Summary
We prospectively compared the ability of neuroradiologists to diagnose medulloblastoma with novice raters using only apparent diffusion coefficient (ADC) values measured on ADC maps. One hundred and three pediatric patients with pre-operative magnetic resonance imaging scans showing a posterior fossa tumor with histological verification were retrospectively identified from a ten-year period at a tertiary care medical center. A single observer measured the lowest ADC values in all tumors to determine the mean minimum ADC (ADCmin) value that provided greatest accuracy in distinguishing medulloblastomas from other tumors, which was determined to be 0.66×10−3 mm2/s. Imaging studies, including ADC maps, from 90 patients were provided to two neuroradiologists, who provided a diagnosis, which was later dichotomized as medulloblastoma or other. Two medical students measured ADCmin within tumors and those with ADCmin < 0.66×10−3 mm2/s were recorded as medulloblastoma; any other value was recorded as other. Diagnostic accuracy was measured. ADCmin values allowed a correct identification of lesions as either medulloblastoma or other in 91% of cases. After diagnoses by the two neuroradiologists were categorized as either medulloblastoma or other, their diagnoses were correct in 90% and 84% of cases, respectively. In 19 cases, at least one neuroradiologist was incorrect; the addition of ADC values to clinical interpretation would have allowed a correct diagnosis in 63% of such cases. Diagnostic accuracy based on ADC values by medical students was comparable to that of subspecialty-trained neuroradiologists. Our findings suggest that the addition of ADC values to standard film interpretation may improve the diagnostic rate for these tumors.
Keywords: apparent diffusion coefficient, tumors, children, posterior fossa
Introduction
Pediatric posterior fossa tumors can be challenging to distinguish on preoperative imaging, even by experienced radiologists. Apparent diffusion coefficient (ADC) maps have shown great promise in distinguishing posterior fossa tumors in the literature, however it is unclear if this will translate to clinical benefit. To elucidate this, we prospectively compared standard of care diagnosis by attending neuroradiologists and diagnosis by ADC alone.
Distinguishing between medulloblastoma, ependymoma, and cerebellar astrocytoma, the three most frequently encountered pediatric posterior fossa tumors, can be challenging using traditional MRI1. Even with extensive training and experience, distinguishing features are often either subtle, making diagnosis more difficult, or absent, making diagnosis impossible. For example all three tumor types may present in the midline with solid and cystic regions in children near five years of age1. Medulloblastoma and ependymoma can be particularly difficult to differentiate as both commonly are located within the fourth ventricle1.
A variety of advancements in MRI, especially the development of diffusion-weighted imaging (DWI) and ADC maps, have been explored as diagnostic adjuncts to traditional MRI2-4. Multiple reports have demonstrated that high tumor cellularity results in restricted diffusion manifested by low ADC values5-7. Thus tumors of different cellularity can be distinguished on the basis of their ADC values2-4,6,8-16. For example, researchers have retrospectively identified thresholds that distinguish high grade tumors, WHO grade III and IV, from low grade tumors, WHO grade I and II, with impressive accuracy14,16.
A variety of methods utilize ADC maps to distinguish different types of tumors. Such strategies include measuring mean tumor ADC9,17,18, minimum tumor ADC (ADCmin)3,5,15,16, normalized tumor ADC (both quantitative3,6,18 and qualitative14,16 ), and ADC histograms11. For this investigation we used the mean ADCmin, i.e., the average of the three lowest ADC values within the tumor, because this measure is less affected by tumor heterogeneity and edema.
Despite some success in using ADC values in retrospective studies, the degree to which clinical radiologists can benefit from utilizing ADC maps in a clinical setting is currently unclear. To evaluate the clinical utility of using restricted diffusion to predict medulloblastoma histology, we compared the diagnostic accuracy of practicing neuroradiologists with that of novice raters who measure ADCmin and subsequently compared their measurements to predefined thresholds to identify medulloblastomas from other tumors. We hypothesized that the simpler more objective ADCmin method, as employed by physicians-in-training, would be comparable to clinical diagnosis by practicing neuroradiologists.
Methods
Case Selection
We obtained IRB approval and a waiver of informed consent to include imaging studies and clinical data at our university-based, tertiary-care facility. One hundred and three pretreatment MR scans with DWI sequences of posterior fossa masses were identified using a retrospective medical record search at our institution between January 1, 2001 and December 31, 2011. To be included, patients had to be 18 years of age or younger at the time of imaging and have had the posterior fossa pathology identified by histology. Our list of search terms included medulloblastoma, ependymoma, astrocytoma, atypical teratoid/rhabdoid tumor (ATRT), and choroid plexus papilloma; those five tumor types representing the vast majority of pediatric posterior fossa tumors.
Seven hundred and fifty-two patients were identified; 367 patients were excluded due to unavailable pathology reports and another 200 patients were excluded because they did not have posterior fossa masses. Eighty-two patients were excluded because they lacked preoperative DWI, leaving 103 patients whose scans and diagnoses could be used to determine an ADC value that could be used as a threshold for distinguishing medulloblastomas from other posterior fossa tumors.
