Summary
We report a 33 year-old woman addicted to chronic unspecified solvents abuse with stupor, respiratory disorders, tetraplegia and severe metabolic acidosis. On admission an unenhanced cranial CT scan showed symmetrical hypodensities of both lentiform nuclei. MR imaging performed 12 hours after stupor demonstrates bilateral putaminal hemorrhagic necrosis, bilateral external capsule, corona radiata and deep cerebellar hyperintensities with right cingulate cortex involvement. DWI reflected bilateral putaminal hyperintensities with restricted water diffusion as to citotoxic edema and development of vasogenic edema in the external capsule recalling a fork. On day twenty, after specific treatments MRI demonstrated a bilateral putaminal marginal enhancement. Bilateral putaminal necrosis is a characteristic but non-specific radiological finding of methanol poisoning. Lentiform Fork sign is a rare MRI finding reported in literature in 22 patients with various conditions characterized by metabolic acidosis. Vasogenic edema may be due to the differences in metabolic vulnerability between neurons and astrocytes. We postulate that metabolic acidosis could have an important role to generate this sign.
Keywords: diffusion-weighted imaging, lentiform fork sign, metabolic acidosis, putaminal necrosis
Introduction
The basal ganglia are particularly vulnerable to a wide range of toxins and metabolic disturbances 1. In the acute setting, bilateral basal ganglia abnormalities on MRI could be non-specific and the differential diagnosis should include hypoglycaemia, cerebral hypoxic encephalopathy, carbon monoxide poisoning, encephalitis, osmotic myelinolysis, toxin exposure, vascular causes and drug abuse 2. The present study describes a rare neuroradiological finding called the lentiform fork sign in a patient with acute metabolic acidosis, bilateral putaminal necrosis, cortical and deep cerebellar hyperintensities. Uremic encephalopathy, diabetes mellitus, methanol and ethylene glycol intoxications, acidopathies such as propionic acidaemia (PA) and pyruvate dehydrogenase deficiency were commonly associated with metabolic acidosis. Literature reviews 3-11 demonstrated that these conditions were also related to a lentiform fork appearance. Methanol is a clear colourless toxic liquid, commonly found in many commercial products of daily use like solvents, antifreeze, varnish and cleaning fluids. Accidental or suicidal oral ingestion causes visual disturbance and central nervous system (CNS) depression leading to respiratory failure and severe metabolic acidosis. Bilateral putaminal necrosis and subcortical white matter lesions were the most frequent neuroimaging findings in patients with methanol intoxication 12. We describe a comatose 33-year-old woman admitted to the emergency department with mild mydriasis and bilaterally unreactive pupils.
Case Report
A 33-year-old woman with fever, vomiting, ocular deviation, dyspnoea and deterioration of consciousness was referred to our emergency department. On admission neurological examination revealed quadriplegia, stupor and bilateral Babinski sign. No extrapyramidal signs, such as resting tremor or rigidity and no asterixis or myoclonus were detected. Glasgow coma scale (GCS) score was 7 at that time. Electrocardiography and chest radiogram were normal. Arterial blood gas analysis showed systemic metabolic acidosis (pH:7.03; PCO2 11 mmHg; P02 122; HCO3 2,9 mmol/L; BE -25 mmol/L) with high anion gap and high osmolar gap. Laboratory evaluation revealed elevated liver enzyme levels (ALT 143 U/L (2-40); AST 340 U/L (2-40)), serum creatinine 2.07 mg/dl (0.5-1.2) and hyperkalaemia 6,8 mmol/L (3.5-5.3). The glucose level was 190 mg/dl (50-110) and the ammonia level was 61 mol/l. Urinalysis demonstrated elevated ketone and protein levels without blood cells. No drug abuse was reported but in the last six months the patient had been addicted to taking diluted alcoholic solutions (disinfectants).
After admission to the intensive care unit, the patient was treated with intubation, plasma expanders and sodium bicarbonate to correct acidosis.
