Summary
Positron emission tomography (PET) imaging with F-18 fluorodeoxyglucose (FDG) can be used as a downstream marker of neuronal injury, a hallmark of neurodegenerative dementias. Characteristic patterns of regional glucose metabolism have been used to classify the dementia subtypes, namely Alzheimer's dementia (AD), frontotemporal dementia (FTD), diffuse Lewy body (DLBD) and vascular dementia (VD). We undertook this study to assess the utility of FDG-PET in the differential diagnosis of dementia subtypes. One hundred and twenty-five patients diagnosed with dementia were referred from cognitive disorders and memory clinics of speciality neurology centres for the FDG-PET study. Imaging-based diagnosis of dementia type was established in 101 patients by visual assessment of individual scans by a PET physician blinded to the clinical diagnosis. The results were compared with an 18-month follow-up clinical assessment made by the specialist neurologist. Concordance of visual evaluation of FDG-PET scans with clinical diagnosis of the dementia type was achieved in 90% of patients scanned. This concordance was 93.4% for AD, 88.8% for FTD, 66.6% for DLBD and 92.3% for the other dementia syndromes. FDG-PET performed after the initial work-up of dementias is useful for supporting the clinical diagnosis of dementia subtype.
Keywords: FDG-PET, AD, FTD, DLBD, vascular dementia
Introduction
The definitive diagnosis of neurodegenerative dementias like Alzheimer’s disease (AD) is based on the post-mortem observation of specific pathological lesions within the brain. The underlying pathologies are associated with neuronal and synaptic losses and with atrophy in specific brain areas. In vivo brain F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) is a minimally invasive diagnostic imaging procedure used to evaluate cerebral glucose metabolism which provides an index of local synaptic activity and the dominant biochemical maintenance processes in this condition. Cerebral glucose hypometabolism on FDG-PET is a downstream marker of neuronal injury and neurodegeneration. Characteristic patterns of regional glucose hypometabolism on FDG-PET have been identified in association with the common neurodegenerative dementias 1-6. Alzheimer’s disease (AD) is by far the commonest of the neurodegenerative dementias comprising more than 60%, while the non-AD subgroup comprises diffuse Lewy body dementia (DLBD), frontotemporal dementia (FTD), vascular dementia (VD) and mixed dementias.
Accurate diagnosis of the dementia type is critical for patient counselling and treatment decisions. In addition, as new disease-specific treatments targeting the underlying pathophysiology emerge there will be an increased need for precise differential diagnosis of dementia syndromes. A purely clinical classification of dementia subtypes may be difficult because of overlapping presentations and imprecise clinical and neuropsychological distinctions. FDG-PET is now approved by the US Food and Drug Administration (FDA) for differentiating FTD from AD 7. The addition of FDG-PET to clinical information alone increases not only diagnostic accuracy but also physicians’ confidence in AD and FTD diagnosis 1. We undertook this study to determine the accuracy of FDG-PET to differentiate AD from non-AD dementias on a single case basis.
Patients and Methods
Subjects
We prospectively enrolled 125 consecutive patients with dementia at the Cognitive Disorders and Memory (CDM) clinics of speciality neurology centres at tertiary care hospitals (All India Institute of Medical Sciences and Institute of Human Behaviour and Allied Sciences) from December 2008 to April 2011 for the FDG-PET study. Each patient underwent a comprehensive clinical and neuropsychological evaluation including a physical and mental status examination. The mental status examination involved the mini mental state examination (MMSE) 8 and clinical dementia rating scale (CDR) performed by the neurophysician (MT, SK) 9. Vitamin B12 and TSH levels were estimated in all patients. Each subject also underwent computed tomography (CT) or magnetic resonance imaging (MRI) at outside centres to rule out any structural abnormality like subdural hematoma, brain tumours or hydrocephalus as the cause for dementia.
Twenty-four patients, including those with elevated serum TSH (above 10 μIU/ml), Vitamin B12 deficiency, subdural hematomas, brain tumour, white matter small vessel disease and hydrocephalus were excluded from the study. All these patients were appropriately managed for these potentially reversible causes of dementia except those with small vessel disease who were managed for vascular risk factors.