Determination of ADCmin Threshold
As part of a separate study, a single observer identified the ADCmin threshold in 103 cases. This individual, who was blinded to diagnosis in each case, measured the three lowest ADC values (ADCmin) within tumors on a PACS workstation and obtained a mean ADCmin value for each tumor. In the event that multiple pretreatment examinations were available, only the scan closest to time of surgery was used. The observer then obtained the pathology diagnosis from the electronic medical record and compared the mean ADCmin value against the diagnosis. This individual calculated the diagnostic accuracy (i.e., the sum of medulloblastoma cases with ADCmin less than a particular threshold and other tumor cases with an ADCmin greater than a threshold divided by the total number of cases) as a function of various ADCmin threshold values. The threshold that provided the maximal accuracy was found to be 0.66 mm2/s, which had the following test characteristics: accuracy of 93.2%, sensitivity of 0.939, specificity of 0.929, positive predictive value of 0.861, and negative predictive value of 0.970.
The observer then transferred the MR scans to a GE Advantage Windows Workstation (version 4.4, GE Healthcare, Waukesha, WI, USA) for review by neuroradiologists and medical students. This transfer from the PACS system was performed in order to eliminate access by readers to film interpretation reports and clinical information that would have been accessible on the PACS workstation and could bias film interpretation. Furthermore, some ADC maps were only available on the GE Advantage Windows workstation. Unfortunately 13 cases could not be transferred to the Advantage Windows workstation due to image formatting incompatibility; the remaining 90 patients, each having a single tumor, were included for analysis. Seventy scans were from our institution and 20 were from other institutions.
Image Analysis
All scans included unenhanced and contrast-enhanced axial T1-weighted axial images, axial T2-weighted images, axial fluid attenuated inversion recovery (FLAIR) images and axial DWI images. All imaging examinations were transferred to a single GE Advantage Workstation for analysis. For cases in which ADC maps were not already provided on our PACS system, ADC maps were created using the Functool ADC program on the workstation.
Four raters analyzed the examinations, which included two neuroradiologists (both with four years' experience as attending radiologists performing solely neuroradiology imaging interpretation) and two third-year medical students. All raters were blinded to tumor diagnosis, which was determined by the lead investigator on review of pathology reports in all cases.
The neuroradiologists were provided with patient age and gender and told that all cases had a posterior fossa mass. For each case, the neuroradiologists were asked to provide the single most-likely diagnosis and to list, in order, the three imaging sequences that were most helpful in making the diagnosis. Subsequently, the principal investigator divided the diagnoses offered by the neuroradiologists into two categories: medulloblastoma and other (i.e., not medulloblastoma).
Students independently examined ADC maps in conjunction with all other MR images and were instructed to place an ROI around each of three regions containing the lowest ADC values within the tumor and record those values. We chose circular 30 mm2 ROIs, similar in size to those used by other investigators15,19,20. ROIs were placed without overlap with one another whenever allowed by tumor size. Fewer ROIs were permitted for eight patients who had very small tumors. ROI placement was guided by the fact that the regions with lowest ADC values are typically the darkest region within the tumor on the ADC map. Thus, the initial placement of ROIs was guided by visual inspection to determine the regions of lowest signal intensity. However, the ADC values within ROIs were also evident on the PACS monitor screen, which allowed the students to compare various ROIs to identify those with the three lowest values.
In order to decrease the likelihood that low signal intensity regions represented areas of susceptibility effect related to hemorrhage or calcification, ADC maps were simultaneously viewed with images from other pulse sequences. The region having the lowest ADC value (hereafter referred to as ADCmin) was recorded. A study coordinator classified those tumors determined by the medical students as having an ADCmin less than the threshold value of 0.66 mm2/s as medulloblastoma; all other cases assessed by the students were classified as “other”.
Tumor Histology
Pathology reports were obtained using the electronic medical record at our institution. All diagnoses were unequivocal. Using the pathology report, the principal investigator divided tumors into one of two categories: medulloblastoma or other (i.e., not medulloblastoma).
Statistical Analysis
Analysis was performed in Rstudio version 0.94.92 and Microsoft Excel 2010. The diagnostic accuracy for all raters (i.e., the frequency with which the rater correctly identified the posterior fossa mass as a medulloblastoma or other tumor type) was calculated. Inter-rater agreement is reported for pairwise rater comparisons using Cohen's Kappa statistic.
Figure 1.
