Summary
Lhermitte-Duclos disease is a rare pathologic condition consisting of a dysplastic gangliocytoma of the cerebellum. Its association with phacomatosis and an autosomal dominant neoplastic syndrome, Cowden's syndrome is also known. Modern neuroimaging contributes to a correct diagnosis and pre- and postoperative evaluation. Here we describe the morphologic and metabolic aspects of the disease as shown by conventional MRI, diffusion imaging and spectroscopy in a 31-year-old woman. In addition, the specific neuroradiologic characteristics are presented and discussed in the light of the main pathologic and clinical features, such as hypertrophy of the cerebellar folia associated with white matter atrophy.
Keywords: CT and MR imaging, Lhermitte- Duclos disease, brain tumor
Introduction
Considered halfway between a glioma with low malignancy grade and a slow progressive malformative-dysplastic disorder, Lhermitte-Duclos disease (LDD) or dysplastic cerebellar gangliocytoma is a rare disease of the cerebellum 1,2.
Since Lhermitte and Duclos's first description of dysplastic cerebellar gangliocytoma in 1920, a number of synonyms have been coined testifying to the different theories on its aetiopathogenesis and pathophysiology, which remain unknown. Dysplastic gangliocytoma of the cerebellum is therefore also known as diffuse ganglioneuroma of the cerebellar cortex, purkinjoma, cerebellar hamartoma, diffuse myelin ganglioneuroma of the cerebellar cortex or hypertrophy of the granule cells of the cerebellum 2,3.
LDD was recently found associated with phacomatosis and, in 40% of cases, Cowden's syndrome, an autosomal dominant neoplastic syndrome characterised by mucocutaneous lesions, systemic hamartomas and malignant neoplastic lesions of the breast, thyroid, genito-urinary and gastrointestinal tracts 4,5.
MRI, associated with diffusion imaging and spectroscopy, enables a definite diagnosis and is useful for post-operative control and follow-up 1,2.
We describe a patient with LDD, studied by conventional MRI, diffusion imaging and spectroscopy.
Case Report
A 31-year-old woman was admitted to our institution for persistent headache associated with paraesthesias on the left side of the face, scotomas, dysarthria, gait ataxia, and dysphagia for solids. A CT scan (Aquilon 64; Thoshiba Medical System, Tokyo, Japan) showed triventricular hydrocephalus. Because of a rapid neurologic worsening, the patient underwent a ventricular shunt. The MRI investigation (Achieva Nova Dual 1.5 T, Philips, Netherlands) revealed a cerebellar malformation with a tonsillar expansion beyond C1. Scans were performed after injection of contrast medium (Gadovist, Bayer-Schering, Netherlands). After one week, a surgical partial resection of the cerebellar dysplastic vermis and bilateral tonsillectomy was performed. The histological examination indicated a dysplastic gangliocytoma of the cerebellum. The postoperative course was characterized by a transitory persistence of the ataxia and the regression of facial sensory symptoms.
Discussion
Lhermitte-Duclos disease (LDD) is a rare condition usually affecting patients aged 30-50 years, though it may occur in infants or adults over 60 years. There is no sex (M:F=1) or race preference 6,7. Lhermitte and Duclos reported the first case of cerebellar ganglion cell tumour in 1920. They performed a histologic examination of the lesion, finding abnormally widened cerebellar folia with abnormal ganglion cells, and labelled it diffuse ganglioneuroma. Since then, many LDD cases have been reported 4.
There is considerable controversy over the cause of this disease: it may have a hamartomatous, neoplastic, or congenital malformative origin 5,7. The condition is often associated with different types of malformation such macrocephaly, megacephaly, syringomyelia, polydactyly, multiple haemangiomas and mucocutaneous lesions as well as breast, thyroid, genitourinary and gastrointestinal malignancies 2. This has suggested a genetic correlation between LDD and Cowden's syndrome, an autosomal dominant syndrome characterised by a genetic aberration 4,5.
Eighty percent of patients with Cowden's syndrome have germline mutations in the PTEN gene at locus 10q23.2, which has been identified as the major susceptibility gene for Cowden's syndrome. Most LDD patients appear to have a germline loss of the PTEN allele and go on to lose the remaining PTEN allele at some point, thereby allowing abnormal growth of the granule cells 5.
