Summary
This study evaluated the relationship of cerebrovascular reactivity in young healthy women with changes in concentrations of circulating ovarian hormones throughout the menstrual cycle. Nineteen healthy nulliparous, right-handed, regularly menstruating women (age 23-25 years) underwent color-coded duplex sonography of the common (CCA), internal (ICA) and external (ECA) carotid arteries on both sides. Mean blood flow velocity values measured before and ten minutes after intravenous administration of 1000 mg acetazolamide (ACE) were assessed in relation to the serum concentration of estrogen and progesterone on days 5, 13 and 26 of the cycle. After ACE administration flow velocity in the right CCA and ICA increased by 23% and 35% on day 5, 12% and 31% on day 13 and 30% and 47% on day 26 respectively, and the changes were significantly larger on the right side (F=6.793 and F=4.098 respectively; both p<0.05). Changes in blood flow velocity in the right CCA and ICA after ACE injection were significantly associated with ovarian hormone concentrations (F=3.828, P=0.028 and F=3.671, P=0.032 respectively). We conclude that cerebrovascular reactivity changes across the menstrual cycle are associated with ovarian steroid hormone changes, and are asymmetric. The results imply that vasculature of the right hemisphere may undergo cyclic vasodilation across the menstrual cycle and this effect should be considered in studies of cerebrovascular reactivity in women with migraine and mood disorders.
Keywords: cerebrovascular reactivity, Doppler, menstrual cycle, sex hormones, women
Introduction
A higher prevalence of migraine and mood disorders in young women compared to men may imply that female sex hormones play a role in their pathogenesis 1,2. Our knowledge of the mechanisms of sex hormone action in the human brain comes predominantly from lower-order animal species and from in vitro studies 3,4. Extrapolations of results from studies of animals to humans, however, are frequently made without a full appreciation that there are inherent species differences. In vitro models, while simplifying the cellular environment, do not mimic the complexity of neurovascular and hemodynamic interactions. A number of studies have demonstrated changes in cerebral artery function in postmenopausal women after long-term exposure to constant doses of exogenous estrogen and progestin 4-6, however, the results of those studies are not necessary applicable to younger premenopausal women, who receive only intermittent pulses of gonadal hormones during a menstrual cycle. The short-term effects of estrogen (E2) and progesterone (PG) on cerebral vessels, resulting from normal cyclic variations of these hormones across the menstrual cycle in women, remain largely unknown.
The menstrual cycle provides a natural paradigm to investigate how steroid ovarian hormones regulate brain vascular physiology 7,8 because of natural substantial fluctuations of sex hormone concentrations in the blood. In addition, the time of exposure to E2 and PG is more than sufficient to produce non-genomic and genomic effects 4,9. Measurements of the ability of vessels to dilate in response to a vasodilatatory stimulus such as acetazolamide (ACE), a carbonic anhydrase inhibitor 10-12, can provide information on the actual level of vasodilatation of brain vessels across the menstrual cycle 13,14. We hypothesize that this ability, named cerebrovascular reactivity (CVR), varies substantially throughout the menstrual cycle along with changes in circulating ovarian hormones.
Subjects and methods
Study population
The University Ethics Committee approved the study protocol, that was compliant with the standards as described in the Declaration of Helsinki and each volunteer gave informed consent. We recruited 19 healthy nulliparous, right-handed, regularly menstruating women (age 23-25 years), with at least six self-reported regular consecutive menstrual cycles ranging from 27 to 32 days. We applied the following exclusion criteria: oral contraceptive use or any other hormonal treatment in the past or present, any other medication for at least three months before and during the study period, history of any type of migraine, premenstrual dysphoric syndrome, any serious disease, particularly head trauma, diabetes, psychiatric, cardiovascular or gynecological disorders, current smoking, alcohol or coffee abuse. Subjects had to be strictly normal on clinical examination by a physician. Their body mass index was <25. Blood cell count, liver function tests, and the concentrations of electrolytes, blood glucose, cholesterol, triglyceride, BUN and creatinine were within normal limits.
