Summary
Gliosarcoma is a rare central nervous system (CNS) neoplasm with biphasic glial and non-glial malignant components. Here we describe the radiologic and histopathologic features observed in five cases of primary gliosarcoma. The mean age at diagnosis in the studied patients was 54.2 years; these patients were predominantly males (male:female ratio = 4:1). At diagnosis all patients had several clinical deterioration. The most common symptoms of presentation were: headache (5/5 cases), seizures (4/5 cases) and hemiparesis (1/5 cases). All the tumors were large (mean major diameter= 4.12±1.64 cm) at diagnosis as evidenced in computer tomography (CT) scans and magnetic resonance images (MRIs), with preferential involvement of the temporal lobe and frequent associated deviation of the midline structures. Other common characteristics identified on CT scans and MRIs were partial contrast medium uptake with annular pattern (5/5 cases), peripheral edema (5/5 cases), and central calcification (3/5 cases). In additional a peak of dye uptake was observed (4/5 cases) on MRI spectrometry. In the histopathology, the glial component showed malignant astrocytes, with high Ki67 (>60%) and p53 positivity; the sarcomatous components displayed pleomorphic spindle cells similarly with p53 positivity and high Ki67 (75-90%) in all cases. Dedifferentiation to pleomorphic sarcoma (two cases), fibrosarcoma (one case), leiomyosarcoma (one case) and MPNST (one case) were documented. All patients received radiotherapy/chemotherapy and had a median overall survival of ten months. The study of radiologic and histopathologic features in primary gliosarcomas of the brain is a priority to achieve early diagnosis that can be translated to better outcomes. Here we describe the radiologic and histopathologic features observed in a group of gliosarcoma patients with variable histopathologic dedifferentiation.
Keywords: glioblastoma multiforme, gliosarcoma, histopathology, radiology, immunohistochemistry, neurosurgery
Introduction
Gliosarcoma is defined as a biphasic neoplasm of glial origin with mixed areas of malignant mesenchymal differentiation 1. Stroebe in 1895 first suggested the diagnosis of this neoplasm and used the term gliosarcoma to describe a tumor composed of a combination of glioblastoma and sarcoma 2. Subsequently, Feign and Gross in 1955 also used the name gliosarcoma to describe this neoplasm, and they suggested a proliferation of newly formed blood vessels as the origin of the sarcomatous component 3. Currently, there are multiple hypotheses regarding gliosarcoma origin. It is mainly believed that the tumor can be the product of a glioblastoma with metaplastic change. Other hypotheses are the malignant transformation of glial cells (astrocytes) that surround a preexisting sarcoma, or are merely the unfortunate coexistence of two separate tumors 2,4.
Here we reviewed the clinical presentation, radiology, histopathology and immunohistochemistry observed in five cases of primary gliosarcoma. We analyze the characteristics of this tumor and correlate them on the basis of previous reports of this rare central nervous system (CNS) neoplasm.
Materials and Methods
Study design, patients and samples
A retrospective observational study was conducted. Clinical, radiologic and pathological information was obtained and tissue samples form histology slides and paraffin-embedded tissue were reviewed. This study was performed at the National Institute of Cancer in Bogotá, Colombia (www.cancer.gov.co), the largest cancer center in Colombia and the national reference institution for the management of cancer.
We conducted a search to identify all cases of primary gliosarcoma in the databases of the Department of Pathology and the Department of Radiology from January 2005 to December 2009. We found five patients diagnosed with primary gliosarcoma, secondary cases were excluded. The clinical information of these patients, radiologic studies and histopathologic slides and paraffin-embedded tissues were retrieved. We evaluate the paraffin-embedded tissues from these patients through histopathology and immunohistochemistry. The study was conducted in accordance with the declaration of Helsinki and received previous approval by the Institutional Review Board of the Departments of Neurosurgery, Radiology and Pathology.
Clinical and radiologic evaluation
We reviewed the clinical records with focus on age, gender, clinical presentation, duration of symptoms, tumor localization, surgical reports, adjuvant treatment, and follow-up. We determined performance status at presentation with the use of the Karnofsky performance status index (KI). We obtained the computed tomography (CT) scans and magnetic resonance images (MRIs) of these patients and a board certified neuroradiologist performed a new reading. The overall survival (OS) was determined by reviewing the clinical data and determining the date of CNS tumor diagnosis to date of death as a result of any cause. The response to treatment was evaluated with the RECIST guidelines for response evaluation criteria version 1.1 5.
