Summary
Angiographic balloon test occlusion (BTO) allows preoperative risk evaluation of patients undergoing permanent therapeutic occlusion of the internal carotid artery (ICA). The sensitivity of the BTO can be increased using different complementary techniques. Transcranial Doppler (TCD) stands out as a non-invasive, bedside method providing real-time monitoring of cerebral haemodynamics, therefore accurately identifying patients at risk of stroke. A case of a 30-year-old woman with a giant intracavernous aneurysm of the left ICA presenting with subacute left VI nerve palsy is described. A pre-operative TCD- and EEG-monitored BTO of the left ICA was performed. The 16.7% drop found in the middle cerebral artery's peak systolic velocity (PSVMCA) predicts clinical and haemodynamic tolerance to the permanent loss of that vessel. This case illustrates the potential of TCD monitoring during temporary BTO of the ICA. It highlights its ability to provide a complete preclinical evaluation of collateralization and autoregulatory adaptation to unilateral ICA occlusion. TCD may also decrease the time of occlusion required for the BTO.
Keywords: balloon test occlusion, giant aneurysm, internal carotid, transcranial Doppler ultrasonography
Introduction
Balloon test occlusion (BTO) is a pre-operative angiographic test used to estimate the risk of stroke after permanent therapeutic occlusion of an internal carotid artery (ICA) involved by neck or skull base tumours and inoperable fistulae or aneurysms 1-3. BTO decreases the risk of post-operative ischaemic stroke from up to 30% in unselected patients 1 to less than 13% in patients passing this test 4,5. Stroke after ICA occlusion can occur by two mechanisms: haemodynamic compromise (predictable by BTO), or thromboembolization after vessel ligation 6.
BTO was first described in the early 1970s 7, and initially only symptomatic patients or those developing neurological deficits during the test were considered at risk of haemodynamic infarction, and therefore candidates for extracranial-intracranial bypass revascularization 6,8,9. Since clinical monitoring alone has a low sensitivity, different complementary techniques have been used to increase prediction accuracy 1,10-16. Among these, transcranial Doppler (TCD) stands out as a non-invasive bedside method providing real-time monitoring of cerebral haemodynamics that can accurately identify patients at risk of delayed ischaemic sequelae 2,17-19.
Despite the advantages of TCD and the absence of a universally accepted method to monitor the BTO, its potential is not yet widely recognized.
Case Report
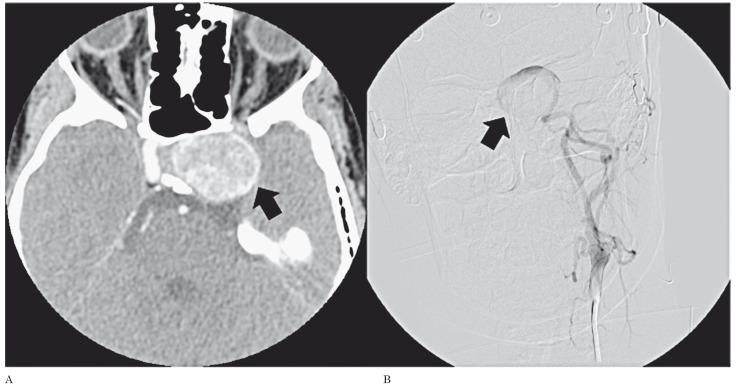
A 30-year-old Caucasian woman presenting with subacute left VI nerve palsy is described. The patient had no relevant medical history and no previous episode of diplopia. The CT scan (Figure 1A) showed a large enhancing mass lesion inside the left cavernous sinus, in close relation to the ICA. Diagnostic digital subtraction angiography (DSA) (Figure 1B) established the definite diagnosis of a giant intra-cavernous aneurysm of the left ICA. As a potential candidate for surgical therapeutic occlusion of the diseased vessel, the patient was referred to the interventional neuroradiology department for risk assessment. A pre-operative TCD- and EEG-monitored BTO of the left ICA was performed.
Figure 1.
Giant aneurysm of the left internal carotid artery, involving its intracavernous segment (arrows). A) Contrast-enhanced CT. B) Digital subtraction angiography.
