Summary
The objective of this study was to determine the accuracy of individual radiologists in detection of vascular injury in patients after penetrating brain injury (PBI) based on head CT findings at admission. We retrospectively evaluated 54 PBI patients who underwent admission head CT and digital subtraction angiography (DSA), used here as a reference standard. Two readers reviewed the CT images to determine the presence or absence of the 29 CT variables of injury profile and quantified selected variables. Four experienced trauma radiologists and one neuroradiologist assigned their own specific scores for each CT variable, a high score indicative of a high probability of artery injury. A sixth set consisted of the average score obtained from the five sets, generated by five experts. Receiver operating characteristic (ROC) curves were constructed for each set to assess the diagnostic performance of an individual radiologist in predicting an underlying vascular injury. The area under ROC curve (AUC) was higher for CT scores obtained from the sixth set (average of five sets of scores) of variable rank score 0.75 (95% CI 0.62-0.88) and for the rest of the data sets, the value ranged from 0.70 (95% CI 0.56-0.84) to 0.74 (95% CI 0.6-0.88). In conclusion, radiologists may be able to recommend DSA with a fair accuracy rate in selected patients, deemed ‘high-risk' for developing intracranial vascular injuries after PBI based on admission CT studies. A better approach needs to be developed to reduce the false positive rate to avoid unnecessary emergency DSA.
Keywords: gunshot injuries to head, digital subtraction angiography, intracranial arterial injuries, civilian penetrating traumatic brain injury, traumatic intracranial pseudo-aneurysms
Introduction
The incidence of intracranial vascular rupture after penetrating brain injury remains largely unknown due to significant mortality caused by severe trauma before admission to a hospital1-7. In those who survive, the aim of the emergent diagnostic strategy is to determine the extent of the intracranial damage and provide crucial information for therapeutic decision-making. Detection of intracranial arterial injuries in patients after penetrating trauma is important because the ruptured artery is a life-threatening condition that can lead to the secondary brain injury and death4. Coexisting vascular injury influences the management of these patients, as the prevention of bleeding from the ruptured artery is the primary goal of the therapy. Most often those patients with suspected arterial injuries undergo diagnostic digital subtraction angiography (DSA) followed by various intravascular neurointerventional procedures if needed8-10.
Identification of patients with intracranial arterial injuries in emergency settings is a diagnostic challenge. DSA is an invasive procedure, carries a risk of stroke, is inconvenient to patients, and can postpone required surgical interventions11. DSA is only justified in patients with a high probability of having an arterial injury, and that probability is usually determined by radiologists based on several signs on emergency CT scans as all patients with penetrating head injury undergo such a diagnostic study at admission. The available literature mentions various risk factors for traumatic intracranial aneurysms (TICA) based on the injury profile of the penetrating wound2,3,5. It is not known how to provide weighted scores for each of the CT signs according to their contribution to the overall risk score of having an underlying vascular injury.
The objective of this study, therefore, was to determine the accuracy of individual radiologists in discrimination of patients with vascular injury after penetrating head injury from those without arterial injury based on hypothetical scoring of the CT signs obtained from admission CT of the head.
Materials and Methods
The study was compliant with the Health Insurance Portability and Accountability Act and permission was obtained from our institutional review board. The study was conducted at a level I trauma center. The inclusion criteria for this study were (1) a history of penetrating trauma to the head with a total width cranial fracture and bullet involvement of the brain parenchyma, between January 2005 and December 2012; (2) acquisition of brain CT at the time of admission and cerebral four vessel angiogram (DSA) during the course of hospitalization; (3) age ≥ 18 years. The following were exclusion criteria: (1) intracranial injuries without bullet penetration of the cranium; and (2) patients with concomitant neck vessel injuries caused either by multiple entry wounds or extracranial trajectory of a single entry wound.
