Summary
In Graves' ophthalmopathy (GO) it is important to distinguish acute inflammation at an early stage, responsive to immunosuppressive treatment, from inactive fibrotic end stage disease, unresponsive to the same treatment. The purpose of this study was to identify the most relevant signal intensities on orbital MR imaging with contrast administration both to classify patients according to their clinical activity score (defined by a cut-off value of 3) and to make a prediction of patient's CAS. Such threshold was considered as widely used in literature. Sixteen consecutive patients with a diagnosis of GO in different phases of thyroid disease based on clinical and orbital MR imaging signs, and six normal volunteers were examined. Orbital MR imaging was performed on a 1.5 Tesla MR Unit. MR scans were assessed by an experienced neuroradiologist, blinded to the clinical examinations. We found a statistical correlation between CAS and both STIR and contrast enhanced T1-weighted sequences. There was also a statistically significant correlation between STIR and contrast-enhanced T1 images disclosing the possibility of avoiding the injection of contrast medium. Our study proved that signal intensity values on STIR sequence increase in the inflammatory oedematous phase of disease. We confirmed the correlation between signal intensities on this sequence and CAS, showing an increase in signal intensity proportional to the CAS value. So we validated MRI use to establish the activity phase of disease more sensitively than CAS alone.
Keywords: Basedow Graves' disease, thyroid, CAS, proptosis
Introduction
Graves' ophthalmopathy (GO) is a chronic inflammatory autoimmune disease of periorbital tissue and conjunctiva, as well as retrobulbar structures, e.g. extraocular muscles (EOM). The diagnosis of GO is often evident, due to the bilateral symmetric aspect of the orbitopathy in patients with a history of Graves' hyperthyroidism. Mandatory objectives in the first evaluation of patients with clinically suspected GO, in order to establish the correct treatment, are: 1) to support the clinical diagnosis of GO by imaging; 2) to identify the disease phase; 3) to assess the disease severity.
The clinical activity score (CAS), proposed by Mourits et al. 1, is a validated scoring system for the identification of disease phase. The system is based on the assessment of inflammatory signs and symptoms (pain, eyelid and conjunctiva erythema, chemosis, eyelid oedema) to distinguish inflammatory from non-inflammatory GO. The system is widely used because of its high predictive value for the outcome of immunosuppressive treatment in GO patients 1. According to the European Groups on Graves' Orbitopathy (EUGOGO) recommendations, CAS≥3 defines active GO with a positive predictive value (PPV) of 65% and negative predictive value (NPV) of 56% for response to radiotherapy 1,3,4. However, the CAS system is an examiner-dependent method, and is inadequate for monitoring changes in clinical manifestations until the symptom completely resolves 5-7. Moreover, in Mourits et al.'s study, many patients with a CAS of 1 or 2 showed a significant response to immunosuppressive treatment 8,7, and the CAS cut-off value of 3, as stated by European Groups, did not prove suitable for the Asian population 5-7. So there is no exact correspondence between CAS alone and the real clinical condition of GO.
Therefore, as largely reported in literature, clinical score should be integrated by imaging modalities to improve the diagnostic accuracy 7,9,10, i.e. orbital ultrasound (US), computed tomography (CT) and magnetic resonance (MR). Orbital MR imaging is widely reported to detect not only other pathologies such as orbital mass, infections or vascular lesions, but also to identify oedema and so distinguish active from inactive GO. This is due to the high contrast resolution of MRI allowing the differentiation between signal intensity of fibrous and inflammatory tissue on strong T2-weighted (w) fat-suppressed images, derived from TIRM (Turbo-Inversion Recovery-Magnitude) sequences 5,7,11-15. Furthermore, many studies report the possibility to quantify different signals using STIR protocols or measuring T2-relaxation time, so realizing an objective assessment 13.
The purpose of our study was to identify the most important MR signal intensities on STIR images and on T1-w imaging after contrast administration, performed on the same day as the patient's clinical evaluation, that best discriminate patients with a CAS>3 (suspected non-inflammatory or inactive GO) from patients with a CAS≤3 (suspected inflammatory or active GO). The diagnostic advantages in the evaluation of GO and the improvement of accuracy in detecting disease activity using orbital MR imaging are also further described.
Materials and Methods
Patient characteristics, protocols and clinical evaluations
Between October 2009 and May 2010, 16 consecutive patients (seven men and nine women; mean age 49.19 years), with a diagnosis of GO in different phases of thyroid disease, and six normal volunteers (three men and three women; mean age 50.12 years) were enrolled.
In particular, patients with ocular symptomatology with a proven history of Graves' thyroid disease or with clinical and laboratory signs of Graves' disease, ascertained during the first clinical evaluation, were enrolled (inclusion criteria). Baseline serum thyroid parameters are reported in Table 1. Patients with other orbit diseases such as trauma, optic neuropathy, other inflammatory diseases of unknown origin, previous orbital radiotherapy or surgery, previous immunosuppressive treatments with steroids and antithyroid treatment for more than 12 weeks before MR examination were excluded. All patients gave informed consent and volunteers gave consent to take part in the contrast-enhanced orbital MR studies. The protocol was approved by the local ethical board of our hospital.
Table 1.
