Summary
The objective of this study was to determine long-term outcomes after stent placement for subclavian artery (SA) obstructive lesions assisted by intraoperative intravascular ultrasound (IVUS). The study included 25 lesions in 24 patients who underwent stent placement assisted by intraoperative IVUS for subclavian artery stenosis or obstruction at our hospital between January 2003 and August 2010. Outcome was evaluated based on the results within 30 postoperative days (technical success rate, improvement in upper extremity ischemia, steal syndrome, left-right blood pressure difference, and perioperative complications) and the results after 30 postoperative days (incidence of vertebrobasilar artery territory infarction and restenosis). Stent placement and vessel dilatation were successful in all patients, without perioperative complications. Upper extremity ischemia, steal syndrome, and left-right blood pressure difference disappeared in all cases. During follow-up observation (6-96 months; median 51 months), no restenosis occurred at the stent placement site in any patient. In one case, four years after initial treatment, stenosis was noted proximal to the stent placement site. Satisfactory long-term as well as short-term outcomes were achieved after stent placement for SA obstructive lesions assisted by intraoperative IVUS evaluation.
Keywords: intravascular ultrasound, atherosclerotic subclavian artery obstructive lesions, stent placement
Introduction
Percutaneous revascularization for subclavian artery (SA) stenosis and obstruction can be performed under local anesthesia and has fewer complications than more highly invasive open surgery1,2. Therefore, percutaneous revascularization has assumed a role in the early treatment of SA lesions.
Since the 1990s, with advances in devices and techniques, recanalization by stent placement can be performed not only for stenosis, but also for complete obstruction of the SA2. Intravascular ultrasound (IVUS) provides greater understanding of blood flow, vessel lumen and media size, and the interface between the vessel wall and the blood stream. Using IVUS has improved our understanding of the arterial disease to be treated and assessment of the completion of treatment. Although feasible early outcomes have already been reported for percutaneous revascularization in SA, there are few reports on long-term outcomes: our literature search failed to disclose any reports of evaluation and revascularization of SA lesions using intraoperative IVUS assistance.
The purpose of the current study was to investigate the short and long-term treatment outcomes of the cases in which stent placement for SA stenosis was undertaken with the assistance of intraoperative IVUS evaluation.
Methods
This study included 24 patients (25 lesions) who underwent stent placement for SA stenosis or obstruction using intraoperative IVUS evaluation at our hospital between January 2003 and August 2010. The indications for treatment were symptomatic lesions or ≥70% stenosis. There were 20 men and four women, ranging in age from 56 to 80 years (median, 69 years). Twenty-one lesions were stenotic (stenosis 70-99%; mean, 82%), and four lesions were completely obstructed. Eleven lesions were symptomatic (seven upper extremity ischemia, two subclavian steal syndrome, one stroke, one transient ischemic attack), and 14 lesions caused only mild arm claudication. The preoperative blood pressure difference was 15-30 mmHg (mean, 21 mmHg).
Patients received two oral antiplatelet drugs (aspirin 100 mg/day; and clopidogrel 75 mg/day or cilostazol 200 mg/day or ticlopidine 200 mg/day) for at least three days before treatment. The endovascular procedure was performed under local anesthesia in all patients, with systemic heparinization to keep the activated coagulation time at two to three times normal. A femoral artery approach and/or brachial artery approach was used (femoral artery approach alone in 18 lesions and combined with the brachial artery approach in six lesions). The procedure was performed with the brachial artery approach alone in one case due to severe aortic tortuosity and bending. In the femoral artery approach, a pull-through technique3 was used in two cases. In the femoral artery approach, a standard 9-Fr guiding catheter was used, and in the brachial artery approach, a 6-Fr guiding sheath was used. Distal protection of vertebral artery (VA) was used in five cases, due to the ipsilateral dominant VA, since the protection devices were available. The VA was protected with a balloon catheter; using Hyperglide (ev3 Neurovascular, Irvine, CA, USA) in four cases and Equinox (MTI, Irvine, CA, USA) in one. Post-dilation was performed using a PTA balloon with a diameter not exceeding the normal luminal diameter just distal to the stenosis, as measured by IVUS.
