Abstract
Fibromyalgia (FM) is a syndrome characterized by chronic pain without known peripheral causes. Previously, we have reported dysfunctional pain inhibitory mechanisms for FM patients during pain administration. In this study we employed a seed correlation analysis, independent component analysis (ICA), and an analysis of fractional amplitude of low frequency fluctuations (fALFF) to study differences between a cohort of female FM patients and an age- and sex-matched healthy control group during a resting-state condition. FM patients showed decreased connectivity between thalamus and premotor areas, between the right insula and primary sensorimotor areas, and between supramarginal and prefrontal areas. Individual sensitivity to painful pressure was associated with increased connectivity between pain-related regions (e.g., insula and thalamus) and midline regions of the default mode network (including posterior cingulate cortex and medial prefrontal cortex) among patients and controls. However, neither ICA nor fALFF revealed any group differences. Our findings suggest that abnormal connectivity patterns between pain-related regions and the remaining brain during rest reflect an impaired central mechanism of pain modulation in FM. Weaker coupling between pain regions and prefrontal- and sensorimotor areas might indicate a less efficient system level control of pain circuits. Moreover, our results show that multiple, complementary analytical approaches are valuable for obtaining a more comprehensive characterization of deviant resting-state activity. In conclusion, our findings show that FM primarily is associated with decreased connectivity, for example, between several pain-related areas and sensorimotor regions, which could reflect a deficiency in pain regulation.
Key words: : brain connectivity, fibromyalgia, fMRI, pain, resting-state
Introduction
Fibromyalgia (FM) is a condition characterized by widespread, long-lasting pain and pain in response to normally nonpainful stimuli (allodynia). FM afflicts around 2% of the population, of which almost 90% are women (Wolfe et al., 1995). In addition to pain, commonly occurring symptoms include cognitive dysfunctions, fatigue, and sleep disturbances, and there are also known comorbidities with conditions such as depression (Weir et al., 2006). The polysymptomatic nature of FM leads to great suffering for FM patients and high costs for society. Unfortunately, current treatments are not very efficient (Carville et al., 2008). Although much remains to be understood regarding the neuronal mechanisms that are involved in FM, the combination of multimodal increase in pain sensitivity (Kosek et al., 1996) and dysfunction of descending pain inhibition (Kosek and Hansson, 1997; Lannersten and Kosek, 2010) suggest the importance of central nervous system mechanisms in the pathophysiology of FM. Hence, a better understanding of the neurophysiological underpinnings of FM is essential to develop new treatments.
In a recent review, Cagnie and colleagues (2014) points out several reoccurring findings of central sensitization in FM as revealed by brain imaging: There is moderate evidence for gray matter reductions in anterior cingulate cortex (ACC) and prefrontal regions (reported by, e.g., Burgmer et al., 2009; Jensen et al., 2013), while overall gray matter volume is unaffected. There also exists moderate evidence for spatially similar but stronger response in pain-related regions (including primary (S1) and secondary (S2) somatosensory cortex, cerebellum, insula, posterior cingulate cortex (PCC), and ACC to experimentally induced pain in patients diagnosed with FM relative to controls. For subjectively equal pain intensity, the response to pain stimuli was similar in the somatosensory areas in FM patients and healthy subjects (Gracely et al., 2002; Jensen et al., 2009). However, FM patients had a reduced pain related activation of areas associated with descending pain inhibition compared with healthy controls (e.g., rACC and thalamus) (Jensen et al., 2009). In a subsequent intervention study, cognitive behavioral therapy was found to increase functional connectivity (FC) between ventral lateral prefrontal cortex and thalamus during pain stimulation, indicating an increased top downregulation (e.g., “reappraisal”) of pain following treatment in FM (Jensen et al., 2012).
Resting-state brain activity constitutes an ecologically valid imaging condition for characterizing brain mechanisms important for the perception of chronic ongoing pain. As such resting-state functional magnetic resonance imaging (fMRI) could be a promising approach for detecting future biomarkers of FM (Fomberstein et al., 2013), complementing studies of brain responses to particular experimental stimuli. Napadow and colleagues (2010) investigated resting-state network associated with FM and showed an increased connectivity between the default mode network (DMN) and insula using independent component analysis (ICA) in FM patients compared with healthy controls. Interestingly, this pattern of hyper connectivity correlated with the individual level of spontaneous pain reported at the time of scanning. In a subsequent longitudinal study, Napadow and colleagues (2012) showed that improvement of symptoms following an acupuncture intervention was correlated with a decreased connectivity between DMN and insula. In another study of resting-state connectivity in FM patients, Cifre and colleagues (2012) investigated connectivity differences between 15 seed regions located in pain-related brain areas. They reported both increases and decreases in resting-state connectivity for FM relative to controls, whereas no connectivity differences correlated with pain inventories. The strongest group differences that they observed included decreased connectivity between insula and thalamus, PCC and superior temporal sulcus, and between ACC and amygdala for FM patients. Conversely, increased resting-state connectivity was observed, for example, between primary motor areas and supplementary areas and ACC and caudate nucleus.