Testing
The cycles were counted from the first day of menses and were standardized to a 28-day period as described in our previous study 15. Subjects were examined on days 5, 13 and 26 of the cycle, and were asked to be fasting at least 12 hours and abstain from vigorous exercise, alcohol intake, and caffeine-containing beverages for at least 24 hours prior to testing. Ultrasound examinations were carried out in a quiet room, with subjects lying in a supine position, after a 15-minute rest period. To avoid inter-observer error and minimize the effect of circadian rhythms on cerebral blood flow, one experienced investigator performed all ultrasonographic examinations between 6:00 am and 8:00 am. On each study day after the baseline sonographic examination, we measured arterial blood pressure (ABP) and heart rate (HR), and sampled 10 cm3 of blood to measure plasma E2 and PG concentrations, using an automated chemiluminescence system (ACS 180:PLUS immunoassay; Bayer, Germany). Precision of measurements, expressed as coefficient of variation, provided by the manufacturer for PG and E2 was less than 12% and 9% respectively. Thereafter, 1000 mg acetazolamide (Diamox®, Lederle, USA) was administered intravenously. We chose the dose of 1000 mg to assure maximal vasodilatatory response as the full inhibition of carbonic anhydrase can be obtained at a dose of 15 mg/kg of body mass 10. As none of our volunteers weighed more than 65 kg, everyone received at least 15.4 mg/kg. Ten minutes after injection we repeated ultrasonographic examination, ABP and HR measurements. Blood flow velocity increase after ACE injection was expressed as a percentage of baseline value according to a formula: Δ=(V2-V1)*100/V1 where V1 and V2 indicate velocity before and after injection, respectively.
Ultrasonographic examination
The common (CCA), internal (ICA) and external (ECA) carotid arteries were examined on both sides with a gray-scale, and pulsed Doppler imaging, using a 7.5 MHz linear array transducer (Toshiba Aplio, Toshiba Medical System Division, Tokyo, Japan). The order of ultrasound measurements as to the side was randomized before and after ACE administration. The sample volume, adjusted to the size of insonated vessels, was placed within the ICA and ECA at 15 mm to 20 mm and 10 mm to 15 mm distal to the CCA bifurcation, respectively. To obtain waveforms from the CCA, the sample was placed at 10 mm to 20 mm below the bifurcation. The mean velocities were obtained by manually tracing the maximum frequency envelope of the Doppler waveform over completed cycles.
Statistical analyses
Data analysis was performed using SYSTAT12 statistical software (SYSTAT, Chicago, IL. USA). We used parametric tests as the distribution of data was normal. To test hypotheses regarding the homogeneity of hemodynamic changes after ACE injection across the cycle we used repeated-measures ANOVA with post-hoc Newman-Keuls multiple comparisons test. To quantify associations of E2 and PG concentrations with velocity measurements, we employed multivariable linear regression analysis. HR and ABP values obtained before and after ACE administration were compared using paired t-test. Probability <0.05 was considered significant.
Results
As expected, PG concentration was low on days 5 and 13 and high on day 26, while E2 concentration was low on day 5, very high on day 13, and relatively high on day 26 (Table 1). Fluctuations of ABP and HR during the cycle were insignificant (Table 1). After ACE injection ABP remained unchanged.
Table 1.
Fluctuations of systolic and diastolic arterial blood pressure (ABP), heart rate (HR), 17-beta estradiol (E2) and progesterone (PG) during the menstrual cycle in 19 young healthy women.