After placement of the guiding catheter at the origin of the SA, IVUS was performed to assess lesion extent, configuration, degree of calcification (defined as none, no calcification; mild, <1/3 circumference; moderate, 1/3 to 2/3 circumference; and severe, >2/3 circumference), site of stenosis, and preoperative vessel diameter correctly. Subsequently, predilation with a 3.0 mm diameter balloon was performed, followed by stent placement. We used the information from IVUS for stent selection as follows. When stenosis involved the SA origin, we used balloon-expandable stent Palmaz stent (Cordis Endovascular, Miami Lakes, FL, USA) in 17 lesions and an Express stent (Boston Scientific, Natick, MA, USA) in two lesions. When the stenosis was ≥1 cm from the SA origin, a self-expandable stent (Smart Control (Cordis Endovascular) (four lesions); Luminex stent (Bard, Murray Hill, NJ, USA) (one lesion); or Easy Wallstent (Boston Scientific) (one lesion) was used. Regarding selection of stent diameter and length for placement, the stent diameter (fully open) was ≥1 mm larger than the internal diameter of the normal proximal SA, and the stent length was sufficient for the stenosis to be covered by the stent.
We evaluated the incidence of perioperative complications (symptomatic/asymptomatic infarction). Short-term outcome were evaluated on the results within 30 postoperative days, including the technical success rate (defined as ≤30% residual stenosis), degree of calcification on IVUS (defined as none, no calcification; mild, <1/3 circumference; moderate, 1/3 to 2/3 circumference; and severe, >2/3 circumference). Rate of improvement in upper extremity ischemia (diagnosed when the patients complained of disabling exertional arm discomfort), steal syndrome (diagnosed by retrograde vertebral artery blood flow on digital subtraction angiography), and left-right BP difference (defined as BP difference of >20 mmHg between the two arms and improvement defined as BP difference of ≤10 mmHg) were also evaluated. Long-term outcome were evaluated on the results after 30 postoperative days, including recurrence of symptoms during clinical follow-up, incidence of infarction on imaging follow-up, and incidence of restenosis (defined as stenosis ≥70%).
Two antiplatelet drugs were continued for at least one month after treatment. Within 12 months of treatment, imaging by computed tomography angiography (CTA) or digital subtraction angiography (DSA) was performed. Thereafter, patients were followed with periodic imaging.
Results
Short-term outcome (within 30 postoperative days)
There were no perioperative complications. Stent placement and vessel dilatation were successful in all patients. Residual stenosis was 0-10% (mean, 2.6%) on DSA and 5-19% (mean, 8.6%) on IVUS (Table 1). The degree of calcification on IVUS was: none, 1 (4%); mild, 23 (92%); moderate, 1(4%); and severe, 0 (0%) (Table 2). In all 24 patients who had been symptomatic, upper extremity symptoms and the steal syndrome, which had been present preoperatively, disappeared. In all patients, the left-right (arm) BP difference also improved (≤10 mmHg).
Table 1.
Lesion characteristics on IVUS and stent device
| Case |
Lesion characteristics |
Pre-MLD (short axis) (mm) |
Stent |
Post-MLD (short axis) (mm) |
Residual stenosis (%) |
| 1 | Obstructive | 0 | Palmaz | 5.5 | 10 |
| 2 | Regular | 2 | Palmaz | 7 | 5 |
| 3 | Regular | 2.8 | Palmaz | 6.1 | 5 |
| 4 | Irregular | 2.6 | EasyWall | 5.9 | 10 |
| 5 | Irregular | 3.1 | Palmaz | 5.8 | 15 |
| 6 | Irregular | 3.3 | Palmaz | 6.6 | 14 |
| 7 | Irregular | 3.4 | Luminex | 4.6 | 5 |
| 8 | Regular | 3.2 | Palmaz | 7.4 | 7 |
| 9 | Irregular | 1.8 | Palmaz | 7.7 | 5 |
| 10 | Irregular | 1.6 | Palmaz | 5.7 | 5 |
| 11 | Irregular | 3.7 | Palmaz | 7.8 | 10 |
| 12 | Regular | 2.3 | Palmaz | 7.1 | 10 |
| 13 | Irregular | 2.2 | SmartControl | 6.7 | 3 |
| 14 | Regular | 3.4 | Palmaz | 6.8 | 5 |
| 15 | Obstructive | 0 | SmartControl | 5.4 | 10 |
| 16 | Irregular | 0.8 | SmartControl | 7.8 | 7 |
| 17 | Irregular | 1.8 | Express | 5.2 | 17 |
| 18 | Irregular | 2 | Palmaz | 8.4 | 0 |
| 19 | Regular | 1.6 | Palmaz | 5.6 | 19 |
| 20 | Irregular | 1.8 | Palmaz | 3.5 | 14 |
| 21 | Regular | 5.4 | Palmaz | 7.7 | 7 |
| 22 | Regular | 2 | Palmaz | 7.7 | 5 |
| 23 | Regular | 0.7 | Express | 6.7 | 10 |
| 24 | Obstructive | 0 | Palmaz | 7 | 0 |
| 25 | Obstructive | 0 | SmartControl | 4.7 | 7 |
| MLD: minimum lumen diameter | |||||
Table 2.