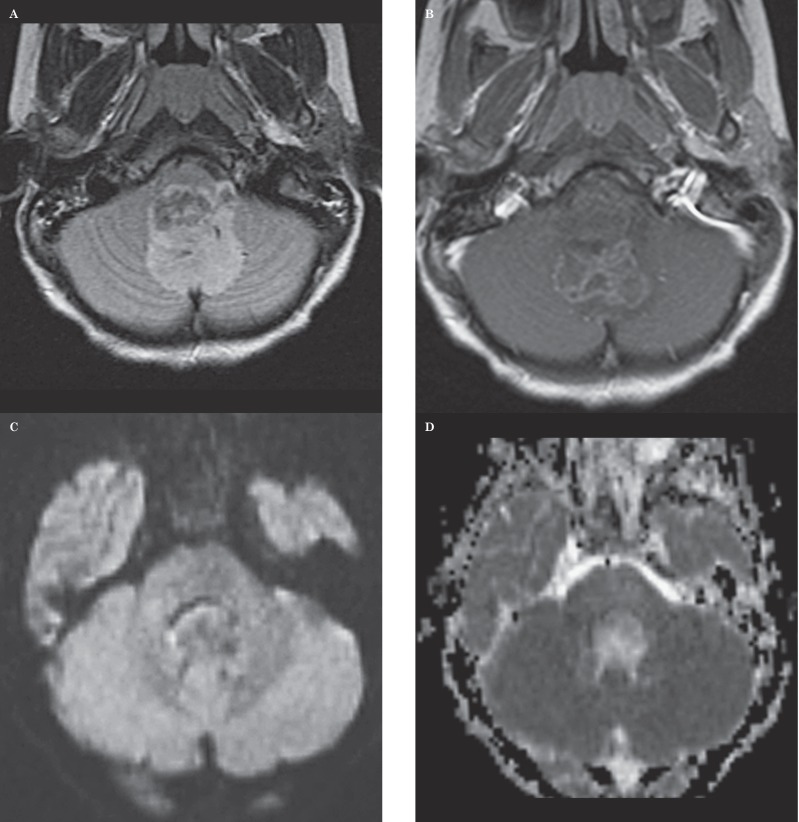
Seven-year-old girl with an anaplastic ependymoma. This case represents an example in which both neuroradiologists provided the correct diagnosis and in which ADC values indicated a correct diagnosis. A) Axial FLAIR image shows a heterogenous mass extending out of the left foramen of Luschka, typical of an ependymoma. B) Axial contrast-enhanced T1-weighted image shows the mass contrast enhances in an inhomogenous manner. C) Axial diffusion-weighted image shows the mass is relatively isointense to normal brain. D) Axial ADC map at the same level as C shows the lesion has a bright signal, consistent with elevated diffusivity. Mean ADCmin value measured by one observer was 1.047 mm2/s and that by the other observer was 1.064 mm2/s, suggesting that the diagnosis was not that of medulloblastoma.
Figure 2.
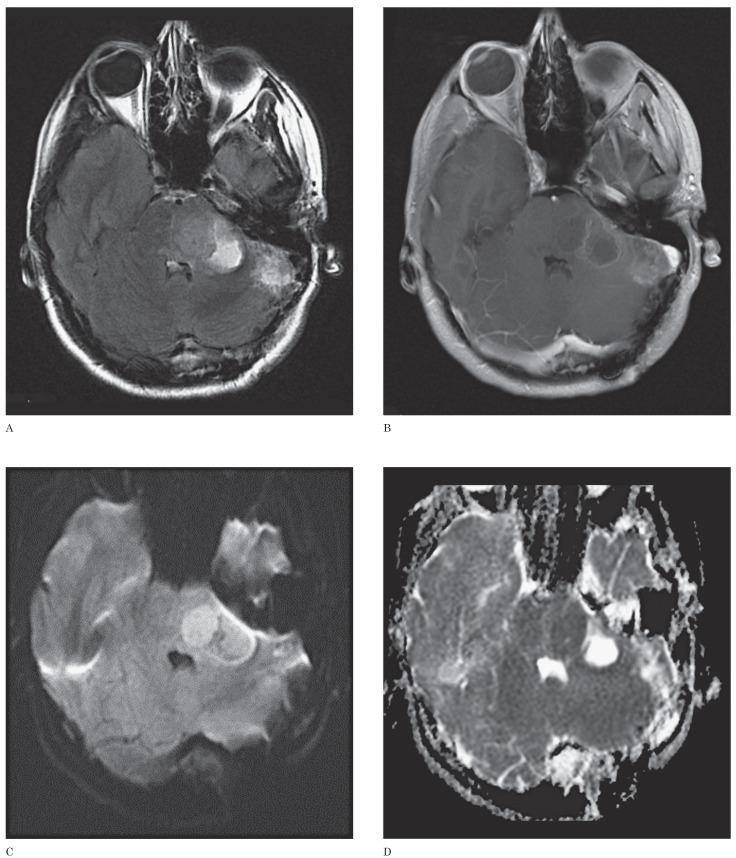
Seventeen-year-old girl with an anaplastic astrocytoma. This case represents the single MR imaging study in which both neuroradiologists provided the correct diagnosis and in which ADC values indicated an incorrect diagnosis. However, the location and appearance are atypical for medulloblastoma. A) Axial FLAIR image shows a complex mass involving the pons, left middle cerebellar peduncle and left cerebellar hemisphere. This case is the sole one in which, of the 6 cases that would have been incorrectly diagnosed by the ADC threshold, the clinical impression of both radiologists would have been correct. This outcome suggests that weighting the ADC threshold more heavily in the interpretation of imaging of PFT would rarely result in a less accurate diagnosis than clinical impression alone. B) Axial contrast-enhanced T1-weighted image shows a contrast-enhancing mass containing multiple cystic regions. C) Axial DWI image shows that the portion of the tumor within the pons has bright signal, possibly indicating restricted diffusion. D) Axial ADC map at the same level as C shows the pontine lesion has a dark signal suggestive of low ADC values. Mean ADC value measured by one observer was 0.615 mm2/s and that by the other observer was 0.624 mm2/s, suggesting the diagnosis of medulloblastoma. However, the imaging features shown in A and B would be atypical for medulloblastoma based on lesion location (i.e., pontine involvement) and the presence of a large cyst.