Clinically, patients may be asymptomatic, or they may present with symptoms of ataxia, headache, cranial nerve palsy, paroxysm of vertigo, psychic deterioration and, in severe cases, signs and symptoms of intracranial hypertension secondary to hydrocephalus. Usually, patients have long-standing symptoms that have been present for years, indicating the slowly progressive nature of this disease 7,8.
Histologic examination reveals disruption of the normal cerebellar cortical cell layers with dysplastic hypertrophied ganglion cells leading to expansion of the granule layer and increased myelination in the molecular layer causing it to widen. There is a loss of Purkinje cells and white matter. Markers of the neoplastic process, such as mitotic activity, necrosis and endothelial proliferation are characteristically absent. No case of malignant transformation has been reported.
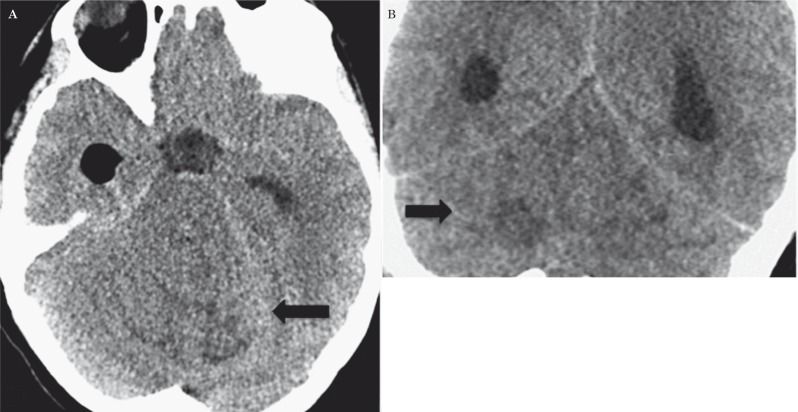
Imaging plays an important role in the diagnostic process. The dysplastic gangliocytoma is hypoattenuated on unenhanced computed tomographic (CT) images (Figure 1): in such cases, the only diagnostic clue may be the mass effect, which manifests as compression of the fourth ventricle, effacement of the cerebellopontine angle cistern and hydrocephalus. No appreciable enhancement is seen on contrast-enhanced CT images. However, CT remains of limited value because of the beam-hardening artifacts caused by the petrous temporal bone that obscure the details.
Figure 1.
Unenhanced CT. Axial (A) and coronal (B) planes showing a hypodense infratentorial lesion with hyperdense streaks, mild edema and mass effect (black arrows).
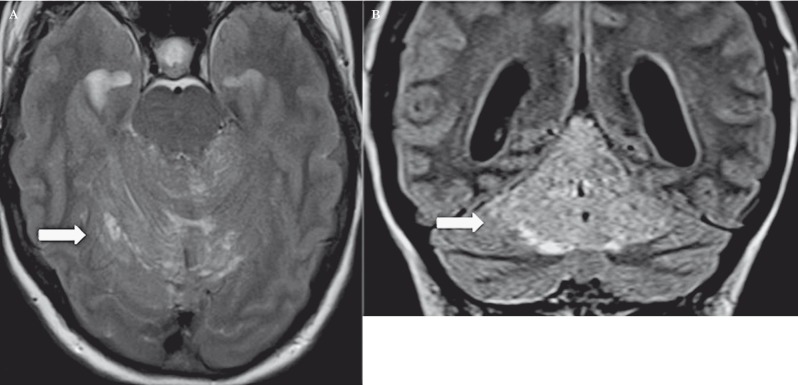
Conventional MRI, associated with diffusion imaging and spectroscopy, is currently the most useful imaging method able to provide a definite diagnosis by showing an expansile lesion that is typically hypointense on T1- and hyperintense on T2-weighted sequences 6. These signal alterations are related to white matter atrophy, thickening of the granule cell layer and enlargement of the cell layers making up the cerebellar cortex (Figure 2). The mass is characterised by parallel hyperintense striations related to thickening of the cerebellar folia due to enlargement of the cortical cells and dysplasia of the sulci, which are considered pathognomonic signs of LDD 1. A “tiger-striped” cerebellar lesion with unilateral hemispheric expansion and preservation of the gyral pattern is reported as a specific sign for LDD and these findings often establish the operative diagnosis 9.
Figure 2.
