Summary
A 74-year-old man was admitted to the Emergency Room of our institution with worsening dysarthria, left-side weakness and hypoesthesia (NIHSS score: 5) since his awakening at 7:30 a.m. The evening before, he had gone to sleep at 10:30 p.m. Brain computed tomography (CT) and cervicocranial CT angiography showed low density attenuation of the right caudate nucleus head and lenticular nucleus and sub-total occlusion of ipsilateral middle cerebral artery (MCA) pre-bi/trifurcation M1 segment. Brain CT perfusion showed an ischemic core in the right striatal region, surrounded by a wide region of ischemic penumbra. Although the onset of symptoms, defined as “time last-seen well”, was 14 hours before presentation, the following worsening of neurological conditions (NIHSS score: 12) and the evidence of cerebral blood flow / cerebral blood volume mismatch at CT perfusion led us to propose neuroendovascular treatment on the basis of an off-label use. Neuroendovascular treatment by Penumbra system was achieved and the right MCA was only partially recanalized. The patient was discharged with NIHSS score of 12. At six months, modified Rankin scale score was 3. To the best of our knowledge, this is the first Italian case report describing a patient who underwent successful neuroendovascular treatment for a “wake-up stroke” without clinical worsening nor major complications and an acceptable clinical outcome. This was possible thanks to an extension of the therapeutic window guided by CT perfusion.
Keywords: neuroendovascular treatment, neuroimaging-based reperfusion therapy, thrombolysis, unclear onset-stroke, wake-up stroke
Introduction
Approximately 25% of patients with ischemic stroke awake with neurological deficits. In these wake-up strokes (WUS), the onset of symptoms is defined as “time last-seen well” (TLSW). Current guidelines do not recommend thrombolysis or neuroendovascular treatment in patients with WUS or in patients with unclear-onset stroke, since the actual time of onset is often unknown 1,2.
Modern multimodal neuroimaging, including computed tomography (CT), CT angiography, CT perfusion, magnetic resonance (MR) imaging, MR angiography, diffusion-weighted imaging, and MR perfusion, may guide reperfusion therapies, allowing the treatment of patients eight hours from onset 3,4. Notably, several trials 5-7 report the safety and efficacy of both intravenous and intra-arterial neuroimaging-guided reperfusion therapies in patients with WUS or unclear-onset strokes.
The purpose of this case report is to describe a patient who underwent successful neuroendovascular treatment for a WUS without major complications and with an acceptable clinical outcome, thanks to an extension of the therapeutic window guided by CT perfusion.
Case Report
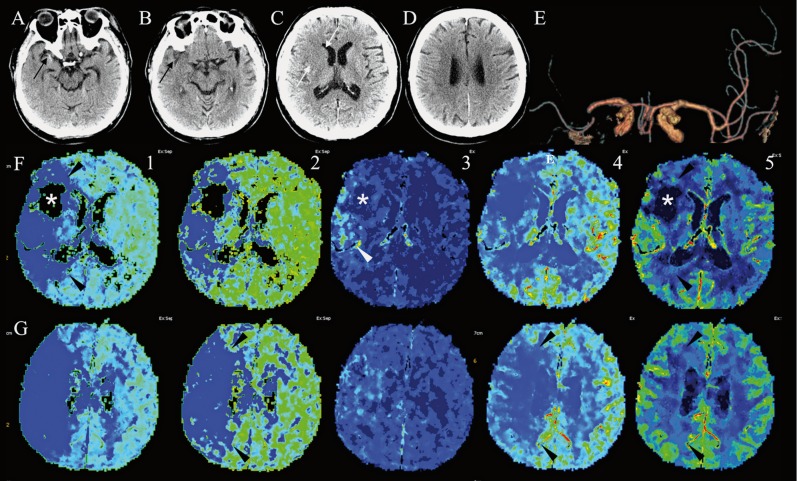
A 74-year-old man was admitted in the Emergency Room of our institution at 9:10 a.m. for dysarthria, left-side weakness and hypoesthesia since awakening at 7:30 a.m. The evening before, he had gone to sleep at 10:30 p.m. Neurological examination showed slight left VII cranial nerve palsy, dysarthria, left hemiparesis and hypoesthesia (National Institute of Health Stroke Scale score, NIHSSs = 5). History revealed silent myocardial infarction 20 years ago and dyslipidemia. The patient had stopped clopidogrel therapy ten days before admission. A standard 12 lead electrocardiogram displayed bradycardia. Two hours after admission, he underwent brain CT and CTA (Figure 1), that showed subtle high density attenuation and dot signs (Figure 1A,B), in the right middle cerebral artery (MCA) low density attenuation of the right caudate nucleus head and lenticular nucleus (Figure 1C,D), thereby fulfilling an Alberta stroke program early CT score 8 of 8, and occlusion of ipsilateral MCA pre-bi/trifurcation M1 segment (Figure 1E).