In this study, we aimed to replicate and expand on previous studies investigating resting-state fMRI in FM. Replication is a cornerstone in science and the issues regarding replication and lack thereof are gaining well deserved attention also in the field of cognitive neuroscience (e.g., see Barch and Yarkoni, 2013). In line with Napadow and colleagues (2010, 2012) we used ICA to investigate group differences in the resting state, focusing on similar networks of intrinsic FC. Based on their findings we expected to observe similar FC increases between DMN and insula and secondary somatosensory cortex, and between right executive attention network (EAN) and intraparietal sulcus in FM. Since the generation of independent components is (largely) data driven, ICA does not allow for regional specific hypotheses of FC changes outside the selected cardinal resting-state networks. We therefore included seed correlation analysis (SCA) in our analytical strategy to explore putative differences in connectivity between healthy controls and FM. Further, we chose to limit the set of seeds used in our SCA to brain regions that are known from the previous literature to be pertinent for the experience and processing of pain. Our main hypothesis was that FM is associated with altered FC between pain-related brain regions and the remaining brain. Furthermore, we aimed to find how pressure pain sensitivity (hyperalgesia) is related to FC, which to the best of our knowledge never has been studied.
Materials and Methods
Subjects
Subjects were recruited by newspaper advertisement to participate in a multicenter experimental study (ClinicalTrials.gov identification number: NCT01226784) where FM patients were randomized to physical exercise or relaxation therapy. The current study was performed in the Stockholm cohort only and relies on baseline data before start of treatment. The study was approved by the Regional Ethics Committee in Stockholm and written informed consent was obtained from all participants (ethical permit number 2010/1121-31/3).
Inclusion criteria for women with FM were they had to be of working age (20–65 years), and meet the American College of Rheumatology (ACR) 1990 classification criteria for FM (Wolfe et al., 1990). Exclusion criteria were high blood pressure (>160/90 mmHg), osteoarthritis in hip or knee, other severe somatic or psychiatric disorders, other primary causes of pain than FM, high consumption of alcohol (Audit >6), participation in a rehabilitation program within the past year, regular resistance exercise training or relaxation exercise training twice a week or more, inability to understand or speak Swedish, and not being able to refrain from analgesics, NSAID, or hypnotics for 48 h prior to examinations. Thirty-one FM patients were initially recruited. Due to technical failures of the MRI scanner and failure to comply with the study protocol, we successfully collected resting-state fMRI and behavioral data from 17 subjects. Data from one subject were rejected due to excessive movement during scanning (a mean frame-wise displacement (FD)>0.35 mm, corresponding to two standard deviations (SDs) from the mean of all the participants), leaving data from 16 subjects for further analyses. One patient was on anticonvulsants and 11 were taking antidepressants (4 tricyclic antidepressants, 4 selective serotonin re-uptake inhibitors, and 3 serotonin-noradrenalin re-uptake inhibitors). All patients had a physical exam by a specialist in rehabilitation medicine and filled in questionnaires regarding the impact of FM [fibromyalgia impact questionnaire (FIQ)] (Bennett, 2005), and health-related quality of life (SF-36) (Contopoulos-Ioannidis et al., 2009). The mean age of the 16 subjects was 48.3 (range 25–64 years) (only females). Mean total FIQ score was 61.2 (SD=13.3) and mean FM duration was 7.6 years (SD=3.8).
For the control cohort, 32 healthy female controls were recruited, of which 24 participants successfully completed the scanning fMRI session. Imaging data from two subjects were rejected due to excessive mean FD, leaving data from 22 subjects for analyses (age 45.7, ranging 20–63 years).
Procedure
An automated, pneumatic, computer-controlled pressure stimulator, with a piston that applies pressure via a 1 cm2 probe was used to assess pressure pain sensitivity at the left thumb. The day before scanning, each subject was calibrated for subjective pain ratings by receiving one ascending and one randomized series of pressure stimuli. Subjects were instructed to rate the intensity of the pain evoked by each stimulus by putting a mark on a 100 mm horizontal visual analogue scale (VAS) ranging from “no pain” to “worst imaginable pain.” The ascending series was used to identify the individual pain threshold and the pressure intensity corresponding to the stimulation maximum (first rating >60 mm VAS). These values were then used to compute the magnitude of five different pressure intensities, delivered in a randomized order. In total 15 stimuli of 2.5 sec duration were delivered in a randomized order at 30 sec intervals. A first order polynomial function was estimated and used to determine each individual's representation of VAS 50 mm, built on the 15 ratings from the randomized series (for further details, see Jensen et al., 2009). Pain sensitivity is here defined as low (or negative correlation with the) P50 measure, and pain resilience as high (or positively correlation with) P50.
The patients had to refrain from analgesics, NSAIDS, and sedatives 48 h before the assessment of pain and 72 h before the fMRI scan.
MRI data acquisition
MR imaging was performed on a 3T General Electric 750 MR scanner installed at the MR Research Center, Karolinska Institute, Stockholm. Anatomical MR imaging was acquired with a high-resolution BRAVO 3D T1-weighted image sequence (1×1×1 mm3 voxel size). For each subject we performed one resting-state scan consisting of 200 volumes, using an echo-planar imaging with TR/TE=2500/30 msec, flip=90°, 49 slices, 96×96 matrix size, FOV=288×288 mm, slice thickness=3 mm, and interleaved slice acquisition. In the resting-state condition, subjects were instructed to lie still and rest, and keep their eyes closed and try not to fall asleep. Prior to the resting-state fMRI data acquisition, subjects underwent two fMRI scans of a pain exposure paradigm (∼7 min each), and two scans of stroop task (∼7 min each). Data from the task-evoked fMRI runs will be reported elsewhere.
Resting-state fMRI data analysis
We employed several analytical approaches to detect group differences in resting-state activity, and to associate brain connectivity to individual estimates of perceived pain (P50).