Histopathologic and immunohistochemical evaluation
All the tissues samples analyzed from the five patients, before being included in this study, were confirmed by two pathologists blinded to the previous diagnosis who reviewed the histologic slides stained with hematoxylin and eosin obtained from tissues previously fixed in 10% buffered formalin and embedded in paraffin.
We prepare and analyzed all the case samples with an immunohistochemistry panel to evaluate glial, mesenchymal, and neuronal elements, and determine mesenchymal dedifferentiation, and exclude metastasis (Table 1).
Table 1.
Immunohistochemistry panel used in the study.
| Stain | Clone | Dilution | Brand | Use |
| GFAP | GF2 | 1:50 | Dako-Cytomation | Glial differentiation |
| Vimentin | V-9 | 1:200 | Biogenes | Mesenchymal differentiation |
| Smooth muscle actin | ZMSA-5 | 1:50 | Dako-Cytomation | Muscle differentiation |
| Muscle specific actin | HHF35 | Prediluted | Dako-Cytomation | Muscle differentiation |
| H-caldesmon | h-cald | 1:200 | Thermo Scientific | Muscle differentiation |
| Neurofilament | 2F11 | 1:50 | Dako Cytomation | Neuronal differentiation |
| Chromogranin A | DAK-A3 | 1:100 | Dako Cytomation | Neuronal differentiation |
| Synapthophysin | SNP88 | 1:100 | BioGenex | Neuronal differentiation |
| PGP 9.5 | 1:50 | Dako Cytomation | Neuronal differentiation | |
| S100 | 4C4.9 | 1:50 | Neomarkers | Neural crest differentiation |
| CD56 | BL56CO4 | 1:50 | Biocare Medical | Neural crest differentiation |
| CD57 (Leu-7) | NK1 | 1:200 | Neomarkers | Neural crest differentiation |
| CD31 | MEM-05 | 1:50 | ZYMED | Blood vessels identification |
| CD34 | QBEND | 1:50 | BioGenex | Blood vessels identification |
| CD68 | KPI | 1:50 | Dako Cytomation | Pleomorphic xanthoastrocytoma |
| CD99/MIC2 | HO36-1.1 | 1:100 | Thermo Scientific | Other grade IV neoplasms* |
| FLI1 | FLI1 | Prediluted | Thermo Scientific | Other grade IV neoplasms* |
| BCL-2 | Bcl2/100/D5 | 1:50 | Novocastra | Molecular marker analyzed |
| p53 | DO.7 | 1:25 | Dako Cytomation | Molecular marker analyzed |
| Ki67 | MIB1 | 1:100 | Dako Cytomation | Molecular marker analyzed |
| EMA | E29 | 1:50 | Dako Cytomation | Epithelial cancer metastasis |
| Keratin | AE1AE3 | 1:100 | Dako Cytomation | Epithelial cancer metastasis |
* Primitive neuroectodermal tumor, medulloblastoma.
The immunohistochemistry panel was composed of: glial fibrillary acid protein (GFAP) to evaluate glial differentiation; vimentin to evaluate mesenchymal differentiation; muscle specific actin (HHF35), desmin, smooth muscle actin (SMA) and h-caldesmon to evaluate muscular differentiation; neurofilament (NF), chromogranin, synaptophysin, and PGP9.5 to evaluate neuronal differentiation; S-100 protein, CD56, CD57 to evaluate neural crest components; CD34 and CD31 to evaluate blood vessels; and CD99, FLI-1, CD68 to exclude another CNS tumor diagnosis such as other grade IV neoplasms, and pleomorphic xanthoastrocytoma. We evaluated in all cases p53 and bcl-2 immunohistochemical expression and determined the proliferation index with Ki67 (MIB-1) expression. Additionally, we performed immunoreactivity for epithelial membrane antigen (EMA) and pankeratin (AE1AE3) to exclude metastatic sarcomatoid carcinoma. All the immunohistochemical panel stains are listed in Table 1.
To prepare the immunohistochemistry slides neutral buffered formalin-fixed paraffin-embedded tissue blocks were processed by routine methods, and 3 μm sections were obtained. Sections were deparaffinized and dehydrated. Sections were microwaved for 15 minutes in 1 mM EDTA (pH 8.0), cooled, and treated with 3% H2O2 in 10% methanol to inhibit endogenous peroxidase activity, blocked for 15 minutes at RT in solution-B (10% horse serum [Vector Laboratories, Burlingame, CA, USA], 5% BSA, and 0.3% Triton X-100 in PBS) and incubated overnight at 4°C with the appropriate primary antibody. Following washing, sections were stained, for one hour, with a goat anti–rabbit biotinylated horseradish peroxidase H-conjugated secondary antibody followed by Avidin. Sections were washed in PBS and incubated for five minutes with substrate for peroxidase. Slides were counterstained with hematoxylin, dehydrated in 95% alcohol and absolute alcohol, cleared in xylene, and mounted in Permount.