Neurological conditions worsened and the patient became lethargic, with increased left sensitive deficits and hemiparesis (NIHSSs = 12). Brain CT perfusion (Figure 1F,G) showed an ischemic core in the right lenticular region, surrounded by a wide area of ischemic penumbra. An increased area of capillary permeability was also evident.
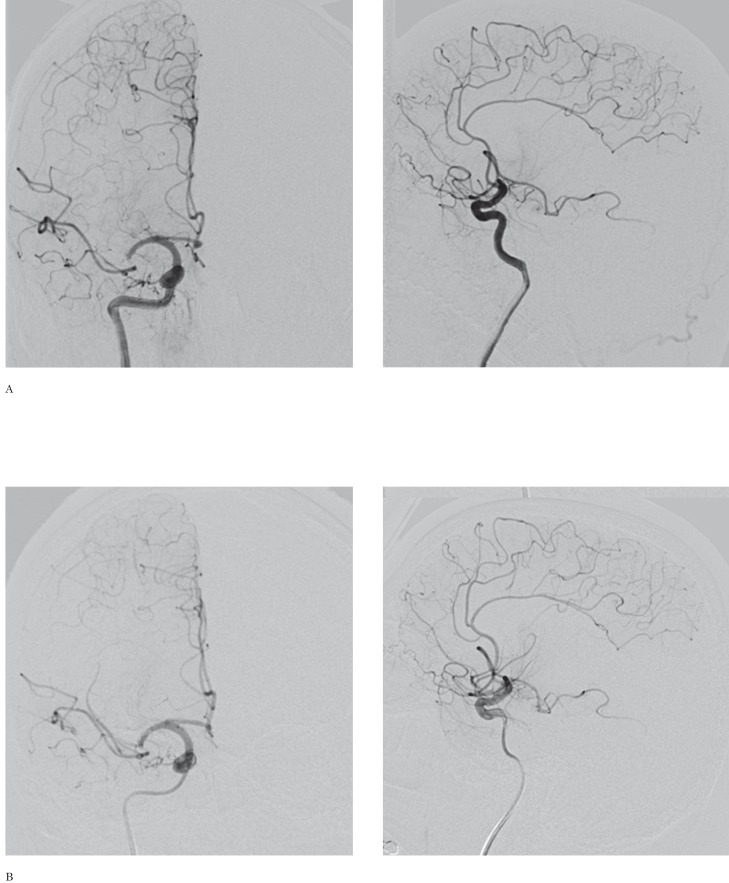
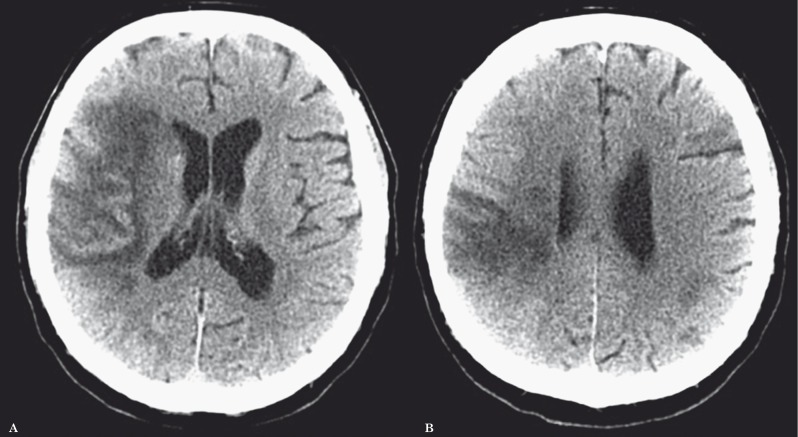
All primary data were acquired using a LightSpeed VCT scanner and post-processed in an Advantage Workstation 4.6 (General Electric Healthcare, Fairfield, CT, USA). Perfusion CT data were analysed by CT perfusion 4D imaging software, evaluating regions of interest on cerebral blood volume (CBV) and cerebral blood flow (CBF) maps 9,10. A CBF of 12.7 mL/100 g/min and CBV of 2.2 mL/100 g/min were considered threshold levels predictive of the infarct core 11. All the remaining perfusion-deficient territory compared to the contralateral hemisphere was considered potentially salvageable. Namely, the penumbra was defined by normal CBV but decreased CBF, i.e. a "mismatch" between the CBV and CBF lesion size 9,10. Although the TLSW was 14 hours, the patient's worsening of neurological conditions and the evidence of "mismatch" between CBV and CBF lesion size at CT perfusion led to rule out systemic thrombolysis and propose neuroendovascular treatment on the basis of an off-label use. Cerebral angiography (Figure 2A), performed by a digital biplanar rotational angiographic suite (Innova 3131IQ, General Electric Healthcare, Fairfield, CT, USA), showed a thrombus in the right MCA M1 segment. Neuroendovascular treatment by the Penumbra system (Penumbra, Inc, Alameda, CA, USA) was performed 70 minutes later (Figure 2B) and the right MCA was only partially recanalized. Then, the patient was treated