FDG-PET procedure
All subjects were asked to come with at least four hours fasting but with liberal water intake on the day of the PET study. Each patient was injected with 185-296 MBq (5-8 mCi) of F-18 FDG intravenously followed by a rest period of 60 minutes with eyes open in a silent, dimly lit room followed by acquisition on a Discovery STE16 camera (General Electric Medical Systems, Milwaukee, WI, USA). This scanner has a transaxial resolution full width half maximum (FWHM) of 5.12 mm for three-dimensional (3D) mode at 1 cm offset from the centre of field of view. Patients were imaged supine with their heads positioned in a headrest. An initial scout of the head was followed by low dose CT for attenuation correction and coregistration. A single bed 3D emission scan was then acquired for 20 minutes in each patient. Scans were reconstructed using the 3D VUE algorithm (GE) and viewed on a Xeleris Workstation (GE) using the volumetric protocol.
‘Visual’ reading
FDG-PET images were displayed scaled to a common maximum in standard colour scale. During visual reading all images from each subject were scaled to his/her own global maximal voxel value. Uniformity of reading was achieved by focusing on the relative intensity between various cortical and subcortical regions rather than absolute values of any particular region. All images were independently reviewed by two nuclear medicine physicians [RS, 15 years’ experience and MT, nine years’ experience] and the final impression was based on consensus between the two. MRI films (without the report) of each patient were made available to the reader for reference when needed. Scans were classified based on regional metabolism patterns reported in literature 1-6 and the characteristic patterns are listed in Table 1.
Table 1.
Patterns of glucose metabolism used for ‘visual’ classification of single cases.
|
Alzheimer’s dementia Hypometabolism in: • Parieto-temporal (symmetrical or asymmetrical), including posterior cingulate and precuneal cortices • With or without frontal hypometabolism (symmetrical or asymmetrical) Preserved metabolism in: • Sensorimotor and visual cortices • Both basal ganglia and thalami • both cerebellar cortices |
|
Frontotemporal dementia Hypometabolism in: • Both frontal cortices (usually symmetrical) and • Anterior temporal cortices |
|
Diffuse Lewy body dementia Hypometabolism in: • Both visual cortices • Posterior cingulate sign |
|
Vascular dementia • Regional cortical hypometabolism corresponding to a vascular territory with • Corresponding signal changes on MRI |
|
Mixed dementia • Parieto-temporal hypometabolism with or without regional cortical defects and ischaemic cortical and subcortical signal changes on MRI |
|
Creutzfeld Jacob Disease • Cortical and subcortical hypometabolism |
Final diagnosis
A total of 101 patients were included in the final analysis. Sixty of the patients included in this study had been included in a previous analysis by our group 10. The final diagnosis of dementia type was based on longitudinal clinical follow-up of at least 18 months at the CDM clinic, after the preliminary diagnosis of dementia by a specialist neurologist. Clinical diagnosis was based on diagnostic systems reported in the literature. Possible or probable AD was diagnosed using the National Institute of Neurological and Communicative diseases and Stroke/Alzheimer’s disease and related disorders association (NINCDS/ADRDA) criteria 11. Lund-Manchester criteria 12 were used for diagnosing FTD. DLBD was diagnosed using consensus criteria for DLBD 13 and National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherche et l'Enseignement en Neurosciences (NINDS-AIREN) criteria were used for VD 14. For the purpose of analysis, patients were divided into the following groups based on the type of dementia, AD, FTD, DLBD and others which included vascular and mixed dementias, Creutzfeld Jacob Disease (CJD) and posterior cortical atrophy (PCA).
Statistical analysis
Chi square test was used for evaluation of qualitative data. ANOVA was done for between group comparison of quantitative data. Concordance of FDG-PET with clinical diagnosis was calculated for the whole group and for individual dementia subtypes. A 2×2 contingency table was used for calculation of sensitivity and specificity for FDG-PET Vs the clinical diagnosis for AD, FTD and DLBD.
