Abstract
The role of oxidative stress during aging is well documented. Evidence is available linking animal life span to the development of oxidative stress. Up to a certain limit of oxidative stress, cells function to counteract the oxidant effects and to restore redox balance by resetting critical homeostatic parameters. Red blood cells (RBCs) offer a very good model to study cellular senescence. In vivo aging of red blood cells is associated with increased cellular density, which corresponds to increased cell age. The present study aims to investigate age-dependent oxidative stress in RBC subpopulations obtained after Percoll density gradient centrifugation from young and old rats. We observe an increase in plasma membrane redox system (PMRS) activity (p<0.001) and lipid peroxidation (p<0.001) between less dense and senescent RBCs in both young and old rats. Our findings provide evidence of a higher level of oxidative stress in senescent erythrocytes, with the effect being more pronounced in old (24-month-old) rats compared to young (4-month-old) rats. The present findings emphasize the role of oxidative stress not only in organismal aging but also in cell senescence.
Introduction
According to the free radical theory of aging, oxidative stress increases with age, resulting in the accumulation of oxidation products of lipids, nucleic acids, and proteins and culminating with cellular dysfunction and making the body prone to external deleterious agents.1 Aerobic metabolism comes with a price; a certain amount of oxidative damage takes place even under normal conditions. Other than this constitutive oxidative damage, certain conditions such as diabetes, cardiovascular diseases, cancer, and aging are accompanied with a higher rate of oxidative stress.2,3 Animal life span has been found to be intricately linked with increased oxidative stress.4 Up to a certain limit of oxidative stress, cells function to counteract the oxidant effects and to restore redox balance by resetting critical homeostatic parameters.5
Erythrocytes, or red blood cells (RBCs), are among the most common type of cells and play an important role in delivering oxygen from the lungs or gills to body tissues via the blood. The mature human RBC has an average life span of 120±20 days,6 whereas rat erythrocytes have been reported to have a life span of only 60 days.7,8 Having a cell structure devoid of cell organelles, particularly a nucleus and mitochondria, the RBC does not have the ability to synthesize amino acids and fatty acids and thus has a limited capacity for metabolism that is barely enough to survive its life span. It is acknowledged that erythrocytes have a definite life span in all animal species investigated so far, and there is little dispute with regard to species-specific life span. This implies that life and death are well regulated for erythrocytes, in spite of their lack of capacity for protein synthesis. As they age, erythrocytes undergo various physicochemical changes, including cell density enhancement.9 Consequently, RBC deformability and aggregation are strongly affected, leading to the disturbance in RBC circulation in blood vessels.10
In vivo aging of RBCs is associated with increased cellular density, which corresponds to increased cell age. A change in the overall redox status toward a more oxidized state has been reported during cellular aging.11 Increased accumulation of oxidized and denatured proteins, particularly hemoglobin, is also documented.12 Studies have shown that erythrocyte aging in vivo is accompanied by a reduction in sodium-potassium adenosine triphosphatase (Na/K-ATPase),13 Gardos channel,14 and calcium (Ca2+)-ATPase with a concomitant significant increase in intracellular calcium.15 Additionally, metabolic and functional changes have been observed for young and old erythrocytes kept outside the blood circulation.16 Dense RBCs exhibit increased rigidity and decreased stability17 and a higher degree of acidosis.18 Studies have shown that young and old cells behave differently when exposed to oxidative stress.15 Despite extensive investigations,11 there are still gaps in the understanding of the role of oxidative stress in cell senescence and also the understanding of the role of organismal aging on the cell senescence process.
Recent findings concerning the important role of the erythrocyte plasma membrane redox system (PMRS) in aging,19 the importance of glutathione (GSH) as a regulator of intracellular redox homeostasis in erythrocytes,20 l-cysteine availability as a rate-limiting step in GSH synthesis, and the implications of age-dependent protein oxidative changes 21 prompted us to study these and other oxidative stress parameters in young and senescent erythrocyte subpopulations from 4-month-old and 24-month-old Wistar strain male rats.
Material and methods
Chemicals
Percoll, sorbitol, reduced GSH (2,4,6-Tris(2-pyridyl)-s-triazine [TPTZ]). 4,7-Diphenyl-1,10-phenanthroline disulfonic acid disodium salt (DPI), and 5, 5′-dithiobis(2-nitrobenzoic acid) (DTNB) were purchased from Sigma Aldrich, USA. All other chemicals were of highest purity available from Merck, India, and HIMEDIA Labs, India.
Animal model and study protocol
The experiment was carried out with two different age groups of male Wistar rats young (4 months, n=12) and old (24 months, n=12). They were housed in a temperature-controlled room (25±5°C) with 12-hr light–dark cycles for at least 1 week. All rats were fed with a normal laboratory diet (nutrient-rich pellets) containing total energy as fat, protein, and carbohydrates, and had free access to drinking water.