Baseline serum thyroid parameters.
| Range (min − max) | Median | Reference values | |
| TSH UI/ml | 0.001-5.45 | 1.23 | 0.4-4.0 |
| FT3 pg/ml | 2.41-9.60 | 3.7 | 1.5-5.9 |
| FT4 pg/ml | 6.8-21.7 | 10.3 | 5.2-15.8 |
| hTRAb* U/l | 0.2-40 | 5.75 | <1.5 |
| N° males: 7 (43.7%); N° females: 9 (56.3%) | |||
Two CAS were assessed for each patient by two independent "blinded" operators, who assigned a rating for each domain that make up the score at issue, according to the scheme reported in Table 2. Therefore, CAS values were derived from the sum of all ratings assigned by each operator. The meaning of "blinded" refers to the fact that the two operators were blinded to the patient's clinical conditions, to prevent certain information leading to conscious or subconscious bias on their part, invalidating the results.
Table 2.
Range of scores attributable to each domain making up the CAS value.
| Domains | Scores |
| Conjunctival chemosis | 0 − 1 − 2 |
| Conjunctival redness | 0 − 1 |
| Eyelid erythema | 0 − 1 |
| Eyelid swelling | 0 − 1 − 2 |
| Spontaneous orbital pain | 0 − 1 |
| Gaze evoked orbital pain | 0 − 1 |
MR imaging analysis
All patients were subjected to resonance on the same day as the clinical evaluation. Orbital MR imaging was performed on a 1.5 Tesla MR Unit (Magnetom Symphony, Siemens Medical Systems, Enlangen, Germany) with an imaging protocol consisting of axial turbo spin echo (TSE) T2-w images (parameters: repetition time/echo time/Nex = 3350/116/2 with a 259×384 matrix, FOV 240 mm and a 5 mm slice thickness with no gap), axial fat-suppressed (FS) TSE T2-w images (parameters: repetition time/echo time/Nex = 3960/115/3 with a 248×320 matrix, FOV 220 mm and a 4 mm slice thickness with a 0.4 mm space between slices), coronal fat-suppressed TSE T2-w images (parameters: repetition time/echo time/Nex = 3960/115/3 with a 248×320 matrix, FOV 210 mm and a 3.6 mm slice thickness with a 0.4 mm gap), coronal fat-suppressed T1-w images (parameters: repetition time/echo time/Nex = 472/15/3 with a 448×512 matrix, FOV 210 mm and a 3 mm slice thickness with no gap), axial 3D fat-suppressed T1-w VIBE images (parameters: repetition time/echo time/Nex = 8/2,46/3 with a 205×256 matrix, FOV 200 mm, 1 slab with 52 slices per slab and 1 mm slice thickness without gap). Coronal fat-suppressed T1-w images were acquired on a plane perpendicular to the optic nerve.
Contrast-enhanced images were obtained immediately after IV administration of 0.1 ml/kg of Gadobutrol 1.0 mol (Gadovist; Bayer Schering Pharma, Berlin, Germany) and consisted in axial 3D fat-suppressed T1-w VIBE images and coronal fat-suppressed T1-w images with the same parameters as the pre-contrast phase. MR scans were assessed by an experienced neuroradiologist, blinded to the clinical examinations. Because GO most frequently involves the inferior and medial rectus muscles 5,11-13, the neuroradiologist positioned ROIs avoiding the magnetic inhomogeneity artifacts from bone/ air of the maxillary sinus. ROIs were drawn around the right and left edges of the above-mentioned muscles on coronal acquisitions, from the anterior origin of the muscles to the orbital cavity apex.
We approved the values in the area of the highest signal intensity as a resulting number without a quantity and representing mean value and standard deviations. Moreover these measured signal intensities were set in proportion to those of the ipsilateral temporal muscle to calculate the signal intensity ratio (SIR), which is a more objective and reproducible indicator. The temporal muscles are considered the reference standard because they are spared from Graves' disease and because of their structural similarity and close anatomical relationship to the EOM 5,11,12. (Figures 1 to 3). Therefore, a wide number of variables were obtained, considering SIR related to FS T2 and gadolinium-enhanced FS T1-w images, for each muscle in both the patient and the volunteer groups.
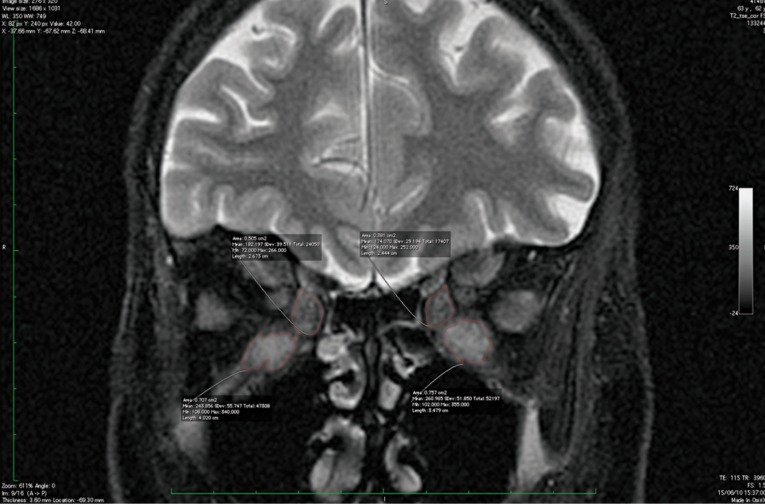
Figure 1.
Coronal T2 FS image showing ROI on extraocular muscles.