Axial (A) and coronal (B) T2-weighted 1.5T MR planes. The lesion is hyperintense with parallel hypointense streaks indicating the thickening of the cerebellar folia and the cortical gyri with dysplastic sulci (white arrows).
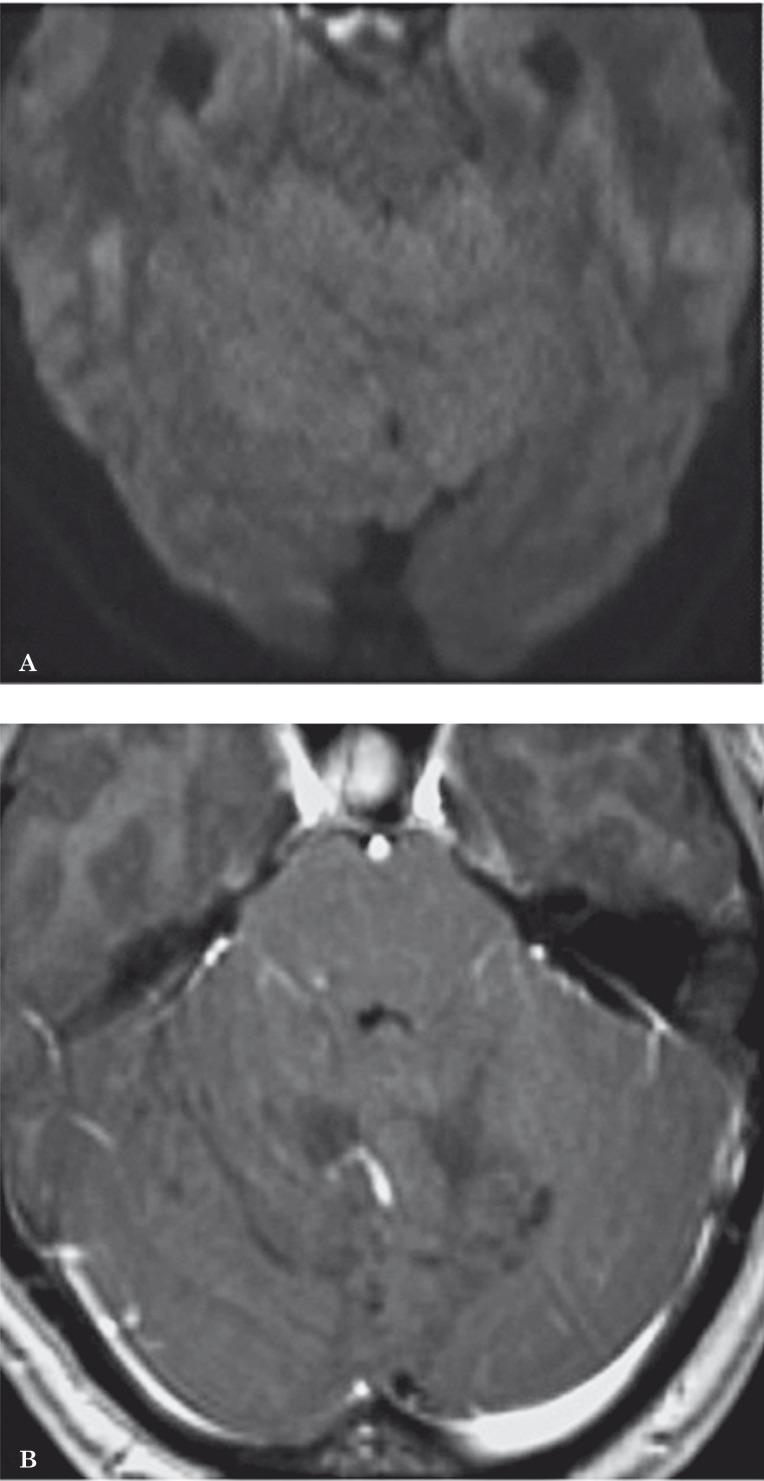
Diffusion MR shows a hyperintense area at the level of the lesion, due to the “residual T2-contrast”. In the apparent diffusion coefficient map, the mass appears isointense to the cerebellar parenchyma since the percentage of free water does not vary (Figure 3A). T2 hyperintensity is due to morphological alterations of the cells making up the internal molecular layer and granular cell layer, associated with atrophy of the cerebellar white matter, thus confirming the dysplastic and malformative origin of LDD 10,11 (Figure 2).
