Summary
Neurogenesis in the adult mammalian brain is active in two areas: the subgranular zone in the dentate gyrus of the hippocampus and the subventricular zone. Cancer stem cells have been isolated from malignant brain tumors and it is widely believed they arise from transformed endogenous stem cells. We sought to determine if the initial location of glioblastoma (GB) as seen on conventional MRI and its relationship to the subventricular zone (SVZ) predicts the pattern of recurrence. We analyzed the initial (prior to any treatment) and last follow-up MR studies in 49 patients with GB. On post contrast images all non-treated GB were divided into three groups according to the relationship of their enhancing margins to the SVZ: Group I (directly in contact with the SVZ), Group II (in the subcortical [SC] region) and Group III (in both the SVZ and SC regions). Recurrences or continuous growth seen as enhancing areas on follow-up studies were characterized as local, spread, or distant according to their contact with the surgical bed and correlated with the locations of the initial tumors. Local and spread patterns of recurrence occurred with nearly equal frequency (45 and 43% each, respectively) and distant in 12%. In Group I, 80% showed a spread pattern, 20% a local pattern, and none a distant pattern. In Group II, 45% showed a spread pattern, 35% a local pattern, and a 20% distant one. In Group III, 58% showed a local pattern, 33% a spread pattern, and 8% distant one. Unlike other reports, the location of GB in relation to the SVZ in our patients did not predict the pattern of tumor recurrence and/or extension in our patients.
Keywords: glioblastoma, magnetic resonance imaging, recurrence, cancer stem cells, subventricular zone
Introduction
Ramon and Cajal postulated that the adult brain is incapable of producing new neurons and this belief was held as dogma during most of the 20th century 1. In 1962, Altman utilized bromodeoxyuridine labeling technology to identify newborn cells in well-defined areas of adult rodent brains which subsequently led to the discovery of adult neurogenesis and in 1998 Eriksson established the existence of adult human neurogenesis 2-4. Neural stem cells (NSC) were isolated, grown, and differentiated into neurons and astrocytes in vitro also in the 1990s 5-7. Today the consensus is that adult neurogenesis is active in two main areas: the subgranular zone (SGZ) of the dentate gyrus (DG) of the hippocampus and the subventricular zone (SVZ) 1-9. NSC have the capacity to self-renew and differentiate into multiple neural cell lineages. The microenvironment that supports and regulates NSC maintenance, self-renewal, development, and fate is called a “niche” and involves soluble factors, anatomic organization, and neuronal activity 4. Cancer stem cells (CSC) have been isolated from malignant brain tumors and these cells may arise from transformed NSC 10-12. Thus, CSC are believed to be adult abnormal NSC, although a clear relationship between stem-like cancer cells and true NSC has not been proven 10-12. Studies show that glioblastomas (GB) tend to arise in association with areas of active neurogenesis and that recurrences are more common in these regions (such as the SVZ). To further investigate this association, we retrospectively analyzed initial and follow-up MR images in patients with GB to assess if their initial site predicted their site of recurrence.
Materials and Methods
Patients
We analyzed the initial (prior to any treatment) and last follow-up MR studies in 49 patients with GB diagnosed at our hospital between January 2001 and January 2011. All patients had histologically confirmed primary GB (WHO IV). No patient had evidence, by clinical history or genetic markers, that the tumors had arisen as low-grade and then transformed into high-grade lesions and all were considered primary or de novo GB.
MRI analysis
Patients underwent MR brain imaging with conventional sequences (T1 and T2-weighted sequences, FLAIR, gradient-echo, and diffusion-weighted sequences) and post contrast three-dimensional MPRAGE T1 images in at least the axial plane with coronal and sagittal reformations. Images were obtained on 1.5T or 3.0T scanners. MR follow-up examinations were performed every three months unless the patient required additional evaluation. For the purposes of this study, we analyzed the T1-post contrast images in initial and last follow-up studies. Measurements were carried out on a clinical PACS system. One neuroradiologist retrospectively reviewed the medical records of all patients with regard to age at presentation, gender, date of first and last follow-up MRI studies, and final pathology diagnosis.