by oral clopidogrel (75 mg/die). At day 9, brain CT (Figure 3) showed frontoparietal infarction with uncertain hemorrhagic transformation, however a wide frontal region was spared. At day 13, the patient was discharged to rehabilitation for left sensory-motor hemiparesis. NIHSSs was 12. Six-month follow-up modified Rankin scale score was 3. Ten months later, the patient was readmitted to our Stroke Unit for a transient ischemic attack characterized by motor aphasia lasting ten minutes. A paroxysmal atrial fibrillation was diagnosed and oral anticoagulant therapy was started.
Figure 1.
Computed tomography (CT) of the brain obtained at diagnosis. Serial consecutive (A,B) and nonconsecutive (C,D) unenhanced axial CT images show (black arrows) subtle high density attenuation and dot sign in the right middle cerebral artery (MCA), associated with subtle low-density attenuation (white arrows) in the ipsilateral fronto-insular region, head of caudate nucleus, and lenticular nucleus, while a scan through the coronae radiatae (D) was negative. Anteroposterior multi-intensity projection CT angiography shows partial occlusion of the right MCA, soon before its genu (E). Axial perfusion CT maps obtained at the level of images A (F) and B (G), indicating Tmax (1), mean transit time (2), capillary permeability (3), cerebral blood flow (4), and cerebral blood volume (5) show a clear ischemic core (asterisk) and a wide area of ischemic penumbra (black arrowheads). Note also that capillary permeability shows alteration (white arrowhead) in the right superficial frontal territory.
Figure 2.
Neuroendovascular treatment. Anteroposterior (left) and lateral (right) early angiograms obtained at 2.30 p.m. (A) and after thrombectomy at 4.10 p.m. (B) show only a subtle reperfusion of the right middle cerebral artery.