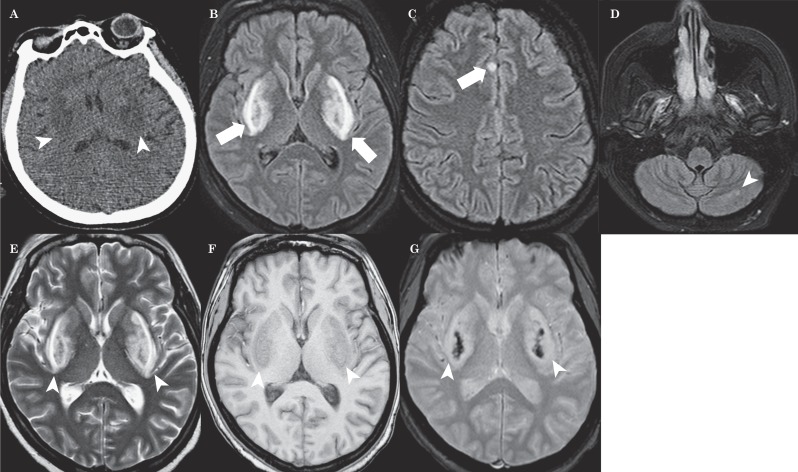
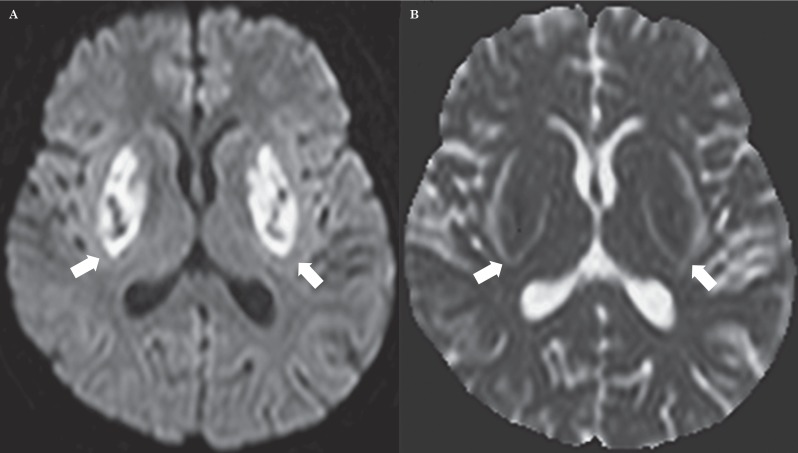
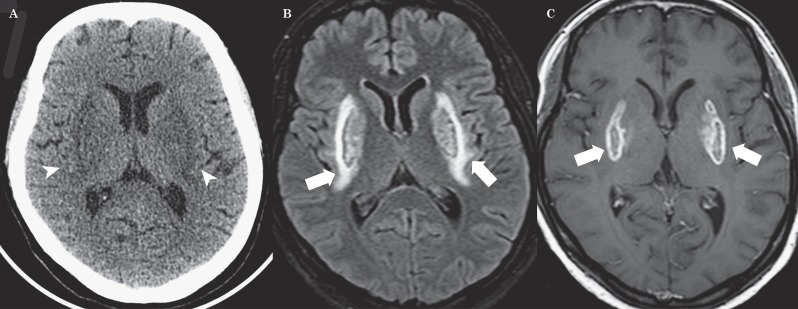
An initial unenhanced CT scan revealed symmetrical hypodensities of both lentiform nuclei (Figure 1A). Twelve hours after admission, brain MRI (Figure 1B-E) revealed bilateral T2 and FLAIR hyperintensities of lentiform nuclei, cerebellum and right cingulum cortex with a brightly hyperintense rim surrounding both putamina that looked like a fork. T1-weighted MRI (Figure 1F) showed hypointensity over the basal ganglia bilaterally. Axial T2 fast field echo (FFE) demonstrated hypointensities on both basal ganglia (Figure 1G). Diffusion-weighted imaging (DWI) showed high signal intensities in the corresponding areas (Figure 2A). The apparent diffusion coefficient (ADC) map showed low signal intensities in the putamen and globus pallidus bilaterally and high signal intensities of both the forks (Figure 2B). On day 10 a brain CT scan showed a soft reduction of bilateral putaminal hypodensities (Figure 3A). Follow-up brain MRI (20 days later) revealed reduction of the lentiform and cerebellar lesions and post-contrast T1 sequences demonstrated bilateral putaminal hyperintensities with marginal enhancement (Figure 3B,C).
Figure 1.
A) Unenhanced CT scan indicates symmetrical hypodensities of both lentiform nuclei (arrowheads). B-D) MRI FLAIR images reveal bilateral hyperintensities involving the lentiform nuclei (short white arrow), right cingulum cortex (long white arrow) and cerebellum (arrowhead) with a brightly hyperintense rim surrounding both putamina resembling a fork. E) The T2 sequence shows bilateral putaminal hyperintensities with the lentiform fork (arrowheads). F) T1 MRI shows hypointensity over the basal ganglia bilaterally (arrowheads). G) Axial T2 Fast Field Echo (FFE) demonstrates hypointensities on both basal ganglia (arrowheads).