Collection of blood and isolation of RBCs
After the end of the experimental period, rats were sacrificed under light anesthesia induced by exposure to diethyl ether. Blood samples were collected by cardiac puncture into 10 units/mL heparin-rinsed anti-coagulant syringes, and then RBCs were pelleted by centrifugation at 800×g for 10 min at 4°C. After the removal of plasma, buffy coat, and the upper 15% of packed RBCs (PRBCs), the RBCs were washed twice with cold phosphate-buffered saline (PBS) (0.9% NaCl and 10 mmol · L−1 Na2HPO4, pH 7.4) and then used for fractionation. All protocols for experiments were approved by the Animal Care and Ethics Committee of University of Allahabad.
Fractionation of erythrocytes according to density/age
Rat erythrocytes were separated on a Percoll density gradient according to an established protocol.22 The packed RBCs were washed twice with RPMI-1640 medium and re-suspended to a 25% hematocrit. The suspension was overlaid onto a Percoll/4% sorbitol (wt/vol) gradient and centrifuged for 20 min at 1075×g on a Hettich Centrifuge Universal 320 R. The separated bands of erythrocytes were collected in two fractions representing young (less dense) fractions, corresponding to a 60% Percoll density grandient, and senescent (dense) fractions, corresponding to a 75% Percoll density gradient.
Assay of acetylcholinesterase
Acetylcholinesterase (AChE) activity was assayed in RBCs following the method of Beutler based on the procedure of Ellman et al.23 Hemoglobin was estimated by the ferricyanide/cyanide method as described by Beutler.24 AChE activity is expressed in i.u. (1 i.u.=μmol of acetylthiocholine iodide hydrolyzed/min) per gram of hemoglobin at 37°C. A molar absorption coefficient of 1.36×10−4 liter · mol−1 · cm−l was used for the thionitrobenzoate ion at 412 nm.
Measurement of erythrocyte PMRS activity
The activity of the erythrocyte PMRS was measured by the reduction of ferricyanide described earlier in Kumar and Rizvi.19 Briefly, packed RBCs (0.2 mL) were suspended in PBS containing 5 mM glucose and 1 mM freshly prepared potassium ferricyanide to a final volume of 2.0 mL. The suspensions were incubated for 30 min at 37°C and then centrifuged at 800×g at 4°C. The supernatant collected was assayed for ferrocyanide content using DPI, and absorption was recorded at 535 nm (c=20,500 M−1 cm−1). The results are expressed in μmol ferrocyanide/mL PRBC per 30 min.
Determination of erythrocyte malondialdehyde content
Erythrocyte malondialdehyde (MDA) was measured according to the method of Esterbauer and Cheeseman,25 with slight modification. Packed erythrocytes (0.2 mL) were suspended in 3 mL of PBS containing 0.5 mM glucose (pH 7.4). The suspension (0.2 mL) was added to 1 mL of 10% trichloroacetic acid (TCA) and 2 mL of 0.67% thiobarbituric acid (TBA), boiled for 20 min at 90–100°C, and then cooled. Subsequently, the mixture was centrifuged at 1000×g for 5 min, and the absorbance of supernatant was read at 532 nm. The concentration of MDA in erythrocytes was calculated using extinction coefficient (ɛ=31,500) and is expressed as nmol · mL−1 of packed erythrocytes.
Determination of erythrocyte reduced GSH
Erythrocyte reduced GSH was measured following the method of Beutler.24 The method is based on the ability of the –SH group to reduce DTNB and form a yellow-colored anionic product whose optical density is measured at 412 nm. Concentration of GSH is expressed in mg · mL−1 PRBCs and was determined from a standard plot.
l-cysteine influx in erythrocyte
The procedure for measuring l-cysteine influx was essentially the same as described earlier.26 A total of 0.25 mL of washed packed erythrocytes (either from “young,” less dense fraction or from “senescent,” dense fraction) were suspended in 1 mL of PBS containing 8 mM glucose and 10 mM l-cysteine and incubated for 1 hr at 37°C in a water bath. The concentration of l-cysteine used for influx studies and the duration of incubation were the same as the values used by Yildiz et al.20 At the end of incubation, erythrocytes were removed and centrifuged and supernatants were discarded. The free sulfhydryl (–SH) concentration in erythrocytes was then determined as described by Sedlak and Lindsay.27 Briefly, 100 μL of erythrocytes were lysed in 100 μL of 10% TCA prepared in sodium phosphate–EDTA buffer (0.01 M sodium phosphate/0.005 M EDTA). The erythrocyte lysates were then centrifuged at 12,000×g for 5 min. At the end of centrifugation, 100 μL of the supernatant was mixed with 1.9 mL of Tris-EDTA buffer containing 0.6 μM/mL DTNB (262 mM Tris base, 13 mM EDTA [pH 8.9]). Samples were allowed to stand for 5 min to develop color. The absorbance of the samples was then measured at 412 nm, and the concentrations of the free –SH were calculated by using the mM extinction coefficient of 13.6. Influx rate was calculated by subtracting the control (erythrocytes incubated in PBS–glucose without l-cysteine for 1 hr at 37°C) free –SH concentration from free –SH concentration obtained following treatment with l-cysteine.