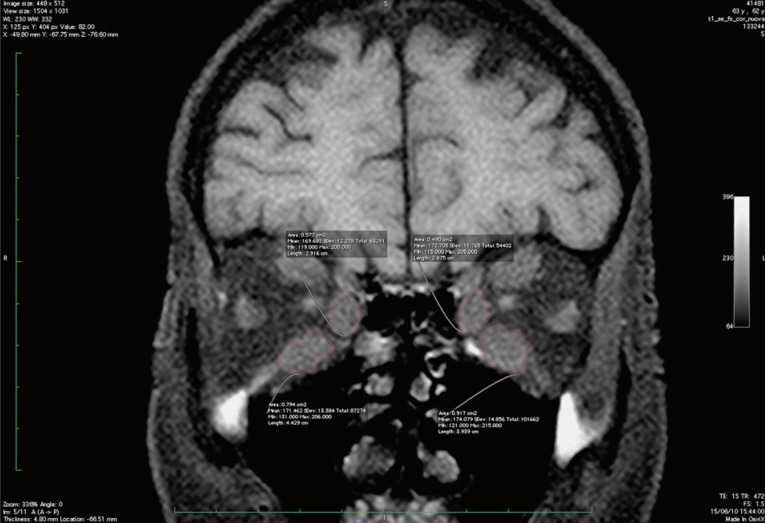
Figure 2.
Coronal FS T1-unenhanced image showing ROI on bilateral inferior and medial rectus muscles.
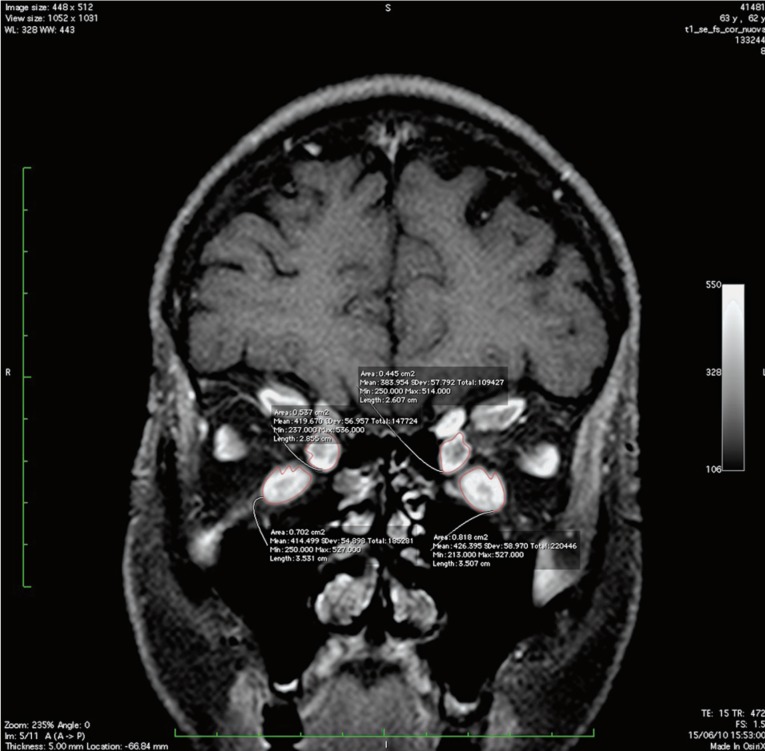
Figure 3.
Coronal FS T1-enhanced image showing ROI on inferior and medial rectus muscles.
Statistical analysis
The most important MR signal intensities that best discriminate a suspected non inflammatory or inactive GO patient (CAS≤3) from a suspected inflammatory or active GO patient (CAS>3) were detected using the new Random Forest (RF) algorithm 16. This approach simultaneously models the relationship between an outcome (i.e. CAS) and multiple potential predictor variables (i.e. MR signal intensities) by building 100,000 trees where, for each tree, a subset of both observations and MR signals were randomly chosen. Moreover, for each MR signal evaluated, RF provided a relative measure of how much this signal should be relevant to discriminate suspected inflammatory from non-inflammatory patients, among the other predictors. Since the RF algorithm scrambles all the available data, by the use of permutation and bootstrapping methods, the most accurate evaluation of relative variable importance was provided for each predictor at issue, even with a small number of observations. To confirm the validity of the RF findings, a discriminatory power of each MR signal intensity was assessed by estimating the area under the receiver operating characteristic (ROC) curve, along with its 95% confidence interval (95%CI) estimated by the DeLong method 17. The optimal cut-off was assessed by jointly maximizing sensitivity and specificity. Sensitivity and specificity, computed at the optimal cut-off, were also reported. An evaluation of the statistical power acquired to detect a specific area under the ROC curve, using a one-sided z-test, was also provided. A p value ≤ 0.05 was considered for statistical significance. All statistical analyses were performed by evaluating left and right MR signal measurements separately, and using R software (Ver. 2.12, random Forest and Diagnosis med and packages).
Results
CAS values assessed in all patients by the two independent operators were perfectly concordant. The reader can find a list of all notations used to define each MR signal intensity in Table 3.
Table 3.
List of MR signal notations.