Results
Demographic data for the patients and their clinical details are presented in Table 2. Chi square for gender differences between AD and the other dementia subgroups (Χ2=0.55,3.5,0.39) was not significant. ANOVA for significance of between group differences in age (p=0.09), disease duration (p=0.7), MMSE (p=0.8) and CDR (p=0.6) was not statistically significant. Follow-up of at least 18 months was possible in all but the two CJD patients in whom survival was less than one year.
Table 2.
Demographic and clinical findings in the patients.
|
Number (M/F) |
Age in years Mean (SD) |
Disease duration at the time of scanning in months Mean (SD) |
MMSE | CDR |
Follow-up after FDG PET scan in months Mean (SD) |
|
| AD | 61(34/27) | 62.8 (11.4) | 24.1 (11.4) | 17.7 (2.9) | 1.9 (0.6) | 24.6 (2.4) |
| FTD | 18 (12/6) | 57.0 (9.5) | 21.6 (7.9) | 17.2! (2.8) | 2.0 (0.5)! | 21.5 (3.3) |
| DLBD | 9 (2/7) | 65.8 (7.5) | 27.6 (19.3) | 18.2! (2.6) | 2.1 (0.6)! | 25.7 (1.5) |
| *OTHERS | 13 (6/7) | 65.7 (11.6) | 22.3 (18.2) | 17.3! (3.7) | 1.5(0.5)! | 23.3 (8.4) |
M=male, F=female, SD=standard deviation, AD=Alzheimer’s dementia, FTD=Fronto-temporal dementia, DLBD=diffuse Lewy body dementia, MMSE=Mini mental state examination, CDR=Clinical dementia rating scale, *OTHERS includes CJD=2 (Creutzfeld Jacob disease), VD=5 (vascular dementia), mixed dementia=5 and PCA=1 (posterior cortical atrophy), != not evaluable in all cases.
Diagnostic classification of single patient scans on FDG-PET
Blinded expert ‘visual reading’ of the FDG-PET images resulted in 90% concordance with the clinical diagnosis in all subjects. Specifically, this concordance was 93.4% for AD, 88.8% for FTD, 66.6% for DLBD and 92.3% for the other syndromes included (Table 3). FDG-PET achieved a sensitivity of 93.4% (95% CI= 84.3% to 97.4%) and specificity of 87.5% (95% CI= 73.8% to 94.5%) for the diagnosis of AD versus the other dementia syndromes. Sensitivity and specificity for FTD versus the remaining subjects was 88.8% (95% CI= 67.2 to 96.9%) and 100% (95% CI= 95.5% to 100%) respectively. The sensitivity of FDG-PET for DLBD was 66.6% while specificity was 98.3%.
Table 3.
Classification matrix of subjects for ‘visual’ reading of FDG PET scans