Distribution of target lesion calcification on IVUS
| None (n, %) | 1 (4) |
| Little (n, %) | 23 (92) |
| Moderate (n, %) | 1 (4) |
| Severe (n, %) | 0 (0) |
| None, no calcification; mild, <1/3 circumference; moderate, 1/3-2/3 circumference; severe, >2/3 circumference | |
Long-term outcome (after 30 postoperative days)
During follow-up observation (6-96 months; median, 51 months), no recurrence of symptoms or no symptomatic/asymptomatic infarctions occurred. During imaging follow-up (CTA 17, DSA 8; 12-80 months; median, 24 months), no restenosis at the stent placement site was observed in any patient (Figures 1 and 2). In one case (5%), four years after initial treatment, stenosis was noted proximal to the stent placement site. The degree of calcification of this restenosis case on IVUS at the initial treatment was moderate. Retreatment (PTA and stent placement) was performed, and the stenosis disappeared (Figure 3).
Figure 1.
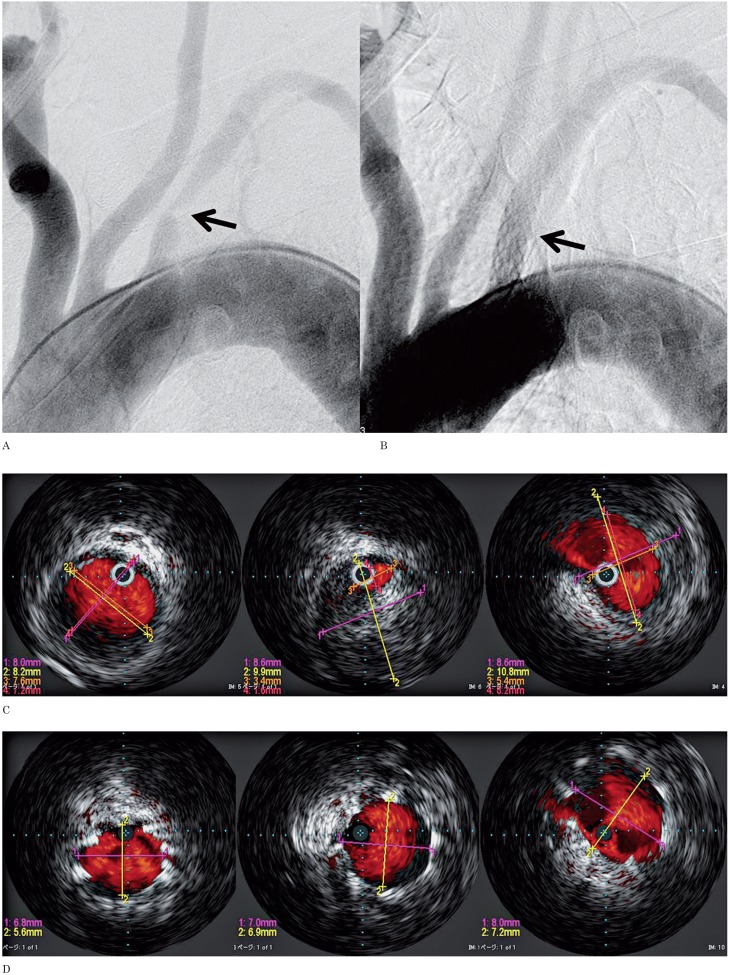
A 56-year-old man with arm ischemia. A) Digital subtraction angiography (DSA) depicts a high grade stenosis at the orifice of left subclavian artery (arrow). B) DSA after placement of a Palmaz stent depicts elimination of the stenosis (arrow). C) The luminal diameter of the site of stenosis and areas proximal and distal to the lesion measured by IVUS. D) After stent placement, IVUS shows the stent to be placed to cover the plaque at the lesion sufficiently. E) CTA after 1 year shows good patency (arrow).