| STIRRinf | Signal intensity value in STIR sequences in inferior rectus muscle |
| STIRRmed | Signal intensity value in STIR sequences in medial rectus muscle |
| T1SNZinf | Signal intensity in T1 without contrast medium in inferior rectus muscle |
| T1SNZmed | Signal intensity in T1 without contrast medium in medial rectus muscle |
| T1CONinf | Enhanced-T1 signal intensity of the inferior rectus muscle |
| T1CONmed | Enhanced-T1 signal intensity of the medial rectus muscle |
| STIRtemp | Signal intensity value in STIR sequences in temporal muscle |
| T1tempSNZ | Signal intensity in T1 without contrast medium in temporal muscle |
| T1tempCON | Signal intensity in T1 with contrast medium in temporal muscle |
| SIRretINF | Signal intensity ratio in inferior rectus muscle |
| SIRretMED | Signal intensity ratio in medial rectus muscle |
| SIRT1retINF | Signal intensity ratio in T1 in inferior rectus muscle |
| SIRT1retMED | Signal intensity ratio in T1 in medial rectus muscle |
| CMSIRretINF | Signal intensity ratio between the signal intensities of inferior rectus muscle and the ipsilateral temporalis muscle |
| CMSIRretMED | Signal intensity ratio between the signal intensities of medial rectus muscle and the ipsilateral temporalis muscle |
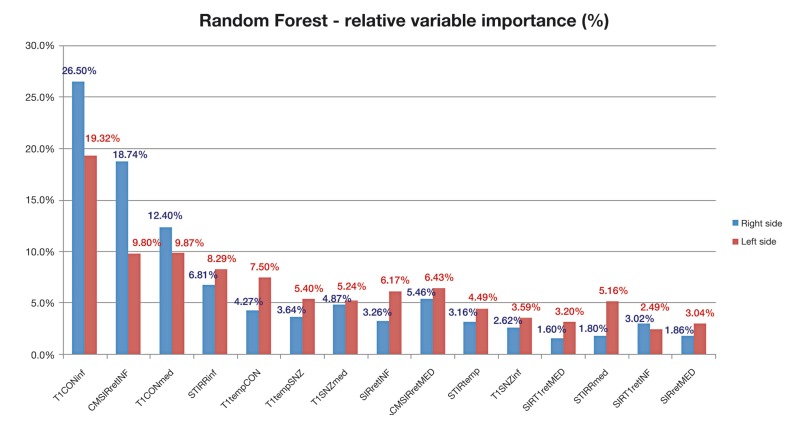
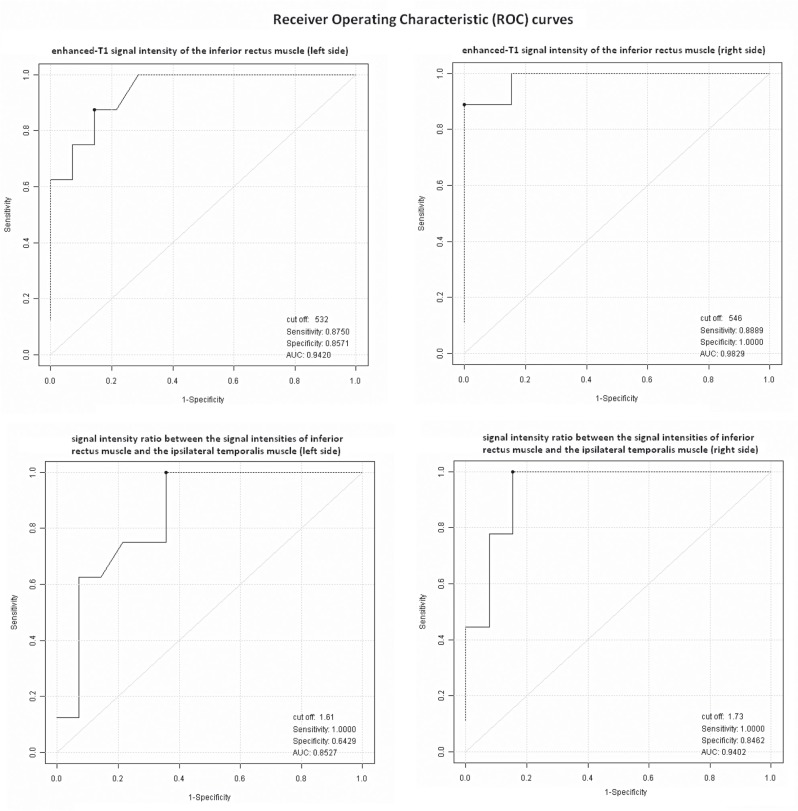
Among all patients that represent the whole sample, 13 had CAS≤3 and represented those with suspected non-inflammatory or "inactive GO" group, whilst nine patients had CAS>3 and represented the others with suspected inflammatory or "active GO" group. The RF algorithm showed that the most important signal that best discriminates the two patient groups was the enhanced-T1 signal intensity of the inferior rectus muscle (T1CONINF): indeed the relative variable importance attributed by the algorithm was 19.3% and 26.5% for the left and right muscle sides, respectively. The next important signal was the signal intensity ratio between the signal intensities of inferior rectus muscle and the ipsilateral temporalis muscle (CMSIRRETINF): a relative variable importance of 9.8% and 18.7% were attributed for the left and right muscle sides, respectively. A graphic representation of all MR signals (measured on left and right sides) relative variable importance, sorted from the highest to the lowest percentages, is also reported in Figure 4. The estimated area under ROC curve (AUC) of all left and right MR signals, along with its 95%CI and the optimal cut-offs are reported in Table 4. As previously found by the RF algorithm, T1CONINF seemed to confirm the most important predictor that best discriminates patients belonging to the "active GO" group from patients belonging to the "inactive GO group", with the highest discriminatory power both for left signals (AUC=0.942, 95%CI = [0.86 - 1.00]) and for right signals (AUC=0.983, 95%CI = [0.94 - 1.00]), acquiring the maximum sensitivity of 0.88 and maximum specificity of 0.86 at the cut-off of 532 for the left signal, whilst the maximum sensitivity of 0.89 and maximum specificity of 1.00 at the cut-off of 546 for the right signal. A sample of nine patients from the "active GO" group and 13 from the "inactive GO" group achieved 83% power to detect an area under ROC curve greater than or equal to 0.80, under the null hypothesis of 0.50 at a significance alpha level of 0.05. The ratio of the standard deviation of the responses in the "active GO" group to the standard deviation of the responses in the "inactive GO" group was assumed to be 1. Furthermore, a graphic representation of the ROC curves for T1CONINF and CMSIRRETINF (left and right) signals, are reported in Figure 5.
