Summary
Desmoplastic infantile ganglioglioma (DIG) is a rare supratentorial tumor in the central nervous system. Definitive diagnosis of this neoplasm is based on histopathologic analysis evaluating distinctive findings such as the fibroblastic differentiation. Here we present a clinical case of DIG with a long follow-up in an eight-year-old boy with a six-month history of recurrent emesis, psychomotor hyperactivity and generalized tonic-clonic seizures. Computed tomography scan and magnetic resonance imaging (MRI) showed a cystic, heterogeneous, mass on the right temporal uncus. A histopathological diagnosis of late presentation DIG was made. We documented the immunohistochemical expression of a molecular soft tissue / muscle differentiation marker (h-CaD) in addition to a low proliferative index (Ki-67) in this case. After surgical intervention, a control MRI showed changes of right frontal-temporal craniotomy and a persistent mass in the anterior and medial temporal lobe with basal extension. Further surgical intervention was performed, completely removing the tumor, which had the same characteristics. The patient is asymptomatic while receiving anticonvulsant therapy (phenytoin) with no evidence of tumor recurrence on MRI after a follow-up of five years. The low grade and soft tissue appearance in images are correlated with the histopathologic and immunohistochemical profile of this tumor, but the rarity of this tumor makes a presumptive diagnosis by images a challenge. The above-mentioned molecular markers or new ones could be used as molecular targets for molecular imaging studies to increase the probability of a pre-operative diagnosis based on molecular features through images.
Keywords: desmoplastic ganglioglioma, radiology, histopathology, immunohistochemistry, children's tumors
Introduction
Desmoplastic infantile ganglioglioma (DIG) is a rare supratentorial tumor of the central nervous system (CNS) accounting 0.1-1.0% of intracranial tumors in children 1,2. DIG belongs to the neuronal/glioneuronal tumor group, and DIG along with children's desmoplastic astrocytoma (ChDA) are the desmoplastic CNS neoplasms 2,3. DIGs typically have a better prognosis than gliomas, and are identified based on the pathologic findings of neoplastic neurons with neuronal clustering, loss of organized distribution, size variability, differing stages of maturation, fibroblastic background and cytological atypia.
DIGs generally appear during the first 18 months of life (although by definition a diagnosis may be made between 0 and 60 months of age), and have been cataloged by the World Health Organization (WHO) classification of tumors as having benign behavior (grade I), in contrast to the malignant behavior seen in the majority (85%) of children's CNS tumors 4.
Over the last decade, significant progress has been made in unraveling the genetic pathways underlying the large variety of glial neoplasms 5. Nevertheless, due to their rarity, the genesis of DIGs has not been well studied. Some reports have shown genetic alterations (9 and 22q array loss and in V600E BRAF mutation) in two cases of DIG 6. Some authors have suggested that these neoplasms can be considered congenital tumors, while others have described cases in older children 7.
The most distinctive histopathological finding in DIG is the fibroblastic differentiation. This characteristic feature allows the formation of a complex extracellular matrix and intracellular cytoskeleton in the neoplastic cells 2-4. Here we describe a new clinical case with a long follow-up of an extremely rare presentation of DIG in a patient older than five years. A similar presentation was first described by Kuchelmeister et al. as non-infantile desmoplastic ganglioglioma 8. In addition, we correlate the histopathology, immunohistochemistry and radiological images.