A retrospective review of the trauma registry containing 6110 adult subjects with penetrating trauma during the study period yielded 182 patients with cerebral or neck vessel angiograms (DSA) performed by the interventional neuroradiology section. Out of the 182 patients, 54 patients underwent DSA for suspected intracranial vascular injuries and all the patients had an accompanying admission CT of the brain. These 54 consecutive patients formed the study group. There were 41 men and 13 women (mean age 32 years; range, 18-73 years). All the initial CT studies were acquired at the time of admission. The mean duration between the admission CT and the initial DSA was 7.9 hours (range, 90 min-18 hours). There were 25 patients with follow-up angiograms, all performed within 13 days of admission (range, 2 days-13 days; mean, 5 days). The mechanism of injury was GSW in 40 patients, stab wounds in six, shotgun injuries in three, nail gun injuries in four and one patient was impaled by a piece of wood.
Variable Construction
We analyzed the relationship of 29 study variables (Table 1). All the variables included were CT findings in patients with penetrating brain injury, and were deemed to be risk factors related to an underlying vascular injury.
Table 1.
CT variables of injury profile and the six sets of scoring created.
| CT variable | R1 | R2 | R3 | R4 | R5 | AVG |
| Entry site: Frontobasal- includes frontal, facial, orbital | 3 | 5 | 4 | 3 | 0 | 3 |
| Entry site: Temporal- pterion, temporal and parietal | 7 | 5 | 3 | 4 | 0 | 3.8 |
| Entry site: Occipital- includes occipital and sub-occipital | 1 | 1 | 3 | 2 | 0 | 1.4 |
| Penetrating injury (only entry wound) | 4 | 1 | 1 | 3 | 2 | 2.2 |
| Perforating injury, both entry and exit wound | 3 | 4 | 3 | 4 | 3 | 3.4 |
| Exit site: Frontobasal- includes frontal, facial and orbital | 2 | 5 | 4 | 3 | 0 | 2.8 |
| Exit site: Temporal- pterion, temporal and parietal | 2 | 5 | 3 | 4 | 2 | 3.2 |
| Exit site: Occipital- includes occipital and sub-occipital | 0 | 1 | 3 | 2 | 1 | 1.4 |
| Monohemispheric injury | 2 | 1 | 2 | 1 | 1 | 1.4 |
| Bihemispheric injury | 4 | 5 | 4 | 4 | 2 | 3.8 |
| Injury to unilateral anterior fossa | 1 | 1 | 1 | 1 | 1 | 1 |
| Injury to bilateral anterior fossa | 2 | 4 | 4 | 3 | 2 | 3 |
| Injury to unilateral middle cranial fossa | 2 | 2 | 2 | 3 | 1 | 2 |
| Injury to bilateral middle cranial fossa | 4 | 5 | 4 | 5 | 2 | 4 |
| Injury to posterior fossa: uni- or bilateral | 1 | 1 | 5 | 1 | 2 | 2 |
| Midline shift, any | 1 | 1 | 2 | 2 | 2 | 1.6 |
| Effacement of basal cisterns | 1 | 1 | 2 | 2 | 1 | 1.4 |
| In 2 cm proximity to the circle of Willis | 8 | 4 | 5 | 3 | 3 | 4.6 |
| Subarachnoid hemorrhage | ||||||
| Small amount in basal cistern and/or convexities (Score 1-6) | 0 | 1 | 1 | 1 | 2 | 1 |
| Moderate amount in basal cistern and/or convexities (Score 7-12) | 1 | 3 | 4 | 3 | 4 | 3 |
| Large amount in basal cistern and/or convexities (Score 13-18) | 5 | 5 | 5 | 5 | 7 | 5.4 |
| Intraventricular hemorrhage | ||||||
| Small amount (Score 1-4) | 0 | 1 | 1 | 1 | 2 | 1 |
| Moderate amount (Score 5-8) | 1 | 3 | 4 | 2 | 4 | 2.8 |
| Large amount (Score 9-12) | 5 | 5 | 4 | 3 | 7 | 4.8 |
| Transventricular trajectory | 2 | 5 | 4 | 3 | 6 | 4 |
| Presence of SDH (subdural hematoma). | 1 | 1 | 1 | 1 | 3 | 1.4 |
| Presence of EDH (epidural hematoma). | 1 | 5 | 1 | 1 | 3 | 2.2 |
| Intraparenchymal hematoma | ||||||
| Size: diameter 0-3 cm | 1 | 1 | 1 | 2 | 4 | 1.8 |