Statistical analysis
Categorical variables are reported as numbers and percentages. Continuous variables were reported as means, standard deviations (SD) and ranges, or medians. The distribution of time-to-event data was estimated using the Kaplan-Meier method. All calculations were performed using STATA for MacOS, release 11, 2010.
Results
Clinical manifestations
In the period of the study, 280 primary tumors of the CNS were diagnosed in the National Institute of Cancer (Colombia). We found 50 grade IV glial tumors (17% [50/280]), consistent with 45 cases of glioblastoma multiforme (16%) and five cases of primary gliosarcoma (1.8%). Patients with primary gliosarcoma corresponded to four males and one female (male: female ratio = 4:1). The mean age at the time of diagnosis was 54.2 years (range 45 to 60 years) (Table 2). All five patients had considerable clinical deterioration at the time of diagnosis with a poor score performance measured by Karnofsky performance scale index on presentation. Symptoms at presentation included headache (n=5), seizures (n=4), and hemiparesis (n=1) (Table 2).
Table 2.
Clinical, surgical and therapeutic characteristics in the study patients with primary brain gliosarcoma.
| Patient | Age | Sex | Clinical presentation |
Localization of the tumor |
Surgery findings |
Adjuvant treatment |
Overall Survival |
| 1 | 57 | M | Generalized tonic-clonic seizures KI= 80 |
Left frontal | 3 × 2 cm solid mass |
Radiotherapy + Chemotherapy |
11 Months |
| 2 | 56 | M | Simple partial seizures, hemiparesis, intracranial hypertension syndrome KI=70 |
Left parietal | 6 cm necrotic mass with diffuse infiltration |
Radiotherapy + Chemotherapy |
11 Months |
| 3 | 45 | F | Simple partial seizures KI=70 |
Left parietal | 4 cm soft infiltrative lesion with necrosis |
Radiotherapy + Chemotherapy |
3 Months |
| 4 | 60 | M | Generalized tonic-clonic seizures KI: 60 |
Right fronto-parietal |
5.5 cm necrotic and hemorrhagic lesion |
Radiotherapy + Chemotherapy |
6 Months |
| 5 | 53 | M | Hemiparesis, intracranial hypertension syndrome KI: 70 |
Left parietal | 2.1 cm solid lesion |
Radiotherapy + Chemotherapy |
10 Months |
Abbreviations: Age in years at diagnosis, M: Male; F: Female; KI: Karnofsky performance status index.
Radiologic aspects
All the patients were studied pre-surgically with CT scans and MRIs, which disclosed large lesions (mean major diameter= 4.12±1.64cm), with heterogeneous morphology (5/5 cases), and preferential involvement of the temporal lobe with accompanying deviation of the midline structures (n=4). All the lesions showed partial contrast medium uptake and annular pattern with peripheral edema, central calcification on the CT scans was identified in three of five cases (Figure 1). The MRIs showed similar findings, and the MRI spectrometry showed a peak of dye uptake in four cases (Figure 1).
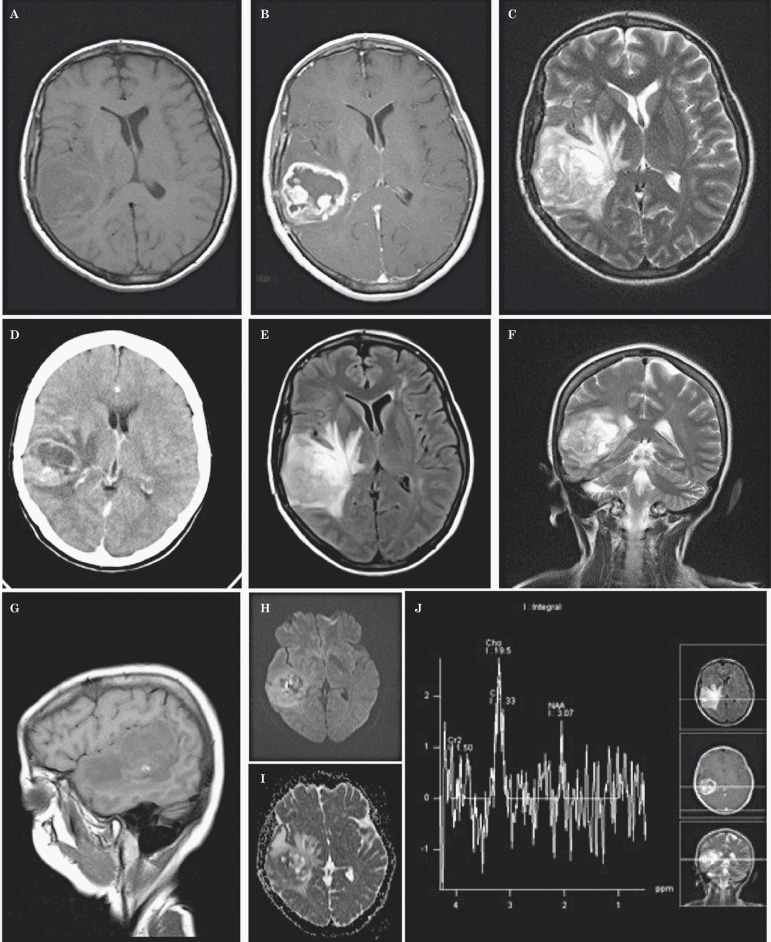
Figure 1.