Case Report
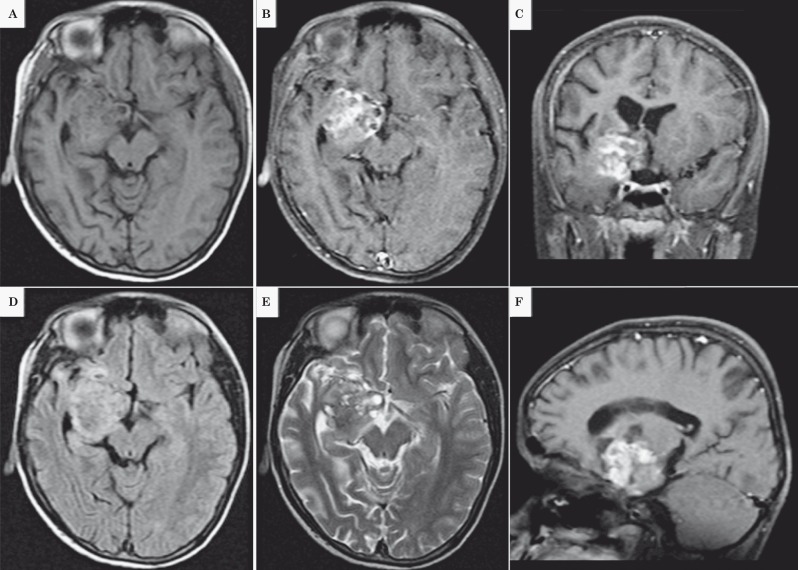
The patient is an eight-year-old boy with a six-month history of recurrent emesis, psychomotor hyperactivity and generalized tonic-clonic seizures. The parents reported no previous medical issues. He was without other physical and neurological abnormalities. Computed tomography (CT) scan showed a cystic, hypodense mass on the right temporal uncus with a peripheral hyperintensity. Magnetic resonance imaging (MRI) T1 scans showed hypodensity on cystic areas and isointensity on solid ones with contrast (Figure 1A-C). On T2 and FLAIR the cystic area was hyperintense and the solid component was heterogeneous (Figure 1D-F).
Figure 1.
A-C) T1 scans showed hypodensity on cystic areas and isointensity on solid ones with contrast. D-E) On T2 and FLAIR the cystic area was hyperintense and the solid component was heterogeneous (Figure 1D, 1E and 1F).
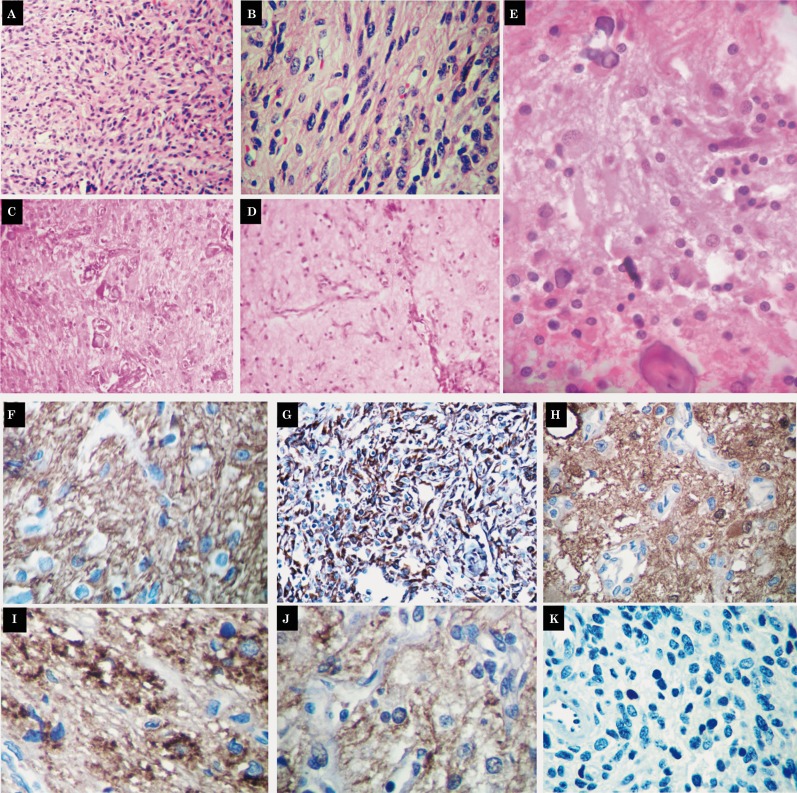
A surgical resection of the tumor was made via craniotomy. A heterogeneous vascularized and infiltrative, cystic tumor was found. Upon histopathological study a glioneuronal tumor was recognized, comprising three distinct components. The first corresponded to desmoplastic stroma with spindled astrocytes positive for glial fibrillary acidic protein (GFAP) and h-caldesmon (dilution 1:200; Thermo ScientificTM) (Figure 2 A-G). The second component was composed of primitive neuroepithelial cells positive for PGP9.5, vimentin and neurofilament. The third component consisted of abnormal neurons positive for synaptophysin, chromogranin, PGP9.5, neurofilament and neuron-specific enolase (Figure 2H-J). CD99, Fli-1, EMA, AE1/AE3 and desmin were negative. The proliferation index (Ki67) was low (1%) (Figure 2K). A diagnosis of late presentation DIG was made.