Rather than defining seed regions that covered the entire brain, we opted to restrict our SCA to previously known pain-related areas in the brain. Hence, we defined 159 seeds located in pain-related brain areas. These pain areas were selected from an automatically generated meta analysis of 316 articles tagged with the term “pain” in the framework of neurosynth (www.neurosyntsh.org): A volume obtained from forward inference (i.e., brain areas commonly activated given the term “pain”) was thresholded at p<0.05 false discovery rate (FDR)-corrected, to construct a binary brain mask constituted by regions that have been robustly associated with perception and modulation of pain. Within these pain regions, spherical seed regions (4 mm radius) were evenly placed out in a 3D grid of 10 mm center to center adding up to a total of 159 seed regions (Supplementary Fig. S1 and Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/brain). This decision was motivated by our aim to keep the number of seed regions as low as possible and thereby minimize loss of sensitivity due to correction of multiple comparisons, while at the same time allowing for an extensive set of seed that lessen the influence of seed selection bias (Cole et al., 2010). Prior to SCA, imaging data were preprocessed using SPM12 (Welcome Trust Center of Neuroimaging, University College London, London, United Kingdom). Image preprocessing included a slice time correction to the middle slice, realignment to the mean image using fourth degree of B-spline interpolation, co-registration of functional and structural images, tissue segmentation of structural images, and normalization of structural and functional scans to the MNI template using the deformation field obtained from the segmentation (fourth degree B-spline function, resampling to 2 mm isotropic voxels). Finally, functional volumes were spatially smoothed using an 8 mm FWHM Gaussian kernel. Subject level SCA analyses were carried out using the Conn toolbox (www.nitrc.org/projects/conn) (Whitfield-Gabrieli and Nieto-Castanon, 2012). Functional volumes were band pass filtered at 0.008–0.09 Hz (default values). Subject specific nuisance regressors included six movement and their time derivatives, and five regressors pertaining to white matter and cerebral spinal fluid (CSF) signals respectively, using a component based noise correction (CompCor) approach (Behzadi et al., 2007). Additionally, images that were regarded as movement outliers were regressed out. Outliers were detected using the ART toolbox (nitrc.org/projects/artifact_detect/) and defined as volumes with FD>0.5 mm or signal intensity changes greater than 3 SDs (default thresholds). The average number of regressed volumes of all participants were 7, with no significant group difference [t(37)=−1.45, p=0.16].
Beta estimates pertaining to the regressors of interest were used in the second level group analyses for each subject and each seed region. All second level group analyses were controlled for mean FD and age. Independent t-tests were used for testing group differences in FC for each seed region. Furthermore, we investigated how pain sensitivity affected FC across subjects in both groups using a measure of pain sensitivity. All reported SCA results are corrected at cluster level significance of FDR p<0.05/159=0.00031, accounting for 159 t-tests using Bonferroni correction. Cluster defining voxel threshold was p<0.001, uncorrected (as recommended in Woo et al., 2014).
For the ICA we used the MELODIC toolbox in FSL (www.fmrib.ox.ac.uk/fsl). Prior to the ICA analysis, functional image data from each subject were motion corrected using MCFLIRT, followed by brain extraction (BET), coregistration to structural images (FSL-FLIRT), spatial smoothing (5 mm FWHM), and then high-pass filtered (f=0.006 Hz). Native space data were normalized to standard space (MNI template provided by FSL) using a 12 degrees of freedom nonlinear transformation. The normalized data were subsequently resampled to 4 mm3 isotropic resolution. Preprocessing parameters were chosen in accordance with Napadow and colleagues (2010).
To evaluate differences in intrinsic brain connectivity between the FM and the healthy cohort, we used dual regression to obtain comparable ICs across subjects by first concatenating data from all subjects. Similar to Napadow and colleagues (2010) the number of estimated IC was set to 25, and the best-fit component for right and left EAN, DMN, and the medial visual network was selected by calculating the average z-score of voxels inside compared to the average z-score outside each binary template mask (kindly supplied by Dr. V. Napadow). Additionally, we chose to include the IC that encompassed the primary sensory/motor network (S1M1) due to the involvement of regions in pain perception. The spatial IC maps obtained from the group-analysis were used to generate subject-specific versions of the ICs and their corresponding time series.
The subject-specific IC maps were brought to the group level and tested for significance using the FSL command randomize. All group-level effects were controlled for mean FD as proposed by Yan and colleagues (2013). We also controlled for age, since Yan and colleagues (2013) recently discovered that age interacted with brain changes associated with FM. Group differences of independent components were identified using threshold-free cluster enhancement method of p<0.05 (Smith and Nichols, 2009).
Additionally, we carried out an analysis of fractional amplitude of low frequency fluctuations (fALFF), given that a previous study had reported changes in the power spectrum of resting-state BOLD signal changes in patients with FM (Kim et al., 2013). Preprocessing of functional fMRI data and fALFF analysis was carried out using Data Processing Assistant for Resting-State fMRI (DPARSF) (Chao-Gan and Yu-Feng, 2010), www.restfmri.net). Image preprocessing included realignment, normalization using DARTEL, and resampling to 3 mm3 isotropic voxels followed by spatial smoothing (6 mm FWHM) and removal of linear trends. The ratio of the power in the frequency window between 0.01 and 0.08 Hz and the entire frequency window was calculated for each voxel. Subjects normalized (i.e., Z-converted) fALFF images were brought to group analysis that was carried out in SPM12 for comparison of FM with healthy controls. All statistically significant brain activity clusters were labeled using the automatic anatomical labeling atlas provided by MRIcroN (www.mccauslandcenter.sc.edu/mricro/mricron/).