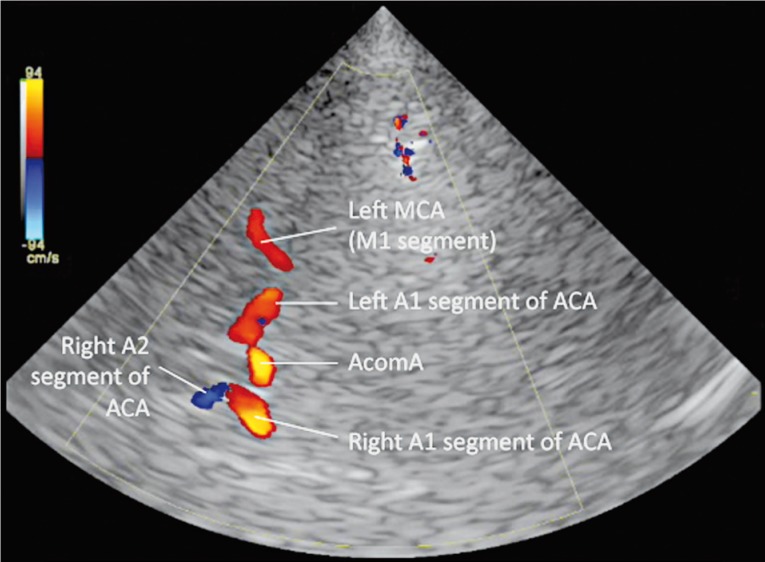
Baseline transcranial color-coded Doppler (TCCD) evaluation revealed blood flow inside the aneurysm (confirming it was not excluded from the circulation), an anterograde left ophthalmic artery and an otherwise normal intracranial arterial and venous circulation. A 6F ENVOY® Guiding Catheter (DePuy Orthopaedics Inc., Raynham, MA, USA) was used to position a 4×20 mm HyperGlide™ Occlusion Balloon System (ev3 Endovascular Inc., Plymouth, MN, USA) just below the aneurysmal segment of the left ICA. Upon balloon inflation, adequate vessel occlusion was angiographically and sonographically documented. During the occlusive period (a total of 30 min), TCCD revealed collateralization through the anterior communicating artery (but not through the ophthalmic artery or the posterior communicating artery), maintaining adequate blood flow to the left middle cerebral artery (MCA) (Figure 2).
Figure 2.
TCD evaluation during ICA occlusion shows collateralization through the anterior communicating artery (AcomA). Note the absence of a posterior communicating artery contribution. ACA - anterior cerebral artery; MCA - middle cerebral artery.
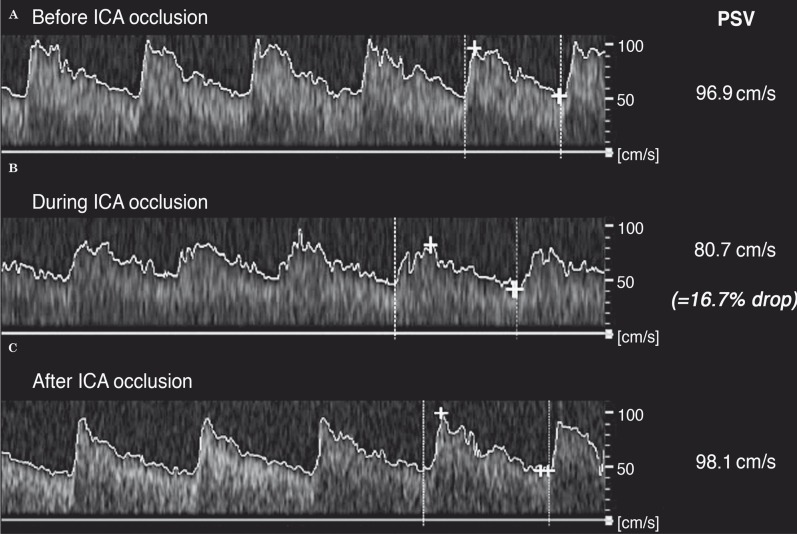
The baseline left MCA’s peak systolic velocity (PSVMCA) measured by TCD was 96.9 cm/s, dropping to a minimum of 80.7 cm/s during the occlusion of the ICA, and quickly returning to 91.8 cm/s after balloon deflation (Figure 3). The patient remained asymptomatic throughout the BTO, and no electroencephalographic changes were recorded. The 16.7% drop in the PSVMCA documented predicts clinical and haemodynamic tolerance to the permanent loss of that vessel. To date, the patient is waiting for neurosurgical intervention.