Statistical analysis
Data are expressed as mean±standard error of the mean (SEM) for at least eight independent experiments. Intra-group variation between un-fractionated, young and senescent erythrocytes was assessed by t-test using PRISM version 5.01 software package for Microsoft Windows. Differences between the groups were assessed by two-way analysis of variance (ANOVA). Post hoc testing was performed for inter-group comparisons using the Bonferroni test. A probability (p) value of less than 0.01 was considered as statistically significant
Results
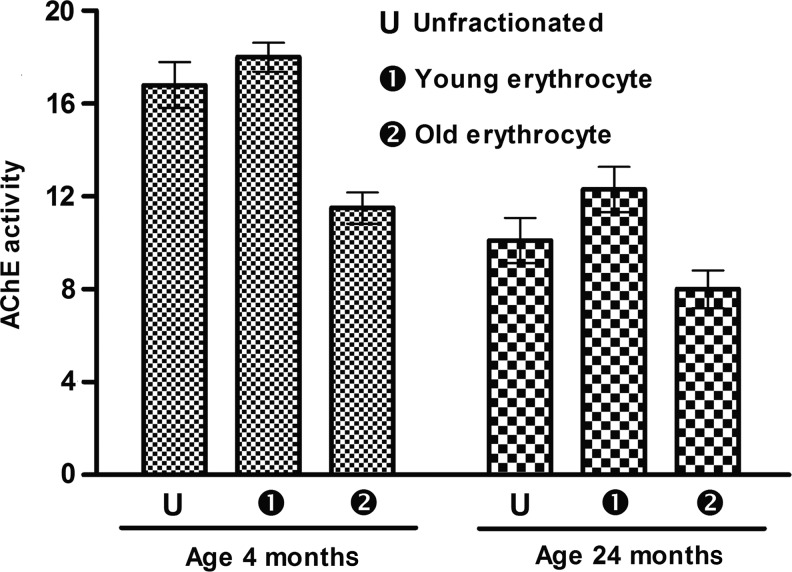
RBC AChE activity was significantly higher (39.88%) in 4-month-old rats compared to 24-month-old rats. After fractionation of erythrocytes by Percoll density gradient centrifugation, the less dense younger erythrocytes showed higher AChE activity in both young and old rats. In young rats, the difference in AChE activity between less dense and senescent erythrocytes was 36.11%, whereas in old rats the difference was (35.83%) (Fig. 1).
FIG. 1.
Red blood cell (RBC) acetylcholinesterase (AChE) activity as a function of rat erythrocyte age. AChE activity is expressed in i.u. (1 i.u.=μmol of acetylthiocholine iodide hydrolyzed/min) per gram of hemoglobin at 37°C. Values are taken mean±standard error of the mean (SEM). Values are mean±SEM. Intra-group variation for 4-month-old rate: U-2, 1-2 (significant at p<0.05), U-1 (not significant). For 24-month-old rats: U-2, 1-2 (significant at p<0.05), U-1 (not significant). Inter-group comparisons measured using a Bonferroni post hoc test (significant at p<0.05).
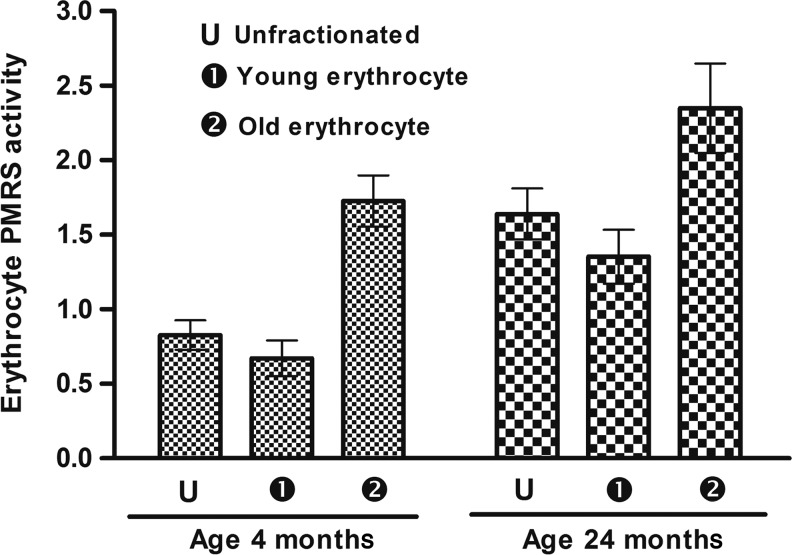
The PMRS activity of un-fractionated RBCs was higher in old rats (24 months old) compared to young rats (4 months old). There was significant difference between PMRS activity in fractionated less dense erythrocytes and high-density senescent erythrocytes from both young and old rats (p<0.001); however, this variation was more pronounced in young rats (157.61%) compared to old rats (73.68%) (Fig. 2).
FIG. 2.