Results
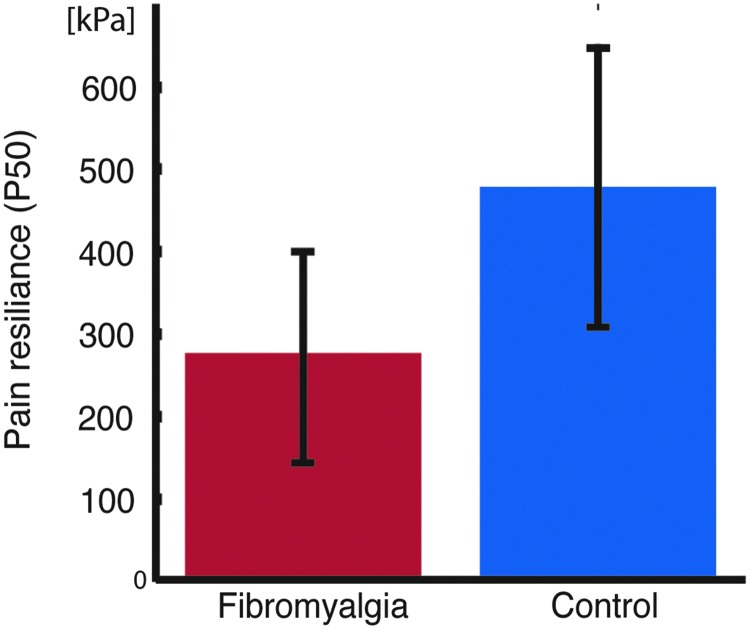
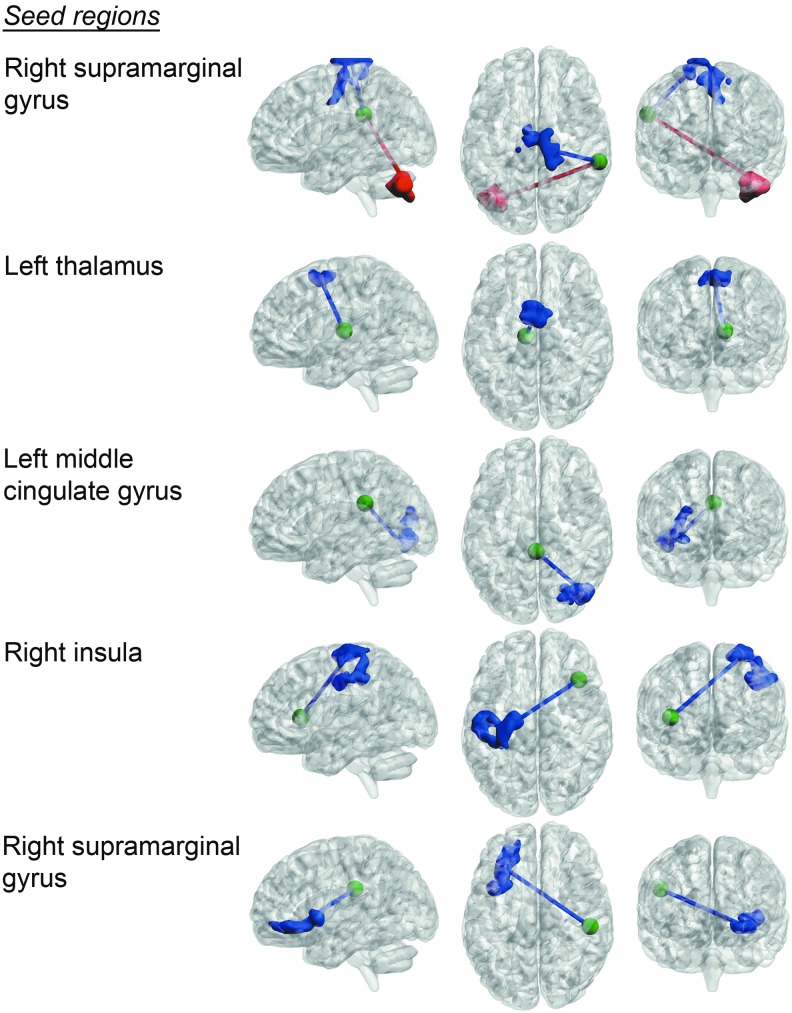
FM patients showed significantly increased pain sensitivity compared with the control group [t(36)=3.98, p=0.00032] (Fig. 1). Seed-based region connectivity analyses revealed a general pattern of a decreased FC between the targeted pain-related brain areas and the remaining brain (Table 1 and Fig. 2). Specifically, FM patients showed a decreased connectivity between the right insula and a cluster of activity that covered the left primary sensorimotor areas. Additionally, FM patients displayed a decrease in intrinsic connectivity between a seed positioned in right supramarginal gyrus and the right primary sensorimotor region. Moreover, a reduced degree of connectivity was observed for the right supramarginal gyrus and left inferior prefrontal cortex (PFC), and between thalamus and medial premotor cortex. The only significant increase in connectivity was found between right supramarginal gyrus and left cerebellum, for which the FC was shifted from a negative to a positive correlation in FM patients compared to healthy controls.
FIG. 1.
Group differences in pain sensitivity. Fibromyalgia patients had lower P50 [pressure corresponding to ratings of 50 mm on a 100 mm visual analogue scale (VAS)] compared with controls [two sample t-test, t(36) = −3.98, p=0.00032]. Error bars denote standard deviations.
Table 1.