Table 4.
Discriminatory power and test characteristics at the optimal cut-off for all MR signals measured both on left and on right sides.
| Left signals | Right signals | |||
| AUC* (95%CI) | Optimal cut-off (SE, SP)** | AUC* (95%CI) | Optimal cut-off (SE, SP)** | |
| STIRRinf | 0.839 (0.66 - 1.00) | 255 (SE = 0.88, SP = 0.71) | 0.846 (0.68 - 1.00) | 244 (SE = 1.00, SP = 0.62) |
| STIRRmed | 0.777 (0.58 - 0.98) | 206 (SE = 1.00, SP = 0.57) | 0.752 (0.54 - 0.96) | 255 (SE = 0.67, SP = 0.77) |
| T1SNZinf | 0.665 (0.43 - 0.90) | 192 (SE = 1.00, SP = 0.50) | 0.718 (0.49 - 0.94) | 211 (SE = 0.78, SP = 0.69) |
| T1SNZmed | 0.772 (0.58 - 0.97) | 183 (SE = 1.00, SP = 0.57) | 0.803 (0.62 - 0.99) | 192 (SE = 1.00, SP = 0.62) |
| T1CONinf | 0.942 (0.86 - 1.00) | 532 (SE = 0.88, SP = 0.86) | 0.983 (0.94 - 1.00) | 546 (SE = 0.89, SP = 1.00) |
| T1CONmed | 0.857 (0.70 - 1.00) | 511 (SE = 0.88, SP = 0.79) | 0.906 (0.78 - 1.00) | 514 (SE = 1.00, SP = 0.77) |
| STIRtemp | 0.696 (0.48 - 0.91) | 81 (SE = 0.88, SP = 0.50) | 0.701 (0.48 - 0.92) | 81 (SE = 0.89, SP = 0.62) |
| T1tempSNZ | 0.777 (0.59 - 0.97) | 158 (SE = 0.88, SP = 0.71) | 0.786 (0.59 - 0.98) | 155 (SE = 1.00, SP = 0.54) |
| T1tempCON | 0.777 (0.57 - 0.98) | 293 (SE = 0.75, SP = 0.79) | 0.769 (0.56 - 0.98) | 302 (SE = 0.67, SP = 0.92) |
| SIRretINF | 0.759 (0.52 - 0.99) | 3.70 (SE = 0.50, SP = 1.00) | 0.778 (0.58 - 0.98) | 2.74 (SE = 0.89, SP = 0.62) |
| SIRretMED | 0.616 (0.34 - 0.89) | 2.68 (SE = 0.63, SP = 0.71) | 0.667 (0.43 - 0.90) | 2.38 (SE = 0.89, SP = 0.46) |
| SIRT1retINF | 0.661 (0.32 -1.00) | 1.22 (SE = 1.00, SP = 0.14) | 0.654 (0.31 - 1.00) | 1.28 (SE = 0.89, SP = 0.31) |
| SIRT1retMED | 0.692 (0.27 - 1.00) | 1.00 (SE = 1.00, SP = 0.00) | 0.671 (0.30 - 1.00) | 1.08 (SE = 1.00, SP = 0.00) |
| CMSIRretINF | 0.853 (0.69 - 1.00) | 1.61 (SE = 1.00, SP = 0.64) | 0.940 (0.84 - 1.00) | 1.73 (SE = 1.00, SP = 0.85) |
| CMSIRretMED | 0.763 (0.56 - 0.96) | 1.60 (SE = 1.00, SP = 0.64) | 0.859 (0.71 - 1.00) | S1.82 (SE = 0.67, SP = 0.92) |
AUC = Area under the receiver operating characteristic curve, along with bootstrap 95% confidence interval. ** SE = Sensitivity, SP = Specificity.
Figure 4.
Relative variable importance attributed by the Random Forest algorithm to all left and right MR signals intensities to discriminate "inactive GO" (CAS≤3) from "active GO" (CAS>3) patient groups.
Figure 5.
Receiver operating characteristic (ROC) curves of enhanced-T1 signal intensity of the inferior rectus muscle (left and right sides) and signal intensity ratio between the signal intensities of the inferior rectus muscle and the ipsilateral temporalis muscle (left and right sides), on discrimination between “active GO” and “inactive GO” patients. The optimal cut-off was assessed by jointly maximizing test sensitivity and specificity.
Discussion
GO is a chronic inflammatory autoimmune disease, more often associated with Graves' hyperthyroidism, but it may exist in patients with euthyroid or hypothyroid chronic autoimmune thyroiditis. The most important pathogenic factors are the TSH receptor auto-antibodies, sharing as targets TSH receptors localized on orbital fibroblasts and adipocytes. The autoimmune attack determines swelling of the extra-ocular muscles in association with an increase in orbital connective tissue and fat volume, with consequent restrictive ophthalmoplegia 5,11,12. The most common GO clinical signs include eyelid erythema and oedema, chemosis, upper eyelid retraction, and proptosis (exophthalmos) 17, restricted ocular motility but also sight-threatening complications of compressive optic neuropathy and corneal ulcerations. The clinical manifestations of GO are evident and severe in only 3-5% of patients. The disease is more often characterized by a subclinical course; sometimes presentation can be heterogeneous because not all features are present 6,8,19.
In 15% of all patients, GO may present predominantly with unilateral eye changes or may precede or follow the onset of Graves' disease 11. Bilateral orbital involvement is found on imaging in 50-75% of GO patients presenting clinically with asymmetric or unilateral eye findings. Especially in the latter cases, it is important to exclude other diseases by orbital imaging 13,20,27.