Erythrocyte plasma membrane redox system (PMRS) measured as marker of oxidative stress during rat aging. The value of PMRS is expressed in micromoles ferrocyanide/mL PRBC per 30 min. Values are mean±standard error of the mean (SEM). Intra-group variation for 4-month-old rats: U-2, 1-2 (significant at p<0.05), U-1 (not significant). For 24-month-old rats: U-1, U-2, 1-2 (significant at p<0.05). Inter-group comparisons measured using a Bonferroni post hoc test (significant at p<0.05).
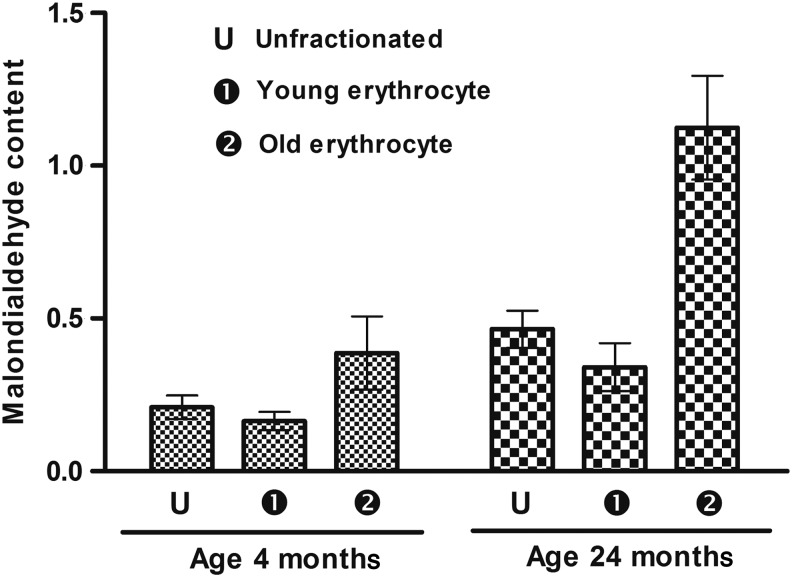
Twenty-four-month-old rats displayed higher lipid peroxidation, measured as MDA, compared to 4-month-old rats in un-fractionated RBCs. There were also significant differences in both the groups between the less dense erythrocytes and more dense (senescent) erythrocytes, although this difference was much higher in old rats (230.38%) compared to young rats (135.36%) (Fig. 3).
FIG. 3.
Erythrocyte malonaldialdehyde (MDA) content as a function of age of rats. MDA level is expressed as nmol · mL−1 of plasma. Values are mean±standard error of the mean (SEM). Intra-group variation for 4-month-old rats: 1-2 (significant at p<0.05), U-1, U-2 (not significant). For 24-month-old rats: U-2, 1-2 (significant at p<0.05), U-1 (not significant). Inter-group comparisons measured using a Bonferroni post hoc test (significant at p<0.05).
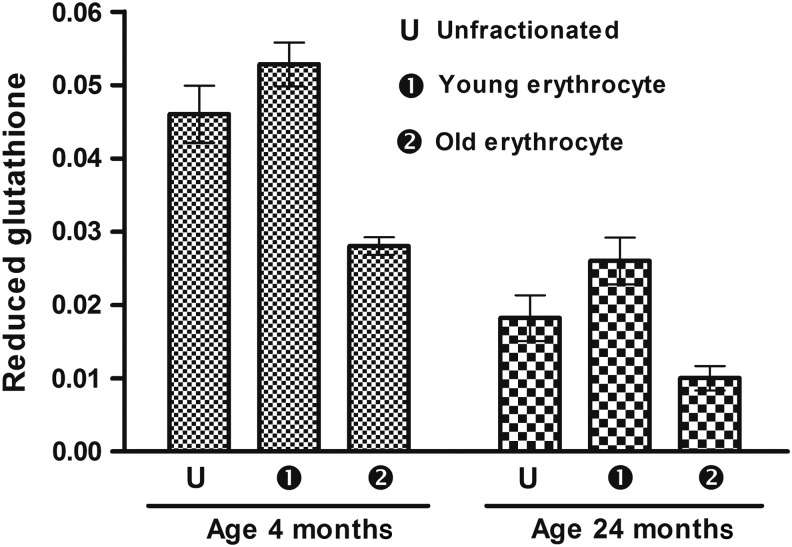
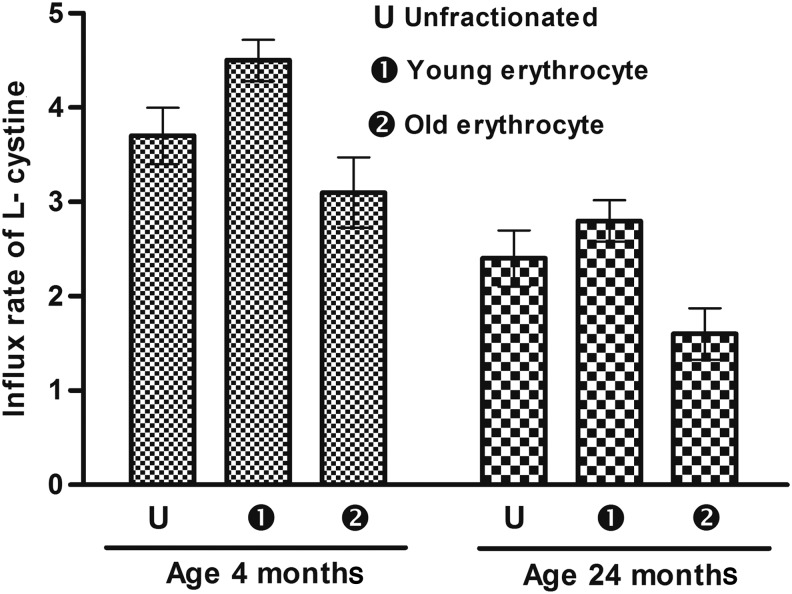
Young rats had higher RBC intracellular GSH levels compared to old (24 month) rats. In fractioned cells, the less dense young erythrocytes had higher GSH levels compared to more dense (senescent) erythrocytes (46.96% and 61.63%, respectively) (Fig. 4). The influx of cysteine in erythrocytes in un-fractionated, less dense, and senescent (old) erythrocytes is shown in Fig. 5. Un-fractionated erythrocytes from young (4-month-old) rats had significantly (p<0.001) higher l-cysteine influx compared to old (24-month-old) rats. In both of the rat groups, the less dense fraction had a higher rate of l-cysteine infux compared to senescent erythrocytes represented by the dense fraction.
FIG. 4.
Erythrocyte reduced glutathione (GSH) content. Concentration of GSH is expressed in milligram per milliliter packed erythrocytes. Values are mean±standard error of the mean (SEM). Intra-group variation for 4-month-old rats: U-2, 1-2 (significant at p<0.05), U-1 (not significant). For 24-month-old rats: U-2, 1-2, U-1 (significant at p<0.05). Inter-group comparisons measured using a Bonferroni post hoc test (significant at p<0.05).
FIG. 5.
l-cysteine influx in erythrocytes as a function of rat age. Influx rate is expressed as μmol/hr per mL of packed red blood cells. Values are mean±standard error of the mean (SEM). Intra-group variation for 4-month-old rats: U-1, 1-2 (significant at p<0.05), U-2 (not significant p<0.05). For 24-month-old rats: U-2, 1-2 (significant at p<0.05), U-1 (not significant p<0.05). Inter-group comparisons were measured using a Bonferroni post hoc test (significant at p<0.05).
Discussion
