Summary
Glioblastoma is a malignant infiltrative glial tumor occurring most often over 50 years of age, with diverse clinical presentations. We describe a case of temporal lobe glioblastoma with a rare presentation as an acute ischemic stroke, discussing the imaging and histopathological findings, and reviewing the literature. A 77-year-old woman had sudden onset of left hemiparesis and hemihypoesthesia. The neuroradiological studies revealed an acute ischemic lesion in the right lenticulostriate arteries territory and a right anterior temporal lobe tumor, enhancing heterogeneously after contrast with enhancement of the right middle cerebral artery wall. Histopathological analysis of the resected temporal lesion revealed a glioblastoma multiforme with tumoral infiltration of the vascular wall. Glioblastoma should be considered in the etiology of acute ischemic stroke, where neuroimaging plays an important diagnostic role, enabling a more immediate therapeutic approach, with a consequent impact on survival.
Keywords: glioblastoma, stroke, vasculopathy, computed tomography, magnetic resonance imaging
Introduction
There are only a few case reports 1-6 in the literature describing acute ischemic stroke secondary to brain tumors, which are not usually considered in the etiology of cerebral infarcts in clinical practice. We describe a histologically proven case of vascular wall infiltration by glial tumoral cells, causing deep middle cerebral artery stroke.
Case Report
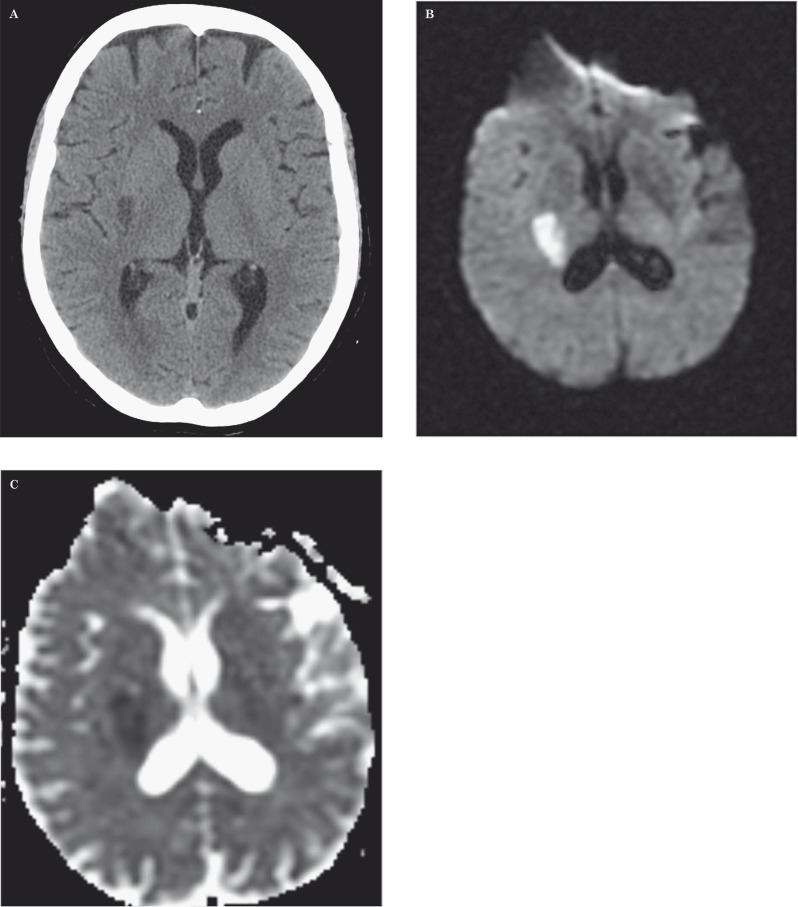
A 77-year-old woman, with a medical history of arterial pre-hypertension 7 and dyslipidemia under medical control, had an acute onset of left hemiparesis and hemihypoesthesia. Initially she had a brain computed tomography (CT) scan, an arterial angiographic CT study and a brain magnetic resonance (MR). The imaging studies revealed a focal right lenticulocapsular lesion, with slight mass effect, hypodense on CT, showing water molecules restricted diffusion on MR (Figure 1). The lesion was suggestive of an acute ischemic stroke in the lenticulostriate arteries territory (perforating branches of the right middle cerebral artery). There coexisted another lesion with pronounced mass effect in the ipsilateral anterior temporal lobe (Figure 2), corticosubcortical, with heterogeneous enhancement, more prominent on the anterior lateral portion but extending into the mesial temporal lobe, and also enhancement of the right middle cerebral artery wall (Figure 3). The angiographic study showed a focal stenosis of the distal M1 portion of the right middle cerebral artery (MCA), as well as a reduced caliber of the right anterior cerebral artery (Figure 4). The histopathological study after anterior temporal partial lobectomy, performed 2.5 weeks after the clinical presentation, confirmed the suspected diagnosis of glioblastoma with vascular wall infiltration of small and medium-sized arteries, including the MCA, that were encompassed by a tumoral mass and also infiltration of leptomeningeal vessels by glial tumoral cells (Figure 5). After surgery the patient remained with permanent left hemiplegia and immediate adjuvant therapy was not implemented.