| Parameter | Day 5 | Day 13 | Day 26 | P value, F statistic |
| Systolic ABP (mmHg) | 117.8±6.2 | 120±7.6 | 118±6.9 | 0.814, 0.207 |
| Diastolic ABP (mmHg) | 77.2±5.0 | 77.1±4.9 | 80.9±5.8 | 0.326, 1.186 |
| HR (min-1) | 74.4±8.5 | 74.7±4.9 | 70.3±5.8 | 0.400, 0.960 |
| E2 (pg/ml) | 33.0±32.6 | 105.5±77.6 | 59.1±45.3 | p < 0.05 |
| PG (ng/ml) | 0.9±0.57 | 1.3±0.9 | 5.9±3.7 | p < 0.05 |
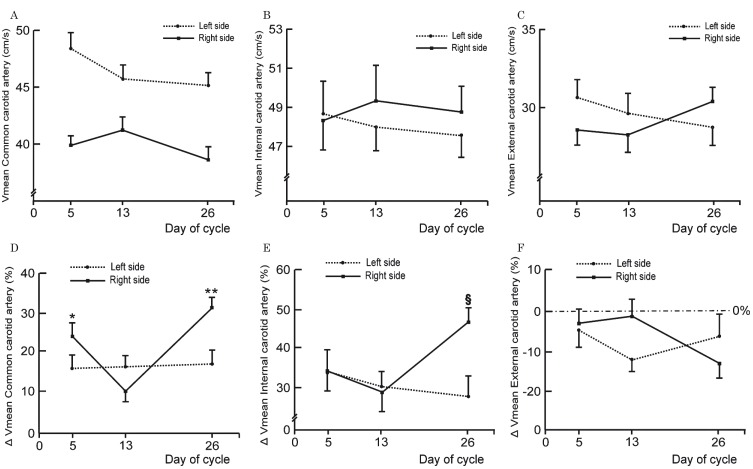
The change in baseline mean flow velocity in carotid vessels of both sides was not significant throughout the menstrual cycle (Figure 1A-C). After ACE administration the velocity in the CCA on the right side increased by 23% on day 5, 12% on day 13, and 30% on day 26. Velocity changes (Δ values) on days 5 and 26 were significantly higher compared to the change on day 13, and compared to the change on the left side (Figure 1D). By contrast, on the left side, fluctuations of Δ values across the cycle were insignificant. Velocities in the right ICA increased after ACE injection on day 5 by 35%, on day 13 by 31% and on day 26 by 47 % (Figure 1E). The increase in flow velocity on day 26 was significantly higher than that on day 13, and the increase in flow velocity on the left side on day 26. The difference between days 5 and 13 showed a trend toward significance (p=0.053). As in the left CCA, the velocity change in the left ICA after ACE was insignificant on all study days. Contrary to the velocity in the ICA and CCA, blood flow velocities in both ECAs dropped after ACE administration in all volunteers on each study day (Figure 1F), but the differences in Δ values were not significant. Using multivariable regression analysis we found significant associations between Δ values of mean blood flow velocity in the right CCA and ICA and E2 and PG concentrations; the respective regression equations are given below: Δ CCA right Vmean = 1.67 PG (ng/ml, P=0.035) - 0.08 E2 (pg/ml, P=0.044) + 22.46;
Δ ICA right Vmean = 1.61 PG (ng/ml P=0.054) - 0.09 E2 (pg/ml, P=0.035) + 39.34.
There was no significant relationship between E2 and PG concentration and Δ values of mean blood flow velocity in the left CCA, left ICA and both ECAs (Table 2).
Table 2.
Contribution of sex hormones to the variability of mean blood flow velocities changes (Δ values in cm/s) in carotid arteries after acetazolamide administration in young healthy women during the menstrual cycle as determined by the multivariable linear regression analysis.
| Variable | Regression model |
| Δ CCA RIGHT | 1.668 PG (p=0.035) - 0.080 E2 (p=0.044) + 22.462 F=3.828; P=0.028 |
| Δ CCA left | 0.371 PG (p=0.577) - 0.006 E2 (p=0.860) + 16.059 F=0.161; P=0.851 |
| Δ ICA RIGHT | 1.607 PG (p=0.054) - 0.088 E2 (p=0.035) + 39.340 F=3.671; P=0.032 |
| Δ ICA left | -1.326 PG (p=0.178) - 0.043 E2 (p=0.379) + 38.489 F=1.560; P=0.219 |
| Δ ECA RIGHT | -0.876 PG (p=0.314) - 0.012 E2 (p=0.775) - 2,645 F=0.622; P=0.541 |
| Δ ECA left | 1.328 PG (p=0.204) - 0.077 E2 (p=0.141) - 5.968 F=1.677; P=0.197 |
CCA, ICA, ECA - common, internal and external carotid arteries, respectively; E2 - 17β-estradiol (pg/ml), PG - progesterone (ng/ml), F - Snedecor's ratio, P - probability.
