Abstract
Significance: Abnormal lung development in the perinatal period can result in severe neonatal complications, including persistent pulmonary hypertension (PH) of the newborn and bronchopulmonary dysplasia. Reactive oxygen species (ROS) play a substantive role in the development of PH associated with these diseases. ROS impair the normal pulmonary artery (PA) relaxation in response to vasodilators, and ROS are also implicated in pulmonary arterial remodeling, both of which can increase the severity of PH. Recent Advances: PA ROS levels are elevated when endogenous ROS-generating enzymes are activated and/or when endogenous ROS scavengers are inactivated. Animal models have provided valuable insights into ROS generators and scavengers that are dysregulated in different forms of neonatal PH, thus identifying potential therapeutic targets. Critical Issues: General antioxidant therapy has proved ineffective in reversing PH, suggesting that it is necessary to target specific signaling pathways for successful therapy. Future Directions: Development of novel selective pharmacologic inhibitors along with nonantioxidant therapies may improve the treatment outcomes of patients with PH, while further investigation of the underlying mechanisms may enable earlier detection of the disease. Antioxid. Redox Signal. 21, 1926–1942.
Introduction
Neonatal respiratory failure affects 2% of live births and contributes significantly to neonatal morbidity and mortality (10). While preterm infants are at a higher risk, a substantial proportion of neonatal respiratory failure occurs in term and near-term infants. Improved detection and treatment of hypoxemic respiratory failure, therefore, requires a better understanding of specific pathophysiology and signaling pathways during fetal and neonatal life. Multiple factors are involved in the progression of neonatal pulmonary vascular diseases. This review will focus on recent progress in identifying underlying causes of neonatal pulmonary hypertension (PH) and the potential therapeutic advantages that this knowledge brings.
Normal and Abnormal Lung Development
In utero, complex signaling pathways regulate normal lung alveolar and vascular development and prepare the lung for the transition to pulmonary gas exchange at birth. Impaired development resulting from intrauterine factors, premature birth, or a failure to decrease pulmonary vascular resistance at birth can lead to PH and respiratory failure.
Perinatal lung development
As the human fetal lung develops, lung septation and alveolarization begin at around 32–36 weeks of gestation and continue well into postnatal life. During this process, vascular growth and branching is tightly coupled with the growth and branching of the airway epithelium (76). Formation of the pulmonary vasculature is dependent on vasculogenesis, or de novo formation of blood vessels, and angiogenesis, the formation of new vessels from preexisting ones. During the final stages of vascular development, the pulmonary capillaries surround the thinning alveolar walls, providing the increased alveolar and capillary surface areas that are necessary for efficient gas exchange at birth. This highly complex structural organization requires the tight regulation of vascularization and alveolarization. Between birth and adulthood, vascular development continues with the expansion of capillary volume and surface area, driven by angiogenesis from pre-existing vessels and intussusceptive growth (48). Antenatal or postnatal events that affect the developmental program of the fetal or newborn lung may contribute to defective pulmonary vascular development.
Vascular endothelial growth factor (VEGF) is expressed in vascular endothelial and smooth muscle cells (SMC) and in airway epithelium in the fetal lung, and it is central to vascular development of the perinatal lung. Two distinct transmembrane tyrosine kinase receptors, VEGF receptor 1 (VEGFR-1) and VEGF receptor 2 (VEGFR-2), are expressed in the vascular endothelium. Experimental inactivation of VEGF or its receptor genes results in embryonic lethality that is characterized by deficient organization of endothelial cells (ECs) and vascularization, and targeted inactivation in the early postnatal period increases mortality and impairs lung vascular development (69). Clinically, decreased VEGF and VEGFR-1 mRNA and protein is evident in the lungs of premature neonates who died with bronchopulmonary dysplasia (BPD) (19), while VEGF expression is decreased in tracheal aspirates from premature infants who develop BPD (111). These data suggest that abnormal VEGF signaling before and after birth contribute to impaired lung vascular and parenchymal development manifest in BPD and other perinatal lung disorders. The inhibition of VEGF receptors in adult rodents also produces abnormalities of lung architecture, suggesting that VEGF signaling remains important after infancy for the maintenance of normal pulmonary vasculature and alveolar structure.
