Summary
Endovascular treatment has assumed a major role in the management of intracranial aneurysms. Although current techniques have proven extremely effective in the embolization of a large number of intracranial aneurysms, wide-necked basilar tip aneurysms represent a subset that continues to pose technical challenges in treatment. This study reports our experience with WEB II, a new embolization device employed in four patients with this type of aneurysm.
Keywords: intracranial aneurysm, basilar artery, endovascular treatment, Woven EndoBridge II (WEB II)
Introduction
Intracranial aneurysms are common, with a prevalence between one and five per cent 1-3. Basilar tip aneurysms account for 7-8% of all intracranial aneurysms 1,4,5. They carry a higher risk of rupture than aneurysms in other locations and are frequently wide-necked.
Endovascular treatment is currently utilized for the embolization of many intracranial aneurysms and several devices, such as Guglielmi detachable coils, stents, flow diverters and balloons, are available.
Despite an ever-growing number of devices and techniques, the treatment of basilar tip aneurysms remains challenging. The endovascular treatment of these aneurysms with coils is safe 6-8, although several studies have shown relatively low complete occlusion rates (from 31.7% to 85% 9-13) and high recurrences and retreatment rates (from 17.4% to 23% 9,10,13). Coil compaction is a frequent complication of coil embolization in basilar tip aneurysms, ranging from 24% to 36% 10,14.
Complex techniques, such as double balloon remodelling 14, Y-stent-assisted coil embolization 15-17 and Waffle-cone stenting 18-20 have been introduced to address this issue. However, these methods are technically difficult to perform and are not suitable in most cases. The use of intraluminal flow diverters in basilar tip aneurysms is limited by the presence of vessels in proximity of the aneurysm neck, which would be virtually occluded by the flow diverter 21,22. Microsurgical clipping can be performed for the occlusion of basilar artery aneurysms, albeit clipping is technically complex and very invasive.
The WEB II (Sequent Medical, Aliso Viejo, CA, USA) is an innovative aneurysm embolization device specifically developed for the treatment of wide-necked bifurcation/terminal aneurysms. It acts as an intrasaccular flow disrupter, creating a block of flow in the aneurysm neck and within its dome, causing thrombus formation and isolation of the aneurysm from the parent vessel. This study reports our initial clinical experience with the WEB II device in the endovascular treatment of wide-necked basilar tip aneurysms.
Materials and Methods
We performed a retrospective analysis of basilar tip aneurysms treated with the WEB II embolization device at the Institute of Neurological Sciences of Bologna, Bellaria Hospital, Bologna and at the Department of Neuroradiology, Baggiovara Hospital in Modena, between June 2011 and June 2012.
Approval from the ethics committee was obtained and all subjects gave their informed consent. Therapeutic alternatives were discussed by a multidisciplinary team of neurointerventional radiologists and neurosurgeons. Site conditions and technical operational guidelines of the World Federation of Interventional and Therapeutic Neuroradiology (WFITN) were followed 23. Aneurysm characteristics and treatment modalities are described in detail below.
Results
In our preliminary experience four wide-necked bifurcation basilar tip aneurysms were treated. In all cases it was possible to deploy the WEB II into the aneurysm dome, obtaining complete cessation of intra-aneurysmal blood flow within minutes after deployment.
Satisfactory and stable occlusion was confirmed up to 12 months follow-up in two cases and up to seven months follow-up in the other two cases.
No intra-operative aneurysm rupture and no thromboembolic complications occurred. No treatment-related mortality or morbidity were observed. The WEB II has a less conformable structure and no morphological modification was observed. No revascularization or growth of the aneurysm was detected, although a longer follow-up is necessary to confirm the stability of occlusion. In one case, the aneurysm height exceeded the maximum WEB II height available. Therefore a combined approach with WEB II and Guglielmi detachable coils, previously described in the literature 24, was applied.
In our experience, this treatment technique appeared to be both safe and effective.
Case Reports
Patient 1
Presentation. A 50-year-old presented with ruptured aneurysm of the left carotid siphon, treated with coils placement in 2003. The patient was followed up for one year after treatment. In June 2011 the patient presented headache during a hypertensive crisis and was admitted to the hospital. CT angiography (CTA) disclosed an aneurysm of the apex of the basilar artery.