Figure 3.
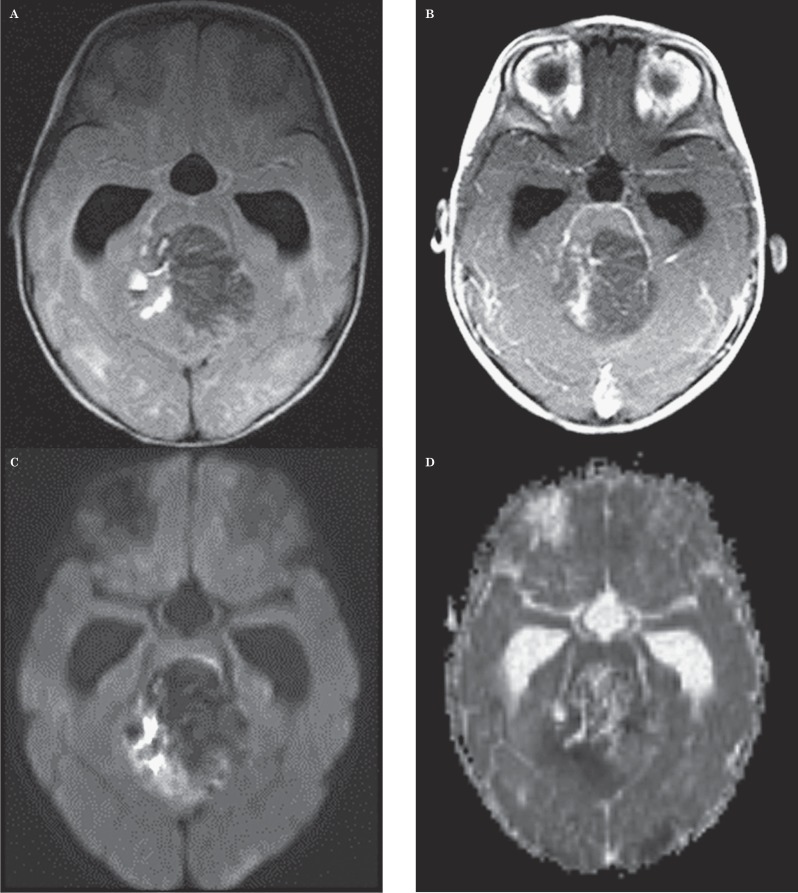
Twelve-month-old girl with medulloblastoma. This case represents the single MR imaging study in which neither neuroradiologist provided the correct diagnosis but in which ADCmin values by both readers indicated a correct diagnosis. A) Axial FLAIR image shows a heterogeneous mass in the fourth ventricle with hyperintense foci and a small fluid-fluid level suggestive of hemorrhage. B) Axial contrast-enhanced T1-weighted image shows small foci of contrast enhancement within a portion of the mass. C) Axial DWI image shows a hyperintense signal within the right half of the lesion, suggestive of low ADC values. D) Axial ADC map at the same level as C shows the lesion has regions of dark signal consistent with restricted diffusion in the right half of the mass. Mean ADCmin value measured by one observer was 0.569 mm2/s and that by the other observer was 0.631 mm2/s, suggesting the diagnosis of medulloblastoma.
Figure 4.
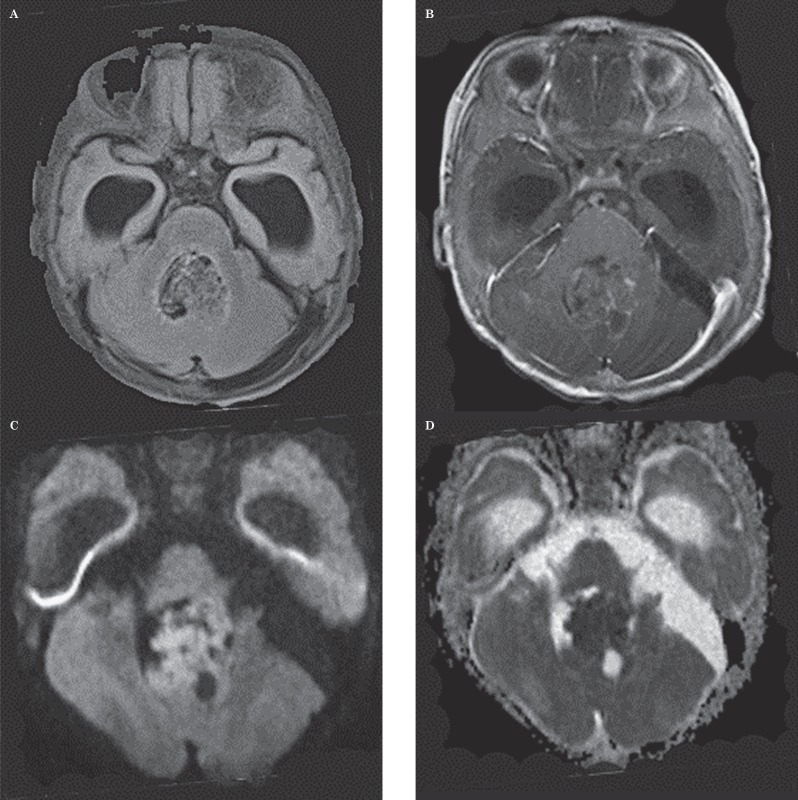
Ten-month-old boy with atypical teratoid rhabdoid tumor. This case represents one of the two MR imaging studies in which neither a neuroradiologist nor the ADC values by a reader indicated the correct diagnosis. This case illustrates the fact that an atypical teratoid rhabdoid tumor can simulate medulloblastoma in location, appearance and diffusion characteristics. A) Axial FLAIR image shows an inhomogeneous mass in the fourth ventricle. B) Axial contrast-enhanced T1-weighted image shows only small foci of contrast enhancement in the lesion. C) Axial DWI image shows large regions of bright signal suggestive of low ADC values. D) Axial ADC map at the same level as C shows the lesion has large regions of dark signal consistent with low ADC values. Mean ADC value measured by one observer was 0.526 mm2/s and that by the other observer was 0.535 mm2/s, suggesting the diagnosis of medulloblastoma. Because ADC values in both medulloblastomas and atypical rhabdoid tumors are low, the use of the ADCmin threshold produced a false-positive result for medulloblastoma.