| Size: diameter 3-6 cm | 2 | 3 | 3 | 4 | 8 | 4 |
| Size: diameter >6 cm | 8 | 5 | 3 | 5 | 14 | 7 |
| Hematoma at entry site | 4 | 2 | 1 | 3 | 4 | 2.8 |
| Hematoma at exit site | 4 | 2 | 1 | 3 | 4 | 2.8 |
| Bone or bullet fragments displaced into parenchyma | 5 | 1 | 4 | 3 | 1 | 2.8 |
| Bone or bullet fragments crossing dural compartments | 5 | 1 | 3 | 5 | 1 | 3 |
| Bone or bullet fragments scattered in paths branching into various directions | 7 | 4 | 4 | 5 | 1 | 4.2 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| R radiologist; AVG: average score from the five sets. | ||||||
Variable Ranking
Variable ranking was performed independently by four experienced trauma radiologists and one neuroradiologist. The ranking was performed in such a way that the rank score sum for all the variables had to equal 100. By convention, a high score for a selected variable is indicative of a significant variable associated with a high probability of penetrating vascular injury. Five data sets were thus created and the sixth set consisted of the average score obtained from the five sets. Hence, a total of six sets was created. The ranking pattern of the selected variables is shown in Table 1.
This method of variable selection might contain many redundant features, since the ranking score is computed independently for each feature, by completely ignoring its correlation with others. Even though variable ranking is not optimal, it may be preferable to other variable subset selection methods because of its computational and statistical scalability.
Image Interpretation and Definitions
Two trauma radiologists (KS and NS) with 20 and two years of experience reviewed the CT images by consensus. The readers were blinded to the imaging findings of DSA and CT angiograms of the brain. CT images included 5mm axial sections. All studies were performed using 16-, 40- or 64-MDCT scanners (Brilliance; Phillips Healthcare, Andover, MA, USA). The reviewers assessed and recorded the presence of various CT findings of craniocerebral trauma. All the variables were given nominal scores based on the presence or absence of each of the CT findings. For subarachnoid hemorrhage (SAH) (Figure 1), intraventricular hemorrhage (IVH) (Figure 1) and parenchymal hematoma (Figures 1-3), quantitative analysis was performed. Once these CT finding were identified, the maximum diameter of the parenchymal hematoma was measured perpendicular to the trajectory of the wound. SAH was quantified using the Hijdra scale with modifications12,13. The modified scoring system is shown in Table 2. The modification accounts for the hemorrhage over the cerebral hemispheres (Figure 1) and the posterior cranial fossa and ranges from 0 to 18. The extent of IVH was quantified using a modified Graeb score, whereby a score 0 (no blood), 1 (sedimentation, < 25% filled) (Figure 1), 2 (moderately filled), and 3 (completely filled) was given to each ventricle for a maximum possible score of 1212,14.
Table 2.
SAH score (Modified Hijdra scale).
| Anatomic location | Score | Amount of blood in cisterns and fissures |
| Basal cisterns | 0-3 | 0, none; 1, small amount; 2, moderately filled; 3, completely filled |
| Sylvian fissures | 0-3 (on each side) | |
| Hemispheres | 0-3 (on each side) | 0, none; 1, seen in 1-5 5 mm CT slices; 2, 6-12 slices; 3, > 12 slices |
| Posterior cranial fossa | 0-3 (uni or bilateral) | |
| Total score | 0-18 |
Figure 1.