A rounded heterogeneous mass is observed in the right temporal region (CT, A) with contrast media uptake and ring enhancement shows calcifications and peripheral vasogenic edema. Also depicted are secondary manifestations of intracranial hypertension, hypodense areas and midline shifting to the left (B). In MRI scans the mass is hypointense with small hyperintense foci and poorly defined in the upper aspect of the right temporal lobe (C). T2 and FLAIR (D, E) scans disclose the same heterogeneous rounded mass, with a hyperintense medial area, showing contrast media uptake in the peripheral border (ring contrast uptake), surrounded by hypointense areas and extensive vasogenic edema (F, G). Diffusion and mass diffusion coefficient were heterogeneous with areas slightly hyperintense and hypointense (H, I). MRI spectroscopy shows a choline (Cho) peak, with normal creatinine (Cr) and low NAA (N-acetyl-aspartate) indicating a primary CNS neoplasm (J).
Neurosurgical characteristics
All patients underwent craniotomy with microsurgery and gross total resection of the tumor. The majority of tumors were primarily in the left temporal lobe (n=3), followed by left parietal (n=1) and right temporal (n=1) lobes. All the tumors were large, with an average size similar to that observed on images.
Histopathology and immunohistochemical aspects
In all cases, we found mixed neoplasm with glial and sarcomatous components. The glial component was classified as high grade and showed malignant astrocytes with nuclear atypia with frequent mitotic figures, including atypical forms, with associated necrotic areas and glomeruloid endothelial vascular proliferation. The sarcomatoid elements showed a fusocellular and anaplastic morphology admixed with areas of glioblastomatous appearance, suggesting gliosarcoma diagnosis (Figure 2) (5/5 cases). The mesenchymal component had morphological and immunophenotypic dedifferentiation to pleomorphic sarcomas in two cases, fibrosarcoma in one case, leiomyosarcoma in one case (smooth muscle actin, HHF35, H-caldesmon and calponin positive) and malignant peripheral nerve sheath tumor in one case (S100 and CD57 positive) (Table 3). All cases had a high Ki67 index, greater than 75%, and all cases demonstrated p53 expression (Table 4 and Figure 3). Endothelial vascular proliferation with CD34 and CD31 expression was observed in both components (Table 4). Negativity for neuronal (NF, chromogranin, synaptophysin, and PGP9.5) markers was documented. Other CNS neoplasms with similar morphology and metastasis were excluded by immunohistochemistry (negativity for CD99, FLI-1, CD68, EMA and AE1AE3).
Table 3.
Glial and mesenchymal differentiation studied by immunohistochemistry in the analyzed cases.
| Patient | Glial component | Mesenchymal component | ||||
| GFAP | Vimentin | GFAP | Vimentin |
Other positive stains |
Mesenchymal differentiation | |
| 1 | Positive | Negative | Negative | Positive | HHF35, SMA, H-caldesmon |
High grade leiomyosarcoma |
| 2 | Positive | Negative | Negative | Positive | HHF35 | High grade fibrosarcoma |
| 3 | Positive | Negative | Negative | Positive | S-100, CD57 | High grade peripheral neural sheath sarcoma |
| 4 | Positive | Negative | Negative | Positive | None | High grade pleomorphic sarcoma |
| 5 | Positive | Negative | Negative | Positive | None | High grade pleomorphic sarcoma |
Abbreviations: GFAP: Glial fibrillary acidic protein. HHF35: Anti-muscle actin antibody. SMA: Smooth muscle actin.
Table 4.