Significant Differences in Resting-State Brain Connectivity Between Fibromyalgia Patients and Healthy Control Subjects
| Seed (Center of sphere) | Target (Peak coordinate) | Cluster size (No. of voxels) | Cluster p-FDR | |
|---|---|---|---|---|
| HC>FM | Insula (40, 24, 8) | S1/M1 (−30, −22, 68) | 1498 | <0.0000001 |
| Supramarginal gyrus (60, −36, 28) | S1/M1 (10, −24, 80) | 1286 | <0.0000001 | |
| Supramarginal gyrus (50, −26, 28) | Inferior PFC (−32, 30, −8) | 812 | 0.000044 | |
| Mid cingulate (0, −36, 28) | Occipital cortex (42, −72, −12) | 731 | 0.00010 | |
| Thalamus (−10, −16, 8) | Premotor cortex (0, 6, 56) | 623 | 0.00028 | |
| FM>HC | Supramarginal gyrus (60, −36, 28) | Cerebellum (−42, −74, −34) | 929 | 0.000015 |
All differences in brain connectivity were significant at the Bonferroni corrected level of p<0.00031, FDR corrected at cluster level.
FDR, false discovery rate; FM, fibromyalgia; PFC, prefrontal cortex.
FIG. 2.
Differences in functional connectivity between fibromyalgia patients and a cohort of healthy subjects projected on a semitransparent template brain [p<0.00031, false discovery rate (FDR) corrected at cluster level]. Blue activations/edges represent decreased connectivity in fibromyalgia patients relative to healthy subjects. Red activations/edges represent increases in resting-state connectivity in fibromyalgia patients compared with healthy subjects. Seed-points for the connectivity analysis are marked as green spheres (for visualization purposes are the radius of the seeds doubled compared to those of the actual seeds).
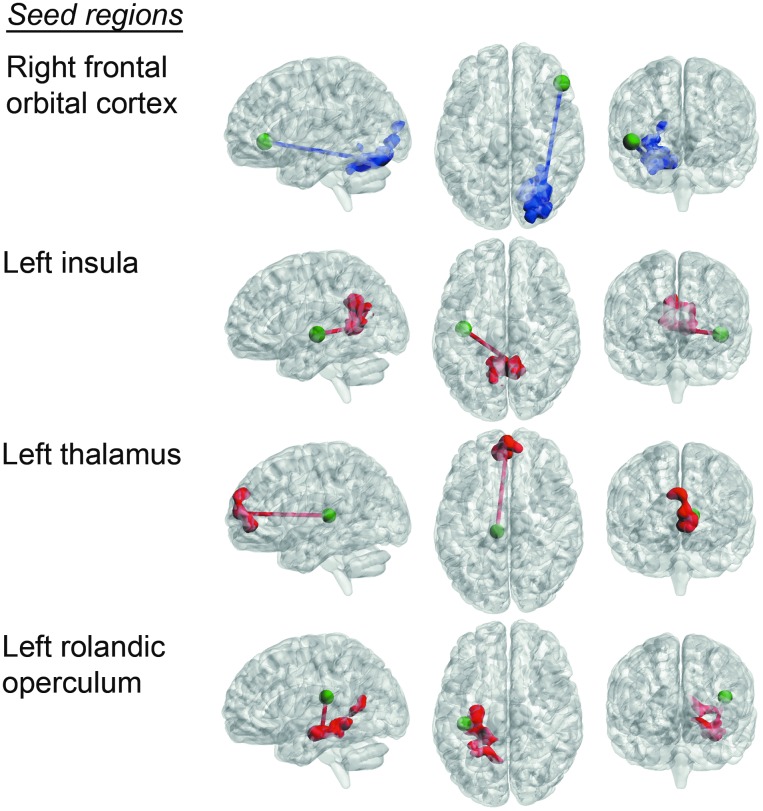
Significant correlations between pain sensitivity and changes in intrinsic connectivity (taken across cohorts) are shown in Figure 3 and Table 2. In detail, a lower P50 score (i.e., increased sensitivity to pain) was associated with increased connectivity between left insula and dorsal PCC. Furthermore, we observed an increased connectivity related to increases in pain sensitivity between left rolandic operculum and left parahippocampus, and thalamic-prefrontal connectivity. These results showed that the strength of intrinsic brain connectivities between pain-related regions and the posteromedial node of the DMN are higher in hyperalgesia. Moreover, resilience to pain (i.e., a positive relationship with the P50 scores) was associated with increased connectivity between right inferior orbital regions and right associative visual cortex. There were also significant interaction effects between the groups and pain sensitivity on connectivity. For healthy controls, high pain resilience (P50) positively correlated with increasing connectivity between middle cingulate cortex (MNI: [−0, −6, 38]) and dorsomedial prefrontal cortex (MNI: [4, 50, 30], cluster size: 2249; p<0.000001) and PCC (MNI: [−10, −50 28], cluster size: 707; p<0.00019), whereas high pain resilience in FM was negatively correlated for these connectivities. Similarly, high pain resilience was associated with stronger connectivities between a supplementary motor area (MNI: [10, 24, 48]) and associative visual areas (MNI: [−34, −74, −14], cluster size: 1368; p<0.000001) and postcentral areas (MNI: [−16, −26, 54], cluster size: 682; p<0.0001) in healthy controls compared to FM patients.
FIG. 3.
Correlations between individual ratings of pain sensitivity and resting-state brain connectivity (p<0.00031, FDR corrected at cluster level). Blue activations/edges represent brain areas that showed significant positive co-variability between pain resilience (P50) and resting-state brain connectivity. Red activations/edges represent brain regions that exhibited a stronger degree of brain connectivity as a function of pain sensitivity. Seed-points for the connectivity analysis are marked as green spheres.
Table 2.