Figure 1.
Baseline values of mean blood flow velocity in carotid arteries measured with Doppler-duplex ultrasound on days 5th, 13th and 26th of the menstrual cycle in young healthy women (n=19) (A-C), and changes in mean blood flow velocity (Δ Vmean) after intravenous injection of 1000 mg of acetazolamide (D-F) expressed as percentages of baseline value. Each circle and square represents mean values. Error bars indicate standard error of the mean. Baseline differences were not significant (repeated measures ANOVA). * P=0.033; ** P=0.002; § P=0.023 - changes were significantly different than registered on day 13 (repeated measures ANOVA followed by Newman-Keuls multiple comparison test).
Discussion
Our study showed that in young healthy women CVR in the territory supplied by the right internal carotid artery (ICA) varied significantly during distinct phases of the menstrual cycle. The CVR changes appear to be associated with oscillations of circulating E2 and PG. As the distribution of receptors for sex hormones in the vessels of both hemispheres is most likely symmetrical, the observed unilateral pattern of CVR changes strongly suggests intermittent interhemispheric differences in neuronal activity across the cycle. This argument is further supported by the lack of significant fluctuations of systemic parameters such as HR and ABP, and the fact that both hemispheres had the same circulating ACE and oxygen content.
One possible explanation for the observed laterality of CVR across the menstrual cycle can be linked to functional vascular adjustment in metabolically demanding active brain regions 16,17. It is tempting to conclude that our regression analysis findings reflect true alterations in neural activity in the face of fluctuations of circulating ovarian steroid hormones 18,19. The fact that significant CVR changes associated with sex hormones were seen only in arteries on the right side speaks clearly against the hypothesis of a purely vascular influence 4, as we would expect similar vascular changes in both hemispheres, regardless of the stimulus and mechanism of its action used to invoke them. A possible disruption of the normal tight coupling documented to exist among hemodynamic response, neuronal metabolism and neuronal activity 16,20,21, is a much less likely alternative explanation in young healthy women with regular menstrual cycles. The few instances in which uncoupling between hemodynamic and neuronal metabolism have been demonstrated involved cases of extreme pathology, such as cerebrovascular disease, increased intracranial pressure, or stroke 12.
If these findings do represent a hormonal modulation of neuronal activity, we should expect our findings to be consistent with asymmetric behavior patterns associated with modulation of cholinergic, serotonergic, noradrenergic, dopaminergic and GABA systems by ovarian steroids 22-25. It has been shown that young women express relatively greater resting right frontal activity during the periovulatory phase compared to the menstrual phase 26. An electroencephalographic study has also shown that the right hemisphere vigilance system can be enhanced by hormonal therapy in women with postmenopausal syndrome 27. Estrogenic effects were reported to occur in the limbic-amygdala-hippocampal structures 5, which can modulate higher perseverance for positive reward during the periovulatory phase 28. Estrogen seems to modulate predominantly the right inferior frontal gyrus, pars opercularis, right lateral occipital cortex, and superior parietal lobule 29.
Our findings are also in agreement with research studies suggesting relatively greater left baseline anterior hemisphere activity during the perimenstrual period (days 5 and 26) 30,31. In addition, increased activation of the right dorsolateral prefrontal and anterior cingulate cortex was associated with E2 in response to positive stimuli and decreased activation to negative stimuli during the luteal phase32. Our findings are also congruent with other brain imaging and mapping findings33 suggesting that estrogen and progesterone asymmetrically modulate neuronal circuitry across the menstrual cycle.