Figure 2.
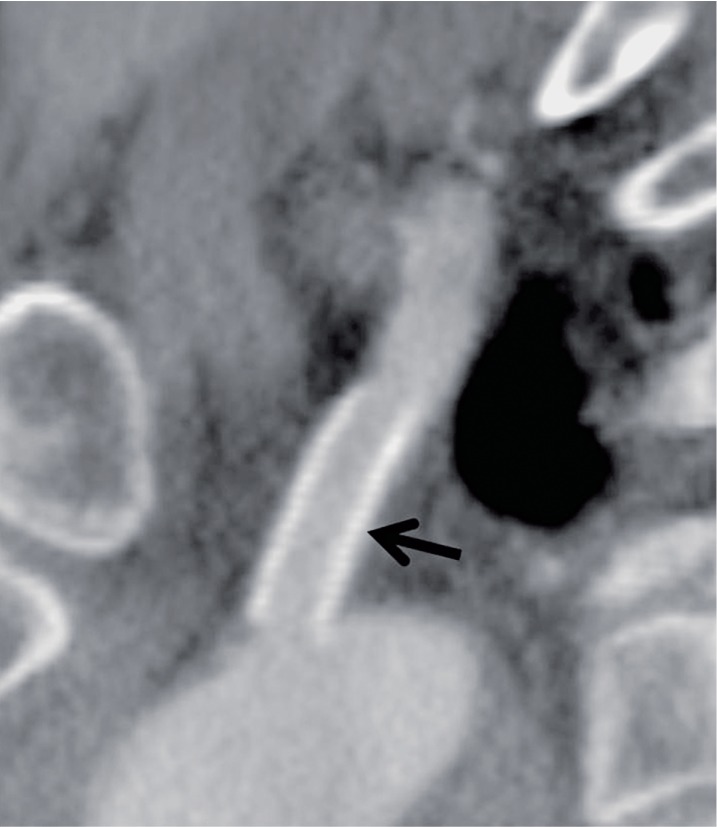
A 67-year-old woman with developing arm ischemia. A,B) DSA depicts total occlusion at the orifice of left subclavian artery (arrow). C) Post-DSA reveals an adequate result after balloon angioplasty and deployment of a Palmaz stent (arrow). D,E) CTA after 3 months shows good patency (arrow).
Figure 3.
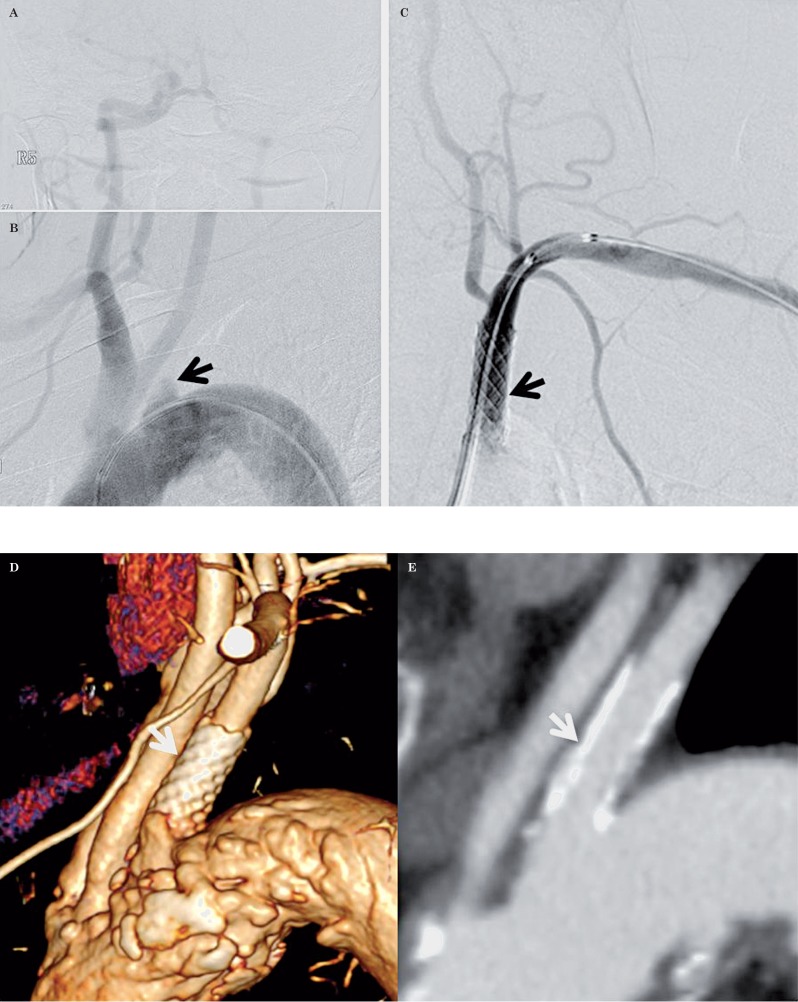
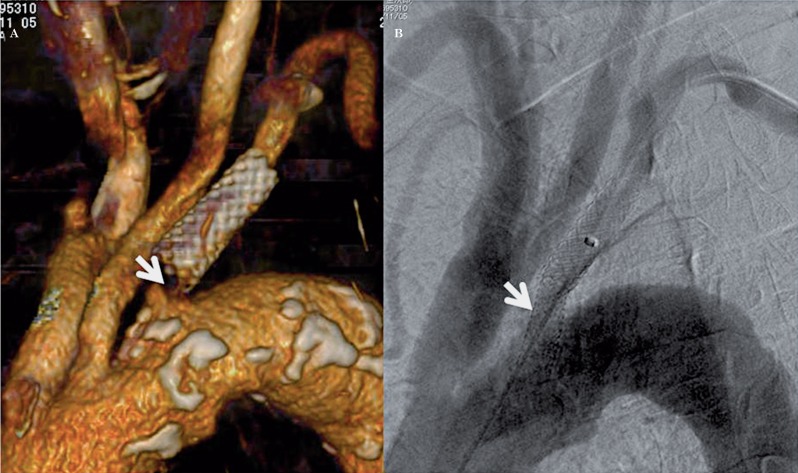
An 80-year-old man performed stent placement for left subclavian artery stenosis. Four years after the first procedure, the stenosis progressed to the ipsilateral subclavian artery orifice. A) CTA shows high grade stenosis proximal to the first stent (arrow). B) Aortic arch DSA after placement of an Express stent depicts elimination of the stenosis (arrow).
Discussion
Some studies on surgical treatment and outcomes in subclavian artery stenosis reported a perioperative mortality rate of 0-1.8%, ischemic complication rate of 0-8%, and a five-year primary patency rate of 83-95%4-8. With surgical treatment, although the incidence is low, perioperative complications and mortality, as well as symptom recurrence, have been reported.
Although PTA is currently the treatment of the choice for subclavian artery (SA) stenosis or occlusion, there are still some complications related to the procedure. Of 107 cases (108 lesions) reported by Sixt et al.9, the technical success rate was 96% (100% for stenosis (78/78) and 87% for total occlusions (26/30)), treatment modalities included PTA alone (13%) or stenting (87%), and the mortality rate and morbidity rate were both 0%. Patel et al.10, in a study of 170 cases (177 lesions), reported a technical success rate of 98.3% (99.4% for stenosis (155/156) and 90.5% for occlusions (26/30)), all underwent primary stent placement, and stroke in one patient (0.6%, not described about distal protection of vertebral artery). Thus, technical success rates were high, and perioperative complication rates were low. In the 25 lesions, the technical success rate was 100%, morbidity was 0%, with no ischemic complications in the current study, and mortality was 0%. Therefore, regarding early outcomes, the present results are similar or better than those reported to date. And in the current study, distal protection of the vertebral artery (VA) with a balloon catheter was performed in five cases via the ipsilateral brachial artery route due to the ipsilateral dominant VA. So this better result might be provided by these cases being included.