Two phases characterize the GO clinical course 19 : 1) "active phase", histologically characterized by mononuclear cell infiltration, proliferating fibroblasts and oedema within the extra-ocular and levator muscles, lachrymal glands and adipose tissue with consequent oedema and enlargement of muscles; 2) "inactive or chronic phase", characterized by fibrosis and fatty infiltrations of muscles causing the extension of fibrous strands into adjacent adipose tissue 5,11,12.
Patients presenting the active phase of disease are more often symptomatic, reporting a dry and gritty ocular sensation, photophobia, excessive tearing, double vision and a pressure sensation behind the eyes.
Specific symptoms of active disease are sudden appearance or change in altered and double vision. In particular, worsening on waking intermittent diplopia eventually associated with pain is strongly suggestive of active GO.
Permanent alterations in visual functions, such as blurred vision or altered colour perception, are potential markers of dysthyroid optic neuropathy (DON) with optic nerve compression in the orbital apex, and they are associated with active disease. However, clinicians should specifically seek these symptoms in all patients suspected of having active disease, as they are not always reported and considering that treatment of DON is obligatory.
Early diagnosis has primary importance in GO management, because an appropriate treatment can avoid permanent injuries (proptosis, sight impairment). Medical therapy by corticosteroids in the early stages of disease is useful to reduce the inflammation and to obtain GO remission, but it is useless in fibrotic end-stages. Early diagnosis and treatment would therefore lead to at least partial remission in up to 65% of cases 22.
The CAS scoring system evaluates disease activity and response to immunosuppressive therapy. Because CAS determination requires two clinical examinations, some authors proposed a modification that allows determination of CAS in a single session 21. This method is widely used because of the reported correlation between pre-treatment CAS and post-immunomodulating therapy outcome in GO patients characterized by a retailed PPV of 80% and a NPV of 64% 1. However, Mourits et al.'s study never demonstrated that patients with CAS<3 have inactive GO 7,11. Moreover, in Mourits et al.'s' report, ten out of 13 patients with CAS<3 were responders to immunosuppressive treatment 7,8,21. Finally, this method does not fully describe the overall status of GO, especially in the seven-item form 7,8. These results, therefore, show that CAS by alone cannot adequately detect active GO 7.
The significant limits of CAS consist in the lacking evaluation of the various levels of inflammation activity, so any improvement or exacerbation does not alter the score until it completely resolves, and low sensitivity and subjectivity because of the operator dependence 5-7. Furthermore the score attributes the same value to the various factors, each of which probably has different importance, thereby missing a diversification. Therefore CAS requires the integration with other modalities 7,9,10.
Clinical assessment and imaging methods like orbital US, CT and MRI are used to determine disease activity and severity. Orbital US is the least expensive and simplest examination to perform and allows good evaluation of muscular diameters and reflectivity, but it is not able to study the orbital apex, so will not yield information on the optic nerve, and is characterized by an apparent inaccuracy because of variability in orbital tissue measurement 23. Orbital CT is useful in detecting enlargement of extra-ocular muscles and in evaluating optic nerve and proptosis degrees. However, irradiation of the crystalline is the greatest limit of CT which prevents its use in follow-up 17. Furthermore, CT does not estimate muscle composition and so it is not able to identify inflammation. In fact, in our study there is no correlation between muscle diameters and CAS, confirming the literature stating that muscle thickness alone is not a measure of the degree of inflammation 23.
MRI gives all the information of both US and CT and in addition is able to detect muscle oedema, because of its capacity to characterize tissues. Many recent studies have validated the utility of MRI in GO because of its features: detailed imaging of orbital anatomy (consisting of high soft tissue contrast with the possibility of characterizing tissues, thin sections and multiplanar reconstructions), lack of ionizing radiations, standardization of study protocol with consequent examination reproducibility. So this modality will differentiate inflammatory from fibrotic alterations of orbital tissue and so discriminate the active from the inactive phase to select the right treatment. STIR sequences, in particular, have been found useful in detecting oedema in extra-ocular muscles which present T2 relaxation times longer than healthy controls 5,7,24. Moreover, T2 sequences allow us to observe the therapeutic effect 25.
An accurate interpretation of pathological alterations of EOM and periorbital tissue can suggest the correct therapeutic approach and can even provide pre-clinical information in patients with Basedow's disease without ocular symptoms. There is growing evidence 6,8,11,13,19,29, in fact, that EOM inflammatory involvement can occur earlier than ocular symptomatology and a recent study reports the involvement of one EOM at least on MR examination in 50% of patients of the presented case series, characterized by subjects in subclinical phase 26.
In agreement with some of the literature, we confirm the probable multifactorial pathogenesis of GO if we consider that we found no correlation between the blood levels of anti-TSH receptor antibodies and MR signal intensity values or CAS 7,23.
Orbital imaging is always necessary, even in patients with mild orbitopathy, presenting with typical subjective complaints such as retrobulbar pain and gritty sensations, as well as objective signs of GO such as lid swelling, lid retraction, motility impairment and proptosis, helping to support the diagnosis and providing a baseline examination with additional information in the decision making for immunomodulating therapy and for follow-up 13.
Imaging is always required in doubtful cases, such as asymmetrical orbital involvement, to exclude any other pathology, the clinical suspicion of optic nerve involvement in GO and to plan orbital decompression 13,28. MRI is able to differentiate the two activity states, demonstrating interstitial oedema on coronal TIRM sequences within the extraocular muscles in active disease: It is therefore the modality of choice to identify active inflammatory changes and to assess treatment response, additionally thanks to its lack of ionizing radiation 13.