Histopathologic features and immunostains in glial and stromal components.
| Patient | Glial component | Mesenchymal component | ||||||
|
Endothelial vascular proliferation * |
P53 | Bcl-2 | Ki67 (%) |
Endothelial vascular proliferation * |
P53 | Bcl-2 | Ki67 (%) | |
| 1 | Present | Positive | Negative | 60 | Present | Positive | Positive | 75% |
| 2 | Present | Positive | Negative | 65 | Present | Positive | Negative | 90% |
| 3 | Present | Positive | Negative | 60 | Present | Positive | Negative | 80% |
| 4 | Present | Positive | Negative | 70 | Present | Positive | Negative | 80% |
| 5 | Present | Positive | Negative | 65 | Present | Positive | Positive | 90% |
* Endothelial vascular proliferation identified by morphology and CD34 and CD31 expression.
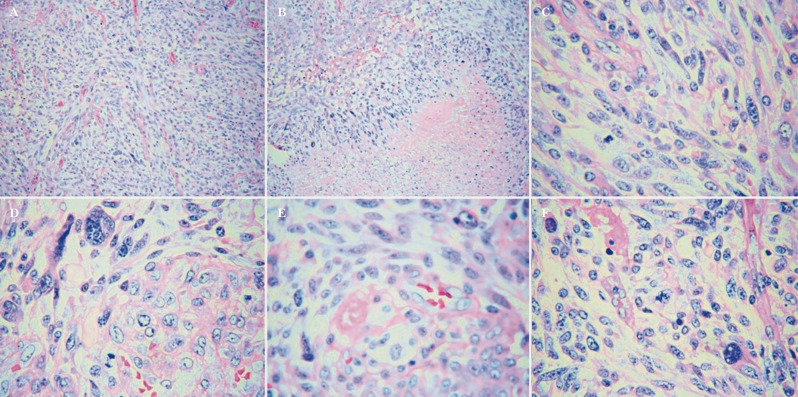
Figure 2.
Histopathological studies of all tumors identified a mixed CNS neoplasm with large areas of spindle cell differentiation (A, 100×) and extensive areas of necrosis (B, 100×). The neoplastic cells showed a highly pleomorphic morphology (C, 100×), some were multinucleated (D, 400×). Mitotic figures and microvascular proliferation were frequently seen in all cases (E, F, 400×).
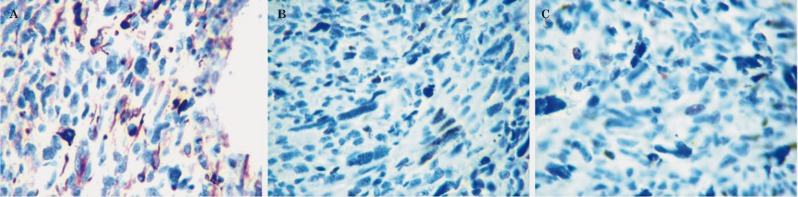
Figure 3.
Two cases presented desmin positivity (A, 400×). All five cases had high ki67 (B, 400×) and p53 (C, 400×) expression.
Treatment and follow-up
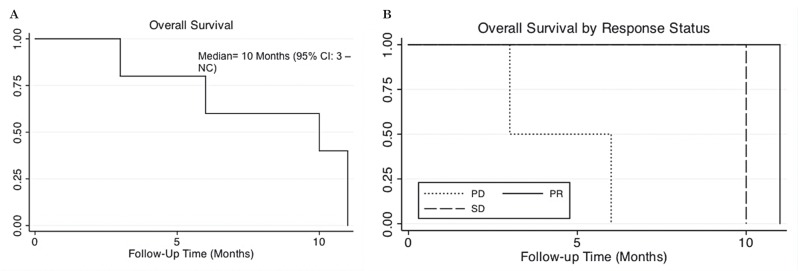
All patients received radiotherapy completing a total mean dose of 5400 cGy each case with weekly vincristine-based chemotherapy. All patients received pain-relieving medications resolving this symptom in all cases. After the surgical treatment, two patients presented occasional seizures that are under treatment with carbamazepine. Two of them achieved partial response (PR), one stable disease (SD), and two progressive disease (PD). The median overall survival (OS) was ten months. Patients who had PD died sooner than those with SD and PR. No complete responses were found (Figure 4).
Figure 4.
Overall survival was low (A) and stratified by response to therapy (B).