Figure 3.
Angle corrected left middle cerebral artery flow variation registered by transcranial Doppler during the procedure. A) Before left ICA occlusion. B) During ICA occlusion. C) After balloon deflation. Note the documented 16.7% decrease of the peak systolic velocity of the middle cerebral artery (PSV), inferior to the 30% threshold.
Discussion
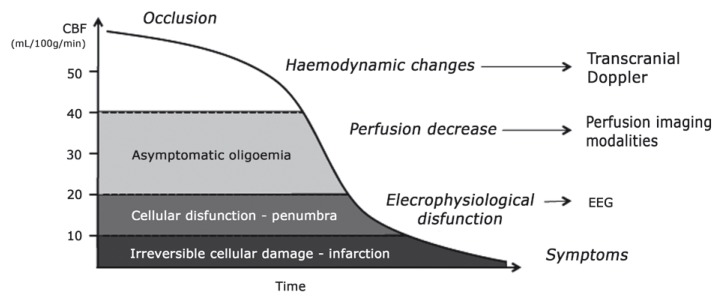
Transcranial Doppler ultrasound (TCD) is an accurate and widely used complementary examination for neurovascular study 20,21. It has been validated for monitoring endovascular carotid balloon occlusion tests for almost 20 years 22 with good results 2,17,19. TCD is characterized by favourable logistics 19, is able to show early events in the ischaemic cascade (Figure 4), allowing the diagnosis of haemodynamic intolerance prior to symptomatic manifestations, and to provide real-time evaluation of the entire intracranial circulation 23. It is also an excellent technique for follow-up 2. Its main limitation is the inadequate temporal bone acoustic window in some patients, which renders TCD impossible to perform 24.
Figure 4.
Events following occlusion of a major cerebral artery, according to the decrease in brain tissue perfusion. Note how haemodynamic disturbances are immediate and can be detected with transcranial Doppler.
The published series report a decrease in PSVMCA < 30% relative to baseline during the occlusive period as a consistent predictive value of clinical tolerance 2,17-19, with no false negatives. The PSVMCA is considered an estimate of regional blood flow 25, specifically during the first seconds after balloon inflation, while MCA diameter and territory are constant and before cerebral autoregulation takes place 2,25,26. A PSVMCA reduction > 40% is thought to correspond to a tissue perfusion of approximately 30 ml/100 g/min, a threshold to symptom occurrence in quantitative CBF studies 4,16,19. To date, no patients with predicted tolerance to ICA occlusion by TCD-monitored BTO (PSVMCA drop < 30%) have suffered haemodynamic infarction after definite occlusion 2,17-19,27. However, the risk of thromboembolism after arterial occlusion warrants antithrombotic prophylaxis 6. Some authors also advocate the use of minimal occlusion times by implementing TCD monitoring 2,18 with the BTO. Since the haemodynamic response to ICA occlusion is immediate and can be evaluated by TCD, the classic extended occlusion times required for clinical symptoms to develop may no longer be necessary. In fact, Sorteberg et al. 18 reported 86% sensitivity and 93% specificity (positive predictive value of 86%) of TCD analysis of the first seconds after carotid occlusion. With shorter occlusion times, procedure safety and patient comfort could be improved 2.
The present case illustrates how TCD is able to document the major mechanisms of adaptation and clinical tolerance to ICA occlusion: collateralization and cerebral vascular autoregulation 17. In our case, TCD also demonstrated the anterograde blood flow in the ophthalmic artery that no other complementary method was able to verify, as well as confirming the intra-aneurysmal blood flow shown by DSA. Together with the baseline evaluation of the intracranial circulation, and the direct visualization of collateralization during the procedure, these data improved risk stratification.
TCD is a convenient reliable method to monitor angiographic BTO of the ICA. Its use increases the procedure’s sensitivity, allowing a preclinical diagnosis of haemodynamic intolerance and therefore shorter occlusion times.