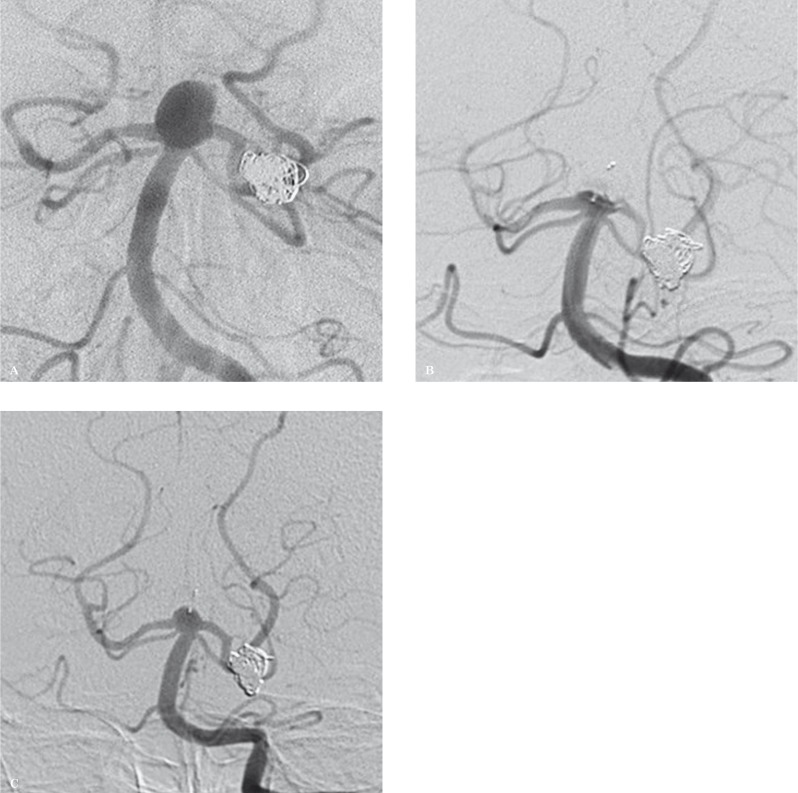
Treatment. Conventional angiography and 3D rotational analysis were performed with a right transfemoral approach and a FargoMax 6F diagnostic catheter (Balt Extrusion, Montmorency, France) which was navigated to the distal cervical portion of the left vertebral artery. At angiography the aneurysm presented a slightly irregularly shape, with an anteroposterior diameter of 11 mm, lateral diameter of 7.7 mm and height of 7.6 mm (Figure 1A). Neck diameters were 7.5 × 6.7 mm and the origin of both posterior cerebral arteries was located in proximity of the aneurysm neck, thereby discouraging the use of coils and an intraluminal flow diverter. The aneurysm was therefore selected for treatment with the WEB II.
Figure 1.
A) DSA showing a slightly irregularly shaped basilar tip wide-necked aneurysm. Both posterior cerebral arteries originate in proximity of the aneurysm neck, thus discouraging the use of coils and intraluminal flow diverters. B) DSA performed 2.5 hours after WEB II deployment. The aneurysm lumen is occluded and the WEB II device appears correctly positioned within the aneurysm. No sign of occlusion of both posterior cerebral arteries can be observed. C) Follow-up DSA performed 6 months after treatment shows a small bulging located at the tip of the basilar artery.
Later a DAC 038 microcatheter (Concentric Medical, Mountain View, CA, USA) was navigated to the proximal portion of the aneurysm lumen. The WEB II (10 × 6 mm) was then carefully delivered into the aneurysm and deployed. Control angiography showed correct device placement and blood flow stagnation. Marked flow stagnation was observed five minutes after device deployment and the WEB II was then detached. Serial control angiograms were performed up to 2.5 hours after device deployment and showed progressive occlusion of the aneurysm lumen, starting distally and proceeding proximally towards the aneurysm neck. No sign of occlusion or narrowing of the posterior cerebral arteries were observed (Figure 1B). Anticoagulant (heparin 5000 IU) and antiplatelet therapy (ASA 1000 mg) were administered throughout the procedure. Haemostasis was achieved with a 6F Angio-Seal device.