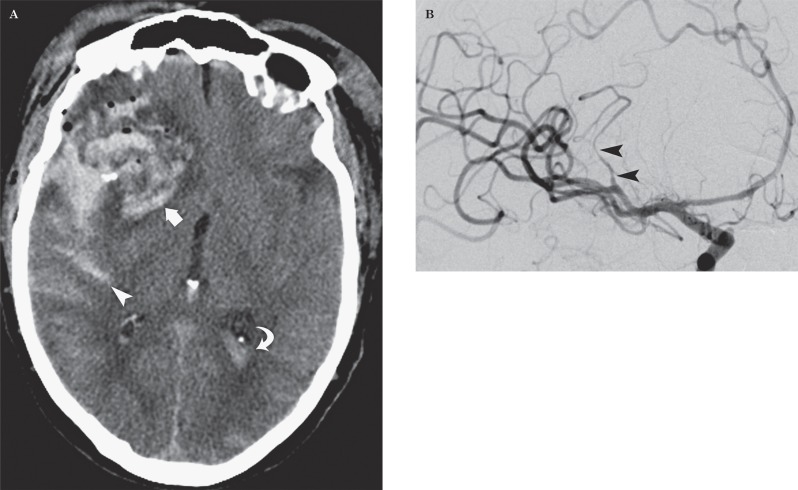
Gunshot injury to the head in a 73-year-old man. A) Axial CT image demonstrates a large parenchymal hematoma (arrow), subarachnoid hemorrhage (arrowhead) and intraventricular blood (curved arrow) from a right pterional entry wound. B) DSA image demonstrates segmental irregularity and narrowing of the sylvian branch of right MCA caused by the arterial dissection.
Figure 2.
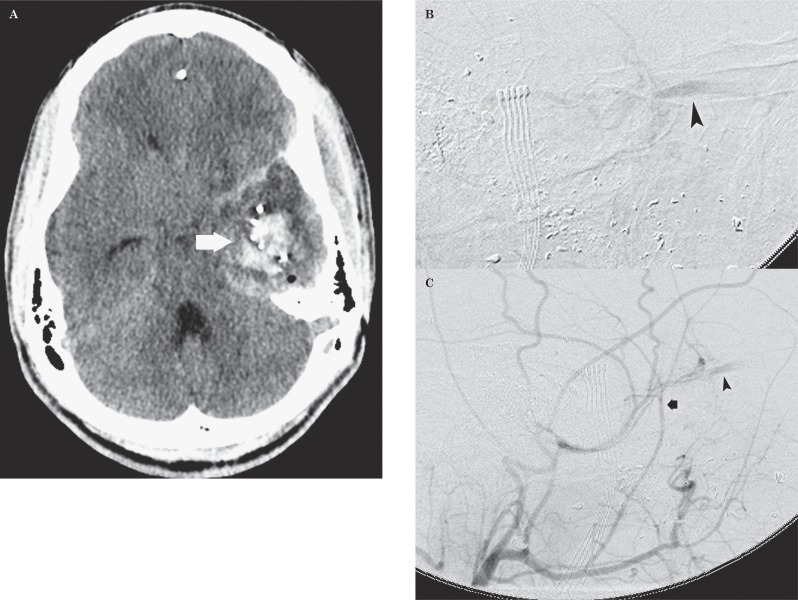
Gunshot injury to the head in a 22-year-old man. A) Axial CT image demonstrates a parenchymal hematoma (arrow) in the left temporal lobe from left facio-orbital entry wound. B,C) Sagittal DSA images demonstrate prompt opacification of the left superior ophthalmic vein (arrowhead) from the middle meningeal artery (arrow) after infusion of contrast through the left external carotid artery.
Figure 3.