Lung VEGF expression is regulated by members of the hypoxia-inducible factor (HIF) family of transcription factors (35, 145). HIFs are heterodimers consisting of oxygen-sensitive α-subunits (HIF-1α, HIF-2α) and constitutively expressed β subunits. Hypoxia stabilizes the α subunit, leading to nuclear accumulation and activation of multiple target genes (165). Conversely, elevated levels of oxygen target the protein for proteasomal degradation, thereby decreasing target gene expression. The deletion of HIF-1 or HIF-2 results in embryonic lethality, and an important recent study demonstrated that SMC-specific knockout of HIF-1α in mice results in PH and increased phosphorylation of myosin light chain under normoxia and after hypoxic exposure (95).
Endothelial nitric oxide synthase (eNOS) is regarded the most important regulator of nitric oxide (NO) production in the perinatal lung vasculature. NO stimulates soluble guanylate cyclase (sGC) activity and increases cyclic guanosine monophosphate (cGMP) in vascular smooth muscle, producing smooth muscle relaxation via mechanisms involving decreased phosphorylation of myosin light chain (130). Lung eNOS mRNA and protein are present in the early fetus, but both increase toward the end of gestation, preparing the lung for pulmonary vasodilation. The role of NO in the regulation of the pulmonary vascular tone in the perinatal period and the pulmonary vascular transition at birth has been established by multiple investigators, and there is substantial evidence that NO also plays a key role in lung vascular growth during fetal and neonatal life. For example, lungs of late fetal and neonatal eNOS-deficient mice have striking abnormalities of vascularization (77), and they are more susceptible to failed vascular growth after exposure to mild hypoxia (14). Recent studies suggest that VEGF-induced lung angiogenesis is, in part, mediated by NO. For instance, the inhibition of VEGF receptors decreased lung eNOS protein expression and NO production, and lung vascular growth could be restored by treatment with inhaled NO (13, 163).
The perinatal period is also distinguished by specific circulatory patterns required for gas exchange. During placental respiration, fetal blood flow bypasses the lungs via the foramen ovale and the ductus arteriosus, thereby directing oxygenated blood to the systemic circulation. At birth, the fetal pulmonary circulation should rapidly adapt to direct blood flow to the lungs as the organ of gas exchange. This is facilitated by a dramatic decrease in pulmonary vascular resistance, regulated by complex physiological and biochemical processes, and resulting in an 8- to 10-fold increase in pulmonary blood flow (38). If this process is altered by abnormal lung vascular development and/or an attenuated decrease in pulmonary vascular resistance at birth, PH and its attendant complications will be the result.
Neonatal pulmonary vascular disease
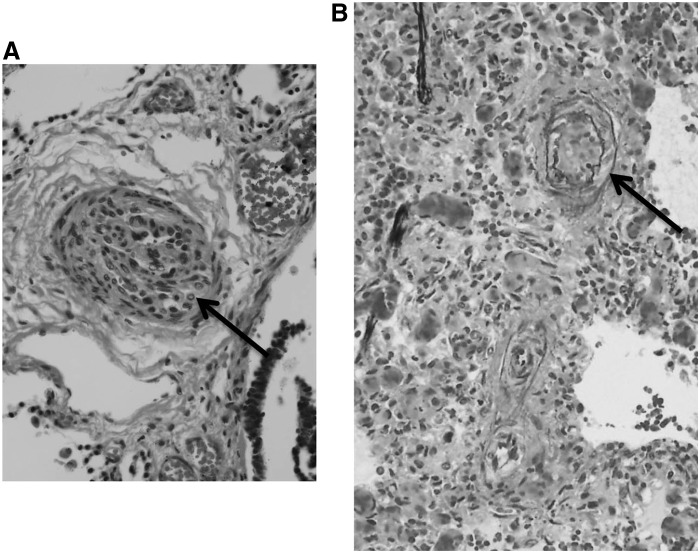
When the pulmonary circulation fails to adapt to postnatal life, the result is persistent pulmonary hypertension of the newborn (PPHN). PPHN is characterized by elevated pulmonary vascular resistance and right-to-left extrapulmonary shunting of deoxygenated blood that produces severe hypoxemia (158). Severe PPHN occurs in 2–6 per 1000 live births, and it carries a significant risk of death, pulmonary morbidity, and neurodevelopmental impairment (97). PPHN can occur idiopathically, with normal lung parenchyma and a severely remodeled pulmonary vasculature. Pathological findings include increased thickness of the smooth muscle layer within small pulmonary arteries (Fig. 1A) and abnormal extension of this muscle to nonmuscular arteries (79). The extent of pulmonary vascular remodeling is associated with the severity of the disease, although the in utero abnormalities that alter the pulmonary circulatory adaptation remain poorly understood.