Figure 1.
CT, diffusion-weighted imaging and ADC maps. Right lenticular focal hypodensity (A) with water molecules restricted diffusion (B,C).
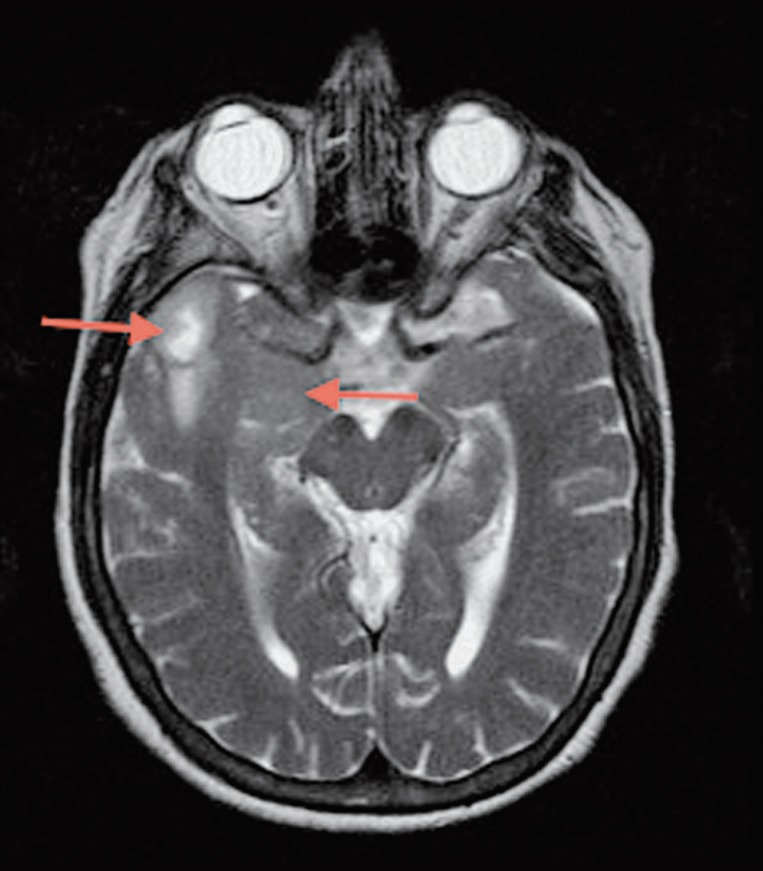
Figure 2.
MR, Ax T2 TSE. Cyst-like lesion in the right lateral anterior temporal lobe, and expansion and slight hyperintensity on right temporal mesial structures.
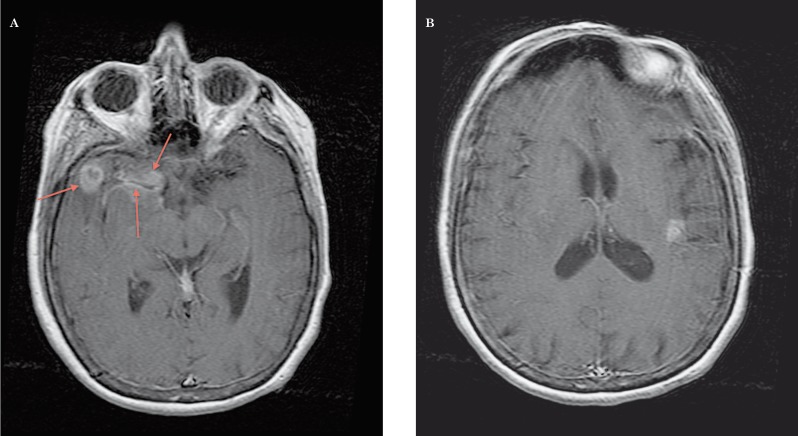
Figure 3.
MR, 3D T1 TFE post-gadolinium. A) Heterogeneous enhancement in the right anterior temporal lobe (ring-like laterally and more diffuse on the mesial portion) and enhancement of the wall of the right middle cerebral artery. B) Focal superficial enhancement in the left insular region.