In GO the standardized MR imaging protocol includes coronal fast spin echo T1-w and T2-w TIRM sequences in axial and coronal planes, with 3 mm slice thickness 13. The signal intensity from inflamed extraocular muscles is known to correlate with the CAS and therefore has an impact on the two treatment options, that are immunosuppressants or surgery 7,13,24,27. CAS alone could not detect active GO sufficiently and orbital MR imaging could predict the response to corticosteroid therapy more accurately than CAS alone 7.
Our study proved that signal intensity values on STIR sequence increase in the inflammatory oedematous phase of disease. We confirmed the correlation between signal intensities on this sequence and CAS, showing an increase in signal intensity proportional to the CAS value. So we validated MRI use to establish the activity phase of disease more sensitively than CAS alone 7. T1-w images after contrast medium injection, associated with fat saturation, are a helpful tool to detect intense enhancement of the extraocular muscles or the eyelid 12,13,15.
However, the role of EOM contrast enhancement on T1 sequences in the acute inflammatory stage of GO remains unclear. Some studies demonstrated decreased contrast enhancement because of microcirculation injury, attributable to muscle enlargement and inflammatory infiltration. On the contrary, our study, in agreement with some previous literature 11, demonstrated a direct proportion between STIR values and enhanced-T1 images, i.e. the increased enhancement during the active phase, as a result of vascular congestion, caused by oedema and interstitial inflammatory infiltration 12,30,31.
Moreover, we found that enhanced T1 signal intensities are the most reliable MR signals to assign a subject to the group with CAS<3 or CAS>3 at the cut-off value of 529. Furthermore, if the signal intensity ratio between the signal intensities of the inferior rectus muscle and the ipsilateral temporalis muscle is linearly combined with the STIR signal intensities of the inferior rectus muscle and the unenhanced-T1 signal intensities of the same muscle, it would most likely predict the exact CAS value.
A fundamental limit of our study is the reproducibility of the data due to the dependence of the signal on specific MR equipment. This limit was in part overcome as the evaluation of the relative importance that each MR signal acquired in predicting the proper CAS group was assessed by means of a robust statistical method 16. Furthermore, since SIR with contrast signals were defined as a ratio, the presence of correlations with CAS would be more reliable as objective and reproducible indicators. Therefore, improvements in reproducibility and the development of a common scale for orbital tissue evaluation, even in different MR systems, currently represents one of the most important priorities. As recommended by Cakirer et al. 12, our report contributes to use and improve this technique in order to predict the severity, type of treatment, and prognosis of the disease.
In conclusion, our study supports the diagnostic accuracy of the CAS and implements its prediction capabilities, in order to fit imaging and clinical evaluation with more strength. Therefore, the final results can be a more objective assessment and fair treatment settings or even pre-clinical information in patients with Graves' disease.
References
- 1.Mourits MP, Prummel MF, Wiersinga WM, et al. Clinical activity score as a guide in the management of patients with Graves' Ophthalmopathy. Clin Endocrinol. 1997;47(1):9–14. doi: 10.1046/j.1365-2265.1997.2331047.x. [DOI] [PubMed] [Google Scholar]
- 2.Mourits MP, Koornneef L, Wiersinga WM, et al. Clinical criteria for the assessment of disease activity in Graves' Ophthalmopathy: a novel approach. Br J Ophthalmol. 1989;73(8):639–644. doi: 10.1136/bjo.73.8.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartalena L, Baldeschi L, Dickinson AJ, et al. Consensus statement of the European group on Graves' orbitopathy (EUGOGO) on management of Graves' orbitopathy. Thyroid. 2008;18(3):333–346. doi: 10.1089/thy.2007.0315. [DOI] [PubMed] [Google Scholar]
- 4.Bartalena L, Baldeschi L, Dickinson A, et al. Consensus statement of the European Group on Graves' orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol. 2008;158(3):273–285. doi: 10.1530/EJE-07-0666. [DOI] [PubMed] [Google Scholar]
- 5.Prummel MF, Gerding MN, Zonneveld FW, et al. The usefulness of quantitative orbital magnetic resonance imaging in Graves' Ophthalmopathy. Clin Endocrinol. 2001;54(2):205–209. doi: 10.1046/j.1365-2265.2001.01220.x. [DOI] [PubMed] [Google Scholar]
- 6.Dickinson AJ, Perros P. Controversies in the clinical evaluation of active thyroid-associated orbitopathy: use of detailed protocol with comparative photographs for objective assessment. Clin Endocrinol. 2001;55(3):224–225. doi: 10.1046/j.1365-2265.2001.01349.x. [DOI] [PubMed] [Google Scholar]
- 7.Tachibana S, Murakami T, Noguchi H, et al. Orbital magnetic resonance imaging combined with clinical activity score can improve the sensitivity of detection of disease activity and prediction of response to immunosuppressive therapy for Graves' Ophthalmopathy. Endocr J. 2010;57(10):853–861. doi: 10.1507/endocrj.k10e-156. [DOI] [PubMed] [Google Scholar]