Follow-up. The post-procedure hospital course was uneventful. Pre-discharge control MR angiography (MRA) performed five days post-treatment showed appropriate positioning of the WEB II within the aneurysm of the basilar artery apex, complete exclusion of the aneurysm from the intracranial blood circulation and no signs of narrowing of the posterior cerebral arteries. Further MRA follow-up examinations performed one, three, six, nine and 12 months post-treatment and digital subtraction angiography (DSA) follow-up performed six months post-treatment showed a small bulging of the tip of the basilar artery, that we considered like a consequence of device morphology (Figure 1C).
The following post-treatment antiplatelet therapy was administered: ticlopidine 500 mg/die and ASA 300 mg/die during the first week after treatment. Subsequently ticlopidine 250 mg/die and ASA 300 mg/die during the second week and ASA 100 mg during the third and fourth weeks after treatment.
Patient 2
Presentation. A 65-year-old man presented with an episode of transient global amnesia lasting five hours in December 2011. Brain CT scan showed an aneurysm of the apex of the basilar artery, which was subsequently studied with MRA.
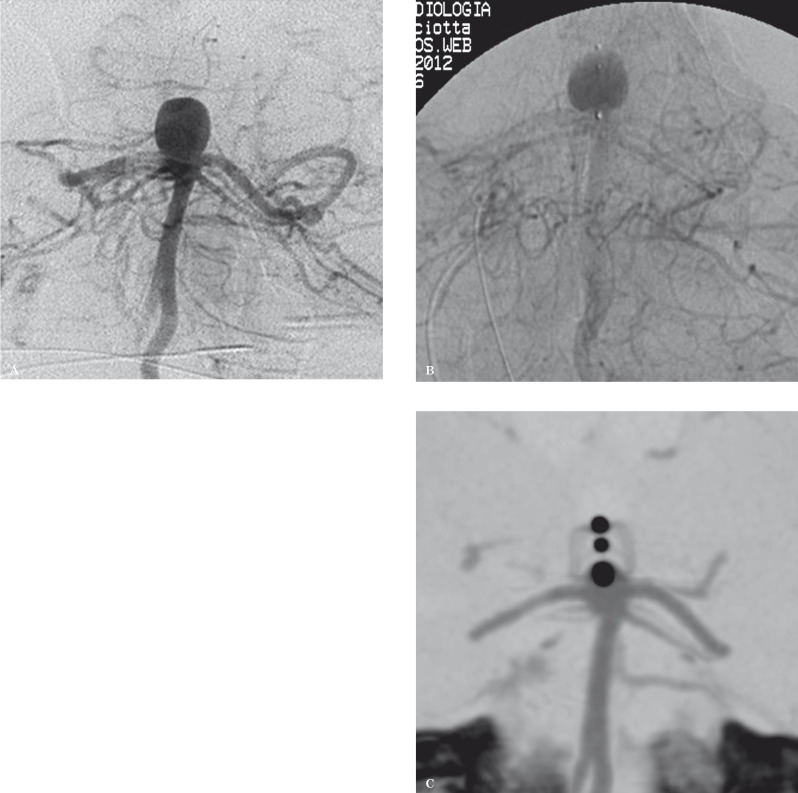
Treatment. Conventional angiography and 3D rotational analysis were performed using a transfemoral approach and a Neuron 070 6F diagnostic catheter (Penumbra Inc., Alameda, CA, USA), which was navigated to the distal cervical portion of the left vertebral artery. The aneurysm presented with anteroposterior diameter of 9.6 mm, lateral diameter of 8.6 mm, height of 10.8 mm and neck diameter of 6.5 mm (Figure 2A). The aneurysm was selected for treatment with the WEB II.
Figure 2.
Wide-necked basilar tip aneurysm. B) Marked blood flow stagnation can be observed within the aneurysm sac 6 minutes after WEB II deployment. C) CTA performed 9 months after treatment shows the WEB II correctly positioned within the aneurysm sac (note the 3 radio-opaque markers aligned with the aneurysm neck and the silhouette of the WEB II itself). Both posterior cerebral arteries appear patent.
Later a microcatheter DAC 038 was navigated to the proximal portion of the aneurysm lumen and the WEB II (10 × 8 mm) was gently delivered into the aneurysm sac and deployed. The device appeared correctly positioned and blood flow stagnation was observed. The WEB II was then detached six minutes after deployment. Final control showed marked flow stagnation without modifications involving the basilar artery and its branches (Figure 2B).