Classification of initial tumors and recurrences
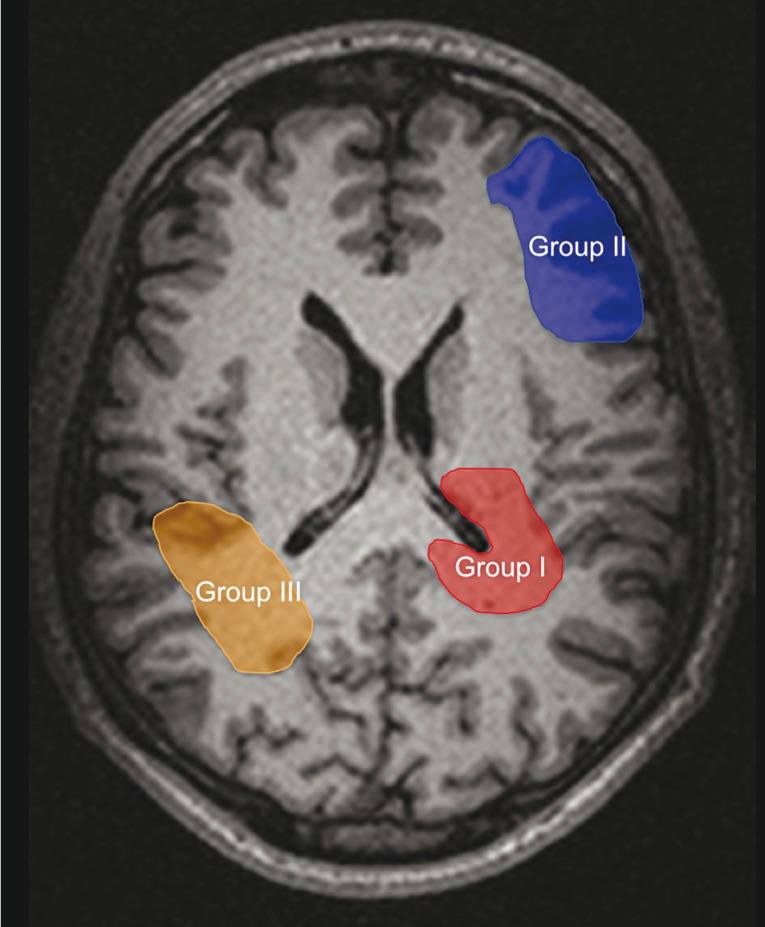
Post contrast images were analyzed and divided into three groups according to the relation between the enhancing margins of the initial tumor and the SVZ (Figure1), as previously described in the literature 13,14 :
− Group I: directly in contact with the SVZ or less than 1 cm away from it.
− Group II: distant to the SVZ in SC regions. GB were > 1 cm distant to the SVZ and in contact with the SC region or < 1 cm away from it.
− Group III: tumors in both the SVZ and SC regions.
Recurrences were categorized as such when new enhancing areas (not related to residual tumor) were found on follow-up studies and characterized as follows:
− Local: when they occurred within the original surgical bed.
− Spread: when they occurred within 1 cm of the original surgical bed.
− Distant: when they did not have any contact with the original surgical bed.
Figure 1.
Schematic drawing of the 3 groups of glioblastoma (GB) according to the relationship of their enhancing margins to the SVZ. Group I, GB directly in contact with the SVZ. Group II, GB in contact to the subcortical (SC) region. Group III, GB contacting both the SVZ and SC regions.
Statistical analysis
Averages and p-values were calculated for all groups and Fisher's exact test was performed on a contingency table.
Results
Clinical findings
Of the 49 patients with GB, 16 were female and 33 were male. Mean ages at presentation were 36.8 ± 20.4 years for Group I, 61.1 ± 14.4 years for Group II, and 54.9 ± 14.2 years for Group III.
Initial location
49% of GB were categorized as Group III that is, being in both the SVZ and SC regions while 41% were categorized as Group II and 10% as Group I.
Patterns of recurrence
The spread and local patterns of recurrence occurred essentially equally, 45% and 43% respectively, and the distant pattern was seen in 12%. In Group I (SVZ only), 80% of tumors showed a spread pattern (Figure 2), while 20% showed a local pattern. In Group II (SC only), 45% showed spread, 35% local, and 20% distant patterns (Figure 3). In Group III (both regions), 58% showed a local pattern (Figure 4), 33% a spread pattern, and 8% a distant one. Table 1 contains the totals for each group. Thus, the pattern and location of recurrences had no significant relation to the location of the initial tumors (p = 0.275). Mean ages at presentation were lower and more heterogeneous for Group I than for Groups II and III.
Table 1.
