty for intracranial arteriosclerotic lesions. Neuroradiology. 1998;40:590–596. doi: 10.1007/s002340050651. [DOI] [PubMed] [Google Scholar]
- 20.Mohammadian R, Najaran A, Sohrabi B, et al. Vertebral artery orifice stenosis: a report of 43 cases from northwest iran treated with angioplasty and stenting. Neuroradiol J. 2011;1:807–815. doi: 10.1177/197140091102400513. [DOI] [PubMed] [Google Scholar]
- 21.Rothwell PM, Eliasziw M, Gutnikov SA, et al. for the Carotid Endarterectomy Trialists Collaboration. Analysis of pooled data from the randomized controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361:107–116. doi: 10.1016/s0140-6736(03)12228-3. [DOI] [PubMed] [Google Scholar]
- 22.Coward LJ, McCabe DJH, Ederle J, et al. on behalf of the CAVATAS Investigators. Long-term outcome after angioplasty and stenting for symptomatic vertebral artery stenosis compared with medical treatment in the Carotid And Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomized trial. Stroke. 2007;38:1526–1530. doi: 10.1161/STROKEAHA.106.471862. [DOI] [PubMed] [Google Scholar]
- 23.Eberhardt O, Naegele T, Raygrotzki S, et al. Stenting of vertebrobasilar arteries in symptomatic atherosclerotic disease and acute occlusion: Case series and review of the literature. J Vasc Surg. 2006;43:1145–1154. doi: 10.1016/j.jvs.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 24.Brott T, Marler JR, Olinger CP, et al. Measurements of acute cerebral infarction: lesion size by computed tomography. Stroke. 1989;20:871–875. doi: 10.1161/01.str.20.7.871. [DOI] [PubMed] [Google Scholar]
- 25.Higashida RT, Tsai FY, Halbach VV, et al. Cerebral percutaneous transluminal angioplasty. Heart Dis Stroke. 1993;2:497–502. [PubMed] [Google Scholar]
- 26.The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Study Group. Prognosis of patients with symptomatic vertebral or basilar artery stenosis. Stroke. 1998;29:1389–1392. doi: 10.1161/01.str.29.7.1389. [DOI] [PubMed] [Google Scholar]
- 27.Liu JM, Hong B, Xu Y. Endovascular stenting for vertebrobasilar artery stenosis. Chin Radiol. 2002;36:1063–1067. [Google Scholar]
- 28.Chang BG, Xue DY, Li W. Intravascular treatment of symptomatic vertebro-basilar artery stenosis: report of 95 cases. Chin J Neurosurg. 2009;25(2):106–109. [Google Scholar]
- 29.Coward LJ, Featherstone RL, Brown MM. Percutaneous transluminal angioplasty and stenting for vertebral artery stenosis. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD000516.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim DJ, Lee BH, Kim DI, et al. Stent-assisted angioplasty of symptomatic intracranial vertebrobasilar artery stenosis: feasibility and follow-up results. Am J Neuroradiol. 2005;26:1381–1388. [PMC free article] [PubMed] [Google Scholar]
- 31.DU B, Dong KH, Xu XT, et al. Stent-assisted angioplasty and long-term results in atherosclerotic vertebral artery ostial stenosis. Zhonghua Nei Ke Za Zhi. 2007;46(3):204–207. Chinese. [PubMed] [Google Scholar]
- 32.Zavala-Alarcon E, Emmans L, Cecena F, et al. Percutaneous vertebral artery intervention: a necessary tool in every interventional cardiologist armamentarium. Cardiovasc Revasc Med. 2007;8:107–113. doi: 10.1016/j.carrev.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Weber W, Mayer TE, Henkes H, et al. Stent-angioplasty of intracranial vertebral and basilar artery stenoses in symptomatic patients. Eur J Radiol. 2005;55:231–236. doi: 10.1016/j.ejrad.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins JS, Patel SN, White CJ, et al. Endovascular stenting for vertebral artery stenosis. J Am Coll Cardiol. 2010;55:538–542. doi: 10.1016/j.jacc.2009.08.069. [DOI] [PubMed] [Google Scholar]
- 35.Dabus G, Gerstle RJ, Derdeyn CP, et al. Endovascular treatment of the vertebral artery origin in patients with symptoms of vertebrobasilar ischemia. Neuroradiology. 2006;48:917–923. doi: 10.1007/s00234-006-0151-7. [DOI] [PubMed] [Google Scholar]
- 36.Edgell RC, Zaidat OO, Gupta R, et al. Multicenter study of safety in stenting for symptomatic vertebral artery origin stenosis: results from the Society of Vascular and Interventional Neurology Research Consortium. J Neuroimaging. 2013;23(2):170–174. doi: 10.1111/j.1552-6569.2011.00665.x. [DOI] [PubMed] [Google Scholar]
- 37.SSYLVIA Study Investigators. Stenting of symptomatic atherosclerotic lesions in the vertebral or intracranial arteries (SSYLVIA): study results. Stroke. 2004;35:1388–1392. doi: 10.1161/01.STR.0000128708.86762.d6. [DOI] [PubMed] [Google Scholar]
- 38.Jiang WJ, Xu XT, Du B, et al. Long-term outcome of elective stenting for symptomatic intracranial vertebrobasilar stenosis. Neurology. 2007;68:856–858. doi: 10.1212/01.wnl.0000256713.23864.be. [DOI] [PubMed] [Google Scholar]
- 39.Lin YH, Liu YC, Tseng WY, et al. The impact of lesion length on angiographic restenosis after vertebral artery origin stenting. Eur J Vasc Endovasc Surg. 2006;32(4):379–385. doi: 10.1016/j.ejvs.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 40.Yu W, Smith WS, Singh V, et al. Long-term outcome of endovascular stenting for symptomatic basilar artery stenosis. Neurology. 2005;64:1055–1057. doi: 10.1212/01.WNL.0000154600.13460.7B. [DOI] [PubMed] [Google Scholar]