Results
Pathology
Nine different tumor types were identified. Forty-six cases were determined to be astrocytomas, 28 cases were medulloblastomas, and seven were ependymomas. The remaining nine cases consisted of three choroid plexus papillomas, two ATRTs, one glioblastoma, one high-grade teratoma, one malignant glioneuronal mass, and one case of Rosai-Dorfman disease (a lesion characterized by massive histiocytosis which, in our patient, presented as a cerebellar mass and was thought preoperatively to represent a tumor).
Diagnostic Accuracy
The two neuroradiologists provided the correct diagnosis in 90% and 84% of cases, respectively. The correct diagnosis, based on the ADCmin measurements independently obtained by both students, was provided in 91% of cases.
Inter-Rater Agreement
Inter-rater agreement is summarized in Table 1. Rate of agreement between students was 89.4% and between radiologist 1 and student 1 and student 2 was 86.3% and 81.3%, respectively. Thus, according to the Landis and Koch classification21, near perfect agreement was seen between radiologist 1 and both students, as well as between both students. However, rates of agreement between radiologist 2 and other raters ranged between 52.4% and 54.5%. Accordingly, only moderate agreement was seen between radiologist 2 and the remaining three raters.
Table 1.
Inter-rater agreement obtained using Cohen's kappa statistic between pairwise raters. R1 indicates radiologist 1, R2 indicates radiologist 2, S1 indicates student 1 and S2 represents student 2. Thus, for example, Cohen's kappa statistic between radiologist 2 and student 1 was 52.4%.
| R1 | R2 | S1 | |
| R2 | 54.5% | ||
| S1 | 86.3% | 52.4 % | |
| S2 | 81.3% | 54.0% | 89.4 % |
Analysis of Errors
Lack of complete agreement by both neuroradiologists and by both measurements using the ADCmin threshold system was seen in 22 cases (24%). Nineteen cases (21%) were misdiagnosed by at least one neuroradiologist and ten cases (11%) were misclassified by the ADCmin threshold system.
The largest category of discrepancies was cases in which the measurements by both students provided the correct diagnosis but only one neuroradiologist made the correct diagnosis (n=11). Other discrepancies are listed in Table 2. Notably, in 19 cases at least one radiologist provided an incorrect diagnosis. In 12 of those cases (53%), both observers using the ADCmin threshold provided measurements indicative of the correct diagnosis. Of the remaining five cases, one observer provided measurements indicative of the correct diagnosis in two cases, and in the remaining three cases neither of the those observers provided measurements indicative of the correct diagnosis. In six cases, neither observer using the ADCmin threshold provided the correct diagnosis (Table 2).
Table 2.
Disagreements between readers and diagnoses indicated by ADC value. The table shows discrepancies between both neuroradiologists' (NRs) diagnosis and diagnosis provided by ADCmin measurements. All values greater than 0.66×10−3 mm2/s were designated as not medulloblastoma. The first column lists the numbers of NRs who were incorrect. The second column shows the number of times the diagnosis provided by the ADCmin measurement was incorrect. In each case, the maximal number of correct answers was two. The shaded boxes represent cases in which specifically an incorrect diagnosis of medulloblastoma was provided. In all other cases, an incorrect diagnosis of a tumor other than medulloblastoma was provided. All ADC values are expressed in units of ×10−3 mm2/s.