Initial types of glioblastoma and types of recurrence.
|
Group I SVZ, (% of group) |
Group II SC, (% of group) |
Group III Both, (% of group) |
|
| Local recurrence | 1 (20%) | 7 (35%) | 14 (58%) |
| Spread recurrence | 4 (80%) | 9 (45%) | 8 (33%) |
| Distant recurrence | 0 (0%) | 4 (20%) | 2 (8.3%) |
| Totals | 5 | 20 | 24 |
SVZ = subventricular zone; SC = subcortical region.
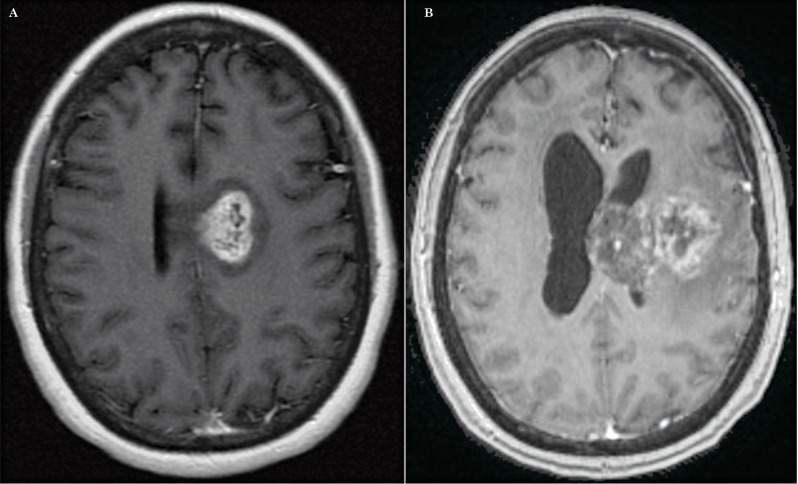
Figure 2.
Axial postcontrast T1-weighted images. A) Initially the study showed an enhancing GB, directly in contact with the left subventricular zone (Group I). B) The post-treatment image shows a ‘spread’ type of recurrence, which occurred within one centimeter of the original surgical bed. This was the most common pattern of recurrence observed in our study. Additionally note the tumor has now spread into the corpus callosum and that there is hydrocephalus.
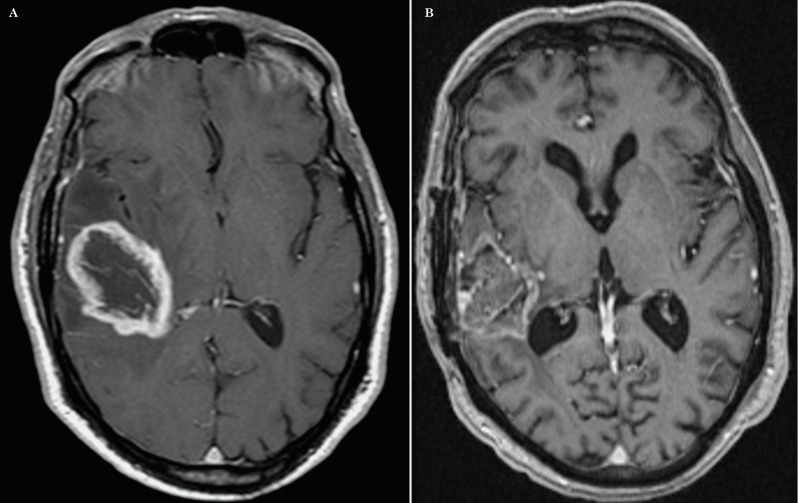
Figure 3.
A) Preoperative axial postcontrast T1-weighted image of an enhancing GB which is in contact with the subcortical fronto-parietal region (Group II). B) Post-treatment T1-weighted image of the same patient demonstrating a ‘distant’ type of recurrence located in the genu of corpus callosum and right frontal lobe that represents the least common type of recurrence found in our analysis
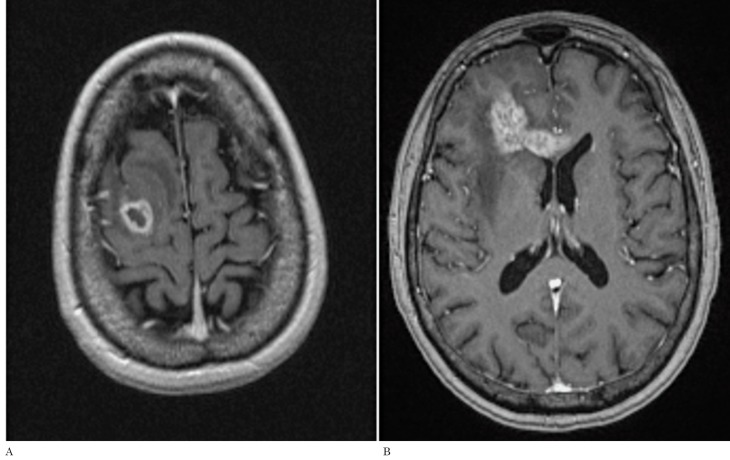
Figure 4.