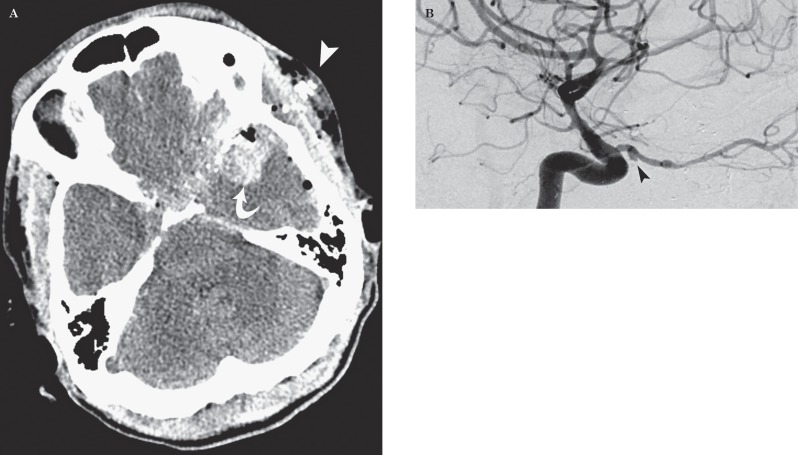
Figure 3 Gunshot injury to the head in a 47-year-old man. A) Axial CT image demonstrates an entry wound over the left pterional region (arrowhead) with a left temporal lobe hematoma (curved arrow). Also seen is the trajectory extending in proximity to circle of Willis. B) DSA image demonstrates a 3 mm TICA (arrowhead) arising from the proximal third of the left ophthalmic artery and irregularity of the intraorbital segment of the artery.
To describe the entry and exit wounds of the ballistics, the cranium is divided into three regions, frontobasal (Figure 2), which also includes orbitofacial injuries, transtemporal injuries which include the pterion (Figures 1 and 3), temporal bone, greater wing of sphenoid bones and third, occipital (Figure 4) or suboccipital injuries. The injuries are further divided into perforating injuries when the profile includes an entry and exit wound with a tract through the brain parenchyma, while penetrating injuries are defined as a projectile penetrating the parenchyma without an exit wound. To describe the injury pattern and location (contusions, lacerations, hematomas and for describing the trajectory of the projectile), the brain was divided into anterior, middle and posterior cranial fossa. Proximity to the circle of Willis (Figure 3) is defined as the trajectory of the wound within 2 cm of the suprasellar cistern, which is the mean length of the M1 segment of the middle cerebral artery (MCA).
Figure 4.
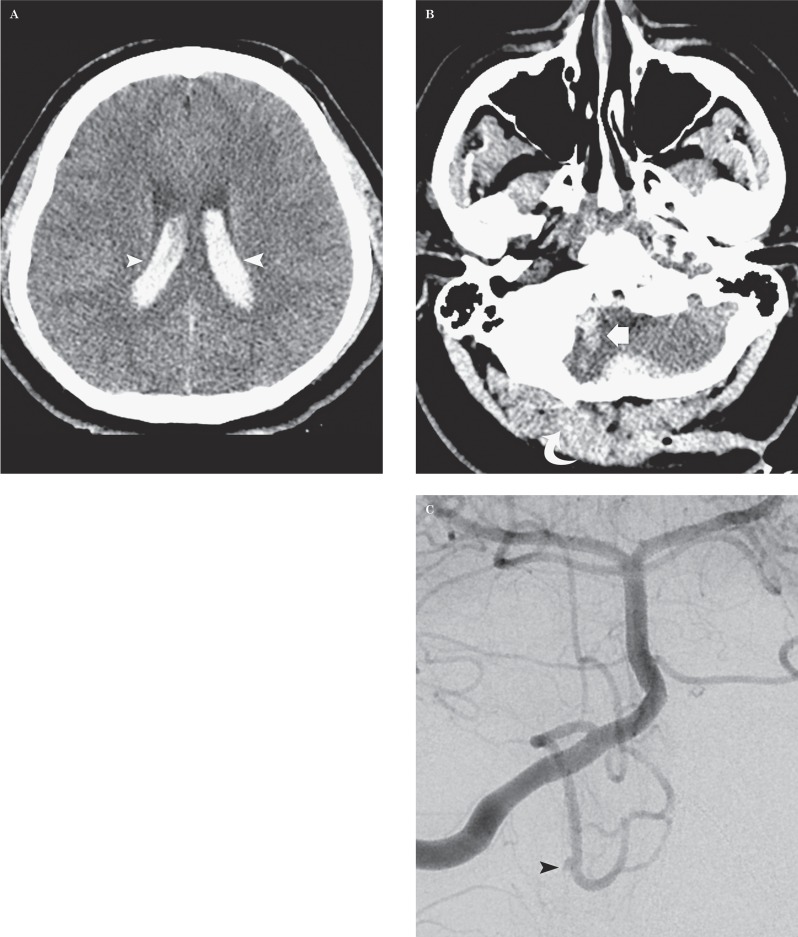
Stab injury to the head in a 22-year-old man. A,B) Axial CT images demonstrate a right occipital penetrating injury (curved arrow) with a large amount of intraventricular hemorrhage (arrowheads) and blood along the right cerebellar trajectory (arrow). C) DSA images on the day of admission demonstrate a 1.5 mm traumatic intracranial aneurysm (TICA) (arrowhead) from a medullary loop of the right posterior inferior cerebellar artery (PICA).
The reference standard was the originally reported interpretation of the DSA performed during the hospital course. The reports were further confirmed by direct review of the images.