|
NRs incorrect |
ADCmin Diagnosis Incorrect |
Number of Cases |
Correct Diagnosis | Explanation of Incorrect ADCmin Diagnosis |
| 1 | 0 | 11 | Medulloblastoma (n=9) | N/A |
| Ependymoma (n=1) Astrocytoma (n=1) | ||||
| 2 | 0 | 1 | Medulloblastoma | N/A |
| 0 | 1 | 2 | Medulloblastoma | ADCmin (0.668) above threshold |
| Well-differentiated astrocytoma |
ADCmin (0.606) below threshold; diagnosed as medulloblastoma |
|||
| 1 | 1 | 1 | Medulloblastoma | ADCmin (1.015) above threshold |
| 2 | 1 | 1 | Medulloblastoma | ADCmin (0.670) above threshold |
| 0 | 2 | 1 | Anaplastic Astrocytoma | ADCmin (0.615 and 0.624) below threshold; diagnosed as medulloblastoma |
| 1 | 2 | 3 | ATRT (n=1) | ADCmin (0.465 and 0.469) below threshold; diagnosed as medulloblastoma |
| Medulloblastoma (n=2) | ADCmin above threshold - (case 1: 0.719 and 0.7570 and case 2: 0.752 and 0.920) |
|||
| 2 | 2 | 2 | ATRT (n=1) | ADCmin (0.528 and 0.535) below threshold; diagnosed as medulloblastoma |
| Medulloblastoma (n=1) | ADCmin values (0.819 and 0.728) above threshold |
The pathologic diagnosis in these cases was medulloblastoma (N=3), ATRT (N=2), and anaplastic astrocytoma (N=1). Of these six cases, both radiologists provided the correct diagnosis in only the anaplastic astrocytoma case.
Six cases of medulloblastoma were misclassified by at least one observer using the ADCmin threshold (Table 2). The ADCmin values in these tumors ranged from 0.668×10−3 mm2/s to 1.015×10−3 mm2/s (mean: 0.783×10−3 mm2 /s). Notably, in two cases, the ADCmin value recorded was minimally above threshold (i.e., 0.668×10−3 mm2/s in one case and 0.670×10−3 mm2/s in another case). Four tumors were misclassified as medulloblastoma using this method. Two cases of ATRT tumors were misclassified as medulloblastoma on the basis of ADC values below the ADCmin threshold.
In one tumor, the mean ADCmin value of both readers was 0.467×10−3 mm2/s and in the other, it was 0.532×10−3 mm2/s. These findings were not surprising, given the fact that ATRTs are known to have similar pathology to medulloblastomas20 and low ADCmin values3,6. In the other two cases of misclassification of a tumor as medulloblastoma based on ADC values below the ADCmin threshold, the diagnosis was astrocytoma. In one case, the mean ADCmin value for both readers was 0.620 and in the other case, the sole ADCmin value below the threshold was 0.606.
Perceived Importance of MRI Sequences
A summary of the three imaging sequences the neuroradiologists considered most valuable in identification of each posterior fossa tumor are summarized in Table 3.
Table 3.
Perceived diagnostic importance of MRI sequences. Table showing the number of cases in which the radiologist indicated a given sequence to be most important, second most important, or third most important for making the correct diagnosis. Rad1 represents radiologist 1 while Rad2 represents radiologist 2, i.e. Rad1 1st indicates the number of times that radiologist 1 found a given sequence to be the most important for diagnosis.
| RANK | DWI/ADC | NONE | SW1 | T1WI | T1WI+C | T2WI |
| Rad 1 | ||||||
| 1st | 21 | 0 | 0 | 2 | 44 | 23 |
| 2nd | 23 | 0 | 0 | 1 | 19 | 47 |
| 3rd | 17 | 0 | 1 | 29 | 23 | 20 |
| Rad 1 total | 61 | 0 | 1 | 32 | 86 | 90 |
| Rad 2 | ||||||
| 1st | 26 | 0 | 0 | 2 | 45 | 17 |
| 2nd | 33 | 0 | 0 | 3 | 31 | 23 |
| 3rd | 18 | 1 | 0 | 12 | 9 | 50 |
| Rad 2 total | 77 | 1 | 0 | 17 | 85 | 90 |
The table indicates that contrast-enhanced T1-weighted images were most commonly deemed most important for diagnosis by both radiologists. For radiologist 1, T2-weighted images were the second most commonly designated as most important (but close in frequency to DWI/ADC images), whereas for radiologist 2, DWI/ADC sequences were the second most commonly designated as the most important for diagnosis.
Discussion
The goal of this study was to compare the accuracy of a quantitative technique for specific diagnosis of posterior fossa tumors against that provided by clinical neuroradiologists. If comparable, then support for use of such a quantitative method as the basis of computer-aided diagnosis of tumors might be found. We found that a simple algorithm, which in the future could be automated, could provide important diagnostic information aiding in clinical diagnosis by neuroradiologists.
This study allows a number of preliminary conclusions that deserve further study. First, quantitative imaging using ADC maps provides information that could serve as the basis for computerized diagnosis of tumors using either DW imaging alone or when combined with anatomic images. In the future, an automated program could be developed to provide ADC values within tumors without prompting by the radiologist. Such a program might be able to provide likelihood ratios for specific tumor types or, alternatively, for likelihood of a tumor (as opposed to another mass lesion) being present. Second, using quantitative data of the sort employed in this study, computerized programs could potentially generate a prioritized list of diagnoses by automatically obtaining, and combining, information provided by various quantitative techniques, such as perfusion imaging, MR spectroscopy and diffusion-weighted imaging.