Figure 3.
A) In the DWI sequences, the lesion appears isointense because the level of free water does not change. B) After gadolinium injection, there is no enhancement because of the absence of brain-blood barrier changes, nor extracellular oedema.
Literature studies with 7T MR report a better identification of the anatomical structures and veins than 1.5T MR due to the increased signal-to-noise ratio. Susceptibility weighted imaging (SWI) in 7T MR can demonstrate in greater detail veins that are even smaller than the voxel size and may help to depict large draining veins and the cerebellar nuclei 9. This information can be very useful for surgical planning. Following administration of paramagnetic contrast material, the lesion shows no enhancement, indicating a non-significant alteration of the brain blood-barrier and the absence of extracellular oedema (Figure 3B).
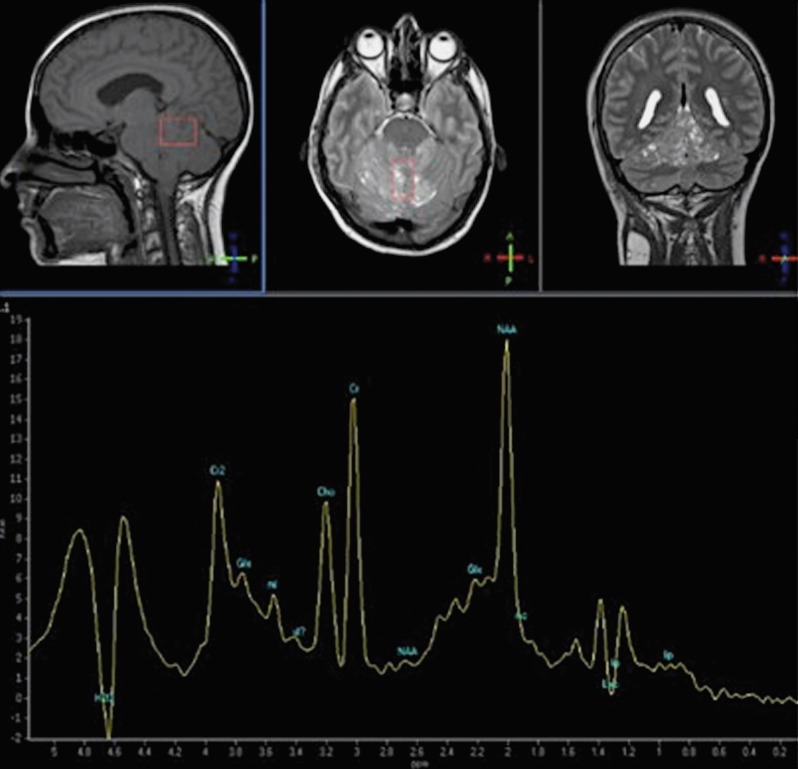
MR spectroscopy reveals a typical spectrum of appearance of a lactate peak and reduction in the choline peak (Figure 4). An alteration in lactate levels in our patient appears to be due to an abnormal increase in anaerobic glycolysis. A reduction in choline levels indicates a cellular turnover associated with demyelination and supports the dysplastic nature of the lesion 6,12,13.
Figure 4.
The spectroscopic analysis reveals an increased Cho/Cr ratio, indicating cellular turnover; the NAA/Cr indicates demyelination and dysplasia; the lactate pick indicates anaerobic glycolysis.
Contrary to other previous reports in the literature 14,15, Moeninghoff et al. demonstrated the Cho, Cr and the resulting Cho/Cr ratio were slightly elevated in the lesion whereas the myoinositol levels were not changed 9.
Finally, the contribution of MR perfusion imaging was also important as it showed an increase in relative cerebral blood volume, relative cerebral blood flow and mean transit time in the lesion 14.
Conclusions
Our case confirms the widely accepted view that MRI, with diffusion imaging and spectroscopy are the most useful techniques in the study of Lhermitte-Duclos disease. They are able to demonstrate pathognomonic features such as thickened cerebellar folia and the behaviour of the mass at diffusion imaging and spectroscopy. Correct recognition of the disease is also particularly important given the possible association with Cowden's syndrome and therefore the need to identify concurrent malignancies.