A) Axial postcontrast T1-weighted image of an enhacing GB prior to any treatment which is in contact with the subventricular zone of the right lateral ventricle atrium and also in contact to the temporal subcortical region (Group III). B). The post-treatment T1-weighted image illustrates the enhancing tumor within the original surgical bed thus being classified as a ‘local’ recurrence type. This was the most common recurrence type for this group.
Discussion
In our patients, tumors belonging to Groups III and I did not show a statistically greater incidence of spread or distant recurrence patterns than did those in Group II. GB located in the SC region (Group II) demonstrated a higher incidence of distant recurrences than those in Groups III and I.
Overall, we did not observe any predictable recurrence pattern linked to the initial anatomical location of the tumors, as has been reported in other series 13-15. Also, unlike other series, our GB arising in close relation to the SVZ did not show any specific recurrence patterns. Thus our observations disagree with that of others who have suggested that different types of recurrences can be predicted by the initial location of GB 14,13. Those authors argue that tumors arising in the SVZ demonstrate more aggressive recurrences, suggesting that these recurrences may be related to the presence of CSC in the SVZ. Although the concept that cancers arise from stem cells was proposed 150 years ago, it is only recently that CSC have been isolated and cultured from human brain tumors 11,16,12. The CSC hypothesis suggests that homeostastic processes are dysregulated and instead of generating NSC and their progenitors they give rise to CSC. Additionally, aberrant differentiation of CSC results in tumor heterogeneity 12. CSC have the capacity for self-renewal, multipotency (ability to generate neurons, astroglia, and oligodendroglia), tumorigenicity, and the ability to migrate, metastasize, and activate telomerase expression and anti-apoptotic pathways. GB have heterogeneous cellular compositions as implied by the older term “multiforme” and some of their cells retain a migratory capacity. This characteristic results in frequent recurrences and extensive tumor involvement by the time of diagnosis 11. Even if NSC are attractive candidates to explain cancer-initiating events, there is still a lack of direct evidence on where exactly this occurs and whether it is related to aberrant neural precursors or differentiated tumorigenic cells 3. The findings in our study were similar to those of Kappadakunnel et al. 17 and agree with their observation that tumors in contact with the SVZ are not more likely to be presumably ‘stem-cell-derived’ than others. Similarly, we also observed that GB in contact with the SC region (Group II) had the highest multifocality rate at both presentation and recurrence. On the other hand, dormant NSC may be present in multiple regions of the adult brain and can become activated by physiological or pathological conditions 18-22. Several authors have found evidence that NSC are widely distributed throughout the human brain suggesting that local environmental factors such as growth factors, nutrients, and other signals are responsible for activating the neurogenesis process 19-22. The occurrence of adult neurogenesis in brain regions other than the SVZ and DG remains controversial but it may be another possible explanation for our results. In other words, since GB may start in CSC and NSC which exist in different parts of the brain and not just in the SVZ then GB and its recurrences may have any or no type of relation to the SVZ. This may imply that the SVZ is not the only site of origin for GB cells. Many reports describe adult neurogenesis in other regions such as neocortex, striatum, amygdala, substantia nigra, olfactory bulb, and spinal cord 19-22.
Our study has several limitations. First, it is a relatively small sample size of 49 patients. Second, there were limited numbers of GB in only the SVZ (Group I). Third, the fact that we use only contrast enhancement as a putative marker of GB when it is well known that non-enhancing areas of edema and even the normal-appearing brain may contain GB. Specifically, we did not use perfusion or MR as these were not available in all patients and the use of MR spectroscopy in this regard is still controversial. In further studies, analysis of a larger number of primary GB arising just from the SVZ and their recurrences may add more information on the behavior of these tumors. There are two known in vivo methods that could potentially assess adult human neurogenesis: MR perfusion and MR spectroscopy. MR perfusion as it applies to tumors relies on cerebral blood volume (CBV) measurements and increases in it have been correlated to neurogenesis-angiogenesis 23. Although controversial, proton MR spectroscopy of the hippocampi which contains stem cells and of transplanted stem cells demonstrates a metabolite that resonates at 1.28 ppm which is believed to be a marker of neurogenesis 4,24. It is clear that MRI will play an important role in the new research field of neurogenesis.