Figure 2.
A) DWI shows diffusion restriction in both basal ganglia, in particular the putamen and globus pallidus. B) ADC map shows low signal intensities in the lentiform nucleus bilaterally and high signal intensities of both the forks.
Figure 3.
A) Brain CT scan on day 10 shows a soft reduction of bilateral putaminal hypodensities (arrowheads). B,C) Follow-up brain MRI (20 days later) reveals a reduction of lentiform hyperintensities (short white arrows) and post-contrast T1 sequences demonstrate bilateral putaminal lesions with marginal enhancement (long white arrows).
Discussion
We describe a case of acute metabolic acidosis related to aspecific solvent abuse that showed unusual bilateral basal ganglia involvement. Conventional MRI demonstrated bilateral putaminal necrosis with a haemorrhagic component in association with another sign recently called lentiform fork sign. Kumar et al. 3 described the constitutive elements of the lentiform fork: 1) the lateral arm, formed by the oedematous external capsule and extending from the anterior end of the putamen to the stem; 2) the stem, created by merging oedematous external and internal capsules at the infero-posterior end of the putamen; 3) the medial arm extended from the stem anteriorly up to one third of the medial edge where it split into two slightly less T2/FLAIR hyperintense branches engulfing the globus pallidus. These two branches are constituted by the oedematous medullary laminae, which divide the lentiform nucleus into three masses (the putamen, globus pallidus interna and externa). DWI showed bilateral putaminal hyperintensities with water restriction and low signal intensities of both the lentiform forks. The apparent diffusion coefficient (ADC) map showed low signal intensities in the putamen and globus pallidus and high signal intensities of the forks bilaterally. Diffusion imaging had the ability to discriminate between cytotoxic and vasogenic oedema showing the different water mobility in the brain regions involved. Bilateral putaminal necrosis had a cytotoxic nature caused by ischaemia or an irreversible damage. The lentiform fork demonstrated increased water mobility caused by vasogenic oedema. Metabolic acidosis was proposed as a trigger in the pathogenesis of this pattern of vasogenic oedema and is essential for generating the lentiform fork sign in several clinical settings. In our patient, vasogenic oedema had partially resolved on follow-up images after intubation and sodium bicarbonate infusion to correct metabolic acidosis. The pathogenetic basis of this characteristic vasogenic oedema may be attributable to the differences in metabolic vulnerability between neurons (that composed basal ganglia) and astrocytes (which formed the surrounding white matter in association with axons and oligodendrocytes). Through changes in vascular reactivity, metabolic acidosis may disrupt the blood–brain barrier leading to vasogenic oedema and later cytotoxic oedema, in relation to the severity of acidosis. Contrast-enhanced MRI confirmed bilateral blood-brain barrier disruption on the putamina. We assumed that putaminal necrosis was associated with alcoholic intoxication such as methanol poisoning, but this finding is not specific and was also seen in a variety of conditions including Wilson and Leigh disease, Kearns-Sayre syndrome and various other neurodegenerative disorders 14-18. The mechanism underlying selective putaminal necrosis is unknown. It has been postulated that the necrosis results from decreased blood flow through the basal veins of Rosenthal secondary to hypotension 19 or from a direct toxic effect of formic acid that shows a higher accumulation in the putamina than in other parts of the brain 20. On the other hand, there was varying sensitivity of striatal neurons to toxic metabolites of methanol 21. Formic acid is responsible for metabolic acidosis which results from the inhibition of cytochrome oxidase, a mitochondrial enzyme required for oxidative phosphorylation. Its inhibition leads to anoxia and, furthermore, to cellular oedema and cell death. It may be difficult to diagnose methanol poisoning if no history of ingestion can be obtained. Therefore methanol poisoning should be considered in every patient with a metabolic acidosis of unknown origin 22. Haemorrhage, confined to the putamina or in the subcortical white matter, has been reported in up to 14% of patients with methanol poisoning 13,23 and has been suggested to indicate a poor prognosis 19,24. Few studies have used DWI in methanol poisoning 19,26. DWI demonstrated restricted diffusion on both putamina such as cytotoxic oedema that caused blood-brain barrier damage. Furthermore, the putamen’s high metabolic demand and its location in the boundary zone of vascular perfusion make it particularly susceptible to vascular damage. We believe that several factors, including cerebral microvascular anatomy, direct methanol metabolite toxicity and severe metabolic acidosis produce this characteristic distribution of pathologic findings.