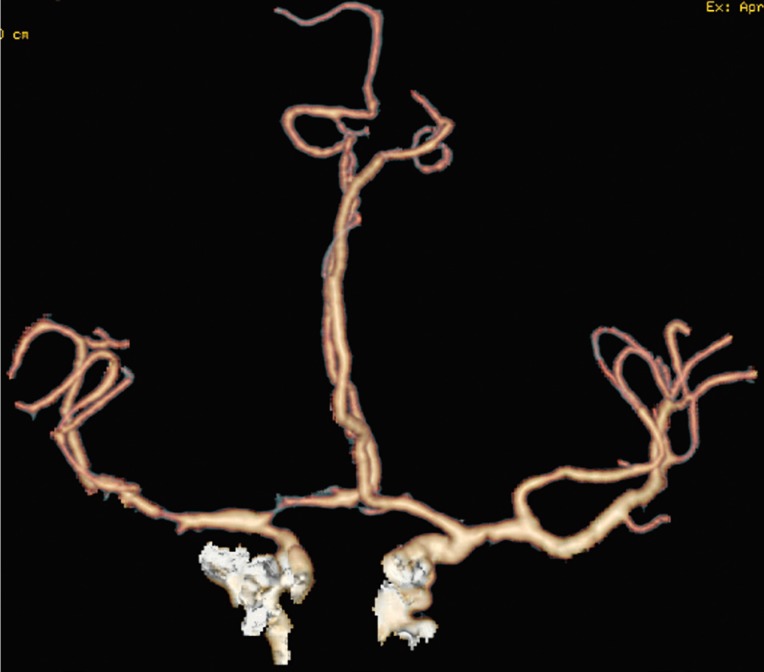
Figure 4.
Arterial angiographic CT study. Stenosis of right middle cerebral artery and narrowing of ipsilateral anterior cerebral artery.
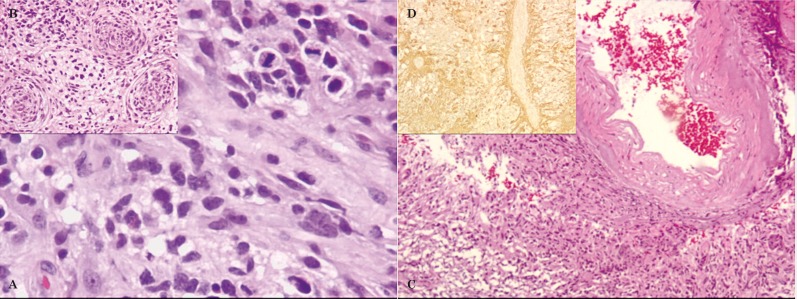
Figure 5.
Histopathology, after partial resection of right anterior temporal lobe (18th day) - glioblastoma multiforme (A-C: HE; D: GFAP). A) Highly cellular neoplasia with hyperchromatic pleomorphic nuclei and mitosis. B) Three tumoral vessels with prominent vascular wall proliferation. C) Tumor involving and infiltrating the leptomeningeal vessels. D) Tumor infiltrating an intracortical vessel.
The follow-up brain CT obtained four weeks after surgery revealed recurrence of right temporal glioblastoma, involution of lenticulocapsular stroke to chronicity and more prominent left insular parenchymal tumoral infiltration. At this point, only palliative treatment was implemented.
Discussion
The clinical presentation of a brain tumor as a sudden alteration of neurological status is frequently associated with intratumoral hemorrhage 8 or tumoral progression, and also commonly presents with seizure 1. Rarely the first clinical presentation is due to an acute cerebral infarct secondary to direct vascular infiltration by the adjacent tumor.
Glioblastoma is a malignant infiltrative glial cell tumor, grade IV in the World Health Organization classification 9, comprising about 16% of primary brain tumors 10 and frequently occurring in patients over 50 years 10. The histopathological study reveals densely compacted tumoral cells, typically with mitosis, vessels and necrosis. The immunohistochemical study shows strong reactivity to glial fibrillary acidic protein in malignant gliomas, with nearly 100% sensitivity as a glial differentiation marker 11. Only seldom does a brain tumor lead to direct occlusion or vascular dissection causing acute cerebral infarct in the corresponding arterial territory. In these rare cases the underlying pathophysiological mechanism is not completely clear, but various explanations include a procoagulant effect mediated by tumor-secreting factors, mechanical compression and/or tumoral cell infiltration causing occlusion or vascular dissection 1,2. Leptomeningeal dissemination giving rise to vasculopathy is also reported, with arterial vessels being encased by the tumor 12. Clinically, patients present with an acute vascular syndrome and imaging studies show an acute ischemic lesion and a distinct tumoral mass that usually arises in the same brain hemisphere.