| Clinical category | ‘Visual’ reading | |||
| AD | FTD | DLBD | Others | |
| AD=61 | 57 (93.4%) | 0 (0 %) | 1 (1.6%) | 3 (4.9%) |
| FTD=18 | 2 (11.1 %) | 16 (88.8%) | 0 (0 %) | 0 (0 %) |
| DLBD=9 | 2 (22.2 %) | 0 (0 %) | 6 (66.6 %) | 1 (11.1 %) |
| Others=13 | 1 (7.6%) | 0 (0%) | 0 (0%) | 12 (92.3 %) |
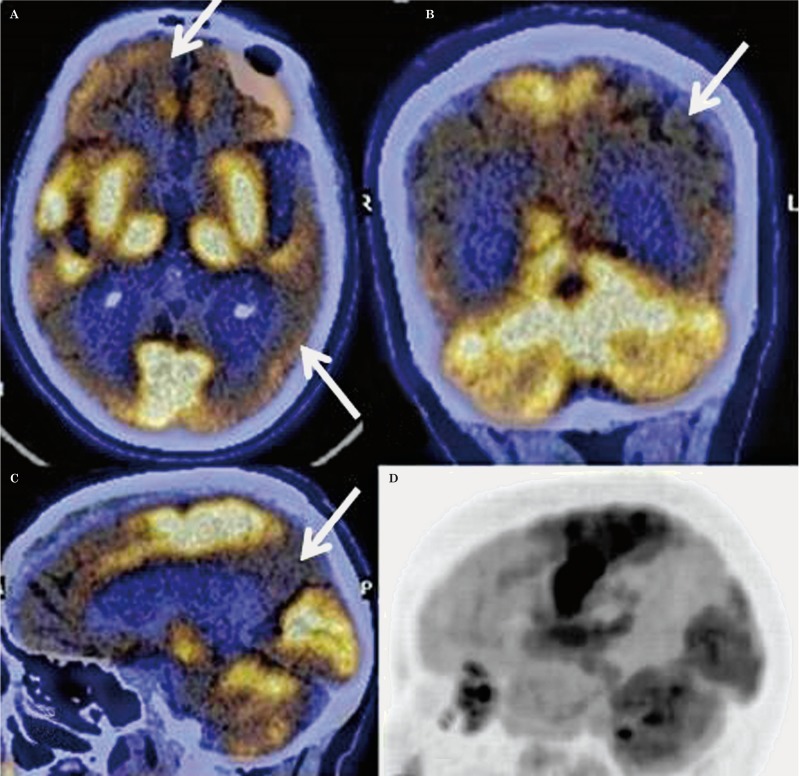
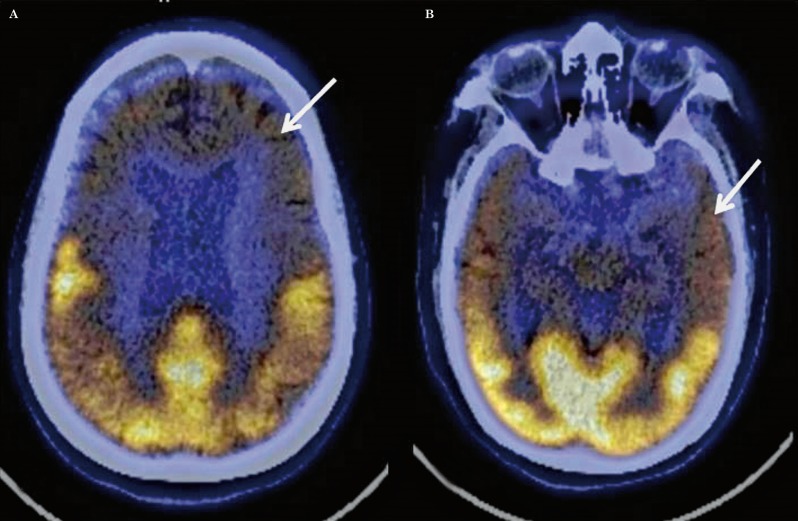
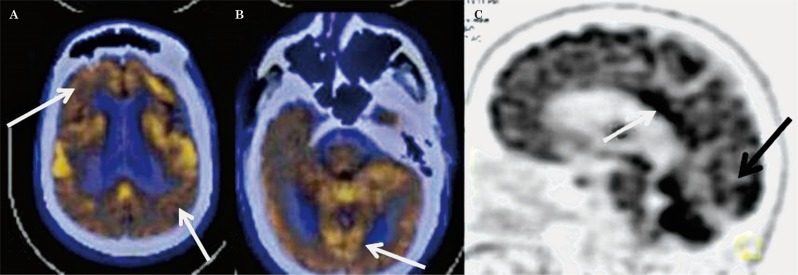
The characteristic pattern of AD was similar to that reported in the literature with symmetrical or asymmetrical parieto-temporal, including posterior cingulate and precuneus hypometabolism with or without frontal hypometabolism (Figure 1). The FTD pattern was characterized by marked frontal and anterior temporal hypometabolism with hypometabolism involving both caudate nuclei anteriorly (Figure 2). This pattern is typical of the behavioural variant of FTD (bv-FTD). Two cases of FTD were reported as semantic variants because of marked anterior temporal hypometabolism more on the left side, and one as progressive non-fluent aphasia (PNFA) with marked left perisylvian hypometabolism. The DLBD pattern was characterized by hypometabolism in the visual cortices in addition to fronto-parieto-temporal hypometabolism (Figure 3). There was relative preservation of uptake in the posterior cingulate cortices (posterior cingulate sign). All cases classified as mixed dementia on FDG-PET had parieto-temporal hypometabolism (AD pattern) in addition to focal/regional areas of hypometabolism which corresponded to hypodense areas on CT with additional signal changes on MRI involving cortical or subcortical grey matter/white matter regions. Hypometabolism corresponding to signal changes in vascular territories on MRI was seen in the cases of VD. One of the two cases of CJD showed global hypometabolism while MRI showed the characteristic pattern of CJD. The other case had parieto-temporo-frontal hypometabolism which was markedly asymmetrical while subcortical hypometabolism was not marked, this case was thus labelled as AD on FDG-PET. FDG-PET in a case of PCA showed marked bilateral occipital hypometabolism extending into bilateral parietal cortices.