References
- 1.American Society of Interventional and Therapeutic Neuroradiology Carotid artery balloon test occlusion. Am J Neuroradiol. 2001;22(8 Suppl):S8–S9. [PMC free article] [PubMed] [Google Scholar]
- 2.Sorteberg A, Bakke SJ, Boysen M, et al. Angiographic balloon test occlusion and therapeutic sacrifice of major arteries to the brain. Neurosurgery. 2008;6(4):651–660. doi: 10.1227/01.NEU.0000325727.51405.D5. discussion 660-661. doi: 10.1227/01.NEU.0000325727.51405.D5. [DOI] [PubMed] [Google Scholar]
- 3.Mathis JM, Barr JD, Jungreis CA, et al. Temporary balloon test occlusion of the internal carotid artery: experience in 500 cases. Am J Neuroradiol. 1995;16(4):749–754. [PMC free article] [PubMed] [Google Scholar]
- 4.Linskey ME, Jungreis CA, Yonas H, et al. Stroke risk after abrupt internal carotid artery sacrifice: accuracy of preoperative assessment with balloon test occlusion and stable xenon-enhanced CT. Am J Neuroradiol. 1994;15(5):829–843. [PMC free article] [PubMed] [Google Scholar]
- 5.Larson JJ, Tew JM, Jr, Tomsick TA, et al. Treatment of aneurysms of the internal carotid artery by intravascular balloon occlusion: long-term follow-up of 58 patients. Neurosurgery. 1995;36(1):26–30. discussion 30. doi: 10.1227/00006123-199501000-00002. [PubMed] [Google Scholar]
- 6.Field M, Jungreis CA, Chengelis N, et al. Symptomatic cavernous sinus aneurysms: management and outcome after carotid occlusion and selective cerebral revascularization. Am J Neuroradiol. 2003;24(6):1200–1207. [PMC free article] [PubMed] [Google Scholar]
- 7.Serbinenko FA. Balloon catheterization and occlusion of major cerebral vessels. J Neurosurg. 1974;41(2):125–145. doi: 10.3171/jns.1974.41.2.0125. doi: 10.3171/jns.1974.41.2.0125. [DOI] [PubMed] [Google Scholar]
- 8.O’Shaughnessy BA, Salehi SA, Mindea SA, et al. Selective cerebral revascularization as an adjunct in the treatment of giant anterior circulation aneurysms. Neurosurg Focus. 2003;14(3):e4. doi: 10.3171/foc.2003.14.3.5. doi: 10.3171/foc.2003.14.3.5. [DOI] [PubMed] [Google Scholar]
- 9.Carter BS, Ogilvy CS, Putman C, et al. Selective use of extracranial-intracranial bypass as an adjunct to therapeutic internal carotid artery occlusion. Clin Neurosurg. 2000;46:351–362. [PubMed] [Google Scholar]
- 10.Monsein LH, Jeffery PJ, van Heerden BB, et al. Assessing adequacy of collateral circulation during balloon test occlusion of the internal carotid artery with 99mTc-HMPAO SPECT. Am J Neuroradiol. 1991;12(6):1045–1051. [PMC free article] [PubMed] [Google Scholar]
- 11.Morioka T, Matsushima T, Fujii K, et al. Balloon test occlusion of the internal carotid artery with monitoring of compressed spectral arrays (CSAs) of electroencephalogram. Acta Neurochir (Wien) 1989;101(1-2):29–34. doi: 10.1007/BF01410065. doi: 10.1007/BF01410065. [DOI] [PubMed] [Google Scholar]
- 12.Murphy KJ, Payne T, Jamadar DA, et al. Correlation of continuous EEG monitoring with [O-l5]H2O positron emission tomography determination of cerebral blood flow during balloon test occlusion of the internal carotid artery. Experience in 34 cases. Interv Neuroradiol. 1998;4(1):51–55. doi: 10.1177/159101999800400106. [DOI] [PubMed] [Google Scholar]