In our series, the location of GB in relation to the SVZ did not predict the types of recurrences observed in other studies. Our findings do not support the observation that different types of recurrences can be predicted according to initial location of GB. Most of our GB recurred or grew in their surgical beds or slightly beyond.
Acknowledgements
CRM is a Damon Runyon-Genentech Clinical Investigator supported in part by a Clinical Investigator Award from the Damon Runyon Cancer Research Foundation (CI-45-09) and grants from the UNC University Cancer Research Fund (UCRF) and Department of Defense (W81XWH-09-2-0042).
References
- 1.Whitman MC, Greer CA. Adult neurogenesis and the olfactory system. Prog Neurobiol. 2009;89(2):162–175. doi: 10.1016/j.pneurobio.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135(3509):1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- 3.Ma DK, Bonaguidi MA, Ming G-L, et al. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009;19(6):672–682. doi: 10.1038/cr.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sierra A, Encinas JM, Maletic-Savatic M, et al. Adult human neurogenesis: from microscopy to magnetic resonance imaging. Front Neurosci. 2011;5:47. doi: 10.3389/fnins.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller MW, Nowakowski RS. Use of bromodeoxyuridine immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988;457(1):44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 7.Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci USA. 1993;90(5):2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chojnacki AK, Mak GK, Weiss S. Identity crisis for adult periventricular neural stem cells: subventricular zone astrocytes, ependymal cells or both? Nat Rev Neurosci. Nat Rev Neurosci. 2009;10(2):153–163. doi: 10.1038/nrn2571. [DOI] [PubMed] [Google Scholar]
- 9.Quiñones-Hinojosa A, Sanai N, Soriano-Navarro M, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494(3):415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- 10.Dirks PB. Brain tumor stem cells: bringing order to the chaos of brain cancer. J Clin Oncol. 2008;26(17):2916–2924. doi: 10.1200/JCO.2008.17.6792. [DOI] [PubMed] [Google Scholar]
- 11.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64(19):7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 12.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea - A paradigm shift. Cancer Res. 2006;66(4):1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. discussion 1895-1896. [DOI] [PubMed] [Google Scholar]
- 13.Barami K, Sloan AE, Rojiani A, et al. Relationship of gliomas to the ventricular walls. J Clin Neurosci. 2009;16(2):195–201. doi: 10.1016/j.jocn.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Lim DA, Cha S, Mayo MC, et al. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neurooncology. 2007;9(4):424–429. doi: 10.1215/15228517-2007-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evers P, Lee PP, DeMarco J, et al. Irradiation of the potential cancer stem cell niches in the adult brain improves progression-free survival of patients with malignant glioma. BMC Cancer. 2010;10:384. doi: 10.1186/1471-2407-10-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vik-Mo EO, Sandberg C, Joel M, et al. A comparative study of the structural organization of spheres derived from the adult human subventricular zone and glioblastoma biopsies. Exp Cell Res. 2011;317(7):1049–1059. doi: 10.1016/j.yexcr.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Kappadakunnel M, Eskin A, Dong J, et al. Stem cell associated gene expression in glioblastoma multiforme: relationship to survival and the subventricular zone. J Neurooncol. 2010;96(3):359–367. doi: 10.1007/s11060-009-9983-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiao J, Chen DF. Induction of neurogenesis in nonconventional neurogenic regions of the adult central nervous system by niche astrocyte-produced signals. Stem Cells. 2008;26(5):1221–1230. doi: 10.1634/stemcells.2007-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arsenijevic Y, Villemure JG, Brunet JF, et al. Isolation of multipotent neural precursors residing in the cortex of the adult human brain. Exp Neurol. 2001;170(1):48–62. doi: 10.1006/exnr.2001.7691. [DOI] [PubMed] [Google Scholar]
- 20.Landgren H, Curtis MA. Locating and labeling neural stem cells in the brain. J Cell Physiol. 2011;226(1):1–7. doi: 10.1002/jcp.22319. [DOI] [PubMed] [Google Scholar]
- 21.Macas J, Nern C, Plate KH, et al. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J Neurosci. 2006;26(50):13114–13119. doi: 10.1523/JNEUROSCI.4667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss S, Dunne C, Hewson J, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16(23):7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pereira AC, Huddleston DE, Brickman AM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. U.S.A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manganas LN, Zhang X, Li Y, et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]