It is important to note that in many subjects the days in the menstrual cycle selected for the study did not correspond with homogenous hormone states given that the length of each cycle varied from woman to woman and cycle to cycle. Based on serial measurements of plasma concentration of sex hormones, we showed that even the standard model of the ovarian cycle in humans, with regular mid-cycle ovulation is inaccurate in predicting the actual plasma hormone concentration 15. The spread of ovulation across the cycle varies widely, and it is only sometimes possible to determine the subjects' accurate ovulation time by relying on the calendar method alone. However, we had to select particular days because serial day-to-day determination of plasma hormone concentration is extremely inconvenient for healthy subjects. Hence, we expected a higher variability in concentrations of steroid ovarian hormones across the cycle and accordingly higher variability of blood flow parameters. Our observations indicate that average group findings cannot be applied to individual women, in whom the exact hormonal make-up on a particular day can be unpredictable. We stress the importance of actual determinations of steroid hormone concentrations in plasma when studying associations with CVR measurements across the natural menstrual cycle.
The ACE-induced blood flow velocity increase in the ICA depends on the degree of baseline peripheral vasodilatation assuming all other rheological parameters remain constant 12. If the peripheral vasculature was constricted at baseline the ACE-induced vasodilatation should substantially decrease the peripheral vascular resistance and increase flow velocity 10,12. However, if at baseline the peripheral vasculature was dilated the administration of ACE should dilate the microvasculature relatively less because its vasodilatatory capacity had already been partly exhausted. Since blood flow through a vessel is directly proportional to the fourth power of its radius, the magnitude of the ACE-induced velocity increase must be a sensitive indicator of the baseline degree of vasodilatation, assuming a constant pressure gradient.
ACE specifically inhibits carbonic anhydrase that catalyzes the reversible hydration of carbon dioxide to bicarbonate and protons, and effectively links blood oxygen delivery to metabolic carbon dioxide production from tissue 34. ACE increases the carbonic anhydrase-catalyzed production of the potent vasodilator nitric oxide from a readily available pool of nitrite 34. This enhancing effect further explains the strong vasodilating properties of ACE, although it has to be noted that further vasodilation is still possible during neuronal activation, even at the maximal vasodilatatory effect of the applied dose of ACE 35.
To demonstrate how sensitive the paradigm with the use of ACE can be in disclosing the baseline degree of vasodilatation, we describe the following hypothetical situation. For instance, if a 0.3 mm vessel diameter decreased by 0.1 mm under influence of a hormone, the blood flow through this vessel drops fivefold accordingly. However, if a 0.2 mm vessel diameter increased by 0.2 mm (i.e. under influence of ACE), the blood flow increases seven times higher than the flow increase resulting from dilation of a 0.3 mm vessel by 0.1 mm. This means that without using ACE we would have to study 153 women to detect the vasomotor alterations at a power similar to that in our study. Our study paradigm therefore enabled us to detect small functional alterations of cerebral micovasculature assuming that the ICA diameter remained unchanged, which is very likely because the ICA is a conductive vessel. Furthermore, if ACE dilated the ICA at the site of velocity sampling, the value of measured velocity should drop accordingly, contrary to what we observed. A slight decrease of blood flow velocity in the ECA after administration of ACE, probably due to redistribution of blood flow from the high resistance vascular ECA bed to the low resistance ICA bed, consistently supports the observed pattern.
Conclusions
Our study showed asymmetric CVR changes across the menstrual cycle in young healthy women in those parts of the brain supplied by the internal carotid arteries. The oscillations of the CVR seemed to be associated with circulating levels of ovarian steroid hormones, E2 and PG. Investigators should be aware of potential substantial CVR changes across the menstrual cycle when designing studies on cerebral reactivity in women with migraine and other neurovascular disorders.