Angiography also showed an irregularly shaped wide-necked aneurysm of the bifurcation of the right middle cerebral artery (MCA), which was considered suitable for microsurgical clipping. Anticoagulant (heparin 5000 IU) and antiplatelet therapy (ASA 1000 mg) were administered during the procedure. Haemostasis was achieved with a FemoSeal device.
Follow-up. A very mild antiplatelet therapy was administered (ASA 300 mg/die for three days after treatment) to allow the patient to undergo microsurgical clipping of the aneurysm of the bifurcation of the right MCA one month after endovascular treatment with WEB II. The post-procedure hospital course was uneventful. Pre-discharge CTA showed correct positioning of the WEB II device within the aneurysm sac, which appeared excluded from the intracranial circulation, and a regular depiction of the posterior cerebral arteries. Further follow-up examinations showed similar findings (Figure 2C).
Patient 3
Presentation. A 73-year-old woman presented with mental confusion in January 2012 and a brain CT scan disclosed a saccular basilar tip aneurysm. The aneurysm was subsequently studied with a CT scan which showed a wide-necked basilar tip aneurysm, whose dome presented with blebs and was oriented upwards and posteriorly. Both posterior cerebral arteries originated from the aneurysm neck. An aneurysm of the bifurcation of the left middle cerebral artery was also detected.
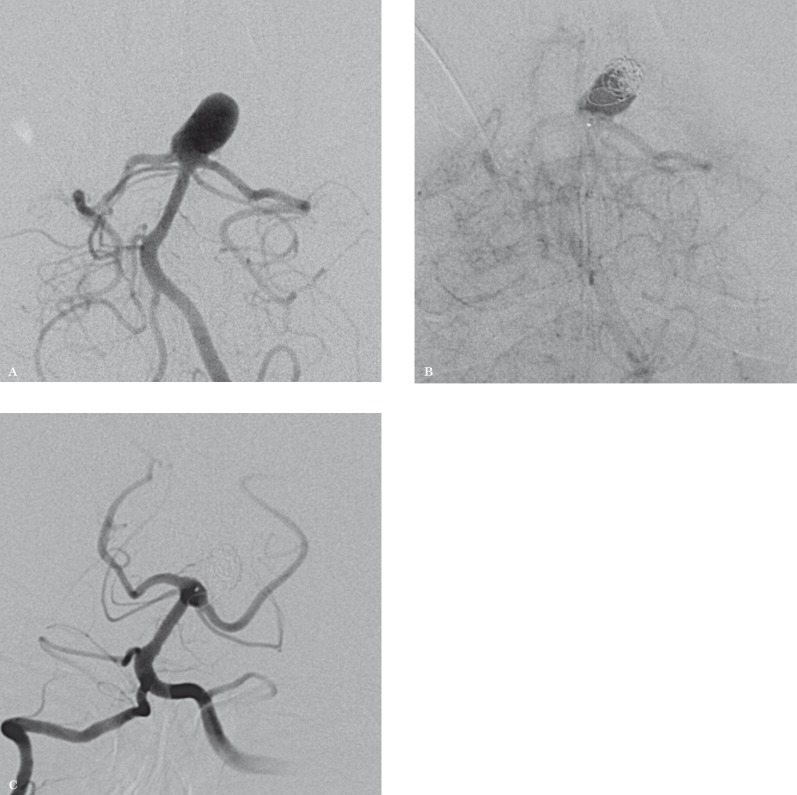
Treatment. In June 2012 angiography determined the aneurysm's exact size. It presented with lateral diameter of 11 mm, height of 14 mm and neck diameter of 6.5 mm (Figure 3A).
Figure 3.
A) Wide-necked basilar tip aneurysm, with height of 14 mm. B) As the aneurysm height exceeded the maximum available height of the WEB II, 2 coils were positioned within the aneurysm after WEB II deployment. This combined technique was described by Pierot et al. (see text for details) and appeared safe and effective. C) DSA performed 9 months after treatment shows complete exclusion of the aneurysm from the intracranial circulation and normal patency of the posterior cerebral arteries.