Figure 1.
A) Transaxial fused FDG-PET/CT images in a case of advanced AD showing symmetrical hypometabolism in both frontal and temporal cortices (arrow). B) Coronal fused FDG-PET/CT image showing hypometabolism in both parietal cortices (arrow). C) Sagittal fused FDG-PET/CT image showing hypometabolism in the precuneus (arrow). D) Lateral maximum intensity projection image showing hypometabolism in the parietal and frontal cortices with preserved metabolism in the sensorimotor, visual and occipital cortices, and both cerebellar hemispheres.
Figure 2.
Transaxial fused FDG-PET/CT images in a case of fronto-temporal dementia showing hypometabolism in both frontal (A, arrow) and both anterior temporal cortices (B, arrow).
Figure 3.
Transaxial fused FDG-PET/CT images in a case of DLBD showing hypometabolism in both frontal and parietal cortices (A) and also in the visual cortices (B, arrow). C) Lateral maximum intensity projection image showing hypometabolism in the visual cortices with preserved metabolism in the posterior cingulate (arrow).
The available MRI reports were recorded for all patients. Changes of cerebral atrophy were reported in 46 patients, marked hippocampal atrophy was reported in seven cases of AD, frontotemporal atrophy was marked in four cases of frontotemporal dementia and one case of AD, vascular signal changes were seen in all cases of VD and mixed dementia (five cases each) and findings of CJD (basal ganglia and cortical hyperintensities) were seen in the two cases of CJD one of whom showed the characteristic pattern on a repeat MRI (DWI) done after a two-week interval.
Discussion
The present study demonstrates the utility of regional patterns of glucose metabolism on the FDG-PET study to differentiate dementias on a single case basis. The regional patterns of metabolism we chose (Table 1) for defining and differentiating AD, FTD, DLBD and mixed dementias were based on patterns described in the literature 1-6. This resulted in 90% concordance with the clinical diagnosis in all subjects. This concordance was highest for AD (93.4%) and lowest for DLBD (66.6%).
The characteristic pattern of AD on FDG-PET was symmetrical or asymmetrical parieto-temporal, including posterior cingulate and precuneus hypometabolism with or without frontal hypometabolism (Figure 1). One case of AD was reported as a DLBD pattern on FDG-PET because of extension of parietal hypometabolism into the lateral occipital cortices. FDG-PET was not concordant with an AD pattern in two cases where the hypometabolism did not pertain to the specific pattern and in one case where it appeared to be involving a vascular territory.
FTD on FDG-PET was characterized by frontal and anterior temporal hypometabolism. This pattern is typical of the behavioural variant of FTD (bv-FTD). Two cases of bv-FTD who showed extension of hypometabolism into the parietal cortices were labelled as AD pattern on FDG-PET.
DLBD on FDG-PET was characterized by hypometabolism in the visual cortices with relative preservation of uptake in the posterior cingulate cortices. Two DLBD were labelled as AD because the parieto-temporal hypometabolism was more marked than visual cortices hypometabolism and the posterior cingulate sign was not marked in these cases.
CJD is characterized by global hypometabolism on FDG-PET with diagnostic findings on MRI. This was well appreciated in one of our cases while the other case had parieto-temporo-frontal hypometabolism which was markedly asymmetrical while subcortical hypometabolism was not marked, this case was thus labelled as AD on FDG-PET. PET had been done as the initial clinical presentation was atypical and MRI was inconclusive at this stage, but MRI with DWI two weeks later was characteristic and showed cortical and subcortical hyperintensities.