Subjective Pain Sensitivity Predicts Functional Connectivity in Fibromyalgia Patients and Healthy Controls
| Contrast | Seed (Center of sphere) | Target (Peak coordinate) | Cluster size (No. of voxels) | Cluster p-FDR |
|---|---|---|---|---|
| Pain resilience (P50) | Frontal Inferior orbital (50, 34, −2) | V2 (32, −70, −20) | 1536 | <0.000001 |
| Pain sensitivity (Anti P50) | Insula (−40, −16, −2) | Dorsal PCC (6, −48, 6) | 1177 | <0.000002 |
| Thalamus (−10, −26, 8) | Dorsal mPFC (0, 60, 10) | 761 | 0.000040 | |
| Rolandic operculum (−40, −26, 18) | Parahippocampus (−30, 22, −12) | 1022 | 0.000005 |
Increased resilience to pain (positive correlation with respect to P50) and increased sensitivity to pain (negative correlation with respect to P50) were associated with seed-based correlation analysis of seeds positioned in the pain mask (Bonferroni corrected at peak level p<0.00031 FDR corrected).
PCC, posterior cingulate cortex.
The ICA did not reveal any group differences in connectivity for the networks of interest. Neither pain resilience (positive correlation with P50), nor pain sensitivity (negative correlation with P50) was significantly correlated with any of the tested ICs. Since previous studies repeatedly had identified certain group differences using ICA (Napadow et al., 2010, 2012), we performed a post hoc analysis by rerunning an ICA with the intent to employ analytical strategies as similar as possible to those that were reported by Napadow and colleagues (2010). This post hoc ICA analysis differed from our original ICA at two stages of the analysis pipeline: First, the subject-specific time series pertaining to ICs of interest were explicitly controlled for white matter and CSF time series using general linear models. Unfortunately, we did not collect cardiac or respiratory data so we could not regress these out, and we had to deviate from Napadow and colleagues (2010, 2012) in this regard. Second, the group comparison was performed using FMRIB Local Analysis of Mixed Effects (FLAME) procedure, in contrast to our original analysis that employed the conventional approach of using the FSL randomize permutation tool. In the post hoc ICA we also omitted to control for age and FD in the group comparisons, to render the group analyses identical to Napadows. This post hoc ICA revealed a significant (if omitting Bonferroni correction with regard to number of group comparisons) increase in connectivity for FM patients compared to healthy controls between the left EAN component and midline DMN regions (mPFC and PCC) (peak voxel at MNI coordinates [2, −50, 26] and [16, 38, 12], cluster size: 1371 and 830 voxels; p=0.0022 and p=0.036 corrected). Additionally, it revealed increased connectivity between the right motor cortex and the postcentral gyrus for FM patients (peak voxel at MNI coordinate [14, −50, 70], cluster size: 815 voxels; p=0.0.028 corrected). Of note, intrinsic connectivity between the primary visual network (included here as a control network) displayed an increased connectivity with two regions adjacent to the supplementary motor areas (peak voxel at MNI coordinates [−28, −6, 36] and [−6, −12, 50], cluster size: 1107 and 785 voxels; p=0.0041 and p=0.0336 corrected). Finally, no brain region showed any significant difference between FM patients and healthy controls with regard to voxel-based measures of the fALFF.
Discussion
The aim of this study was to characterize resting-state brain activity in FM compared to healthy subjects. The main findings are decreased intrinsic connectivities between the seed points in pain-related areas and clusters in sensorimotor-, prefrontal-, and occipital cortical regions. In earlier studies of brain activity in response to pain administration, we have observed reduced brain activation in dorsal anterior cingulate cortex (dACC) and thalamus (Jensen et al., 2009), and increased connectivity between prefrontal areas and thalamus following a nonpharmacological intervention in FM patients (Jensen et al., 2012). These results indicated neuronal deficiency in inhibiting pain in FM. Our current findings add evidence to this notion, by showing a reduced intrinsic coupling between pain-related brain regions and the primary sensorimotor areas in the absence of externally applied pain. Cifre and colleagues (2012) reported stronger correlations and anticorrelations among pain regions in FM, whereas our whole brain analysis instead revealed a general reduction of coupling in FM compared to controls. The neurophysiological meaning of resting-state connectivity is commonly thought to reflect coactivation of anatomically separated brain areas. The diminished resting-state connectivity between pain-related areas and primary sensory cortices could reflect a state of mind for which brain areas that are central for the processing and perception of pain are operating under less constrains by other brain networks. In the noninvasive electrophysiological domain, several studies have linked chronic pain to thalamic-cortical dysregulation (for a commentary, see Jones, 2010). Although the relationship between the hemodynamics of the BOLD fMRI response and electrophysiological signals (such as magnetoencephalography) is still not fully understood, our finding of decreased resting-state connectivity between the thalamus and sensorimotor regions suggest that thalamo-cortical dysregulation is also manifested as disrupted resting-state BOLD connectivity. This disturbed thalamo-cortical connectivity in FM patients could reflect a hampered gate-keeping function of the thalamus (Crick, 1984) leading to enhanced pain perception.
Our finding of a decreased connectivity between right anterior and sensorimotor areas can be viewed in the light of a previous model of neuropathic pain, which proposes that the interoceptive homeostasis that is subserved by insula is disturbed (Craig, 2009). The anterior insula has been implicated in a wide range of behaviors and is proposed to have a pivotal role for the neuronal representation of interoception (Craig, 2009) by integrating stimulus-driven and top-down driven information (Gu et al., 2013). A lesion study (Starr et al., 2009) found that damage to the insula was associated with higher pain intensity ratings during experimental noxious stimuli, and interestingly, that pain activations in primary somatosensory cortex was elevated ipsilaterally to the damaged insula. Speculatively, our observation of an reduction of FC between sensorimotor areas and anterior insula could be interpreted as if the “interoceptive thermostat” in the anterior insular cortex is impaired in terms of integrating and modulating signals from sensory cortex, thus contributing to central pain sensitization in FM patients.