Through a right radial artery approach a Shuttle-SL 5F long introducer (Cook Medical Inc., Bloomington, IN, USA) was inserted in the right vertebral artery. An Evolution microcatheter (Boston scientific, Natick, MA, USA) with a Synchro-14 guidewire (Boston scientific) was then navigated to the proximal portion of the aneurysm lumen and the WEB II (10 × 8 mm) was gently positioned within the proximal portion of the aneurysm sac and detached. As the height of WEB II device was insufficient to fill the aneurysm dome, two MicroPlex 10-system coils (MicroVention Inc., Tustin, CA, USA) (6 mm × 18 cm and 5 mm × 22 cm) were subsequently positioned by the jailing technique in the aneurysm fundus.
At the end of the procedure a marked reduction of intra-aneurysmal flow was observed and both posterior cerebral arteries appeared patent (Figure 3B). Pre-treatment antiplatelet therapy was administered for three days before treatment, including clopidogrel 75 mg/die and ASA 100 mg/die. During the embolization procedure anticoagulant (heparin 5000 IU) therapy was administered.
Follow up. The post-procedure hospital course was uneventful. CTA performed the day following the embolization showed a correct placement of the WEB II device within the aneurysm sac, which appeared excluded from the intracranial circulation. The posterior cerebral arteries appeared normally patent. Further follow-up examination, including angiography performed nine months after embolization, showed similar findings (Figure 3C). The following post-treatment antiplatelet therapy was administered: clopidogrel 75 mg/die and ASA 100 mg/die for the following nine months.
Patient 4
Presentation. A 60-year-old woman underwent microsurgical clipping of a ruptured middle cerebral artery aneurysm in 1991. A follow-up CT scan performed in 1999 showed an aneurysm of the apex of the basilar artery with a diameter of roughly 8 mm. In December 2011 the patient presented with a subarachnoid haemorrhage. CTA showed an unruptured wide-necked basilar tip aneurysm and a ruptured left carotid siphon aneurysm. The latter was treated with microsurgical clipping, which was complicated by vasospasm. Angiography was then performed and 3 mg of nimodipine were administered, leading to a significant reduction of vasospasm.
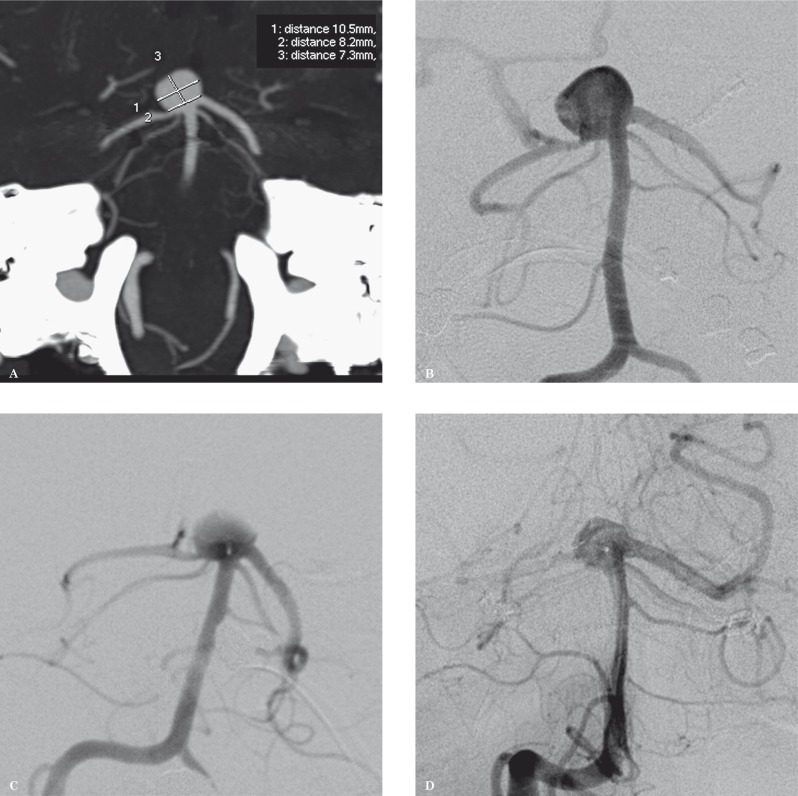
Treatment. In June 2012 angiography allowed a detailed study of the basilar artery aneurysm. The aneurysm was oriented anteriorly and laterally to the right and measured 10.5 × 7.3 mm. A stenosis of the right posterior cerebral artery was observed prior to endovascular treatment (Figure 4A,B). The aneurysm was irregularly shaped, showing some blebs on its surface and the posterior cerebral arteries and superior cerebellar arteries originated in proximity of the aneurysm neck. The right vertebral artery presented a slight dissection enlargement at C5 level.