The initial clinical impression in our case of PCA was corticobasal ganglionic degeneration but was subsequently revised to PCA (independent of the PET report).
MRI provided a conclusive diagnosis in only 23 of our cases. This was possibly because the scans were done at outside centres and the specific area of atrophy had not been looked for on appropriate sequences. A direct comparison with MRI was, however, not a part of this study.
Parietotemporal hypometabolism, the characteristic FDG-PET pattern for AD, resulted in 93.4% sensitivity and 87.5% specificity for distinguishing AD from the other dementias. Misclassifications of AD on FDG-PET occurred when the hypometabolism extended into the lateral occipital cortices or when it was felt to involve a vascular territory. A false positive pattern for AD resulted when frontal hypometabolism extended to involve the parietal cortices in FTD (FTD was classified as AD) and when visual cortices hypometabolism was not appreciated in DLBD (was classified as AD). It has been reported that 35% of FTD patients and 29% of DLBD patients can show cortical deficits similar to AD patients thereby resulting in lower specificities 3. The sensitivity and specificity of FDG-PET for AD reported by us was comparable to that reported by other studies 1,2,3,15,16. In the largest study with the diagnosis of AD confirmed on histopathology (reference standard), among 97 patients, the sensitivity of FDG-PET for diagnosing AD was 94% and specificity among 41 patients without AD was 73% 3. Another study using autopsy confirmation for diagnosis of AD found FDG-PET to have a sensitivity of 97% and a specificity of 86% for distinguishing AD from FTD 1. Specificity of FDG-PET for AD could be improved by the use of pathophysiology-based agents that could image pathology in vivo like amyloid agents (to rule out AD in cases suspected with FTD) or dopaminergic agents (to rule out AD in cases with suspected DLBD). Further studies with the inclusion of these agents along with FDG-PET in doubtful cases would be useful to document this benefit.
The diagnosis of dementia is still largely a clinical diagnosis based on the history, the course of the disease and laboratory tests supported by imaging to rule out reversible causes. Neuroimaging has largely a supportive role and the true value of FDG-PET in the day-to-day challenge of dementia diagnosis was put forward by a recent study showing that PET lowered the number of unclear diagnoses from 39% to 16% and this mainly resulted because 30% of these were found to have a hypometabolism pattern pertaining to AD. This study addressed the clinician’s impression of the contribution of FDG-PET in the diagnostic process. FDG-PET oriented the diagnosis in 56% of cases; confirmed the clinical impression in 16% of cases and had no impact in 28% of cases 17.
FDG-PET had a 100% specificity for diagnosis of FTD while sensitivity was around 88%. The specificity of FDG-PET for the differential diagnosis of dementias reported by Panegyres et al. was also greater than 95% in the primary care setting 16. Our specificity was better because patient referrals were from a tertiary care centre. Three of the cases with marked language changes were correctly classified as semantic and PNFA variants of FTD based on the patterns of hypometabolism, similar to that reported in literature 6,18.
Visual cortices hypometabolism on FDG-PET differentiated DLBD from the other dementias with a sensitivity of 66.6% and specificity of 98%. In the series by Minoshima et al. 2, occipital hypometabolism distinguished DLBD from AD with 90% sensitivity and 80% specificity (using post-mortem diagnostic validation). Only nine patients with DLBD were included in our series, we therefore feel that it would not be correct to comment on the sensitivity and specificity values we obtained and inclusion of a larger series is warranted. Though DLBD has been recognized as the second most frequent type of neurodegenerative dementia, our series had FTD as the second commonest type. This may suggest that DLBD remains an underdiagnosed dementia type - all the more so because clinical criteria have a low sensitivity (22-75%) for the diagnosis of DLBD 19. Dopaminergic imaging is included in the supportive criteria for clinical diagnosis of DLBD and its inclusion and availability could probably improve the sensitivity of functional imaging for the diagnosis of DLBD. The current availability of dopaminergic imaging is limited to very few centres in India because of associated costs and expertise needed for its production.