Erythrocytes have been used as a biological probe in exploring the aging process. It is well known that RBCs increase in density as they age and can be fractionated into different age groups using density gradient centrifugation techniques.28,29 The development of Percoll-based density gradient has provided a methodology employing low viscosity, low osmotic pressure, non-toxicity, and easy adjustability to physiological conditions. The Percoll gradient allows RBCs to migrate better on the basis of their actual density.22 Our separation of erythrocytes into young and old zones corresponding to young and senescent erythrocytes from both 4-month-old and 24-month-old rats was validated with the assay of RBC AChE, which is an established marker of RBC age in vivo.29
AChE in erythrocytes is one of the typical extra-neural enzymes and plays an essential role in acetylcholine-mediated neurotransmission. It is present in the cholinergic synapses in the central nervous system as well as in neuromuscular synapses, where it rapidly hydrolyzes acetylcholine.30 The determination of AChE activity in the erythrocyte membrane has been used as a free radical–mediated oxidative stress parameter during aging in humans.31 Our results clearly show that the activity of AChE in the erythrocyte is significantly decreased in dense (senescent) erythrocyte (fraction 2) of young (4-month-old) rats in comparison to fraction 1 and un-fractionated erythrocytes of young rats. The pattern of decrease in AChE activity in young and senescent erythrocytes was the same in 24-month-old rats, but the level of enzyme activity was much lower in old rats compared to 4-month-old rats.
An increase in RBC oxidative stress has been reported in many pathological conditions associated with various diseases, including renal failure,32 Alzheimer's disease,33 idiopathic thrombocytopenic purpura,34 and sickle cell, thalassemia, and hemolytic anemia,35 in addition to cellular aging and organism aging.19 RBCs undergo multiple changes while they age in vivo. Some of these remain hidden within RBCs, and others affect the properties of the cell directly, like the loss of cations and the loss of membrane with some hemoglobin by vesiculation that results in an increased cellular density.11
It has been reported that several hematologic parameters are altered during aging in mammals. Erythrocytes from aged individuals have been reported to have a reduced life span9 and a significant increase in membrane cholesterol and phospholipid content, causing a reduction of membrane fluidity.36 The PMRS cumulatively contributes to the maintenance of cellular redox status via the maintenance of NAD(P)+/NAD(P)H ratios. This ultimately is believed to limit oxidative stress37,38 and perhaps therefore is protective for the cell.39 In this way, the PMRS helps the cells to respond to changes in redox potential, thereby regulating a variety of physiological functions, including cell metabolism, ion channels, growth, and death.40
The activity of PMRS has been shown to be elevated in erythrocytes of aged rats19 and also in rats subjected to oxidative stress in vivo.41 In view of the important role of erythrocyte PMRS in providing protection against oxidative stress during aging39 and the hypothesis that the activity of PMRS may be an important determinant of animal life span,42 the present observation of an increase in PMRS activity in senescent cells signifies increased oxidative stress. The increase in PMRS activity in senescent cells is more pronounced in old (24-month-old) rats, which again substantiates the higher oxidative stress in old rats.19 This is the first report regarding the involvement of PMRS during cell senescence and provides evidence for the role of oxidative stress during cellular senescence.