Acknowledgments
This study was supported by the American Heart Association (AHA) Established Investigator Award grant [044099N]; and by Polish State Committee for Scientific Research [3P05C 009 22].
References
- 1.Stewart WF, Lipton RB, Celentano DD, et al. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267(1):64–69. [PubMed] [Google Scholar]
- 2.Silberstein S, Merriam G. Sex hormones and headache 1999 (menstrual migraine) Neurology. 1999;53(4) Suppl 1:S3–13. [PubMed] [Google Scholar]
- 3.Florian M, Lu Y, Angle M, Magder S. Estrogen induced changes in Akt-dependent activation of endothelial nitric oxide synthase and vasodilation. Steroids. 2004;69(10):637–645. doi: 10.1016/j.steroids.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Krause DN, Duckles SP, Pelligrino DA. Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol. 2006;101(4):1252–1261. doi: 10.1152/japplphysiol.01095.2005. [DOI] [PubMed] [Google Scholar]
- 5.Bain CA, Walters MR, Lees KR, et al. The effect of HRT on cerebral haemodynamics and cerebral vasomotor reactivity in post-menopausal women. Hum Reprod. 2004;19(10):2411–2414. doi: 10.1093/humrep/deh396. [DOI] [PubMed] [Google Scholar]
- 6.Maki PM, Dumas J. Mechanisms of action of estrogen in the brain: insights from human neuroimaging and psychopharmacologic studies. Semin Reprod Med. 2009;27(3):250–259. doi: 10.1055/s-0029-1216278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silberstein SD, Merriam GR. Physiology of the menstrual cycle. Cephalalgia. 2000;20(3):148–154. doi: 10.1046/j.1468-2982.2000.00034.x. [DOI] [PubMed] [Google Scholar]
- 8.Krejza J, Siemkowicz J, Sawicka M, et al. Oscillations of cerebrovascular resistance throughout the menstrual cycle in healthy women. Ultrasound Obstet Gynecol. 2003;22(6):627–632. doi: 10.1002/uog.907. [DOI] [PubMed] [Google Scholar]
- 9.McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 10.Dahl A, Russell D, Rootwelt K, et al. Cerebral vasoreactivity assessed with transcranial Doppler and regional cerebral blood flow measurements. Dose, serum concentration, and time course of the response to acetazolamide. Stroke. 1995;26(12):2302–2306. doi: 10.1161/01.str.26.12.2302. [DOI] [PubMed] [Google Scholar]
- 11.Okazawa H, Yamauchi H, Sugimoto K, et al. Effects of acetazolamide on cerebral blood flow, blood volume, and oxygen metabolism: a positron emission tomography study with healthy volunteers. J Cereb Blood Flow Metab. 2001;21(12):1472–1479. doi: 10.1097/00004647-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Rudzinski W, Swiat M, Tomaszewski M, et al. Cerebral hemodynamics and investigations of cerebral blood flow regulation. Nucl Med Rev Cent East Eur. 2007;10(1):29–42. [PubMed] [Google Scholar]
- 13.Shinoura N, Yamada R. Decreased vasoreactivity to right cerebral hemisphere pressure in migraine without aura: a near-infrared spectroscopy study. Clin Neurophysiol. 2005;116(6):1280–1285. doi: 10.1016/j.clinph.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Akin A, Bilensoy D, Emir UE, et al. Cerebrovascular dynamics in patients with migraine: near-infrared spectroscopy study. Neurosci Lett. 2006;400(1-2):86–91. doi: 10.1016/j.neulet.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Krejza J, Mariak Z, Huba M, et al. Effect of endogenous estrogen on blood flow through carotid arteries. Stroke. 2001;32(1):30–36. doi: 10.1161/01.str.32.1.30. [DOI] [PubMed] [Google Scholar]
- 16.Takano T, Tian GF, Peng W, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9(2):260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 17.Gordon GR, Choi HB, Rungta RL, et al. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456(7223):745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belfort MA, Saade GR, Snabes M, et al. Hormonal status affects the reactivity of the cerebral vasculature. Am J Obstet Gynecol. 1995;172(4 Pt 1):1273–1278. doi: 10.1016/0002-9378(95)91492-7. [DOI] [PubMed] [Google Scholar]