MRI is the primary modality for evaluation of vascular dementia, and FDG-PET is useful in cases with a clinical suspicion of an added dementia type like Alzheimer's where FDG-PET is quite useful based on the typical pattern of parieto-temporal hypometabolism along with the focal/territorial hypometabolic regions 18. In our series, FDG-PET was concordant with clinical diagnosis in all cases of mixed dementia (vascular +AD).
Only two cases of CJD were included in our study which makes the number small to derive any conclusion. However, it has been suggested that FDG-PET is able to detect CJD at an earlier stage and with greater sensitivity than DW-MRI 21.
In a previous study we reported encouraging results using Tc-99m ECD brain perfusion SPECT for the differential diagnosis of dementia subtypes 22. Although SPECT has been more broadly available, studies show PET has a higher diagnostic accuracy by approximately 15-20%, suggesting it may be more beneficial in the early detection of neurodegenerative diseases 23.
Temporoparietal hypometabolism on FDG-PET thus increases the certainty that the clinical dementia syndrome is AD. Further, the absence of the classical pattern of hypometabolism of FTD or DLBD can effectively rule out the possibility of these dementia syndromes. Our data suggest that the use of imaging techniques like FDG-PET to support a clinical diagnosis at initial patient presentation may provide accurate differential diagnosis in patients with dementia. The availability of amyloid imaging agents and dopaminergic analogues for imaging can further improve the performance of functional imaging techniques such as PET for the differential diagnosis of dementia syndromes.
The limitations of this study were the use of an imperfect reference standard 23 but in India diagnosis of AD remains clinical and histopathology is very difficult to obtain. Clinical diagnosis has a low specificity for AD and low sensitivity for the diagnosis of FTD and DLBD 24. The presence of a referral bias could limit the generalizability of study results. A long-term follow-up to verify the dementia subtype could also have been more useful. In addition, we did not take into account the fact that only visual interpretation of FDG-PET images was performed which could be influenced by reader expertise.
Conclusion
Characteristic patterns of hypometabolism on FDG-PET imaging are useful for the differential diagnosis of dementias on a single case basis. Parieto-temporal hypometabolism on FDG-PET has a high sensitivity for differentiating AD from the other dementia subtypes. This approach can in no way replace a high quality clinical assessment but can probably add value to the diagnostic evaluation.
References
- 1.Foster NL, Heidebrink JL, Clark CM, et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain. 2007;130(Pt 10):2616–2635. doi: 10.1093/brain/awm177. doi: 10.1093/brain/awm177. [DOI] [PubMed] [Google Scholar]
- 2.Minoshima S, Foster NL, Sima AA, et al. Alzheimer’s disease versus dementia with Lewy bodies: cerebral metabolic distinction with Autopsy confirmation. Ann Neurol. 2001;50(3):358–365. doi: 10.1002/ana.1133. doi: 10.1002/ana.1133. [DOI] [PubMed] [Google Scholar]
- 3.Silverman DH, Small GW, Chang CY, et al. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA. 2001;286(17):2120–2127. doi: 10.1001/jama.286.17.2120. doi: 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- 4.Albin RL, Minoshima S, D’Amato CJ, et al. Fluoro-deoxyglucose positron emission tomography in diffuse Lewy body disease. Neurology. 1996;47(2):462–466. doi: 10.1212/wnl.47.2.462. doi: 10.1212/WNL.47.2.462. [DOI] [PubMed] [Google Scholar]
- 5.Ishii K, Sakamoto S, Sasaki M, et al. Cerebral glucose metabolism in patients with frontotemporal dementia. J Nucl Med. 1998;39(11):1875–1878. [PubMed] [Google Scholar]
- 6.Diehl-Schmid J, Grimmer T, Drzezga A, et al. Longitudinal changes of cerebral glucose metabolism in semantic dementia. Dement Geriatr Cogn Disord. 2006;22:346–351. doi: 10.1159/000095624. doi: 10.1159/000095624. [DOI] [PubMed] [Google Scholar]