Furthermore, we could relate reported pain sensitivity to interindividual changes in resting-state brain connectivity. Generally speaking, our finding of an association of pain sensitivity ratings with brain connectivity, primarily in the insula and dorsal PCC, is similar to the previous findings of Napadow and colleagues (2010). In that study, they observed a co-variability in ratings of spontaneous pain and the strength of intrinsic connectivity between the DMN and the insula (among other regions). The seed region used in the current study was localized in the insula, which has been implicated in pain, whereas the dorsal PCC coordinate, according to reverse inference performed by neurosynt.org, primarily is associated with the term “autobiographical” (for a recent review on PCC function, see Leech and Sharp, 2014). Furthermore, we found that increased sensitivity to pain correlated with higher resting-state connectivity between other pain regions (insula, thalamus, and rolanidc peraculum) and brain areas commonly associated with DMN (PCC, mPFC, and hippocampal formation). This finding is in line with the previous report by Napadow and colleagues (2010), who found an increased connectivity between the insula and the DMN that correlated with reports of increased pain in FM patients. Corroborating evidence of elevated coupling between DMN and insula in pain is further provided by a study on chronic back pain using arterial spin labeling (Loggia et al., 2013).
In our original ICA we did not observe any differences in intrinsic connectivity between cohorts. However, in our post hoc ICA, which was carried out to maximally resemble the procedure of analysis described in Napadow and colleagues (2010), we detected an increased connectivity between the midline regions of the DMN and the right EAN in FM patients. Notably, we could not detect any increases in intrinsic connectivity between the DMN and the insula, even at an uncorrected, exploratory level of significance (p<0.1). Moreover, we could not observe any significant difference in connectivity between the EAN and the intraparietal sulcus (Napadow et al., 2010). It is unlikely that these observed discrepancies are due to difference in statistical power, since the sizes of the cohorts essentially were the same. However, increased power of fMRI-studies would generally enhance the overlap of true positive findings (David et al., 2013), and although both studies had cohort sizes within the range of what commonly are used in fMRI studies, the observed discrepancies could reflect a lack of sensitivity that led to the detection of two relatively separate subsets of the true group differences. The divergent findings could possibly also in part be due to the difference in experimental design between the study of Napadow and colleagues (2010) and this study (i.e., here resting-state scans were preceded by task fMRI that potentially introduced spillover effects).
The difference in the results of our original ICA (where we controlled for age in the group analysis) and the post hoc ICA (were age was not controlled for), points to the notion that age might be an important factor to take into account in resting-state connectivity analysis of FM. Interestingly, a recent study by Ceko and colleagues (2013) showed that there exists a strong interaction between age and local measures of gray matter volumes in FM patients. In their combined voxel-based morphometry and resting-state connectivity study, younger FM patients (<50 years of age) exclusively displayed increases in gray matter volume, whereas both increases and decreases in resting-state connectivity was found. On the other hand, older patients showed only decreased regional gray matter changes, and only decreased levels of resting-state connectivity. The mean age of the FM patients in the current study was 48 years compared to 39 years in the study by Napadow and colleagues (2010), which potentially could be a major contribution to the discrepancy.
The large number of performed seed correlations together with the usage of Bonferroni correction, imposed a rather conservative threshold of the smallest size of clusters of activity to be considered to be significant. Hence, it could be argued that this favored the sensitivity for spatially extended connectivity changes at the expanse of potentially more spatially localized group differences. However, we argue that a trade-off between maximizing statistical sensitivity while minimizing seed selection bias is unavoidable when performing a comprehensive exploratory SCA, and that we have balanced the two given the constraints of this study. Second, medications are potential modulators of resting-state brain activity (see, e.g., Flodin et al., 2012). Since we included subjects on antidepressants not to bias the cohort toward, for example, less severe cases, medication could potentially have constituted a general confound. A third limitation in this study is the lack of estimates of spontaneous pain at the time of scanning, which could have complemented the measures of pain sensitivity (Napadow et al., 2010).
The existing literature on putative neurophysiological mechanisms that are involved in the FM are to date far from conclusive. Functional neuroimaging studies that aim to characterize deviant brain functioning in FM are still often exploratory. Future research that employ multiple analytical approaches for characterizing FM physiology in high-powered studies could provide promising features for diagnostic classification using multivariate pattern analysis, which until now has been proven difficult (Sundermann et al., 2014).
In conclusion, by making use of the great body of the existing literature of fMRI studies on pain, we defined pain relevant seed regions of which several displayed a general pattern of functional decoupling in FM patients compared with controls. We further identified connectivity changes associated with pain sensitivity that included increased coupling between seeds in pain region and midline DMN nodes.
Supplementary Material
Acknowledgments
This study was supported by grants from the Swedish Rheumatism Association, the Stockholm County Council, the Swedish Foundation for Strategic Research, the Swedish Research Council K2013-52X-22199-01-3, and the Karolinska Institute Foundation. Fransson was supported by a grant from the Swedish Research Agency.
Author Disclosure Statement
No competing financial interests exist.