FIG. 1.
Representative histology of pulmonary vessels from infants who died with severe pulmonary hypertension (PH). (A) Small pulmonary vessel from an infant with asphyxia and pulmonary hypertension of the newborn (PPHN), demonstrating dramatic smooth muscle cell thickening around pulmonary arteries (arrow). (B) Lung photomicrograph with elastin staining from an infant with BPD-associated PH and organizing pneumonia. A thickened medial layer, double elastic lamina, and modest proliferation of the adventitia are noted (arrow). Both examples indicate a lack of the intimal proliferation that characterizes adult PH.
Chronic hypoxia in utero due to factors including high altitude increases the risk of PH (132). During chronic hypoxia, sustained constriction of pulmonary arteries causes pulmonary arterial hypertension, leading to right ventricular hypertrophy. In addition, acute perinatal asphyxia or alveolar hypoxia arising from lung parenchymal disorders such as meconium aspiration syndrome (MAS), respiratory distress syndrome, and pneumonia can cause structurally normal pulmonary vessels to constrict.
Approximately 13% of all live births are associated with meconium-stained fluid, although only a small fraction will go on to develop the MAS. Aspiration of meconium either before or during delivery can obstruct small airways, cause severe pneumonitis, and induce inflammatory changes in the lung. All of these abnormalities will impair oxygenation after birth and acutely constrict the pulmonary vasculature. Meconium also induces the release of pulmonary vasoconstrictors such as endothelin, thromboxane, and prostaglandin E2, all of which promote the development of PH and the proliferation of vascular smooth muscle that will lead to antenatal and postnatal vascular remodeling. In the most severe cases, severe hypertensive structural remodeling of small intraacinar arteries is observed, presumably in response to chronic intrauterine hypoxia or lung injury (129). Recent studies have also shown depressed expression of eNOS in umbilical venous ECs in infants who have severe PPHN in association with meconium-stained amniotic fluid (175).
Congenital diaphragmatic hernia (CDH) affects approximately 1 in 2000–3000 births, and it is the most common cause of pulmonary hypoplasia in the neonate. CDH is characterized by a variable degree of pulmonary hypoplasia that is accompanied by a decrease in the cross-sectional area of the pulmonary vasculature, increased muscularization of the intra-acinar pulmonary arteries, and, in the most severe cases, left ventricular hypoplasia. Pulmonary capillary blood flow is decreased because of the small cross-sectional area of the pulmonary vascular bed, and flow may be further decreased by abnormal pulmonary vasoconstriction. Mortality and morbidity are high, and severe PH often persists well beyond neonatal life (92).
Maternal medication usage may increase the likelihood of neonatal PH. Many studies have reported that in utero exposure to nonsteroidal anti-inflammatory drugs increases the risk of PPHN (173), potentially via constriction of the ductus arteriosus (120, 176), but recent studies have questioned this association. Serotonin (5-HT) is a potent pulmonary vasoconstrictor (50, 51), and the antenatal use of selective 5-HT reuptake inhibitors has been shown to increase the risk of PPHN in animal studies (40, 65) as well as in some human epidemiologic reports (25).
PH may also arise later in the neonatal period. PH often complicates the course of BPD, the most common chronic lung disease of infancy. BPD occurs most frequently in extremely preterm infants born before 28 weeks of gestation, and it is characterized by alveolar simplification (fewer and larger alveolae with loss of septation), loss of small pulmonary arteries, and decreased capillary density (165). PH and right-sided heart failure complicate the course of a subset of infants with BPD (8, 18, 28), and significantly worsen its clinical course, morbidity, and mortality (93). Over time, BPD-associated PH contributes to ongoing hypoxemia, which induces further vascular remodeling (Fig. 1B) and right ventricular hypertrophy. In the most severe cases, right ventricular hypertrophy progresses to right ventricular failure, cor pulmonale, and death (84). PH is often not diagnosed until the disease is advanced and associated with severe right ventricular cardiac dysfunction (16).
PH and its associated increased vascular reactivity may develop in association with congenital heart disease with a systemic-to-pulmonary communication and increased pulmonary blood flow (86), such as truncus arteriosus, atrioventricular canal, or large ventricular septal defect. Sustained increases in pulmonary blood flow can generate progressive structural and functional abnormalities of the pulmonary vascular bed, which may result in destruction of the pulmonary vascular bed and death secondary to severe cyanosis and myocardial failure (80, 81, 83, 126, 144).