GSH plays an important role in detoxification of free radicals. In erythrocytes, it is a major anti-oxidant protecting important proteins such as spectrin, the oxidation of which may lead to change in membrane fluidity.43 Besides augmentation of the anti-oxidant defense, GSH also plays an important role in maintenance of –SH groups in hemoglobin and other enzymes in reduced state. The importance of GSH during conditions of oxidative stress is thus critical for the cell.11 Our observation of a decrease in GSH in senescent RBC fractions signifies higher oxidative stress in aged erythrocytes, with the condition being more significant in old rats compared to young rats. The decrease in GSH in aged erythrocytes could be due to the reported 30–40% down-regulation of the Embden–Meyerhof pathway.29,44
The functional free –SH group, which plays a critical role in the anti-oxidant role of GSH, is provided by l-cysteine. Although three amino acids are required for GSH synthesis, the rate of GSH synthesis is determined only by l-cysteine availability.45 Rates of l-cysteine influx into erythrocytes have been reported that are concentration and time-dependent.20 Our earlier report has provided evidence of a decreased l-cysteine influx into erythrocytes as a function of age in humans.26 Our observation of a decreased influx of l-cysteine in erythrocytes from old (24-month-old) rats compared to young (4-month-old) rats is the first such report in rats and corroborates findings obtained in humans.26 Less dense, younger erythrocytes show a higher rate of l-cysteine influx compared to senescent erythrocytes in both young and old rats, and this observation explains, in part, the decrease in intracellular GSH in senescent erythrocytes. The decreased influx of l-cysteine, besides influencing GSH synthesis, may also contribute to altered intracellular redox status. Numerous reports confirm the beneficial effects of l-cysteine supplementation in age-dependent conditions.46 It has been hypothesized that aging could be a cysteine deficiency syndrome.47 In view of the above, our findings of an altered l-cysteine influx in young and senescent erythrocytes substantiate reported benefits of l-cysteine supplementation as an effective anti-aging strategy.48
ROS-mediated lipid peroxidation may lead to loss of membrane integrity and cell death.49 MDA has been shown to cross-link erythrocyte phospholipids and proteins, leading to loss of membrane integrity and functions and ultimately diminished cell life span.50 MDA accumulation can also affect anion transport and the band 3–associated enzymes, phosphofructokinase and glyceraldehyde 3-phosphate dehydrogenase.50 Several reports confirm an increase in MDA during aging in erythrocytes in humans.4 An age-dependent increase in MDA levels has been reported in rats.19,51 Our results on fractionated erythrocytes of rats also show increased MDA levels as a function of age. The observation of increase in MDA in lower fraction erythrocytes (old) reflects increased oxidative stress during this period of life and corroborates with the levels of other oxidative stress markers
Conclusion
On the basis of parameters indicative of intracellular oxidative stress, our findings provide evidence of higher oxidative stress in senescent erythrocytes, the effect being more pronounced in old (24-month-old) rats compared to young (4-month-old) rats. We show a critical role of l-cysteine influx in erythrocytes, which is significantly reduced in senescent erythrocytes. The present findings emphasize the role of oxidative stress not only in organismal aging but also in cell senescence.