Our patient had an acute ischemic infarct in the perforating branches of the right middle cerebral artery territory (lenticulostriate arteries), which were in close proximity to the anterior temporal lobe tumor. Brain MR with gadolinium disclosed enhancement of the right middle cerebral artery wall (Figure 3), and there are associated changes in the arterial angiographic CT study showing a focal stenosis of M1 segment of the middle cerebral artery (Figure 4). During surgery, arterial vessels with apparent endoluminal thrombus were encased by the tumoral mass, including the right MCA, and the histopathological findings confirmed tumoral cell infiltration of the wall of medium and small-sized arteries, as well as leptomeningeal vessels (Figure 5).
The cause of the ischemic stroke was in our case a thromboembolic effect of the tumoral cell infiltration of the vascular wall of MCA, and we do not exclude possible vascular compression by the tumoral mass. Leptomeningeal dissemination is also an assumed concurrent etiological mechanism as there is a focal superficial enhancement on the contralateral hemisphere (on the left insular region), also confirmed on the histopathological examination, and extension of glial tumors into the leptomeninges has been described before 2-4,11,12.
Chen et al. 3 described the most similar case to ours, but they did not show the arterial wall imaging on a T1-weighted image after gadolinium injection, which in our case may be correlated with the histopathological finding of arterial wall infiltration by tumoral cells. This same arterial wall imaging feature was not present in the cases reported by Obeid et al. 1 and Rojas-Marcos et al. 2, who show patients with glioblastoma multiforme who developed infarcts several months after biopsy or tumoral resection and implementation of adjuvant radiotherapy/chemotherapy. Hence, the etiology of the infarct was not explicitly related to the tumor, and radiotherapy is a main concurrent cause for arterial occlusion.
Aoki et al. 4 described a patient presenting with a right MCA infarct and a parenchymal tumor in the corresponding area of the infarct appearing after a three-month interval, suggesting the possibility of a malignant glial tumor growing in an infarcted area. There is a recognized association of gliosarcomas with infarcts, and it remains to be clarified if the tumoral cells first infiltrate the vessels causing infarct and then subsequent growth of tumor mass occurs 6.
The case reported by Hart et al. 5 disclosed vascular invasion by tumoral cells in an anatomopathological specimen but MR features were not shown.
We describe a case of glioblastoma whose initial manifestation was an acute ischemic syndrome, with imaging and histological proof of arterial wall invasion of the MCA and other smaller vessels, in a live patient, that would still fit the recommendation criteria for treatment with radiotherapy or chemotherapy, improving survival.
Our assumption of the acute cerebral infarct being secondary to glioblastoma in this patient is supported by the fact that vascular risk factors were under good medical control, there was no evidence of significant atherosclerosis in cervical and cerebral arteries, a cardioembolic source was lacking and the patient had not received previous radiotherapy.
The definite diagnosis is histopathological, but surgical macroscopic evaluation is also relevant. However, the neuroradiological studies (CT, arterial angiography and MR) disclosed the coexistence of distinct focal lesions, characterizing the enhancing patterns and giving the clue for suspicion of a tumoral infiltrative lesion underlying an acute cerebral infarct by direct influence on vascular structures in the vicinity. Imaging of the arterial wall with T1 post-gadolinium was an important resource.
Imaging follow-up after treatment provides information on the evolution to chronicity of the cerebral infarct, documenting the extension of tumoral resection, the progression or tumoral relapse and possible post-surgical complications, contributing to the management of survival expectations.
The treatment of choice is directed at the neoplastic process and the most common therapeutic approaches are surgical resection, radiotherapy and chemotherapy. In these patients there is no need for administration of recombinant human tissue-type plasminogen activator, as usually indicated for strokes within three hours of the onset of symptoms 13, as it may promote intratumoral hemorrhage.
The main concern of treatment in this case was directed to the leading cause of the symptoms, i.e., prompt treatment of the glioblastoma as soon as its diagnosis was recognized in a patient presenting with an acute ischemic syndrome.
The best treatment for glioblastomas is surgical resection of the tumor, combined with radiation therapy and chemotherapy that doubles the two-year survival rate of these patients, despite the overall prognosis remaining poor 14. Current recommendations for the treatment of glioblastoma multiforme in elderly people advise the use of hypofractionated radiotherapy or temozolomide only with deferred radiotherapy (balancing the risk-benefit of radiotherapy towards the clinical status of the patient) 15,16. The rarity of presentation of a glioblastoma as an acute ischemic stroke and the need for a histological examination led neurosurgeons in our case to opt for tumoral resection, delaying implementation of adjuvant therapy, ending in rapid tumor recurrence.