Conclusions
Bilateral putaminal necrosis is a characteristic but non-specific radiological finding of methanol poisoning. It is probably a consequence of direct formic acid toxicity on the basal ganglia. The lentiform fork sign is a rare MRI finding reported in the literature in 22 patients with various conditions characterized by metabolic acidosis. Vasogenic oedema may be due to the differences in metabolic vulnerability between neurons and astrocytes. Metabolic acidosis could have an important role in generating this sign.
References
- 1.Albin RL. Basal ganglia neurotoxins. Neurol Clin. 2000;18(3):665–680. doi: 10.1016/s0733-8619(05)70217-6. doi: 10.1016/S0733-8619(05)70217-6. [DOI] [PubMed] [Google Scholar]
- 2.Beltz EE, Mullins ME. Radiological reasoning: hyperintensity of the basal ganglia and cortex on FLAIR and diffusion-weighted imaging. Am J Roentgenol. 2010;195(3 Suppl):S1–8. doi: 10.2214/AJR.07.7089. (Quiz S9-11). doi: 10.2214/AJR.07.7089. [DOI] [PubMed] [Google Scholar]
- 3.Kumar G, Goyal MK. Lentiform fork sign: a unique MRI picture. Is metabolic acidosis responsible? Clin Neurol Neurosurg. 2010;112(9):805–812. doi: 10.1016/j.clineuro.2010.06.006. doi: 10.1016/j.clineuro.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Wang HC, Cheng SJ. The syndrome of acute bilateral basal ganglia lesions in diabetic uremic patients. J Neurol. 2003;250(8):948–955. doi: 10.1007/s00415-003-1122-0. doi: 10.1007/s00415-003-1122-0. [DOI] [PubMed] [Google Scholar]
- 5.Yoon CH, Seok JI, Lee DK, et al. Bilateral basal ganglia and unilateral cortical involvement in a diabetic uremic patient. Clin Neurol Neurosurg. 2009;111(5):477–479. doi: 10.1016/j.clineuro.2009.01.007. doi: 10.1016/j.clineuro.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Lee EJ, Park JH, Ihn Y, et al. Acute bilateral basal ganglia lesions in diabetic uraemia: diffusion-weighted MRI. Neuroradiology. 2007;49(12):1009–1013. doi: 10.1007/s00234-007-0299-9. doi: 10.1007/s00234-007-0299-9. [DOI] [PubMed] [Google Scholar]
- 7.Kim TK, Seo SI, Kim JH, et al. Diffusion-weighted magnetic resonance imaging in the syndrome of acute bilateral basal ganglia lesions in diabetic uremia. Mov Disord. 2006;21(8):1267–1270. doi: 10.1002/mds.20932. doi: 10.1002/mds.20932. [DOI] [PubMed] [Google Scholar]
- 8.Li JY, Yong TY, Sebben R, et al. Bilateral basal ganglia lesions in patients with end-stage diabetic nephropathy. Nephrology (Carlton) 2008;13(1):68–72. doi: 10.1111/j.1440-1797.2007.00838.x. doi: 10.1111/j.1440-1797.2007.00838.x. [DOI] [PubMed] [Google Scholar]
- 9.Sheu YL, Cheng SJ, Chen YM, et al. The syndrome of bilateral basal ganglia lesions in diabetic uremic patients presenting with a relapsing and remitting course: a case report. Acta Neurol Taiwan. 2007;16(4):226–230. [PubMed] [Google Scholar]
- 10.Johnson JA, Le KL, Palacios E. Propionic acidemia: case report and review of neurologic sequelae. Pediatr Neurol. 2009;40(4):317–320. doi: 10.1016/j.pediatrneurol.2008.10.021. doi: 10.1016/j.pediatrneurol.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Moore MM, Kanekar SG, Dhamija R. Ethylene glycol toxicity: chemistry, pathogenesis, and imaging. Radiol Case Rep. 2008;3(1):1–5. doi: 10.2484/rcr.v3i1.122. doi: 10.2484/rcr.v3i1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arora V, Nijjar IB, Multani AS, et al. MRI findings in methanol intoxication: a report of two cases. Br J Radiol. 2007;80(958):e243–246. doi: 10.1259/bjr/40137535. doi: 10.1259/bjr/40137535. [DOI] [PubMed] [Google Scholar]