Pathogenesis of neonatal pulmonary vascular disease
The fetal lung is programmed to develop in a low-oxygen intrauterine environment that favors multiple growth factor signaling pathways. The VEGF and NO signaling pathways are among those critical for antenatal and postnatal lung vascular growth. At birth, successful transition of the pulmonary circulation requires normal structural and functional development of the vasculature in utero, as well as the coordinated regulation of vasodilators and vasoconstrictors. Perinatal pulmonary vascular tone is regulated by a complex interaction of vasoactive substances produced by the vascular endothelium, including NO and endothelin-1 (ET-1) (64, 68, 78, 192). Disruption of any of these pathways either before or after birth may produce PH.
An increase in oxygen tension usually occurs at the time of birth. Preterm birth produces a lung that is ill-equipped to cope with the oxidant stress associated with increased ambient oxygen concentrations. Exposure to hyperoxia during this developmentally sensitive period disrupts normal parenchymal and vascular lung development processes (165). High concentrations of supplemental oxygen are routinely used to treat hypoxemia and reverse pulmonary vasoconstriction in infants with neonatal respiratory failure (56). However, the optimal amount of supplemental oxygen and risks of hyperoxic ventilation are not well understood. Hyperoxic ventilation may increase oxidant stress via the formation of reactive oxygen species (ROS), small molecules derived from molecular oxygen that can serve as a potential source of vascular injury. Long-term oxygen therapy is often required in preterm infants with BPD, but exposure of the immature lung to elevated levels of oxygen may have adverse effects on lung development and may contribute to BPD and PH.
Many factors associated with PH, including oxidant stress, have the capacity to perturb eNOS function even if protein levels are sufficient. Presumably, this is because the normal catalytic function of eNOS depends on numerous post-translational modifications, including association with the chaperone protein Hsp90 and the availability of essential substrates and cofactors including L-arginine, tetrahydrobiopterin (BH4), NADPH, and calcium/calmodulin. The depletion of Hsp90 or biopterin will reduce the production or bioavailability of NO, resulting in attenuated vasodilation. Potential roles for impaired NO signaling in PH have been investigated in animal models and are discussed in later sections of this review.
Pulmonary vascular remodeling is a common feature in animal models of PH and in patients with PPHN or BPD. Increased pulmonary arterial muscularization can contribute to hypertension by altering vasoreactivity. Narrowing and stiffening of both the proximal and distal pulmonary arteries also contribute to PH by reducing the size of the vessel lumen and decreasing compliance, resulting in increased right ventricular afterload (179). ROS, including H2O2 and superoxide, stimulate fetal pulmonary artery smooth muscle cells (PASMC) growth (185), and growth factors mitogenic for vascular smooth muscle increase ROS (20, 186). Conversely, antioxidants attenuate serum-induced cell proliferation, and at high doses, they induce apoptosis in PASMC (184, 185).
In addition to smooth muscle remodeling, adventitial changes contribute to pulmonary vascular disease. The proliferation of adventitial fibroblasts is stimulated by stressors, including hypoxia, and adventitial remodeling is evident in patients with idiopathic PH and in animal models of PH (159). ROS generated in the adventitia contribute to “outside-in” effects on pulmonary vascular remodeling, and adventitial ROS levels are regulated by enzyme systems discussed in Sources of ROS in Neonatal PH section, including NADPH oxidases (Nox) and superoxide dismutases (SOD) (159). Identification of the specific sources of elevated ROS in PH may enable earlier detection of the onset of disease, and antioxidant therapy or targeting of specific signaling molecules may prevent or reverse pulmonary vascular remodeling.