Discussion
Clinical manifestations and histopathology
The World Health Organization (WHO) 4 classifies gliosarcoma as a high-grade tumor (Grade IV) similar to glioblastoma. Gliosarcomas represent approximately 2% of grade IV glial neoplasms and present a similar age distribution as glioblastoma 1-4. Gliosarcoma and glioblastoma also share multiple morphologic, radiologic and clinical characteristics and their definitive differentiation is based only on histopathology 4,9. The most common presentation of gliosarcomas is as secondary tumors, frequently arising after cerebral radiotherapy to treat a primary glial neoplasm, with very few cases reported of primary origin 1,2. This study, the first study on this neoplasm performed in Colombia, describes the characteristics of five cases of primary gliosarcoma. In our cases we observed similar clinical, radiological and pathological characteristics to reports from other latitudes.
None of our cases had metastases in accordance with previous descriptions reporting that extracranial metastases are rare, but not exceptional as seen in glioblastomas 6,7. The most common sites of metastasis are: skull bones, lungs (72%), liver (41%) and lymph nodes (18%) 7.
Other biphasic malignant tumors with mixed mesenchymal elements and ependymal or oligodendroglial components, named oligosarcomas and ependymosarcomas, have also been described, but these entities are not currently recognized as particular subtypes in the WHO classification 2,8, and are reported only as a rare combination of findings. In gliosarcomas the mesenchymal component can present high variability, to any mesenchymal tissue mainly fibrosarcomatous (fibrosarcoma dedifferentiation), but cases of liposarcomatous, muscular, melanocytic and chondromatous elements have been reported 1,3,5,8-12. We identified muscular dedifferentiation in one case (case 1) and peripheral nerve differentiation in another (case 3). The other three cases presented fibrosarcomatous dedifferentiation.
All our patients were adults aged between 45 and 60 years, close to the reported mean age of 52 years 4,15,16. We observed a male predominance in accordance with other studies, however some studies have reported no gender predilection 8. Our cases were localized more frequently in the in the supratentorial area: temporal lobes similar to previous reports of a distribution by lobes as follows: temporal (44%), parietal (28%), frontal (17%) and occipital (11%) 7,13,14.
In the clinical evaluation we found a rapid progression, with secondary development of intracranial hypertension and mass effect symptoms, characteristics also described by other groups 15,16.
At microscopic examination, the gliosarcomas were characterized by biphasic morphology composed of neoplastic astrocytes (pleomorphic and atypical), placed in a fibrillary stroma rich in blood vessels, with endothelial vascular proliferation similar to that seen in glioblastoma multiforme 1-5.
Molecular features
With respect to the molecular signatures few molecular studies have analyzed this neoplasm, mutations in PTEN and p53 genes have been reported in both components, which may suggest a monoclonal origin 5. Other studies using immunohistochemistry have documented positivity to glial fibrillary acid protein (GFAP) in almost all glial components evaluated with negativity in most of the sarcomatous areas 5. Currently, gliosarcomas are considered to be the product of a metaplastic change in a glioblastoma 4,6. Constant changes in chromosome 7, loss of chromosome 10, and alterations in chromosome 3 has been reported and may suggest a likely monoclonal origin 6,7, but more studies are needed to confirm this theory. The molecular mechanisms of development of sarcomatous components in gliosarcomas have not been elucidated. It is thought that multiple genetic alterations are involved, but few genetic alteration studies have been conducted; p53 gene abnormalities have been described in at least a quarter of gliosarcomas. These alterations are seen also in multiple epithelial tumors with mesenchymal differentiation, but are probably only one reason for the common development of the neoplasms and not the origin of the sarcomatous differentiation. So this finding does not conclude that p53 is the cause of the sarcomatous differentiation 12,13. Other molecular alterations, such as retinoblastoma gene (RB) anomalies, have also been implicated in the development of the mesenchymal differentiation 12. Overexpression of the epidermal growth factor receptor (EGFR), frequently reported in glioblastomas, has not been reported typically in gliosarcomas. Therefore more research is needed in this area 4.
The neoplastic cells demonstrated positive immune reactivity for GFAP in glial components and vimentin positivity in mesenchymal areas. There was, however, little GFAP expression in minimal mesenchymal dedifferentiated areas with complete GFAP loss in areas with complete dedifferentiation into pure sarcoma, a finding reported by other groups 5,8. Ki67 expression was constantly high in all the cases in accordance with previous reports 5,15. We found p53 expression in all the tumors evaluated. This expression was independent of the type of dedifferentiation and response to therapy, in accordance with reports associating anomalies in this gene with the etiology of this neoplasm 16. Other markers and molecular techniques to exclude other anomalies were not evaluated due to technical limitations.