Through a right femoral artery approach a Shuttle-SL 5F long introducer (Cook Medical) was inserted into the right vertebral artery. An evolution microcatheter (Boston Scientific) with Synchro-14 Guidewire (Boston Scientific) was then navigated to the proximal portion of the aneurysm lumen, the WEB II (11 × 6 mm) was gently positioned within the aneurysm sac and detached. At the end of the procedure a marked reduction of intra-aneurysmal flow was observed and patency of both posterior cerebral arteries and superior cerebellar arteries was preserved (Figure 4C). At the end of treatment procedures, an enlargement of the right vertebral artery pseudoaneurysm was observed, with formation of a fistula with cervical epidural venous plexus.
Figure 4.
A,B) CTA and DSA showing an irregularly shaped basilar tip wide-necked aneurysm, oriented anteriorly and laterally to the right. The posterior cerebral arteries and superior cerebellar arteries originate in proximity of the aneurysm neck. C) After WEB II deployment, a marked decrease of intra-aneurysmal blood flow can be observed. The patency of both posterior cerebral arteries and superior cerebellar arteries is maintained. D) DSA performed 8 months after treatment shows a small residual neck in the basilar tip aneurysm as well as preserved patency of all the blood vessels originating in proximity of the aneurysm neck.
Pre-treatment antiplatelet therapy was administered for three days before treatment, including clopidogrel 75 mg/die and ASA 100 mg/die. During the embolization procedure anticoagulant (heparin 5000 IU) therapy was administered.
Follow up. Follow-up CT scan performed the day after aneurysm embolization showed correct placement of the WEB II and exclusion of the basilar tip aneurysm from the intracranial circulation. Four days after treatment the patient complained of paraesthesia in the right upper limb and a CT scan was subsequently performed, which appeared substantially negative. Angiography was performed seven days after treatment, confirming the presence of minimal dissection of the right vertebral artery in the middle cervical part. CTA and DSA, performed seven and eight months after embolization respectively, confirmed correct device placement as well as patency of the posterior intracranial circulation. A small residual neck was detected in the basilar tip aneurysm (Figure 4D). A minimal dissection located in the middle cervical tract of the right vertebral artery was also observed, in the absence of fistulae.
Nine months after treatment the patient presented with seizures, restlessness and loss of consciousness, although no modifications of previous findings were observed on CT and CTA scans.
The following post-treatment antiplatelet therapy was administered: clopidogrel 75 mg/die and ASA 100 mg/die for the following eight months.
Discussion
WEB II is a new aneurysm embolization device which acts with an innovative approach. It is designed to be positioned inside the aneurysm dome to create a stable, high-density mesh across the aneurysm neck at the parent artery–aneurysm interface. This metallic mesh shapes the flow towards the parent vessel and induces flow stagnation within the aneurysm dome, leading to thrombus formation. The device also serves as a scaffold to promote and support neoendothelium formation, which contributes to permanently exclude the aneurysm from the intracranial circulation 24-28.
Treatment with WEB II requires single device deployment. Compared with coils, this reduces the number of instruments and their limited manipulation could determine a higher level of safety, shorter duration of the procedure and a lower level of radiation exposure.
Compared with intra-luminal flow diverters, WEB II acts by blocking the aneurysm blood flow, without the need to place devices inside the parent vessel. Therefore, if the WEB II is correctly positioned, it will not affect vessels originating in proximity of the aneurysm neck and does not need aggressive antiplatelet therapy. Moreover, we detected flow stagnation in about five minutes after device deployment and progressive and fast aneurysm occlusion. It is particularly important to clarify the potential use of WEB II for the treatment of ruptured intracranial aneurysms. Unlike intraluminal flow diverters, the WEB II achieves fast flow stagnation and rapid subsequent thrombosis which could represent a considerable advantage, particularly in the treatment of ruptured aneurysms.