- 7.Medicare National Coverage Determinations. Centers for Medicare and Medicaid Services Web site. Manual: Chapter 1, Part 4 (Sections 200-310.1) - Coverage Determinations. Available at: http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/ncd103c1_Part4.pdf. [Google Scholar]
- 8.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 9.Berg L. Clinical Dementia Rating (CDR) Psychopharmacol Bull. 1988;24(4):637–639. [PubMed] [Google Scholar]
- 10.Sharma R, Tripathi M, D’Souza MM, et al. Spectrum of neurocognitive dysfunction in Indian population on FDG PET/CT imaging. Indian J Nucl Med. 2011;26(2):67–77. doi: 10.4103/0972-3919.90255. doi: 10.4103/0972-3919.90255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimers Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- 12.Miller BL, Ikonte C, Ponton M, et al. A study of Lund-Manchester research criteria for frontotemporal dementia: Clinical and single-photon emission CT correlations. Neurology. 1997;48(4):937–942. doi: 10.1212/wnl.48.4.937. [DOI] [PubMed] [Google Scholar]
- 13.Román GC, Tatemichi TK, Erkinjuntti T. Vascular dementia: Diagnostic criteria for research studies - A report on the NINDS-AIREN international workshop. Neurology. 1993;43(2):250–260. doi: 10.1212/wnl.43.2.250. doi: 10.1212/WNL.43.2.250. [DOI] [PubMed] [Google Scholar]
- 14.Laforce R, Jr, Buteau JP, Paquet N, et al. The value of PET in mild cognitive impairment, typical and atypical/unclear dementias: a retrospective memory clinic study. Am J Alzheimers Dis Other Demen. 2010;25(4):324–332. doi: 10.1177/1533317510363468. doi: 10.1177/1533317510363468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mc Keith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 16.Panegyres PK, Rogers JM, McCarthy M, et al. Fluorodeoxyglucose-positron emission tomography in the differential diagnosis of early-onset dementia: a prospective, community-based study. BMC Neurol. 2009;9:41. doi: 10.1186/1471-2377-9-41. doi: 10.1186/1471-2377-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jagust W, Reed B, Mungas D, et al. What does fluorodeoxyglucose PET imaging add to a clinical diagnosis of dementia? Neurology. 2007;69(9):871–877. doi: 10.1212/01.wnl.0000269790.05105.16. doi: 10.1212/01.wnl.0000269790.05105.16. [DOI] [PubMed] [Google Scholar]
- 18.Hodges JR, Patterson K, Oxbury S, et al. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(Pt 6):1783–1806. doi: 10.1093/brain/115.6.1783. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- 19.Ransmayr G, Wenning GK, Seppi K, et al. Dementia with Lewy bodies. Nervenarzt. 2000;71(12):929–935. doi: 10.1007/s001150050689. doi: 10.1007/s001150050689. [DOI] [PubMed] [Google Scholar]
- 20.Mielke R, Heiss WD. Positron emission tomography for diagnosis of Alzheimer’s disease and vascular dementia. J Neural Transm Suppl. 1998;53:237–250. doi: 10.1007/978-3-7091-6467-9_21. doi: 10.1007/978-3-7091-6467-9_21. [DOI] [PubMed] [Google Scholar]
- 21.Xing XW, Zhang JT, Zhu F, et al. Comparison of diffusion-weighted MRI with 18F-fluorodeoxyglucose-positron emission tomography/CT and electroencephalography in sporadic Creutzfeldt-Jakob disease. J Clin Neurosci. 2012;19(10):1354–1357. doi: 10.1016/j.jocn.2011.11.035. doi: 10.1016/j.jocn.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 22.Tripathi M, Tripathi M, Vibha D, et al. Tc-99m ethylcysteinate dimer SPECT in the differential diagnosis of dementias. Neurol India. 2010;58:857–862. doi: 10.4103/0028-3886.73745. doi: 10.4103/0028-3886.73745. [DOI] [PubMed] [Google Scholar]
- 23.Patwardhan MB, McCrory DC, Matchar DB, et al. Alzheimer disease: operating characteristics of PET—A meta-analysis. Radiology. 2004;231(1):73–80. doi: 10.1148/radiol.2311021620. doi: 10.1148/radiol.2311021620. [DOI] [PubMed] [Google Scholar]
- 24.Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1143–1153. doi: 10.1212/wnl.56.9.1143. doi: 10.1212/WNL.56.9.1143. [DOI] [PubMed] [Google Scholar]