- 13.Glazer M, Dross P. Necrosis of the putamen caused by methanol intoxication: MR findings. Am J Roentgenol. 1993;160(5):1105–1106. doi: 10.2214/ajr.160.5.8470586. doi: 10.2214/ajr.160.5.8470586. [DOI] [PubMed] [Google Scholar]
- 14.Hsu HH, Chen CY, Chen FH, et al. Optic atrophy and cerebral infarcts caused by methanol intoxication: MRI. Neuroradiology. 1997;39(3):192–194. doi: 10.1007/s002340050391. doi: 10.1007/s002340050391. [DOI] [PubMed] [Google Scholar]
- 15.Medina L, Chi TL, Devivo DC, et al. MR findings in patients with subacute necrotizing encephalomyelopathy (Leigh syndrome): correlation and biochemical defect. Am J Neuroradiol. 1990;11(2):379–384. [PMC free article] [PubMed] [Google Scholar]
- 16.Lawler GA, Pennock JM, Steiner RE, et al. Nuclear magnetic resonance imaging in Wilson disease. J Comput Assist Tomogr. 1983;7(1):1–8. doi: 10.1097/00004728-198302000-00001. doi: 10.1097/00004728-198302000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Seidenwurm D, Novotny E, Marshall W, et al. MR and CT in cytoplasmically inherited striatal degeneration. Am J Neuroradiol. 1986;7(4):629–632. [PMC free article] [PubMed] [Google Scholar]
- 18.Starosta-Rubinstein S, Young AB, Kluin K, et al. Clinical assessment of 31 patients with Wilson's disease. Correlations with structural changes on magnetic resonance imaging. Arch Neurol. 1987;44(4):365–370. doi: 10.1001/archneur.1987.00520160007005. doi: 10.1001/archneur.1987.00520160007005. [DOI] [PubMed] [Google Scholar]
- 19.Feaney MB, Anthony DC, Frosch MP, et al. August 2000: Two cases with necrosis and hemorrhage in the putamen and white matter. Brain Pathol. 2001;11(1):121–122. [PubMed] [Google Scholar]
- 20.Pelletier J, Habib MH, Khalil R, et al. Putaminal necrosis after methanol intoxication. Neurol Neurosurg Psychiatry. 1992;5(3):234–235. doi: 10.1136/jnnp.55.3.234. doi: 10.1136/jnnp.55.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeWitt PA, Martin SD. Dystonia and hypokinesis with putaminal necrosis after methanol intoxication. Clin Neuropharmacol. 1988;11(2):161–167. doi: 10.1097/00002826-198804000-00007. doi: 10.1097/00002826-198804000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Hovda KE, Hunderi OH, Rudberg N, et al. Anion and osmolal gaps in the diagnosis of methanol poisoning: clinical study in 28 patients. Intensive Care Med. 2004;30(9):1842–1846. doi: 10.1007/s00134-004-2373-7. doi: 10.1007/s00134-004-2373-7. [DOI] [PubMed] [Google Scholar]
- 23.Phang PT, Passerini L, Mielke B, et al. Brain hemorrhage associated with methanol poisoning. Crit Care Med. 1988;16(2):137–140. doi: 10.1097/00003246-198802000-00008. doi: 10.1097/00003246-198802000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Gaul HP, Wallace CJ, Auer RN, et al. MR findings in methanol intoxication. Am J Neuroradiol. 1995;16(9):1783–1786. [PMC free article] [PubMed] [Google Scholar]
- 25.Aquilonius SM, Bergström K, Enoksson P, et al. Cerebral computed tomography in methanol intoxication. J Comput Assist Tomogr. 1980;4(4):425–428. doi: 10.1097/00004728-198008000-00001. doi: 10.1097/00004728-198008000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Server A, Hovda KE, Nakstad PH, et al. Conventional and diffusion-weighted MRI in the evaluation of methanol poisoning. Acta Radiol. 2003;44(6):691–695. doi: 10.1080/02841850312331287779. doi: 10.1080/02841850312331287779. [DOI] [PubMed] [Google Scholar]
- 27.Deniz S, Oppenheim C, Lehéricy S, et al. Diffusion-weighted magnetic resonance imaging in a case of methanol intoxication. Neurotoxicology. 2000;21(3):405–408. [PubMed] [Google Scholar]