Moreover, when utilizing intraluminal flow diverters, no device is positioned within the aneurysm sac. This allows the formation of the thrombus which, attracting water by means of osmolarity, can determine a further increase in both pressure inside the aneurysm and the size of the lesion. This can lead to hazardous complications, such as hydrocephalus, particularly when located in the basilar artery 21.
Conclusions
The WEB II device, acting as an endosaccular flow-disrupter, presents considerable advantages compared to traditional aneurysm embolization techniques.
Our study confirms that use of the WEB II as aneurysm embolization device is technically feasible and preliminary results in four patients suggest that the WEB II is a suitable device for endovascular treatment of unruptured wide-necked basilar tip aneurysms. Further follow-up of these patients is required to confirm our initial results and to assess any long-term complications that might arise, particularly aneurysm regrowth.
References
- 1.Brisman JL, Song JK, ewell DW. Cerebral aneurysms. New Engl J Med. 2006;355:928–939. doi: 10.1056/NEJMra052760. [DOI] [PubMed] [Google Scholar]
- 2.Caranci F, Briganti F, Cirillo L, et al. Epidemiology and genetics of intracranial aneurysms. Eur J Radiol. 2013;82(10):1598–1605. doi: 10.1016/j.ejrad.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 3.Wiebers DO, Whisnant JP, Huston J III, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 4.Campos C, Churojana A, Rodesch G, et al. Basilar tip aneurysms and basilar tip anatomy. Interv Neuroradiol. 1998;4(2):121–125. doi: 10.1177/159101999800400203. [DOI] [PubMed] [Google Scholar]
- 5.Peluso JPP, Van Rooij WJ, Sluzewski M, et al. Coiling of basilar tip aneurysms: results in 154 consecutive patients with emphasis on recurrent haemorrhage and re-treatment during mid- and long-term follow-up. J Neurol Neurosurg Psychiatry. 2008;79:706–711. doi: 10.1136/jnnp.2007.127480. [DOI] [PubMed] [Google Scholar]
- 6.Molyneux AJ, Kerr RS, Yu LM, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and . Lancet. 2005;366:809–817. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 7.Moscato G, Cirillo L, Dall’olio M, et al. Management of unruptured brain aneurysms: retrospective analysis of a single centre experience. Neuroradiol J. 2013;26(3):315–319. doi: 10.1177/197140091302600311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henkes H, Fischer S, Mariushi W, et al. Angiographic and clinical results in 316 coil-treated basilar artery bifurcation aneurysms. J Neurosurg. 2005;103:990–999. doi: 10.3171/jns.2005.103.6.0990. [DOI] [PubMed] [Google Scholar]
- 9.Sekhar LN, Tariq F, Morton RP, et al. Basilar tip aneurysms: a microsurgical and endovascular contemporary series of 100 patients. Neurosurgery. 2013;72:284–299. doi: 10.1227/NEU.0b013e3182797952. [DOI] [PubMed] [Google Scholar]
- 10.Sung-Chul J, Jae SA, Byung-Duk K, et al. Analysis of clinical and radiological outcomes in microsurgical and endovascular treatment of basilar apex aneurysms. J Korean Neurosurg Soc. 2009;45:224–230. doi: 10.3340/jkns.2009.45.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tateshima S, Murayama Y, Gobin YP, et al. Endovascular treatment of basilar tip aneurysms using Guglielmi detachable coils: anatomic and clinical outcomes in 73 patients from a single institution. Neurosurgery. 2000;47:1332–1339. discussion 1339-1342. [PubMed] [Google Scholar]
- 12.Uda K, Murayama Y, Gobin P, et al. Endovascular treatment of basilar artery trunk aneurysms with Guglielmi detachable coils: clinical experience with 41 aneurysms in 39 patients. J Neurosurg. 2001;95:624–632. doi: 10.3171/jns.2001.95.4.0624. [DOI] [PubMed] [Google Scholar]
- 13.Vallée JN, Aymard A, Vicaut E, et al. Endovascular treatment of basilar tip aneurysms with Guglielmi detachable coils: predictors of immediate and long-term results with multivariate analysis – 6-year experience. Radiology. 2003;226:867–879. doi: 10.1148/radiol.2263011957. [DOI] [PubMed] [Google Scholar]