Of 39 cases followed up after endovascular treatment and after mid- and long-term outcomes, Brountzos et al.2 reported that 10.2% required retreatment for restenosis during the follow-up period. Patel et al.10, of 151 cases followed up, reported that during a mean observation period of 35.2 months, 14.6% required retreatment. Sixt et al., of 97 cases followed up, reported that during a mean observation period of 29 months 12% required retreatment. The primary patency rate was 88% after 12 months. Thus, restenosis and retreatment rates were about 10%. Of the present cases, in which IVUS assistance was applied, none had restenosis. In one case (4%), four years after initial treatment, 85% stenosis proximal to the stent placement site was noted. For this stenosis, PTA and stent placement were performed, and the stenosis disappeared. During subsequent follow-up (20 months), restenosis had not occurred. Therefore, after treatment, follow-up observation is necessary not only at the stent placement site, but also at the vessel proximally and distally.
Amor et al.11 compared direct stenting (22 cases) of lesions with mild calcification and a relatively preserved lumen and stent placement after predilation (54 cases) for obstruction or stenosis associated with severe calcification and tortuosity. Restenosis with direct stenting occurred in one case (4.76%), whereas with stent placement after predilation, restenosis occurred in 14 cases (28.5%). They assumed that sufficient dilatation was difficult to achieve in the lesions with severe calcification, making restenosis more likely in such lesions. Motarjeme et al.12, in a study of 13 cases of complete obstruction, reported a technical success rate of 46% and a very high restenosis rate of 50% after one year, whereas among cases where good dilation was achieved immediately after dilation, there was no restenosis. On the other hand, de Vries et al.4, of 102 cases that included 20 complete obstructions, reported that during a mean follow-up period of 34 months, there was significant restenosis (≥70%) in eight cases (7.8%).
However, for obstructed lesions, no significant restenosis was observed, so they stated that the factors causing restenosis were unknown. In our literature search, no reports of evaluation and revascularization of SA lesions using intraoperative IVUS assistance were found.
In the cases of the current study, including 21 stenotic lesions and four obstructed lesions, stent placement was performed after predilation, and restenosis at the stent placement site did not occur in any case during follow-up observation. Among all stenotic lesions and obstructed lesions, severe calcific stenosis, which would make stent placement difficult, was not observed by IVUS in our cases. In all cases, the site of stenosis and areas proximal and distal to the lesion were assessed by IVUS, and a stent was placed to cover the plaque sufficiently at the stenotic site. The degree of dilation after stent placement was assessed not only by DSA, but also by IVUS. Residual stenosis was 0-10% (mean, 2.6%) on DSA and 5-19% (mean, 8.6%) on IVUS. Good dilation was confirmed in all cases.
We believe that one reason for the absence of restenosis in any of the present cases was that the entire stenotic site could be sufficiently covered by stent placement, and good dilation could be confirmed using intraoperative IVUS. The absence of lesions with severe calcification, which make stent placement difficult, may be one of the reasons for the low restenosis rate in our population. If more patients who had obstructive lesions with moderate or severe calcification were enrolled, the outcomes might be different from the present results.
Guidelines published by the American Heart Association (AHA) in 201113 generally recommend conservative treatment for SA obstructive lesions and state that the indications for a revascularization procedure should be carefully considered. However, in patients with severe upper extremity ischemia, subclavian steal syndrome, or vertebrobasilar artery ischemia, and when blood supply from the collateral circulation cannot be expected, revascularization should be considered to improve ischemic symptoms and prevent stroke. This study includes asymptomatic cases treated before AHA guidelines were published. Although this study showed no perioperative complications and good long-term outcomes, indication for stent placement in asymptomatic SA stenosis should be considered carefully.
There are a number of limitations in this study. 1) This study was retrospective with a non-randomized design and a rather small sample size.
2) This study did not make a comparison between using and not using the IVUS technique.