Based on the ranking in order of importance of individual pulse sequences by the neuroradiologists in our study (Table 3), it is clear that these raters relied to a relatively large degree on DWI data. However, the neuroradiologists did not have knowledge of the ADCmin threshold used in the study. Given the fact that our study was performed at a tertiary medical center that has a large brain tumor center, the neuroradiologists who served as raters may not be representative of radiologists interpreting brain tumor imaging studies at facilities other than university-based medical centers. Thus, it is possible that the quantitative techniques used in our study could provide even greater advantages at smaller medical centers and to radiologists who are not experienced in interpreting a high volume of brain tumor studies or who do not practice in a research environment in which various applications of DWI are emphasized and routinely discussed.
One question worth addressing is the extent to which the addition of ADC values to the interpretation regimen used by radiologists would alter the diagnosis. In our study, application of the ADCmin threshold to ADC maps would have allowed the correct diagnosis to be reached in 12 of the 19 cases in which at least one radiologist provided the incorrect diagnosis. Such a combined use of routine clinical image interpretation and use of a quantitative measure would be expected to have an even more profound effect on the interpretations of radiologists who do not have subspecialty neuroradiology training and who, furthermore, are requested to interpret pediatric brain tumor studies less frequently in a setting other than a tertiary care medical center. By relying more heavily on the ADC values, however, one would want to be careful to avoid replacing correct diagnoses reached by the radiologist's clinical experience with incorrect diagnoses (i.e. false positives or false negatives) generated by the use of the ADCmin threshold. Our data suggest that this event would be uncommon; we found only one case where the ADCmin threshold indicated the wrong diagnosis but both radiologists provided the correct diagnosis. Together, these observations suggest that an increasing reliance on the ADC values measured within a tumor would increase the radiologist's overall accuracy.
We recognize that the application of ADC values specifically to pediatric posterior fossa tumors may meet with the objection that the specific tissue diagnosis in such tumors does not have a practical value because identification of a specific tissue type will not alter surgery. However, we chose this group of tumors because ample preliminary data from published studies were available for comparison with our data. We envision this investigation as a feasibility study; if use of ADC values proved successful, then application of the principles we employed might later be shown to be valuable in more complex clinical settings in which preoperative determination of specific tumor type might indeed be important in altering surgery.
As with all studies, our study is subject to limitations. The first limitation is the relatively small number of raters involved. As already noted, our sample of neuroradiologists may not be truly representative of neuroradiologists in general, given their positions in a tertiary medical center in which much research in diffusion-weighted imaging is being conducted. In addition, given their specific training and subsequent clinical work solely in neuroradiology, these raters are not representative of general radiologists who interpret neuroradiology imaging studies as part of their clinical work. The expected effect of adding general radiologists to the pool of raters would be to lessen the diagnostic accuracy of radiology raters with the potential outcome that the method of using solely ADC values for diagnosis would have a higher diagnostic accuracy compared to radiologists. Another limitation is that only two students measured ADCmin values. However, the fact that they performed similarly to each other and the fact that their accuracy was comparable to previous reports utilizing similar methods is reassuring16. A third potential limitation is that the cases were selected in a retrospective manner, which could possibly subject our study to selection bias. However, we took great care in selecting a representative sample of cases at one tertiary care medical center by attempting to analyze all cases during a ten year period in order to avoid selection bias. We believe our results to be applicable to other similar institutions. A prospective trial in which pediatric posterior fossa tumor patients were entered into the study as they presented clinically would have taken many years to complete, given the relatively low frequency of such tumors, even at a tertiary care medical facility. Nonetheless, we cannot exclude the possibility that a truly prospective study design could provide different results from the present study. Finally, it could be argued that the scenario in which neuroradiologists evaluated the imaging studies was somewhat artificial because they were provided with solely the age and gender of the patient. However, this limited clinical information would generally be considered sufficient to allow them to generate an appropriate differential diagnosis.
Conclusion
We have shown that the DWI method executed by raters untrained in film interpretation, despite its simplicity, can provide prediction of tumor type that is not inferior to standard of care diagnostic interpretation by expert neuroradiologists. Future work should focus on replicating these results with larger samples as well as comparing the DWI method to interpretation by radiologists who are not specifically training in neuroimaging.