For every patient, an individual sum score was generated by assigning scores to presenting signs on CT images from sets of CT scores predefined by the radiologist. Scores for every patient can differ among radiologists as scores depended on relative weights assigned to each CT sign.
After obtaining the CT scores for all 54 patients, ROC curves were constructed for each set to assess the diagnostic performance of an individual radiologist in predicting an underlying vascular injury.
Statistical Analysis
ROC curves, which serve as a graphic representation of accuracy of the variable scoring system, were generated for each radiologist and for the average score from all radiologists. The AUC and 95% confidence intervals (CIs) were generated. AUC were compared among the six data sets using statistical software (Analyse-it Software Method Evaluation edition edition for Microsoft Excel, version 2.30 [www.analyse-it.com]).
Results
There were 21 patients with arterial injuries out of the total 54 patients with penetrating brain injury. Of these, five patients had multiple injuries.
Two patients had three different sites of injuries and three patients had two sites of injuries. A total of 28 arterial injuries were identified in these patients with 11 TICAs (Figures 3 and 4), eight arterial dissections (Figure 1), six arterial occlusions, two carotid-cavernous fistulas and one middle meningeal artery to ophthalmic vein fistula (Figure 2). The injuries were located at the middle cerebral artery (n=10), the internal carotid artery (ICA) (n=8), the anterior cerebral artery (ACA) (n=7), the ophthalmic artery (n=2) and posterior inferior cerebellar artery (n=1).
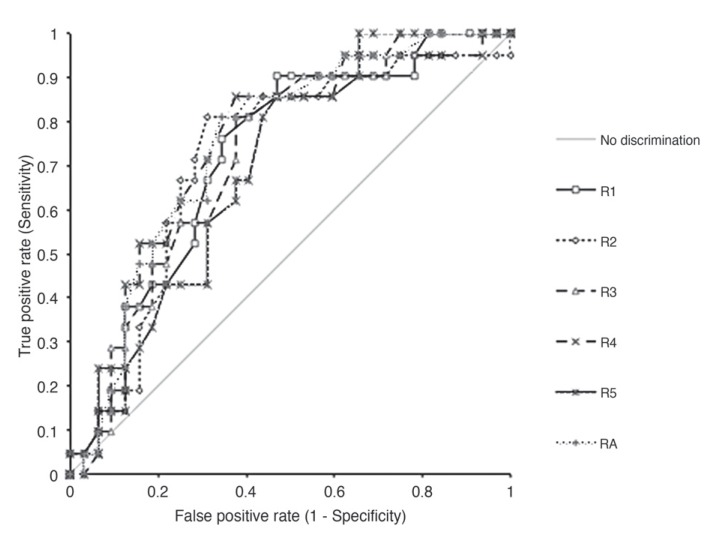
The ROC curves are shown in Figure 5. The AUC was higher for CT scores obtained from the sixth set of the variable rank score (0.75), which consisted of average scores from the five data sets from the individual radiologist. The AUCs for the remaining data sets ranged from 0.70 to 0.74. The individual AUCs and 95% CIs are shown in Table 3. The performance and comparison of individual sets of scorings showed no statistically significant differences in the AUCs (p >0.05), or a trend towards improved accuracy with experience of the radiologists.