Figure 3.
Computed tomography (CT) of the brain obtained 9 days later. Serial nonconsecutive unenhanced axial CT images obtained at the levels of Figures 1C (A) and 1D (B) show sparing of most of the low perfusion area in the right cerebral hemisphere and only uncertain partial subtle hemorrhagic transformation.
Discussion
More than one third of ischemic strokes occur during sleep and generally these WUS are excluded from thrombolytic therapy, since the actual time from symptom onset is often unknown 1,2. However, in daily clinical practice it has been noted that evaluation of the potential benefits and risks of reperfusion therapy results not only from the clinical assessment and the optimization of stroke-to-hospital or door-to-needle times, but also from multimodal advanced neuroimaging, that allows the extension of the time window for therapy. Notably, non contrast-enhanced CT, MR imaging including diffusion-weighted imaging, CT and/or MR angiography and perfusion will identify the volume of the ischemic lesion, the presence and site of intracranial arterial occlusion and potentially salvageable brain tissue, as well as the risk for hemorrhagic transformation after reperfusion 3,5-7. This is possible without treatment delay 4.
Within this context, several papers have demonstrated that CT perfusion can accurately detect the ischemic core and penumbra, thus representing a useful tool for identifying acute stroke patients suitable for treatment 9,10. However, there is still controversy about the accuracy (and thus, the need for) of CT perfusion imaging in acute ischemic stroke imaging in daily clinical practice 12-15.
A recent review 15 from the experience and evidence-based approach of The Massachusetts General Hospital acute stroke imaging algorithm questioned the role of CT perfusion in patients with anterior circulation occlusion in whom the primary indications for intravenous thrombolysis are an elapsed time since onset of 4.5 h or less and an absence of hemorrhage or large infarct on neuroimaging. In this subset of patients, CT perfusion is considered a Level 3/Class IIb method for early estimation of both the infarct core and penumbra, mainly for a not yet standardized method of acquisition and post-processing and the great variability in the interpretation of results. Notably, perfusion CT is considered to have no proven role in selecting patients with anterior circulation occlusion for intravenous thrombolysis or neuroendovascular therapy, but to be limited to i) research patients, ii) patients for whom diffusion-weighted imaging is not available, and iii) other purposes such as hypertensive therapy, although there are still scant data on this application 15.
However, trials on WUS or unclear-onset strokes, including multimodal neuroimaging, proved that the actual onset time of WUS is close to the wake-up time and that stroke may be the cause of wake-up 1. Furthermore, both intravenous 5 and intra-arterial 6,7 neuroimaging-guided reperfusion therapies have been demonstrated to be safe and feasible more than eight hours from TLSW in patients with WUS or unclear-onset strokes, such as occurred in the patient reported herein. Finally, ultra-early diffusion-weighted imaging is not available at our institution as the MRI magnet is not placed in the Emergency Department, and thus in many cases we have to shift to the potential offered by CT perfusion.
To the best of our knowledge, this is the first Italian case report describing a patient who underwent neuroendovascular treatment for WUS by a CT perfusion-guided wide extension of the therapeutic window for neuroendovascular therapy. This occurred without clinical worsening nor major complications and resulted in an acceptable clinical outcome.
Conclusions
We have confirmed the role of neuroimaging-guided neuroendovascular therapy in a patient with WUS beyond eight hours after TLSW. Only a few years ago, this patient would have been considered not eligible for reperfusion. Although this is a single case report, we believe that in future patients with WUS or unclear-onset stroke will be candidates for neuroimaging-guided reperfusion therapies, and notably neuroendovascular treatment. Prospective randomized controlled studies comprising large series of patients are required to determine if this strategy is really feasible. We agree that this is the era in which we are shifting from “time is brain” to “physiology is brain” 3,10,15.