Another concern in this kind of patients is to be aware of the possibility of the malignant transformation of an infarct, thus requiring decompressive craniectomy. For this, short-interval sequential imaging in an acute and subacute phase is important.
In conclusion, although rare, cerebral tumors should be considered in the etiology of acute cerebral infarct. Our case has exceptional imaging characterization of the infarct, tumor and imaging features pointing towards the cause of infarct, such as the arterial wall enhancement. We emphasize the relevance of MR imaging characteristics in elucidating the pathophysiological mechanism of stroke and tumor spread. Imaging also plays a pivotal role in the differential diagnosis between stroke alone or coexistent stroke and tumor, allowing proper clinical management and an appropriate therapeutic approach.
References
- 1.Obeid M, Ulane C, Rosenfeld S. Pearls & Oy-sters: Large vessel ischemic stroke secondary to glioblastoma multiforme. Neurology. 2010;74(13):e50–51. doi: 10.1212/WNL.0b013e3181d7d66a. doi: 10.1212/WNL.0b013e3181d7d66a. [DOI] [PubMed] [Google Scholar]
- 2.Rojas-Marcos I, Martin-Duverneuil N, Laigle-Donadey F, et al. Ischemic stroke in patients with glioblastoma multiforme. J Neurol. 2005;252(4):488–489. doi: 10.1007/s00415-005-0665-7. doi: 10.1007/s00415-005-0665-7. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Cebula H, Schott R, et al. Glioblastoma multiforme presenting with ischemic stroke: case report and review of the literature. J Neuroradiol. 2011;38(5):304–307. doi: 10.1016/j.neurad.2011.01.008. doi: 10.1016/j.neurad.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Aoki N, Sakai T, Oikawa A, et al. Dissection of the middle cerebral artery caused by invasion of malignant glioma presenting as acute onset of hemiplegia. Acta Neurochir (Wien) 1999;141(9):1005–1008. doi: 10.1007/s007010050408. doi: 10.1007/s007010050408. [DOI] [PubMed] [Google Scholar]
- 5.Hart MN, Byer JA. Rupture of middle cerebral artery branches by invasive astrocytoma. Neurology. 1974;24:1171–1174. doi: 10.1212/wnl.24.12.1171. doi: 10.1212/WNL.24.12.1171. [DOI] [PubMed] [Google Scholar]
- 6.Züchner S, Kawohl W, Sellhaus B, et al. A case of gliosarcoma appearing as ischaemic stroke. J Neurol Neurosurg Psychiatry. 2003;74(3):364–366. doi: 10.1136/jnnp.74.3.364. doi: 10.1136/jnnp.74.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 8.Kondziolka D, Berstein M, Resh L, et al. Significance of hemorrhage into brain tumors: clinicopathological study. J Neurosurg. 1987;67(6):852–857. doi: 10.3171/jns.1987.67.6.0852. doi: 10.3171/jns.1987.67.6.0852. [DOI] [PubMed] [Google Scholar]
- 9.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolecek TA, Propp JM, Stroup NE, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. 2012;14(Suppl 5):v1–49. doi: 10.1093/neuonc/nos218. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brat DJ, Prayson RA, Ryken TC, et al. Diagnosis of malignant glioma: role of neuropathology. J Neurooncol. 2008;89(3):287–311. doi: 10.1007/s11060-008-9618-1. doi: 10.1007/s11060-008-9618-1. [DOI] [PubMed] [Google Scholar]
- 12.Herman C, Kupsky WJ, Rogers L, et al. Leptomeningeal dissemination of malignant glioma simulating cerebral vasculitis. Case report with angiographic and pathological studies. Stroke. 1995;26(12):2366–2370. doi: 10.1161/01.str.26.12.2366. doi: 10.1161/01.STR.26.12.2366. [DOI] [PubMed] [Google Scholar]
- 13.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 14.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–1850. doi: 10.1001/jama.2013.280319. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 15.Mason WP, Maestro RD, Eisenstat D, et al. Canadian recommendations for the treatment of glioblastoma multiforme. Curr Oncol. 2007;14(3):110–117. doi: 10.3747/co.2007.119. doi: 10.3747/co.2007.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chamberlain MC. Treatment of newly diagnosed malignant glioma in the elderly people: new trials that impact therapy. Int J Clin Pract. 2013;67(12):1225–1227. doi: 10.1111/ijcp.12258. doi: 10.1111/ijcp.122589. [DOI] [PubMed] [Google Scholar]