Acknowledgments
Dileep Kumar is a D. Phil. Scholar in the Department of Biochemistry, University of Allahabad, India. This work was supported by grant no. SR/SO/HS-0147/2010 from SERB- Department of Science Technology, Government of India, to S.I.R.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Harman D. Aging, a theory based on free radical and radiation chemistry. J Gerontol 1956;11:298–300 [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B, Gutteridge JMC. Cellular responses to oxidative stress: Adaptation, damage, repair, senescence and death. In: Free Radicals in Biology and Medicine, 4th ed. Clarendon Press, Oxford UK, 2007, pp. 187–267 [Google Scholar]
- 3.Lykkesfeldt J. Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clin Chim Acta 2007;380:50–58 [DOI] [PubMed] [Google Scholar]
- 4.Salmon AB, Richardsona A, Pérez VI. Update on the oxidative stress theory of aging: Does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med 2010;48:642–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rattan SIS. Theories of biological aging: Genes, proteins and free radicals. Free Radic Res 2006;40:10–12 [DOI] [PubMed] [Google Scholar]
- 6.Sivilotti ML. Oxidant stress and haemolysis of the human erythrocyte. Toxicol Rev 2004;23:169–188 [DOI] [PubMed] [Google Scholar]
- 7.Belcher EH, Harriss EB. Studies of red cell life span in the rat. J Physiol 1959;146:217–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derelanko MJ. Determination of erythrocyte life span in F-344, Wistar, and Sprague-Dawley rats using a modification of the [3H]diisopropylfluorophosphate ([3H]DFP) method. Fundamemtal Appl Toxicol 1987;9:271–276 [PubMed] [Google Scholar]
- 9.Kosower NS. Altered properties of erythrocytes in the aged. Am J Hematol 1993;42:241– 247 [DOI] [PubMed] [Google Scholar]
- 10.Sutera SP, Gardner RA, Boylan CW, Carroll GL, Chang KC, Marvel JS, Kilo C, Gonen B, Williamson JR. Age-related changes in deformability of human erythrocytes. Blood 1985;65:275–282 [PubMed] [Google Scholar]
- 11.Lutz HU, Bogdanova A. Mechanisms tagging senescent red blood cells for clearance in healthy humans. Front Physiol 2013;387:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rifkind JM, Nagababu E. Hemoglobin redox reactions and red blood cell aging. Antioxid Redox Signal 2013;18:2274–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blostein R, Grafova E. Factors affecting transport changes associated with reticulocyte maturation. Biomed Biochim Acta 1987;46:S172–S176 [PubMed] [Google Scholar]
- 14.Tiffert T, Daw N, Etzion Z, Bookchin RM, Lew VL. Age decline in the activity of the Ca2+ sensitive K+ channel of human red blood cells. J Gen Physiol 2007;129:429–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devi SA, Reddy CSSS, Subramanyam MV. Peroxyl-induced oxidative stress in aging erythrocytes of rat. Biogerontology 2011;12:283–292 [DOI] [PubMed] [Google Scholar]
- 16.Reinhart SA, Schulzki T, Bonetti PO, Reinhart WH. Studies on metabolically depleted erythrocytes. Clin Hemorheol Microcirc 2013;156:161–173 [DOI] [PubMed] [Google Scholar]
- 17.Clark MR, Mohandas N, Shohet SB. Deformability of oxygenated irreversibly sickled cells. J Clin Invest 1980;65:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devi SA, Reddy CSSS, Subramanyam MV. Oxidative stress and intracellular pH in the young and old erythrocytes of rat. Biogerontology 2009;10:659–669 [DOI] [PubMed] [Google Scholar]
- 19.Kumar D, Rizvi SI. A critical period in lifespan of male rats coincides with increased oxidative stress. Arch Gerontol Geriatr 2014;58:427–433 [DOI] [PubMed] [Google Scholar]
- 20.Yildiz D, Uslu C, Cakir Y, Oztas H. L-Cysteine influx and efflux: A possible role for red blood cells in regulation of redox status of the plasma. Free Radic Res 2006;40:507–512 [DOI] [PubMed] [Google Scholar]
- 21.Cebe T, Atukeren P, Yanar K, Kuruç AI, Ozan T, Kunbaz A, Sitar ME, Mirmaroufizibandeh R, Aydın S, Cakatay U. Oxidation scrutiny in persuaded aging and chronological aging at systemic redox homeostasis level. Exp Gerontol 2014;28:132–140 [DOI] [PubMed] [Google Scholar]
- 22.Rowley PT, Siddiqui WA, Geiman QM. Separation of malarial parasites according to age by density gradient centrifugation. J Lab Clin Med 1967;70:933–937 [PubMed] [Google Scholar]
- 23.Ellman GL, Courtney KD, Andres V, Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961; 7, 88–95 [DOI] [PubMed] [Google Scholar]
- 24.Beutler E. A Manual of Biochemical Methods. Grune & Stratton, New York, 1984 [Google Scholar]
- 25.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: Malondialdehyde and 4-hydroxynonenal. Method Enzymol 1990;186:407–413 [DOI] [PubMed] [Google Scholar]
- 26.Rizvi SI, Maurya PK. l-cysteine influx in erythrocytes as a function of human age. Rejuvenation Res 2008;11:661–665 [DOI] [PubMed] [Google Scholar]