References
- 1.Kang DW, Kwon JY, Kwon SU, et al. Wake-up or unclear-onset strokes: are they waking up to the world of thrombolysis therapy? Int J Stroke. 2012;7:311–20. doi: 10.1111/j.1747-4949.2012.00779.x. [DOI] [PubMed] [Google Scholar]
- 2.Moradiya Y, Janjua N. Presentation and outcomes of "wake-up strokes" in a large randomized stroke trial: analysis of data from the International Stroke Trial. J Stroke Cerebrovasc Dis. 2012 doi: 10.1016/j.jstrokecerebrovasdis.2012.07.016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.González RG. Imaging-guided acute ischemic stroke therapy: From “time is brain”; to “physiology is brain”. Am J Neuroradiol. 2006;27:728–735. [PMC free article] [PubMed] [Google Scholar]
- 4.Salottolo KM, Fanale CV, Leonard KA, et al. Multimodal imaging does not delay intravenous thrombolytic therapy in acute stroke. Am J Neuroradiol. 2011;32:864–868. doi: 10.3174/ajnr.A2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barreto AD, Martin-Schild S, Hallevi H, et al. Thrombolytic therapy for patients who wake-up with stroke. Stroke. 2009;40:827–832. doi: 10.1161/STROKEAHA.108.528034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Natarajan SK, Karmon Y, Snyder KV, et al. Prospective acute ischemic stroke outcomes after endovascular therapy: a real-world experience. World Neurosurg. 2010;74:455–464. doi: 10.1016/j.wneu.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 7.Jovin T, Liebeskind D, Gupta R, et al. Imaging-based endovascular therapy for acute ischemic stroke due to proximal intracranial anterior circulation occlusion treated beyond 8 hours from time last seen well. Stroke. 2011;42:2206–2211. doi: 10.1161/STROKEAHA.110.604223. [DOI] [PubMed] [Google Scholar]
- 8.Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–1614. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 9.Wintermark M, Flanders AE, Velthuis B, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006;37:979–985. doi: 10.1161/01.STR.0000209238.61459.39. [DOI] [PubMed] [Google Scholar]
- 10.Wintermark M, Albers GW, Alexandrov AV, et al. Acute stroke imaging research roadmap. Stroke. 2008;39:1621–1628. doi: 10.1161/STROKEAHA.107.512319. [DOI] [PubMed] [Google Scholar]
- 11.Schaefer PW, Roccatagliata L, Ledezma C, et al. First-pass quantitative CT perfusion identifies thresholds for salvageable penumbra in acute stroke patients treated with intra-arterial therapy. Am J Neuroradiol. 2006;27:20–25. [PMC free article] [PubMed] [Google Scholar]
- 12.Goyal M, Menon BK, Derdeyn CP. Perfusion imaging in acute ischemic stroke: let us improve the science before changing clinical practice. Radiology. 2013;266:16–21. doi: 10.1148/radiol.12112134. [DOI] [PubMed] [Google Scholar]
- 13.Sharma M, Pelz DM. CT Perfusion in acute stroke: added value or waste of time? Stroke. 2013;44(9):e115. doi: 10.1161/STROKEAHA.113.002355. [Epub 2013 Aug 15] [DOI] [PubMed] [Google Scholar]
- 14.Wintermark M, Zhu G, Patrie JT, et al. Response to letter regarding article, “CT perfusion in acute stroke: added value or waste of time?”. Stroke. 2013;44(9):e116. doi: 10.1161/STROKEAHA.113.002401. [Epub 2013 Aug 15] [DOI] [PubMed] [Google Scholar]
- 15.González RG, Copen WA, Schaefer PW, et al. The Massachusetts General Hospital acute stroke imaging algorithm: an experience and evidence based approach. J Neurointerv Surg. 2013;5(Suppl 1):i7–i12. doi: 10.1136/neurintsurg-2013-010715. [DOI] [PMC free article] [PubMed] [Google Scholar]