- 13.Sato K, Shimizu H, Inoue T, et al. Angiographic circulation time and cerebral blood flow during balloon test occlusion of the internal carotid artery. J Cereb Blood Flow Metab. 2014;34(1):136–143. doi: 10.1038/jcbfm.2013.176. doi: 10.1038/jcbfm.2013.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segal DH, Sen C, Bederson JB, et al. Predictive value of balloon test occlusion of the internal carotid artery. Skull Base Surg. 1995;5(2):97–107. doi: 10.1055/s-2008-1058940. doi: 10.1055/s-2008-1058940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Struffert T, Deuerling-Zheng Y, Engelhorn T, et al. Monitoring of balloon test occlusion of the internal carotid artery by parametric color coding and perfusion imaging within the angio suite: first results. Clin Neuroradiol. 2013;23(4):285–892. doi: 10.1007/s00062-013-0208-z. doi: 10.1007/s00062-013-0208-z". [DOI] [PubMed] [Google Scholar]
- 16.Witt JP, Yonas H, Jungreis C, et al. Cerebral blood flow response pattern during balloon test occlusion of the internal carotid artery. Am J Neuroradiol. 1994;15(5):847–856. [PMC free article] [PubMed] [Google Scholar]
- 17.Schneweis S, Urbach H, Solymosi L, et al. Preoperative risk assessment for carotid occlusion by transcranial Doppler ultrasound. J Neurol Neurosurg Psychiatry. 1997;62(5):485–489. doi: 10.1136/jnnp.62.5.485. doi: 10.1136/jnnp.62.5.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorteberg A, Sorteberg W, Bakke SJ, et al. Cerebral haemodynamics in internal carotid artery trial occlusion. Acta Neurochir (Wien) 1997;139(11):1066–1073. doi: 10.1007/BF01411562. doi: 10.1007/BF01411562. [DOI] [PubMed] [Google Scholar]
- 19.Eckert B, Thie A, Carvajal M, et al. Predicting hemodynamic ischemia by transcranial Doppler monitoring during therapeutic balloon occlusion of the internal carotid artery. Am J Neuroradiol. 1988;19(3):577–582. [PMC free article] [PubMed] [Google Scholar]
- 20.Newell DW, Aaslid R. Transcranial Doppler: clinical and experimental uses. Cerebrovasc Brain Metab Rev. 1992;4(2):122–143. [PubMed] [Google Scholar]
- 21.Baumgartner RW. Transcranial color duplex sonography in cerebrovascular disease: a systematic review. Cerebrovasc Dis. 2003;16(1):4–13. doi: 10.1159/000070108. doi: 10.1159/000070108. [DOI] [PubMed] [Google Scholar]
- 22.Giller CA, Mathews D, Walker B, et al. Prediction of tolerance to carotid artery occlusion using transcranial Doppler ultrasound. J Neurosurg. 1994;81(1):15–19. doi: 10.3171/jns.1994.81.1.0015. doi: 10.3171/jns.1994.81.1.0015. [DOI] [PubMed] [Google Scholar]
- 23.Sorteberg A, Sorteberg W, Lindegaard KF, et al. Cerebral haemodynamic considerations in obstructive carotid artery disease. Acta Neurochir (Wien) 1996;138(1):68–75. doi: 10.1007/BF01411727. discussion 75-76. doi: 10.1007/BF01411727. [DOI] [PubMed] [Google Scholar]
- 24.Krejza J, Swiat M, Pawlak MA, et al. Suitability of temporal bone acoustic window: conventional TCD versus transcranial color-coded duplex sonography. J Neuroimaging. 2007;17(4):311–314. doi: 10.1111/j.1552-6569.2007.00117.x. doi: 10.1111/j.1552-6569.2007.00117.x. [DOI] [PubMed] [Google Scholar]
- 25.Kofke WA, Brauer P, Policare R, et al. Middle cerebral artery blood flow velocity and stable xenon-enhanced computed tomographic blood flow during balloon test occlusion of the internal carotid artery. Stroke. 1995;26(9):1603–1606. doi: 10.1161/01.str.26.9.1603. doi: 10.1161/01.STR.26.9.1603. [DOI] [PubMed] [Google Scholar]
- 26.Vazquez Añon V, Aymard A, Gobin YP, et al. Balloon occlusion of the internal carotid artery in 40 cases of giant intracavernous aneurysm: technical aspects, cerebral monitoring, and results. Neuroradiology. 1992;34(3):245–251. doi: 10.1007/BF00596347. doi: 10.1007/BF00596347. [DOI] [PubMed] [Google Scholar]
- 27.Sorteberg W, Sorteberg A, Lindegaard KF, et al. Transcranial Doppler ultrasonography-guided management of internal carotid artery closure. Neurosurgery. 1999;45(1):76–87 discussion 87-88. doi: 10.1097/00006123-199907000-00019. doi: 10.1097/00006123-199907000-00019. [DOI] [PubMed] [Google Scholar]