Radiologic features
Grossly, gliosarcomas showed a common morphology with glioblastoma that do not allow differentiation; both tumors have grey-white cut surfaces with areas of necrosis 4. In our cases, we found a pattern characterized by contrast medium uptake, with intense peripheral or irregular ring enhancement, large lesions, calcification and a high peak on MRI that could be useful in the evaluation of these neoplasms, also reported by Machuca et al. 15, Han et al. 17 and Zhang et al. 18. However, causes of other well-defined and homogeneous hyperdense masses need to be excluded 2,8,17. In a previous study, Zhang et al. 18 reported that gliosarcomas are characterized as well-demarcated irregular masses (on both CT and MRIs), with a smooth external wall well-demarcated from the surrounding brain parenchyma regardless of peripheral edema. Peripheral edema was highly prevalent in their study similar to other series 15,19. These characteristics may be due to the slower growth pattern of gliosarcoma 20. Multiple reports have shown variable characteristics on CT and MRIs. Han et al. 17 described gliosarcoma as a tumor with predominant low attenuation or inhomogeneous isoattenuation on CT scans, and generally hypointense tumors on T1-weighted MRIs and hyperintense on T2-weighted images compared with white matter 17,18.
Differential diagnosis
The key to diagnosis of gliosarcoma is to keep in mind a broad differential clinical diagnosis, in addition to the immunohistochemistry panel useful to prove or exclude glial, neuronal, and mesenchymal cell differentiation. Here we show that with a basic panel available in many institutions it is possible to develop a correct diagnosis. That differential diagnosis varies depending on the dedifferentiation and therefore needs to include primary or metastatic neoplasms like malignant fibrous histiocytoma, osteosarcoma, chondrosarcoma, rhabdomyosarcoma, liposarcoma, and angiosarcoma, as well as other sarcomas 1-3,8,15. In addition, a diagnosis of glioblastoma multiforme and gliofibroma need to be excluded in all cases of gliosarcoma. Gliofibromas lack necrosis, atypia and other high degree characteristics 5,16.
Treatment and prognosis
In all our cases surgical resection was performed with post-operative chemotherapy and radiotherapy as the treatment of choice, which are the most intensive therapeutic regimens commonly used in these tumors 20,21. Despite this aggressive treatment, our cases, similar to other published cases of gliosarcoma, had negative prognosis. We found a median OS of ten months, similar to the OS described in Europe and United States 20,22,23. None of our cases had extracranial metastases, though there have been published reports of metastases to lungs, liver, spleen, and bone 24. We found aggressive features in all the cases evaluated in accordance with previous reports. Our cases presented a catastrophic course with a survival of less than one year despite surgical and adjuvant treatment.
Conclusions
Primary gliosarcomas can be identified with careful clinical, radiologic and pathologic correlation. The histopathologic study was based on morphology and required an immunohistochemical panel to discriminate and prove the type of dedifferentiation. Furthermore, we found p53 expression in gliosarcomas, regardless of the type of dedifferentiation, supporting the hypothesis of a likely monoclonal origin of this biphasic tumor.
References
- 1.Barresi V, Cerasoli S, Morigi F, et al. Gliosarcoma with features of osteoblastic osteosarcoma: a review. Arch Pathol Lab Med. 2006;130(8):1208–1211. doi: 10.5858/2006-130-1208-GWFOOO. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez FJ, Scheithauer BW, Jenkins R, et al. Gliosarcoma arising in oligodendroglial tumors (“oligosarcoma”): a clinicopathologic study. Am J Surg Pathol. 2007;31(3):351–362. doi: 10.1097/01.pas.0000213378.94547.ae. [DOI] [PubMed] [Google Scholar]
- 3.Schittenhelm J, Erdmann T, Maennlin S, et al. Gliosarcoma with chondroid and osseous differentiation. Neuropathology. 2007;27(1):90–94. doi: 10.1111/j.1440-1789.2006.00747.x. [DOI] [PubMed] [Google Scholar]
- 4.Miller CR, Perry A. Glioblastoma. Arch Pathol Lab Med. 2007;131(3):397–406. doi: 10.5858/2007-131-397-G. [DOI] [PubMed] [Google Scholar]