References
- Barch DM. Yarkoni T. 2013. Introduction to the special issue on reliability and replication in cognitive and affective neuroscience research. Cogn Affect Behav Neurosci 13:687–689 [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37:90–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. 2005. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol 23:154–162 [PubMed] [Google Scholar]
- Burgmer M, Pogatzki-Zahn E, Gaubitz M, Wessoleck E, Heuft G, Pfleiderer B. 2009. Altered brain activity during pain processing in fibromyalgia. Neuroimage 44:502–508 [DOI] [PubMed] [Google Scholar]
- Cagnie B, Coppieters I, Denecker S, Six J, Danneels L, Meeus M. 2014. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin Arthritis Rheum DOI: 10.1016/j.semarthrit.2014.01.001 [DOI] [PubMed] [Google Scholar]
- Carville SF, Arendt-Nielsen S, Bliddal H, Blotman F, Branco JC, Buskila D, Choy EH. 2008. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis 67:536–541 [DOI] [PubMed] [Google Scholar]
- Ceko M, Bushnell MC, Fitzcharles MA, Schweinhardt P. 2013. Fibromyalgia interacts with age to change the brain. Neuroimage Clin 3:249–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao-Gan Y, Yu-Feng Z. 2010. DPARSF: A MATLAB Toolbox for “Pipeline” Data analysis of resting-state fMRI. Front Syst Neurosci 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifre I, Sitges C, Fraiman D, Muñoz MÁ, Balenzuela P, González-Roldán A, Martínez-Jauand M, Birbaumer N, Chialvo DR, Montoya P. 2012. Disrupted functional connectivity of the pain network in fibromyalgia. Psychosom Med 74:55–62 [DOI] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. 2010. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contopoulos-Ioannidis DG, Karvouni A, Kouri I, Ioannidis JPA. 2009. Reporting and interpretation of SF-36 outcomes in randomised trials: systematic review. BMJ 338:a3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig ADB. 2009. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70 [DOI] [PubMed] [Google Scholar]
- Crick F. 1984. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci 81:4586–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Ware JJ, Chu IM, Loftus PD, Fusar-Poli P, Radua J, Munafò MR, Ioannidis JP. 2013. Potential reporting bias in fMRI studies of the brain. PLoS One 25;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flodin P, Gospic K, Petrovic P, Fransson P. 2012. Effects of L-dopa and oxazepam on resting-state functional magnetic resonance imaging connectivity: a randomized, cross-sectional placebo study. Brain Connect 2:246–253 [DOI] [PubMed] [Google Scholar]
- Fomberstein K, Qadri S, Ramani R. 2013. Functional MRI and pain. Curr Opin Anaesthesiol 26:588–593 [DOI] [PubMed] [Google Scholar]
- Gracely RH, Petzke F, Wolf JM, Clauw DJ. 2002. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum 46:1333–1343 [DOI] [PubMed] [Google Scholar]
- Gu X, Hof PR, Friston KJ, Fan J. 2013. Anterior insular cortex and emotional awareness. J Comp Neurol 521:3371–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Ingvar M. 2009. Evidence of dysfunctional pain inhibition in fibromyalgia reflected in rACC during provoked pain. Pain 144:95–100 [DOI] [PubMed] [Google Scholar]
- Jensen KB, Kosek E, Wicksell R, Kemani M, Olsson G, Merle JV, Kadetoff D, Ingvar M. 2012. Cognitive Behavioral Therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain 153:1495–503 [DOI] [PubMed] [Google Scholar]
- Jensen KB, Srinivasan P, Spaeth R, Tan Y, Kosek E, Petzke F, Kong J. 2013. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis Rheum 65:3293–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. 2010. Thalamocortical dysrhythmia and chronic pain. Pain 150:4–5 [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim SH, Seo J, Kim SH, Han SW, Nam EJ, Chang Y. 2013. Increased power spectral density in resting-state pain-related brain networks in fibromyalgia. Pain 154:1792–1797 [DOI] [PubMed] [Google Scholar]
- Kosek E, Ekholm J, Hansson P. 1996. Sensory dysfunction in fibromyalgia patients with implications for pathogenic mechanisms. Pain 68:375–383 [DOI] [PubMed] [Google Scholar]
- Kosek E, Hansson P. 1997. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain 70:41–51 [DOI] [PubMed] [Google Scholar]
- Lannersten L, Kosek E. 2010. Dysfunction of endogenous pain inhibition during exercise with painful muscles in patients with shoulder myalgia and fibromyalgia. Pain 151:77–86 [DOI] [PubMed] [Google Scholar]
- Leech R, Sharp DJ. 2014. The role of the posterior cingulate cortex in cognition and disease. Brain 137:12–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, Wasan AD, Napadow V. 2013. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain 154:24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, Kim J, Clauw DJ, Harris RE. 2012. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum 64:2398–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. 2010. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum 62:2545–2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, Coghill RC. 2009. Roles of the insular cortex in the modulation of pain: insights from brain lesions. J Neurosci 29:2684–2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. 2009. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98 [DOI] [PubMed] [Google Scholar]
- Sundermann B, Burgmer M, Pogatzki-Zahn E, Gaubitz M, Stüber C, Wessolleck E, Pfleiderer B. 2014. Diagnostic classification based on functional connectivity in chronic pain: model optimization in fibromyalgia and rheumatoid arthritis. Acad Radiol 21:369–377 [DOI] [PubMed] [Google Scholar]
- Weir PT, Harlan GA, Nkoy FL, Jones SS, Hegmann KT, Gren LH, Lyon JL. 2006. The incidence of fibromyalgia and its associated comorbidities: a population-based retrospective cohort study based on International Classification of Diseases, 9th Revision codes. J Clin Rheumatol 12:124–128 [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. 2012. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125–141 [DOI] [PubMed] [Google Scholar]
- Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. 1995. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 38:19–28 [DOI] [PubMed] [Google Scholar]
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Cark P. 1990. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 33:160–172 [DOI] [PubMed] [Google Scholar]
- Woo CW, Krishnan A, Wager TD. 2014. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage 91:412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Milham MP. 2013. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage 76:183–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.