After surgical intervention, a control MRI showed changes of right frontal-temporal craniotomy and a persistent mass in the anterior and medial temporal lobe. Further surgical intervention was performed three months later, completely removing the tumor, which had the same characteristics as in previous histopathologic and immunohistochemical study. Currently, the patient is asymptomatic while receiving anticonvulsant therapy (phenytoin) with no evidence of tumor recurrence on MRI after a follow-up of five years.
Figure 2.
Histopathological study disclosed a central nervous tumor with desmoplastic stroma (A,B, HE 50×) and spindled or elongated astrocytes (C {HE 200×}, D {HE 200×}, E {HE 400x}) positive for GFAP (F, 400×) and h-caldesmon (G, 400×). Additional findings included primitive neuroepithelial cells positive for PGP9.5 (H, 400×), and abnormal neurons positive for synaptophysin (I, 400×), PGP9.5 (H, 400×), and neurofilament (J, 400×). The proliferation index (Ki67) was 1% (K, 400×). Microcalcifications (C,E) and angiomatoid blood vessels (C,D) were recognized.
Discussion
Taratuto et al. first described DIG in 1984 in six patients with brain tumors, as a rare, benign lesion that generally affects children. They typified this tumor as a “superficial cerebral astrocytoma attached to dura mater”, suggesting the probability of a new tumor previously unrecognized 9. In 1987, Vandenberg et al. used the term desmoplastic ganglioglioma to report 11 cases of ChDA and DIG, and recognized these tumors as a particular group of entities 7. These desmoplastic tumors were included in the WHO classification in 1993 as desmoplastic infantile astrocytoma. In the same year, Kuchelmeister et al. published the first two cases of late presentation DIG 8. According to our literature search, approximately 70 cases of DIG have been reported, thus making it one of the rarest CNS tumors. The late presentation of DIG is more infrequent, with only 20 cases reported after five years of age. The current WHO classification classifies these desmoplastic tumors as low-grade superficially located (cortical) neuroepithelial childhood tumors with two subtypes, ChDA and DIG, based on the absence of the neuronal component in the ChDA 4.
DIG is slightly more frequent in boys (male-female ratio of 1.7:1). The most common clinical manifestation is macrocephaly secondary to hydrocephalus (40%), followed by seizures (20%), bulging fontanelles, bony bossing, visual disturbance, and paresis 1,2,10. There is also a reported case in which an intracranial hemorrhage manifested in a nine-day-old neonate 11.
DIG has distinctive macroscopic features: large size, with uni or multiloculated cysts filled with clear or xanthochromic fluid, affecting the temporal, frontal and parietal lobes, without hemispheric predilection. There have also been cases reported involving the occipital lobe, brain stem, thalamus and suprasellar region 2,3. Multilobar presentation is frequently observed (nearly 60% of cases) 3,4. The above gross pathology characteristics, are important when evaluating neuroimaging studies, and may aid in the diagnosis of DIG, but can also be seen in other CNS neoplasms 1. On CT scans these tumors are voluminous with a mixed, solid and cystic appearance, usually have dura mater compromise and moderate to low edema, hypodense or slightly hyperdense in the superficial portion that extends to the overlying meninges, and show intense contrast enhancement 1. The cystic portion is usually located deep, whereas the solid portion is peripheral. MRI is the most useful radiological diagnostic method for DIG, often showing a large cystic supratentorial tumor generally involving more than one lobe, which is hypointense in T1 and hyperintense in T2, with solid areas at times demonstrating ossification and calcification 3.
Histopathologically, DIG is characterized by a desmoplastic stroma in which neuroepithelial elements are found in varying amounts with fibroblastic differentiation 4. The neuronal elements can be observed in different stages of maturation, demonstrating small and medium size ganglion cells displayed in a diffuse form or in groups within the desmoplastic stroma, which may contain eosinophilic granular bodies and perivascular lymphocytic infiltrate 3,4. The presence of gemistocytic bodies has also been reported. Additionally, these tumors can possess undifferentiated cells with mitotic and necrotic figures, which can be anaplastic areas. The presence of this rare particular characteristic, as well as necrosis and endothelial proliferation, may indicate a less favorable prognosis 12.
The immunohistochemical profile of DIG shows that the fusiform cells are positive for GFAP as in the case presented here, the neurons are positive for synaptophysin, NeuN, PGP9.5, neurofilament, and specific neuronal enolase, while the stroma presents a prominent reticulin rete positive with Masson trichrome. The proliferation index (Ki67) is generally low (0.5-15%) 4,10 and correlated with some low-grade features observed on radiologic images.