- 14.Pierot L, Cognard C, Spelle L, et al. Safety and efficacy of balloon remodeling technique during endovascular treatment of intracranial aneurysms: critical review of the literature. Am J Neuroradiol. 2012;33(1):12–15. doi: 10.3174/ajnr.A2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorell WE, Chow MM, Woo HH, et al. Y-configured dual intracranial stent-assisted coil embolization for the treatment of wide-necked basilar tip aneurysms. Neurosurgery. 2005;56:1035–1040. [PubMed] [Google Scholar]
- 16.Akgul E, Aksungur E, Balli T, et al. Y-stent-assisted coil embolization of wide-neck intracranial aneurysms. Interv Neuroradiol. 2011;17:36–48. doi: 10.1177/159101991101700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez-Arjona E, Fessler RD. Basilar artery to bilateral posterior cerebral artery “Y-stenting” for endovascular reconstruction of wide-necked basilar apex aneurysms: report of three cases. Neurol Res. 2004;26:276–281. doi: 10.1179/016164104225013969. [DOI] [PubMed] [Google Scholar]
- 18.Horowitz M, Levy E, Sauvageau E, et al. Intra/extraaneurysmal stent placement for management of complex and wide-necked bifurcation aneurysms: eight cases using the waffle cone technique. Neurosurgery. 2006;58(4) Suppl 2:ONS–258-262. doi: 10.1227/01.NEU.0000204713.24945.D2. discussion ONS-262. [DOI] [PubMed] [Google Scholar]
- 19.Lavine SD, Meyers PM. Application of new techniques and technologies: stenting for cerebral aneurysm. Clinical Neurosurg. 2007;54:64–69. [PubMed] [Google Scholar]
- 20.Yang TH, Wong HF, Yang MS, et al. “Waffle cone” technique for intra/extra-aneurysmal stent placement for the treatment of complex and wide-necked bifurcation aneurysm. Interv Neuroradiol. 2008;4(Suppl 2):49–52. doi: 10.1177/15910199080140S210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cirillo L, Leonardi M, Dall’Olio M, et al. Complications in the treatment of intracranial aneurysms with silk stents: an analysis of 30 consecutive patients. Interv Neuroradiol. 2012;18:413–425. doi: 10.1177/159101991201800407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Briganti , Napoli M, Tortora F, et al. Italian multicenter experience with flow-diverter devices for intracranial unruptured aneurysm treatment with periprocedural complications - A retrospective data analysis. Neuroradiology. 2012;54(10):1145–1152. doi: 10.1007/s00234-012-1047-3. [DOI] [PubMed] [Google Scholar]
- 23.Choi IS, Lasjaunias P, Picard L. Standards of practice in interventional neuroradiology or endovascular neurosurgery. WFITN site conditions and technical operational guidelines. Interv Neuroradiol. 2006;12:7–8. doi: 10.1177/159101990601200102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierot L, Liebig T, Sychra V, et al. Intrasaccular flow-disruption treatment of intracranial aneurysms: preliminary results of a multicenter clinical study. Am J Neuroradiol. 2012;33:1232–1238. doi: 10.3174/ajnr.A3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding YH, Lewis DA, Kadirvel R, et al. The Woven EndoBridge: a new aneurysm occlusion device. Am J Neuroradiol. 2011;32:607–611. doi: 10.3174/ajnr.A2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klisch J, Sychra V, Strasilla C, et al. The Woven EndoBridge cerebral aneurysm embolization device (WEB II): initial clinical experience. Interv Neuroradiol. 2011;53:599–607. doi: 10.1007/s00234-011-0891-x. [DOI] [PubMed] [Google Scholar]
- 27.Lubicz B, Mine B, Collignon L, et al. WEB device for endovascular treatment of wide-neck bifurcation aneurysms. Am J Neuroradiol. 2013;34:1209–1214. doi: 10.3174/ajnr.A3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierot L, Klisch J, Cognard C, et al. Endovascular WEB Flow Disruption in Middle Cerebral Artery Aneurysms: Preliminary Feasibility, Clinical, and Anatomical Results in a Multicenter Study. Neurosurgery. 2013;73:27–35. doi: 10.1227/01.neu.0000429860.04276.c1. [DOI] [PubMed] [Google Scholar]