- 8.Stiebel-Kalish H, Robenshtok E, Hasanreisoglu M, et al. Treatment modalities for Graves' ophthalmopathy: systematic review and meta-analysis. Clin Endocrinol Metab. 2009;94(8):2708–2716. doi: 10.1210/jc.2009-0376. [DOI] [PubMed] [Google Scholar]
- 9.Bahn RS. Graves' ophthalmopathy. N Engl J Med. 2010;362(8):726–738. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoang JK, et al. What's in a name? Eponyms in head and neck imaging. Clin Radiol. 2010;65(3):237–245. doi: 10.1016/j.crad.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Kirsch EC, Kaim AH, De Oliveira MG, et al. Correlation of signal intensity ratio on orbital MRI-TIRM and clinical activity score as a possible predictor of therapy response in Graves' orbitopathy − A pilot study at 1.5 T. Neuroradiology. 2010;52(2):91–97. doi: 10.1007/s00234-009-0590-z. [DOI] [PubMed] [Google Scholar]
- 12.Cakirer S, Cakirer D, Basak M, et al. Evaluation of extraocular muscles in the edematous phase of Graves Ophthalmopathy on contrast-enhanced fat-suppressed magnetic resonance imaging. J Comput Assist Tomogr. 2004;28(1):80–86. doi: 10.1097/00004728-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Kirsch E, Hammer B, von Arx G. Graves' orbitopathy: current imaging procedures. Swiss Med Wkly. 2009;139(43-44):618–623. doi: 10.4414/smw.2009.12741. [DOI] [PubMed] [Google Scholar]
- 14.Kahaly GJ. Imaging in thyroid-associated orbitopathy. Eur J Endocrinol. 2001;145:107–118. doi: 10.1530/eje.0.1450107. [DOI] [PubMed] [Google Scholar]
- 15.Ott M, Breiter N, Albrecht CF, et al. Can contrast enhanced MRI predict the response of Graves' ophthalmopathy to orbital radiotherapy? Br J Radiol. 2002;75(894):514–517. doi: 10.1259/bjr.75.894.750514. [DOI] [PubMed] [Google Scholar]
- 16.Breiman L. "Random forests". Mach Learn. 2001;45(1):5–32. [Google Scholar]
- 17.Strianese D, Piscopo R, Elefante A, et al. Unilateral proptosis in thyroid eye disease with subsequent contralateral involvement: retrospective follow-up study. BMC Ophthalmol. 2013;13:21. doi: 10.1186/1471-2415-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elisabeth R, DeLong DM, DeLong Dl L, et al. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 19.Dickinson AJ, Perros P. Thyroid-associated orbitopathy: who and how to treat. Endocrinol Metab Clin N Am. 2009;38(2):373–388. doi: 10.1016/j.ecl.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Caranci F, Cicala D, Cappabianca S, et al. Orbital fractures: role of imaging. Semin Ultrasound CT MRI. 2012;33(5):385–439. doi: 10.1053/j.sult.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 21.European Group on Graves' Orbitopathy (EUGOGO) Wiersinga WM, Perros P, et al. Clinical assessment of patients with Graves' orbitopathy: the European Group on Graves' Orbitopathy recommendations to generalists, specialists and clinical researchers. Eur J Endocrinol. 2006;155(3):387–389. doi: 10.1530/eje.1.02230. [DOI] [PubMed] [Google Scholar]
- 22.Ceccarelli C, Bencivelli W, Vitti P, et al. Outcome of radioiodine-131 therapy in hyperfunctioning thyroid nodules: a 20 years' retrospective study. Clinical Endocrinology. 2005;62(3):331–335. doi: 10.1111/j.1365-2265.2005.02218.x. [DOI] [PubMed] [Google Scholar]
- 23.Nagy EV, Toth J, Kaldi I, et al. Graves' ophthalmopathy: eye muscle involvement in patients with diplopia. Eur J Endocrinol. 2000;142(6):591–597. doi: 10.1530/eje.0.1420591. [DOI] [PubMed] [Google Scholar]
- 24.Mayer EJ, Fox DL, Herdman G, et al. Signal intensity, clinical activity and cross sectional areas on MRI scans in thyroid eye disease. Eur J Radiol. 2005;56(1):20–24. doi: 10.1016/j.ejrad.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 25.Utech CI, Khatibnia U, Winter PF, et al. MR T2 relaxation time for the assessment of retrobulbar inflammation in Graves' ophthalmopathy. Thyroid. 1995;5(3):185–193. doi: 10.1089/thy.1995.5.185. [DOI] [PubMed] [Google Scholar]
- 26.Lennerstrand G, Tian S, Isberg B, et al. Magnetic resonance imaging and ultrasound measurements of extraocular muscles in thyroid-associated ophthalmopathy at different stages of the disease. Acta Ophthalmol Scand. 2007;85(2):192–201. doi: 10.1111/j.1600-0420.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 27.Yokoyama N, Nagataki S, Uetani M, et al. Role of magnetic resonance imaging in the assessment of disease activity in thyroid-associated ophthalmopathy. Thyroid. 2002;12(3):223–227. doi: 10.1089/105072502753600179. [DOI] [PubMed] [Google Scholar]
- 28.Elefante A, Caranci F, Del Basso De Caro ML, et al. Paravertebral high cervical chordoma. A case report. Neuroradiol J. 2013;26(2):227–232. doi: 10.1177/197140091302600214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mourits MP. Diagnosis and differential diagnosis of Graves' orbitopathy. In: Wiersinga WM, Kahaly GJ, editors. Graves' orbitopathy: a multidisciplinary approach. Basel: Karger; 2007. pp. 66–77. [Google Scholar]
- 30.Amano Y, Amano M, Kumazaki T. Normal contrast enhancement of extraocular muscles: fat-suppressed MR findings. Am J Neuroradiol. 1997;18(1):161–164. [PMC free article] [PubMed] [Google Scholar]
- 31.Taoka T, Iwasaki S, Uchida H, et al. Enhancement pattern of normal extraocular muscles in dynamic contrast-enhanced MR imaging with fat suppression. Acta Radiol. 2000;41(3):211–216. doi: 10.1080/028418500127345361. [DOI] [PubMed] [Google Scholar]