In this particular case we observed the expression of h-CaD in the fusiform cells. Currently h-CaD immunohistochemical expression is very useful to distinguish smooth muscle cells/lesions, epithelioid mesothelioma, gastrointestinal stromal tumor, glomus tumor, myopericytoma and perivascular epithelioid cell tumors 13. CaD is an actin-binding protein, which stabilizes actin filaments and participates in the regulation of the actomyosin ATPase 14,15. Two isoforms, h-CaD (high molecular weight CaD) and l-CaD (low molecular weight CaD), are preferentially expressed in smooth muscle and non-muscle cells. CaD isoforms are most likely generated from a single gene by alternative splicing. CaD has been recognized as a substrate for many kinases 16. Both extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein (MAP) kinase phosphorylated h-CaD. These kinases dynamically regulate phosphorylation during cell proliferation 16,17.
In addition, the ERK MAPK-CaD pathway may modulate PDGF-stimulated cell migration. Recently, CaD has been implicated in the regulation of cell remodeling and cell motility 17. Generally, CaD expression has been shown to be downregulated in transformed cells, which may contribute to increased motility and invasiveness. Cell migration is a multi-staged process requiring dynamic cytoskeletal rearrangements 17. This may be a cause of the benign behavior observed in many DIGs.
The above-mentioned molecular characteristics can be used as targets to develop markers for molecular image studies based on MRI or CT, with the development of antibodies or other minimal sub-unit markers ligated with contrast agents for ex-situ analysis.
To confirm the hypothesis of h-CaD's potential utility in DIG diagnosis, reproducibility and performance studies need to be conducted. On the other hand, other tumors considered in the differential diagnosis of DIG need to be evaluated for h-CaD expression, such as neuronal-glioneuronal tumors (i.e. pleomorphic xanthoastrocytoma), PNET, typical gangliogliomas, supratentorial ependymomas, astrocytomas, and atypical rhabdoid teratoid tumors.
Surgical resection is possible in 70% of DIG cases. Complete resection provides better outcomes than partial resection, even if partial resection is combined with adjuvant therapy, with illness-free periods of 8.3 to 20 years 1,4,10. Occasionally, spontaneous regression has been described 18. In a few cases, DIG can behave aggressively and may present with adjacent parenchymal infiltration, with tumors demonstrating anaplasia, necrosis, and a high proliferation index. Such cases may be rapidly fatal, and may benefit from adjuvant chemotherapy 19. Only one malignant transformation to glioblastoma multiforme, in a two-year-old girl, has been reported 20. Radiotherapy is avoided in the management of DIG as it can interfere with encephalic development in such young patients 10,12.
In conclusion, an adequate correlation of clinical manifestations, and radiologic/histopathologic features is vital for correct diagnosis and management. New molecular imaging studies that allow the identification of molecular markers for low-grade and soft tissue differentiation in ex-situ studies could increase the odds for a presumptive diagnosis.