References
- 1.Poretti A, Meoded A, Huisman TA. Neuroimaging of pediatric posterior fossa tumors including review of the literature. J Magn Reson Imaging. 2012;35(1):32–47. doi: 10.1002/jmri.22722. doi: 10.1002/jmri.22722. [DOI] [PubMed] [Google Scholar]
- 2.Yamasaki F, Kurisu K, Satoh K, et al. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology. 2005;235(3):985–991. doi: 10.1148/radiol.2353031338. doi: 10.1148/radiol.2353031338. [DOI] [PubMed] [Google Scholar]
- 3.Rumboldt Z, Camacho DL, Lake D, et al. Apparent diffusion coefficients for differentiation of cerebellar tumors in children. Am J Neuroradiol. 2006;27(6):1362–1369. [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z, Ma L, Lou X, et al. Diagnostic value of minimum apparent diffusion coefficient values in prediction of neuroepithelial tumor grading. J Magn Reson Imaging. 2010;31(6):1331–1338. doi: 10.1002/jmri.22175. doi: 10.1002/jmri.22175. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita Y, Kumabe T, Higano S, et al. Minimum apparent diffusion coefficient is significantly correlated with cellularity in medulloblastomas. Neurol Res. 2009;31(9):940–946. doi: 10.1179/174313209X382520. doi: 10.1179/174313209X382520. [DOI] [PubMed] [Google Scholar]
- 6.Gauvain KM, McKinstry RC, Mukherjee P, et al. Evaluating pediatric brain tumor cellularity with diffusion tensor imaging. Am J Roentgenol. 2001;177(2):449–454. doi: 10.2214/ajr.177.2.1770449. doi: 10.2214/ajr.177.2.1770449. [DOI] [PubMed] [Google Scholar]
- 7.Humphries PD, Sebire NJ, Siegel MJ, et al. Tumors in pediatric patients at diffusion-weighted MR imaging: apparent diffusion coefficient and tumor cellularity. Radiology. 2007;245(3):848–854. doi: 10.1148/radiol.2452061535. doi: 10.1148/radiol.2452061535. [DOI] [PubMed] [Google Scholar]
- 8.Kotsenas AL, Roth TC, Manness WK, et al. Abnormal diffusion-weighted MRI in medulloblastoma: does it reflect small cell histology? Pediatr Radiol. 1999;29(7):524–526. doi: 10.1007/s002470050636. doi: 10.1007/s002470050636. [DOI] [PubMed] [Google Scholar]
- 9.Rodallec M, Colombat M, Krainik A, et al. Diffusion-weighted MR imaging and pathologic findings in adult cerebellar medulloblastoma. J Neuroradiol. 2004;31(3):234–237. doi: 10.1016/s0150-9861(04)97000-9. doi: 10.1016/S0150-9861(04)97000-9. [DOI] [PubMed] [Google Scholar]
- 10.Quadery FA, Okamoto K. Diffusion-weighted MRI of haemangioblastomas and other cerebellar tumours. Neuroradiology. 2003;45(4):212–219. doi: 10.1007/s00234-003-0951-y. doi: 10.1007/s00234-003-0951-y. [DOI] [PubMed] [Google Scholar]
- 11.Bull JG, Saunders DE, Clark CA. Discrimination of paediatric brain tumours using apparent diffusion coefficient histograms. Eur Radiol. 2012;22(2):447–457. doi: 10.1007/s00330-011-2255-7. doi: 10.1007/s00330-011-2255-7. [DOI] [PubMed] [Google Scholar]
- 12.Guo AC, Cummings TJ, Dash RC, et al. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology. 2002;224(1):177–183. doi: 10.1148/radiol.2241010637. doi: 10.1148/radiol.2241010637. [DOI] [PubMed] [Google Scholar]
- 13.Higano S, Yun X, Kumabe T, et al. Malignant astrocytic tumors: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology. 2006;241(3):839–846. doi: 10.1148/radiol.2413051276. doi: 10.1148/radiol.2413051276. [DOI] [PubMed] [Google Scholar]
- 14.Kan P, Liu JK, Hedlund G, et al. The role of diffusion-weighted magnetic resonance imaging in pediatric brain tumors. Childs Nerv Syst. 2006;22(11):1435–1439. doi: 10.1007/s00381-006-0229-x. doi: 10.1007/s00381-006-0229-x. [DOI] [PubMed] [Google Scholar]
- 15.Jaremko JL, Jans LB, Coleman LT, et al. Value and limitations of diffusion-weighted imaging in grading and diagnosis of pediatric posterior fossa tumors. Am J Neuroradiol. 2010;31(9):1613–1616. doi: 10.3174/ajnr.A2155. doi: 10.3174/ajnr.A2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji YM, Geng DY, Huang BC, et al. Value of diffusion-weighted imaging in grading tumours localized in the fourth ventricle region by visual and quantitative assessments. J Int Med Res. 2011;39(3):912–919. doi: 10.1177/147323001103900325. doi: 10.1177/147323001103900325. [DOI] [PubMed] [Google Scholar]
- 17.Schneider JF, Viola A, Confort-Gouny S, et al. Infratentorial pediatric brain tumors: the value of new imaging modalities. J Neuroradiol. 2007;34(1):49–58. doi: 10.1016/j.neurad.2007.01.010. doi: 10.1016/j.neurad.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Gimi B, Cederberg K, Derinkuyu B, et al. Utility of apparent diffusion coefficient ratios in distinguishing common pediatric cerebellar tumors. Acad Radiol. 2012;19(7):794–800. doi: 10.1016/j.acra.2012.03.004. doi: 10.1016/j.acra.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Lee EJ, Lee SK, Agid R, et al. Preoperative grading of presumptive low-grade astrocytomas on MR imaging: diagnostic value of minimum apparent diffusion coefficient. Am J Neuroradiol. 2008;29(10):1872–1877. doi: 10.3174/ajnr.A1254. doi: 10.3174/ajnr.A1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burger PC, Yu IT, Tihan T, et al. Atypical teratoid/rhabdoid tumor of the central nervous system: a highly malignant tumor of infancy and childhood frequently mistaken for medulloblastoma: a Pediatric Oncology Group study. Am J Surg Pathol. 1998;22(9):1083–1092. doi: 10.1097/00000478-199809000-00007. doi: 10.1097/00000478-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. doi: 10.2307/2529310. [PubMed] [Google Scholar]