References
- 1.Nowak DA, Trost HA. Lhermitte-Duclos disease (dysplastic cerebellar gangliocytoma): a malformation, hamartoma or neoplasm? Acta Neurol Scand. 2002;105(3):137–145. doi: 10.1034/j.1600-0404.2002.1r127.x. [DOI] [PubMed] [Google Scholar]
- 2.Patel S, Barkovich AJ. Analysis and classification of cerebellar malformations. Am J Neuroradiol. 2002;23(7):1074–1087. [PMC free article] [PubMed] [Google Scholar]
- 3.Kulkantrakorn K, Awwad EE, Levy E, et al. MRI in Lhermitte-Duclos disease. Neurology. 1997;48(3):725–731. doi: 10.1212/wnl.48.3.725. [DOI] [PubMed] [Google Scholar]
- 4.Murray C, Shipman P, Khangure M, et al. Lhermitte-Duclos disease associated with Cowden’s syndrome: case report and literature review. Australas Radiol. 2001;45(3):343–346. doi: 10.1046/j.1440-1673.2001.00933.x. [DOI] [PubMed] [Google Scholar]
- 5.Miller C. Cowden disease (multiple hamartoma syndrome) In: Elmets C, Vinson R, Libow L, Quirk C, James W W, et al., editors. EMEDICINE instant access to the minds of medicine. LU; 2002. [Google Scholar]
- 6.Nagaraja S, Powel T, Griffiths PD, et al. MR imaging and spectroscopy in Lhermitte-Duclos disease. Neuroradiology. 2004;46(5):355–358. doi: 10.1007/s00234-004-1201-7. [DOI] [PubMed] [Google Scholar]
- 7.Buhl R, Barth H, Hugo HH, et al. Dysplastic gangliocytoma of the cerebellum: rare differential diagnosis in space occupying lesions of the posterior fossa. Acta Neurochir (Wien) 2003;145(6):509–512. doi: 10.1007/s00701-003-0040-3. [DOI] [PubMed] [Google Scholar]
- 8.Bero V, Sajko T, Talan-Hranilovic J, et al. Intracranial hypertension due to Lhermitte Duclos disease: case report. Acta Clin Croat. 2000;39:181–186. [Google Scholar]
- 9.Moeninghoff C, Kraff O, Schalamann M, et al. Assessing a dysplastic cerebellar gangliocytoma (Lhermitte-Duclos disease) with 7T MR imaging. Korean J Radiol. 2010;11(2):244–248. doi: 10.3348/kjr.2010.11.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moonis G, Ibhaim M, Melhem ER. Diffusion-weighted MRI in Lhermitte-Duclos disease: report of two cases. Neuroradiology. 2004;46(5):351–354. doi: 10.1007/s00234-004-1190-6. [DOI] [PubMed] [Google Scholar]
- 11.Gessaga EC. Lhermitte-Duclos disease (diffuse hypertrophy of the cerebellum). Report of two cases. Neurosurg Rev. 1980;3(2):151–158. doi: 10.1007/BF01644067. [DOI] [PubMed] [Google Scholar]
- 12.Klish J, Juengling F, Spreer J, et al. Lhermitte-Duclos disease: assessment with MR imaging, positron emission tomography, single-photon emission CT, and MR spectroscopy. Am J Neuroradiol. 2001;22(5):824–830. [PMC free article] [PubMed] [Google Scholar]
- 13.Spaargaren L, Cras P, Bomhof MA, et al. Contrast enhancement in Lhermitte-Duclos disease of the cerebellum: correlation of imaging with neuropathology in two cases. Neuroradiology. 2003;45(6):381–385. doi: 10.1007/s00234-003-0984-2. [DOI] [PubMed] [Google Scholar]
- 14.Thomas B, Krishnamoorthy T, Radhakrishnan VV, et al. Advanced MR imaging in Lhermitte-Duclos disease: moving closer to pathology and pathophysiology. Neuroradiology. 2007;49(9):733–738. doi: 10.1007/s00234-007-0241-1. [DOI] [PubMed] [Google Scholar]
- 15.Klisch J, Juengling F, Spreer J, et al. Lhermitte-Duclos disease: assessment with MR imaging, positron emission tomography, single-photon emission CT, and MR spectroscopy. Am J Neuroradiol. 2001;22(5):824–830. [PMC free article] [PubMed] [Google Scholar]