3) This study was performed in a single center and all procedures were performed by the same experienced interventional team. Despite its limitations, the effectiveness of stent placement assisted by IVUS evaluation for SA obstructive lesions was suggested by the good long-term results. A prospective comparative study involving a greater number of patients may be needed in the future to assess these results properly.
Conclusion
With IVUS evaluation and stent placement for SA obstructive lesions, our long-term treatment outcomes were very good. Assessment of lesion extent and lesion characteristics using intraoperative IVUS may be useful to reduce the incidence of restenosis.
References
- 1.Wang KQ, Wang ZG, Yang BZ, et al. Long-term results of endovascular therapy for proximal subclavian arterial obstructive lesions. Chin Med J (Engl) 2010;123(1):45–50. [PubMed] [Google Scholar]
- 2.Brountzos EN, Petersen B, Binkert C, et al. Primary stenting of subclavian and innominate artery occlusive disease: a single center’s experience. Cardiovasc Intervent Radiol. 2004;27(6):616–623. doi: 10.1007/s00270-004-0218-y. doi: 10.1007/s00270-004-0218-y. [DOI] [PubMed] [Google Scholar]
- 3.Henry M, Henry I, Polydorou A, et al. Percutaneous transluminal angioplasty of the subclavian arteries. Int Angiol. 2007;26(4):324–340. [PubMed] [Google Scholar]
- 4.De Vries JP, Jager LC, Van den Berg JC, et al. Durability of percutaneous transluminal angioplasty for obstructive lesions of proximal subclavian artery: long-term results. J Vasc Surg. 2006;41(1):19–23. doi: 10.1016/j.jvs.2004.09.030. doi: 10.1016/j.jvs.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 5.Vitti MJ, Thompson BW, Read RC, et al. Carotid-subclavian bypass: a twenty-two-year experience. J Vasc Surg. 1994;20(3):417–418. doi: 10.1016/0741-5214(94)90140-6. doi: 10.1016/0741-5214(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 6.Perler BA, Williams GM. Carotid-subclavian bypass--a decade of experience. J Vasc Surg. 1990;12(6):722–723. doi: 10.1067/mva.1990.24577. doi: 10.1067/mva.1990.24577. [DOI] [PubMed] [Google Scholar]
- 7.Edwards WH, Jr, Tapper SS, Edwards WH, Sr, et al. Subclavian revascularization. A quarter century experience. Ann Surg. 1994;219(6):673–678. doi: 10.1097/00000658-199406000-00010. doi: 10.1097/00000658-199406000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowell RC, Mills JL. Critical evaluation of axillo axillary artery bypass for surgical management of symptomatic subclavian and innominate artery occlusive disease. Cardiovasc Surg. 1993;1(5):530–535. [PubMed] [Google Scholar]
- 9.Sixt S, Rastan A, Schwarzwälder U, et al. Results after balloon angioplasty or stenting of atherosclerotic subclavian artery obstruction. Catheter Cardiovasc Interv. 2009;73(3):395–403. doi: 10.1002/ccd.21836. doi: 10.1002/ccd.21836. [DOI] [PubMed] [Google Scholar]
- 10.Patel SN, White CJ, Collins TJ, et al. Catheter-based treatment of the subclavian and innominate arteries. Catheter Cardiovasc Interv. 2008;71(7):963–968. doi: 10.1002/ccd.21549. doi: 10.1002/ccd.21549. [DOI] [PubMed] [Google Scholar]
- 11.Amor M, Eid-Lidt G, Chati Z, et al. Endovascular treatment of the subclavian artery: stent implantation with or without predilatation. Catheter Cardiovasc Interv. 2004;63(3):364–370. doi: 10.1002/ccd.20173. doi: 10.1002/ccd.20173. [DOI] [PubMed] [Google Scholar]
- 12.Motarjeme A. Percutaneous transluminal angioplasty of supra-aortic vessels. J Endovasc Surg. 1996;3(2):171–181. doi: 10.1177/152660289600300209. doi: 10.1583/1074-6218(1996)003<0171:PTASAV>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS Guideline on the management of patients with extracranial carotid and vertebral artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. J Am Coll Cardiol. 2011;57(8):e16–94. doi: 10.1016/j.jacc.2010.11.006. doi: 10.1016/j.jacc.2010.11.006. [DOI] [PubMed] [Google Scholar]