- 5.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Romero-Rojas AE, Diaz-Perez JA, Amaro D, et al. Glioblastoma metastasis to parotid gland and neck lymph nodes: fine-needle aspiration cytology with histopathologic correlation. Head Neck Pathol. 2013;7(4):409–415. doi: 10.1007/s12105-013-0448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawar R, Fabiano AJ, Qiu J, et al. Secondary gliosarcoma with extra-cranial metastases: a report and review of the literature. Clin Neurol Neurosurg. 2013;115(4):375–380. doi: 10.1016/j.clineuro.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Behling E, Birbe R, Veznadaroglu E, et al. Gliosarcoma arising from an anaplastic ependymoma: a case report of a rare entity. Hum Pathol. 2004;35(4):512–516. doi: 10.1016/j.humpath.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda T, Yasumichi K, Suzuki T. Immunohistochemistry of gliosarcoma with liposarcomatous differentiation. Pathol Int. 2008;58(6):396–401. doi: 10.1111/j.1440-1827.2008.02242.x. [DOI] [PubMed] [Google Scholar]
- 10.Dulai MS, Moes GS, Briley AL, et al. Gliosarcoma with melanocytic differentiation. Acta Neuropathol. 2008;115(3):357–361. doi: 10.1007/s00401-007-0232-7. [DOI] [PubMed] [Google Scholar]
- 11.Ho LT, Wassef H, Henderson R, et al. F-18 FDG PET-CT imaging in recurrent cerebral gliosarcoma. Clin Nucl Med. 2009;34(3):153–154. doi: 10.1097/RLU.0b013e3181966fc6. [DOI] [PubMed] [Google Scholar]
- 12.Borota OC, Scheie D, Bjerkhagen B, et al. Gliosarcoma with liposarcomatous component, bone infiltration and extracranial growth. Clin Neuropathol. 2006;25(4):200–203. [PubMed] [Google Scholar]
- 13.Reis RM, Könü-Lebleblicioglu D, Lopes JM, et al. Genetic profile of gliosarcomas. Am J Pathol. 2000;156(2):425–432. doi: 10.1016/S0002-9440(10)64746-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han SJ, Yang I, Otero JJ, et al. Secondary gliosarcoma after diagnosis of glioblastoma: clinical experience with 30 consecutive patients. J Neurosurg. 2010;112(5):990–996. doi: 10.3171/2009.9.JNS09931. [DOI] [PubMed] [Google Scholar]
- 15.Machuca TN, Prevedello DM, Pope LZ, et al. Gliosarcoma: report of four cases with immunohistochemical findings. Arq Neuropsiquiatr. 2004;62(3A):608–612. doi: 10.1590/s0004-282x2004000400008. [DOI] [PubMed] [Google Scholar]
- 16.Sreenan JJ, Prayson RA. A study of 13 tumors, including p53 and CD34 immunohistochemistry. Arch Pathol Lab Med. 1997;121(2):129–133. [PubMed] [Google Scholar]
- 17.Han SJ, Yang I, Ahn BJ, et al. Clinical characteristics and outcomes for a modern series of primary gliosarcoma patients. Cancer. 2010;116(5):1358–1366. doi: 10.1002/cncr.24857. [DOI] [PubMed] [Google Scholar]
- 18.Zhang BY, Chen H, Geng DY, et al. Computed tomography and magnetic resonance features of gliosarcoma: a study of 54 cases. J Comput Assist Tomogr. 2011;35(6):667–673. doi: 10.1097/RCT.0b013e3182331128. [DOI] [PubMed] [Google Scholar]
- 19.Lutterbach J, Guttenberger R, Pagenstecher A. Gliosarcoma: a clinical study. Radiother Oncol. 2001;61(1):57–64. doi: 10.1016/s0167-8140(01)00415-7. [DOI] [PubMed] [Google Scholar]
- 20.Moon SK, Kim EJ, Choi WS, et al. Gliosarcoma of the cerebellar hemisphere: a case report and review of the literature. Korean J Radiol. 2010;11(5):566–570. doi: 10.3348/kjr.2010.11.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romeike BF, Chen Y, Walter J, et al. Diagnostic utility of IDH1- and p53-mutation analysis in secondary gliosarcoma. Clin Neuropathol. 2011;30(5):231–234. doi: 10.5414/np300375. [DOI] [PubMed] [Google Scholar]
- 22.Prados MD, Chang SM, Butowski N, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol. 2009;27(4):579–584. doi: 10.1200/JCO.2008.18.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galanis E, Buckner JC, Dinapoli RP, et al. Clinical outcome of gliosarcoma compared with glioblastoma multiforme: North Central Cancer Treatment Group results. J Neurosurg. 1998;89(3):425–430. doi: 10.3171/jns.1998.89.3.0425. [DOI] [PubMed] [Google Scholar]
- 24.Beaumont TL, Kupsky WJ, Barger GR, et al. Gliosarcoma with multiple extracranial metastases: case report and review of the literature. J Neurooncol. 2007;83(1):39–46. doi: 10.1007/s11060-006-9295-x. [DOI] [PubMed] [Google Scholar]