- 19.Berman KF, Schmidt PJ, Rubinow DR, et al. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci USA. 1997;94(16):8836–8841. doi: 10.1073/pnas.94.16.8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devor A, Ulbert I, Dunn AK, et al. Coupling of the cortical hemodynamic response to cortical and thalamic neuronal activity. Proc Natl Acad Sci USA. 2005;102(10):3822–3827. doi: 10.1073/pnas.0407789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malonek D, Dirnagl U, Lindauer U, et al. Vascular imprints of neuronal activity: relationships between the dynamics of cortical blood flow, oxygenation, and volume changes following sensory stimulation. Proc Natl Acad Sci USA. 1997;94(26):14826–14831. doi: 10.1073/pnas.94.26.14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demaree HA, Everhart DE, Youngstrom EA, et al. Brain lateralization of emotional processing: historical roots and a future incorporating “dominance”. Behav Cogn Neurosci Rev. 2005;4(1):3–20. doi: 10.1177/1534582305276837. [DOI] [PubMed] [Google Scholar]
- 23.Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27(9):2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savic I, Lindstrom P. PET and MRI show differences in cerebral asymmetry and functional connectivity between homo- and heterosexual subjects. Proc Natl Acad Sci USA. 2008;105(27):9403–9408. doi: 10.1073/pnas.0801566105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fink M, Wadsak W, Savli M, et al. Lateralization of the serotonin-1A receptor distribution in language areas revealed by PET. Neuroimage. 2009;45(2):598–605. doi: 10.1016/j.neuroimage.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 26.Hwang RJ, Wu CH, Chen LF, et al. Female menstrual phases modulate human prefrontal asymmetry: a magnetoencephalographic study. Horm Behav. 2009;55(1):203–209. doi: 10.1016/j.yhbeh.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Saletu B, Anderer P, Saletu-Zyhlarz GM, et al. Identifying target regions for vigilance improvement under hormone replacement therapy in postmenopausal syndrome patients by means of electroencephalographic tomography (LORETA) Psychopharmacology (Berl) 2005;178(4):389–399. doi: 10.1007/s00213-004-2029-x. [DOI] [PubMed] [Google Scholar]
- 28.Dreher JC, Schmidt PJ, Kohn P, et al. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci USA. 2007;104(7):2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krug R, Plihal W, Fehm HL, et al. Selective influence of the menstrual cycle on perception of stimuli with reproductive significance: an event-related potential study. Psychophysiology. 2000;37(1):111–122. [PubMed] [Google Scholar]
- 30.Protopopescu X, Pan H, Altemus M, et al. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proc Natl Acad Sci USA. 2005;102(44):16060–16065. doi: 10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson DC, Mueller CJ, Dolski I, et al. Now you feel it, now you don’t: frontal brain electrical asymmetry and individual differences in emotion regulation. Psychol Sci. 2003;14(6):612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- 32.Amin Z, Epperson CN, Constable RT, et al. Effects of estrogen variation on neural correlates of emotional response inhibition. Neuroimage. 2006;32(1):457–464. doi: 10.1016/j.neuroimage.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein JM, Jerram M, Poldrack R, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005;25(40):9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aamand R, Dalsgaard T, Jensen FB, et al. Generation of nitric oxide from nitrite by carbonic anhydrase: a possible link between metabolic activity and vasodilation. Am J Physiol Heart Circ Physiol. 2009;297(6):H2068–2074. doi: 10.1152/ajpheart.00525.2009. [DOI] [PubMed] [Google Scholar]
- 35.Yonai Y, Boms N, Molnar S, et al. Acetazolamide-induced vasodilation does not inhibit the visually evoked flow response. J Cereb Blood Flow Metab. 2010;30(3):516–521. doi: 10.1038/jcbfm.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]