Table 3.
Discriminatory ability of the individual radiologists and the average score of five sets shown as area under the ROC curves (AUC).
| AUC | 95% CI | |
| R1 | 0.72 | 0.59 to 0.85 |
| R2 | 0.72 | 0.58 to 0.87 |
| R3 | 0.73 | 0.59 to 0.86 |
| R4 | 0.74 | 0.60 to 0.88 |
| R5 | 0.7 | 0.56 to 0.84 |
| RA | 0.75 | 0.62 to 0.88 |
| R: individual radiologists; RA: AUC obtained from average of five sets of scores. | ||
Figure 5.
Comparison of discrimination ability of the individual radiologists and average score by ROC curves.
Discussion
Early recognition and appropriate management of intracranial vascular injuries after penetrating brain injury could result in a significant reduction in morbidity and mortality. Until now, these vascular injuries have traditionally been diagnosed by DSA. However, it is an invasive technique and is associated with complications. Hence, DSA is recommended in select patient groups, deemed ‘high-risk' for developing intracranial vascular injuries.
Our results demonstrate that radiologists may have a fair accuracy (AUC=0.75) in predicting the risk of vascular injuries by using the CT findings of injury profile after penetrating head injury.
Evaluation of the individual accuracy rate of the radiologists did not demonstrate an improved performance based on their clinical experience.
Studies from the Iran-Iraq conflict and Lebanon have suggested the risk factors for the development of intracranial pseudo-aneurysms, based on entry and exit sites, presence of intracerebral hematoma, wound profile crossing dural compartments, presence of retained fragments, absence of exit wound, presence of hematoma in the distal portion of the trajectory, and presence of delayed parenchymal bleed or SAH2,5. Similarly, Amirjamshidi et al. have suggested angiography in patients with missile passage through Reil's triangle or transhemispheric injuries, large hematoma at the entry site and multiple shells and bone fragments scattered in paths that branch into various directions3. Identification of such risk factors and formulating a scoring system based on CT findings (i.e., a high score to a valuable variable) can help clinicians prompt DSA in high-risk patient groups.
Our study has several limitations. First, it is a retrospective review. Second, the variable ranking is performed hypothetically by the individual radiologists without prior statistical data or reports. Use of two sets of patient population, a training set and another validation set would be optimal for such type of variable ranking.
Conclusions
Radiologists may be able to recommend DSA with a fair accuracy rate in select patients, deemed ‘high-risk' for developing intracranial vascular injuries after penetrating traumatic brain injury based on admission CT studies. The ‘high-risk' patients may be identified by the variable scoring system. However, the CT scoring needs to be improved to increase the accuracy of the system in order to become clinically relevant and decrease the number of unnecessary invasive DSA procedures.