References
- 1.Taranath A, Lam A, Wong CK. Desmoplastic infantile ganglioglioma: a questionably benign tumour. Australas Radiol. 2005;49(5):433–437. doi: 10.1111/j.1440-1673.2005.01479.x. [DOI] [PubMed] [Google Scholar]
- 2.Bhardwaj M, Sharma A, Pal HK. Desmoplastic infantile ganglioglioma with calcification. Neuropathology. 2006;26(4):318–322. doi: 10.1111/j.1440-1789.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 3.Nikas I, Anagnostara A, Theophanopoulou M, et al. Desmoplastic infantile ganglioglioma: MRI and histological findings case report. Neuroradiology. 2004;46(12):1039–1043. doi: 10.1007/s00234-004-1283-2. [DOI] [PubMed] [Google Scholar]
- 4.Brat DJ, VandenBerg SR, Figarella-Branger D, et al. Desmoplastic infantile astrocytoma and ganglioglioma. In: Louis DN, et al., editors. WHO Classification of tumours of the central nervous system. Lyon: Intl. Agency for Research; 2007. 4th ed. [Google Scholar]
- 5.Zheng PP, Hop WC, Sillevis Smitt PA, et al. Low-molecular weight caldesmon as a potential serum marker for glioma. Clin Cancer Res. 2005; 11(12):4388–4392. doi: 10.1158/1078-0432.CCR-04-2512. [DOI] [PubMed] [Google Scholar]
- 6.Dougherty MJ, Santi M, Brose MS, et al. Activating mutations in BRAF characterize a spectrum of pediatric low-grade gliomas. Neuro Oncol. 2010;12(7):621–630. doi: 10.1093/neuonc/noq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.VandenBerg SR, May EE, Rubinstein LJ, et al. Desmoplastic supratentorial neuroepithelial tumors of infancy with divergent differentiation potential (“desmoplastic infantile gangliogliomas”). Report on 11 cases of a distinctive embryonal tumor with favorable prognosis. J Neurosurg. 1987;66(1):58–71. doi: 10.3171/jns.1987.66.1.0058. [DOI] [PubMed] [Google Scholar]
- 8.Kuchelmeister K, Bergmann M, von Wild K, et al. Desmoplastic ganglioglioma: report of two non-infantile cases. Acta Neuropathol. 1993;85(2):199–204. doi: 10.1007/BF00227768. [DOI] [PubMed] [Google Scholar]
- 9.Taratuto AL, Monges J, Lylyk P, et al. Superficial cerebral astrocytoma attached to dura. Report of six cases in infants. Cancer. 1984;54(11):2505–2512. doi: 10.1002/1097-0142(19841201)54:11<2505::aid-cncr2820541132>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 10.Gelabert-Gonzalez M, Serramito-García R, Arcos-Algaba A. Desmoplastic infantile and non-infantile ganglioglioma. Review of the literature. Neurosurg Rev. 2010;34(2):151–158. doi: 10.1007/s10143-010-0303-4. [DOI] [PubMed] [Google Scholar]
- 11.Tekkök IH, Ventureyra EC. Spontaneous intracranial hemorrhage of structural origin during the first year of life. Childs Nerv Syst. 1997;13(3):154–165. doi: 10.1007/s003810050061. [DOI] [PubMed] [Google Scholar]
- 12.Al-Sarraj ST, Bridges LR. Desmoplastic cerebral glioblastoma of infancy. Br J Neurosurg. 1996;10(2):215–219. doi: 10.1080/02688699650040412. [DOI] [PubMed] [Google Scholar]
- 13.Comin CE, Dini S, Novelli L, et al. h-Caldesmon, a useful positive marker in the diagnosis of pleural malignant mesothelioma, epithelioid type. Am J Surg Pathol. 2006;30(4):463–469. doi: 10.1097/00000478-200604000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Wang CL. Caldesmon and the regulation of cytoskeletal functions. Adv Exp Med Biol. 2008;644:250–272. doi: 10.1007/978-0-387-85766-4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang R, Grabarek Z, Wang CL. Differential effects of caldesmon on the intermediate conformational states of polymerizing actin. J Biol Chem. 2010;285(1):71–79. doi: 10.1074/jbc.M109.065078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng PP, Sieuwerts AM, Luider TM, et al. Differential expression of splicing variants of the human caldesmon gene (CALD1) in glioma neovascularization versus normal brain microvasculature. Am J Pathol. 2004;164(6):2217–2228. doi: 10.1016/S0002-9440(10)63778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirzapoiazova T, Kolosova IA, Romer L, et al. The role of caldesmon in the regulation of endothelial cytoskeleton and migration. J Cell Physiol. 2005;203(3):520–528. doi: 10.1002/jcp.20244. [DOI] [PubMed] [Google Scholar]
- 18.Takeshima H, Kawahara Y, Hirano H, et al. Postoperative regression of desmoplastic infantile gangliogliomas: report of two cases. Neurosurgery. 2003;53(4):979–983. doi: 10.1227/01.neu.0000084165.60662.6d. [DOI] [PubMed] [Google Scholar]
- 19.De Munnynck K, Van Gool S, Van Calenbergh F, et al. Desmoplastic infantile ganglioglioma: a potentially malignant tumor? Am J Surg Pathol. 2002;26(11):1515–1522. doi: 10.1097/00000478-200211000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Loh JK, Lieu AS, Chai CY, et al. Malignant transformation of a desmoplastic infantile ganglioglioma. Pediatr Neurol. 2011;45(2):135–137. doi: 10.1016/j.pediatrneurol.2011.04.001. [DOI] [PubMed] [Google Scholar]