- 27.Sedlak J, Lindsay RH. Determination of sulfhydryl groups in biological samples. Anal Biochem 1963;25:192–205 [DOI] [PubMed] [Google Scholar]
- 28.Pinkofsky HB. The effect of donor age on human erythrocyte density distribution. Mech Ageing Dev 1997;97:73–79 [DOI] [PubMed] [Google Scholar]
- 29.Cohen NS, Ekholm JE, Luthra MG, Hanahan DJ. Biochemical characterization of density-separated human erythrocytes. Biochim Biophys Acta 1976;419:229–242 [DOI] [PubMed] [Google Scholar]
- 30.Prall YG, Gambhir KK, Ampy FR. Acetylcholinesterase: An enzymatic marker of human red blood cell aging. Life Sci 1998;63:177–184 [DOI] [PubMed] [Google Scholar]
- 31.Jha R, Rizvi SI. Age-dependent decline in erythrocyte acetylcholinesterase activity: Correlation with oxidative stress. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2009;153:195–198 [DOI] [PubMed] [Google Scholar]
- 32.Caimi G. Erythrocyte peroxide metabolism, plasma lipid pattern and hemorheological profile in chronic renal failure. J Nephrol 2002;15:104–108 [PubMed] [Google Scholar]
- 33.Baldeiras I, Santana I, Proenca MT, Garrucho MH, Pascoal R, Rodrigues A, Duro D, Oliveira CR. Peripheral oxidative damage in mild cognitive impairment and mild Alzheimer's disease. J Alzheimers Dis 2008;15:117–128 [DOI] [PubMed] [Google Scholar]
- 34.Polat G, Tamer L, Tanriverdi K, Gurkan E, Baslamisli F, Atik U. Levels of malondialdehyde, glutathione and ascorbic acid in idiopathic thrombocytopaenic purpura. East Afr Med J 2002;79:446–449 [DOI] [PubMed] [Google Scholar]
- 35.Fibach E, Rachmilewitz E. The role of oxidative stress in hemolytic anemia. Curr Mol Med 2008;8:609–619 [DOI] [PubMed] [Google Scholar]
- 36.Parmahamsa M, Reddy KR, Varadacharyulu N. Changes in composition and properties of erythrocyte membrane in chronic alcoholics. Alcohol Alcohol 2004;39:110–112 [DOI] [PubMed] [Google Scholar]
- 37.Cadenas E. Antioxidant and prooxidant functions of DT-diaphorase in quinine metabolism. Biochem Pharmacol 1995;49:127–140 [DOI] [PubMed] [Google Scholar]
- 38.Landi L, Fiorentini D, Galli MC, Segura-Aguilar J, Beyer RE. DT-Diaphorase maintains the reduced state of ubiquinones in lipid vesicles thereby promoting their antioxidant function. Free Radic Biol Med 1997;22:329–335 [DOI] [PubMed] [Google Scholar]
- 39.Rizvi SI, Jha R, Maurya PK. Erythrocyte plasma membrane redox system in human aging. Rejuvenation Res 2006;9:470–474 [DOI] [PubMed] [Google Scholar]
- 40.Hyun DH, Emerson SS, Jo DG, Mattson MP, de Cabo R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci USA 2006;103:19908–19912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar D, Singh S, Singh AK, Rizvi SI. Pomegranate (Punica granatum) peel extract provides protection against mercuric chloride-induced oxidative stress in Wistar strain rats. Pharm Biol 2013;51:441–446 [DOI] [PubMed] [Google Scholar]
- 42.Rizvi SI, Kumar D, Chakravarti S, Singh P. Erythrocyte plasma membrane redox system may determine maximum life span. Med Hypotheses 2011;76:547–549 [DOI] [PubMed] [Google Scholar]
- 43.Carroll J, Raththagala M, Subasinghe W, Baguzis S, D'amico Oblak T, Root P, Spence D. An altered oxidant defense system in red blood cells affects their ability to release nitric oxide stimulating ATP. Mol Biosyst 2006;2:305–311 [DOI] [PubMed] [Google Scholar]
- 44.Magnani M, Piatti E, Serafini N, Palma F, Dacha M, Fornaini G. The age-dependent metabolic decline of the red blood cell. Mech Ageing Dev 1983;22:295–308 [DOI] [PubMed] [Google Scholar]
- 45.Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med 1992;27:922–935 [DOI] [PubMed] [Google Scholar]
- 46.Blouet C, Mariotti F, Azzout-Marniche D, Mathe V, Mikogami T, Tome D, Huneau JF. Dietary cysteine alleviates sucrose-induced oxidative stress and insulin resistance. Free Rad Biol Med 2007;42:1089–1097 [DOI] [PubMed] [Google Scholar]
- 47.Dröge W. Oxidative stress and ageing: Is ageing a cysteine deficiency syndrome? Phil Trans R Soc B 2005;360:2355–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sekhar RV, Patel SG, Guthikonda AP, Reid M, Balasubramanyam A, Taffet GE, Jahoor F. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Am J Clin Nutr 2011;94:843–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niki E. Biomarkers of lipid peroxidation in clinical material. Biochimica Biophysica Acta 2014;1840:809–817 [DOI] [PubMed] [Google Scholar]
- 50.Dumaswala UJ, Zhuo L, Jacobsen DW, Jain SK, Sukalski KA. Protein and lipid oxidation of banked human erythrocytes: Role of glutathione. Free Radic Biol Med 1999;27:1041–1049 [DOI] [PubMed] [Google Scholar]
- 51.Van der Loo B, Bachschmid M, Spitzer V, Brey L, Ullrich V, Luscher TF. Decreased plasma and tissue levels of vitamin C in a rat model of aging: Implication for antioxidative defense. Biochem Biophys Res Commun 2003;303:483–487 [DOI] [PubMed] [Google Scholar]