References
- 1.Aarabi B. Traumatic aneurysms of brain due to high velocity missile head wounds. Neurosurgery. 1988;22(6):1056–1063. doi: 10.1227/00006123-198806010-00014. doi: 10.1227/00006123-198806010-00014. [DOI] [PubMed] [Google Scholar]
- 2.Aarabi B. Management of traumatic aneurysms caused by high-velocity missile head wounds. Neurosurg Clin N Am. 1995;6(4):775–797. [PubMed] [Google Scholar]
- 3.Amirjamshidi A, Rahmat H, Abbassioun K. Traumatic aneurysms and arteriovenous fistulas of intracranial vessels associated with penetrating head injuries occurring during war: principles and pitfalls in diagnosis and management. A survey of 31 cases and review of the literature. J Neurosurg. 1996;84(5):769–780. doi: 10.3171/jns.1996.84.5.0769. doi: 10.3171/jns.1996.84.5.0769. [DOI] [PubMed] [Google Scholar]
- 4.Bell RS, Vo AH, Roberts R, et al. Wartime traumatic aneurysms: acute presentation, diagnosis, and multimodal treatment of 64 craniocervical arterial injuries. Neurosurgery. 2010;66(1):66–79. doi: 10.1227/01.NEU.0000361285.50218.A8. doi: 10.1227/01.NEU.0000361285.50218.A8. [DOI] [PubMed] [Google Scholar]
- 5.Haddad FS, Haddad GF, Taha J. Traumatic intracranial aneurysms caused by missiles: their presentation and management. Neurosurgery. 1991;28(1):1–7. doi: 10.1097/00006123-199101000-00001. doi: 10.1227/00006123-199101000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Jinkins JR, Dadsetan MR, Sener RN, et al. Value of acute-phase angiography in the detection of vascular injuries caused by gunshot wounds to the head: analysis of 12 cases. Am J Roentgenol. 1992;159(2):365–368. doi: 10.2214/ajr.159.2.1632358. doi: 10.2214/ajr.159.2.1632358. [DOI] [PubMed] [Google Scholar]
- 7.Levy ML, Rezai A, Masri LS, et al. The significance of subarachnoid hemorrhage after penetrating craniocerebral injury: correlations with angiography and outcome in civilian population. Neurosurgery. 1993;32(4):532–540. doi: 10.1227/00006123-199304000-00007. doi: 10.1227/00006123-199304000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Aarabi B, Alden T, Chestnut R. Vascular complications of penetrating brain injury. J Trauma. 2001;51(2 suppl):S26–S28. [PubMed] [Google Scholar]
- 9.Salehi MG, Ghanaati H, Abedini M, et al. Traumatic dissecting posterior cerebral artery aneurysm. A case report and review of the literature. Neuroradiol J. 2012;25(5):563–568. doi: 10.1177/197140091202500509. [DOI] [PubMed] [Google Scholar]
- 10.Takeshita T, Hayashi K, Horie N, et al. Endovascular treatment of intractable bleeding from a traumatic pseudoaneurysm of the internal maxillary artery. Neuroradiol J. 2012;25(4):469–474. doi: 10.1177/197140091202500409. [DOI] [PubMed] [Google Scholar]
- 11.Sliker CW, Shanmuganathan K, Mirvis SE. Diagnosis of blunt cerebrovascular injuries with 16-MDCT: accuracy of whole-body MDCT compared with neck MDCT angiography. Am J Roentgenol. 2008;190(3):790–799. doi: 10.2214/AJR.07.2378. doi: 10.2214/AJR.07.2378. [DOI] [PubMed] [Google Scholar]
- 12.Hijdra A, Brouwers PJ, Vermeulen M, et al. Grading the amount of blood on computed tomograms after subarachnoid hemorrhage. Stroke. 1990;21(8):1156–1161. doi: 10.1161/01.str.21.8.1156. doi: 10.1161/01.STR.21.8.1156. [DOI] [PubMed] [Google Scholar]
- 13.Nayak S, Kunz AB, Kieslinger K, et al. Classification of non-aneurysmal subarachnoid haemorrhage: CT correlation to the clinical outcome. Neuroradiol J. 2011;24(5):715–725. doi: 10.1177/197140091102400508. [DOI] [PubMed] [Google Scholar]
- 14.Graeb DA, Robertson WD, Lapointe JS, et al. Computed tomographic diagnosis of intraventricular hemorrhage. Etiology and prognosis. Radiology. 1982;143(1):91–96. doi: 10.1148/radiology.143.1.6977795. [DOI] [PubMed] [Google Scholar]