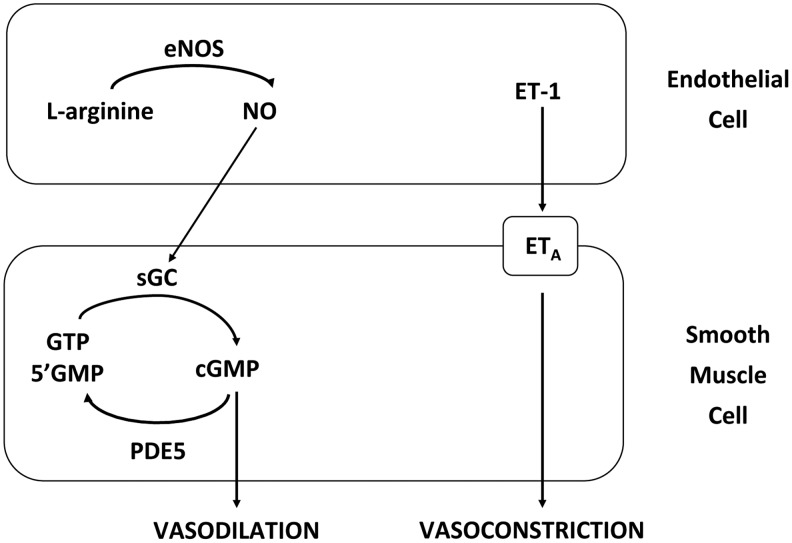
ET-1, a 21-amino-acid polypeptide produced by vascular ECs, has potent vasoactive properties and is mitogenic for vascular SMC (78, 185, 196). ET-1 stimulates smooth muscle contraction via activation of ETA receptors. Previous studies have demonstrated an increase in plasma ET-1 in infants with PPHN (99), CDH (92), and congenital heart disease (197), suggesting that impaired ET-1 signaling contributes to pulmonary vasoconstriction in the neonatal period. Figure 2 shows the regulation of vascular tone by NO and ET-1. These data illustrate some of the complex signaling mechanisms that are involved in maintaining normal vascular tone, and they suggest that the impairment at multiple points within the pathways may contribute to the pathogenesis of PPHN.
FIG. 2.
Mechanisms of pulmonary artery vasodilation and vasoconstriction. Endothelial nitric oxide synthase (eNOS) generates the vasodilator nitric oxide (NO) from L-arginine in endothelial cells (ECs), which activates soluble guanylate cyclase (sGC) in adjacent smooth muscle cells. Active sGC converts guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP), which triggers vasodilation via an enzyme phosphorylation cascade. Conversely, phosphodiesterase type 5 (PDE5) hydrolyzed cGMP to the inactive GMP, thus attenuating the cascade. Endothelin (ET-1) released by ECs triggers a cascade leading to vasoconstriction by activating type a receptors (ETA) on smooth muscle cells.
Experimental Models of Neonatal Pulmonary Vascular Disease
Understanding the mechanisms that produce abnormal lung vascular and parenchymal development and function is important in improving early detection and treatment strategies for infants with PH. Since it is not feasible to study the processes in the human infant, much of our current knowledge is derived from animal models.
Animal models of PPHN
In fetal lambs, ligation, mechanical compression, or pharmacological constriction of the ductus arteriosus produces fetal and neonatal PH (2, 21, 128, 193). Similar to newborns who die of PPHN, these lambs have an increase in the thickness of smooth muscle within the small pulmonary arteries, complete muscularization of normally partially muscularized pulmonary arteries, and extension of muscle to nonmuscularized arteries. PPHN lambs also exhibit impaired NO-cGMP signaling at multiple points in the pathway, including decreased eNOS and sGC expression, and increased PDE5 expression and activity (21). Increased ET-1 levels are also elevated in PPHN lambs (21, 88), while ETA receptor blockade decreases PH and attenuates vascular remodeling (88). These data suggest that attenuated NO-mediated pulmonary vasodilation and enhanced ET-1-mediated vasoconstiction are major contributors to the pathogenesis of PPHN.
Recent studies found elevated production of the 5HT in PPHN lambs (41), which causes fetal pulmonary vasoconstriction via mechanisms involving Rho kinase (40). Conversely, infusion with Rho kinase inhibitors causes pulmonary vasodilation in fetal lambs, suggesting a key role for Rho kinase signal transduction pathways in elevated pulmonary vascular resistance in the fetal pulmonary circulation (137). Further, Rho kinase activity is elevated in pulmonary artery endothelial cells (PAEC) isolated from PPHN lambs (71) in an ET-1-dependent fashion (72). Together, these data suggest that dysregulation of multiple pathways contributes to impaired pulmonary vasodilation in PPHN lambs.
Hypoxia-induced PH
Exposure to high-altitude chronic hypoxia increases pulmonary arterial pressure in newborn lambs (82). Exposure to chronic hypoxia (10% O2) in an airflow chamber induces PH and vascular remodeling in adult mice that correlates with an increase in plasma ET-1 levels, while hypertension and remodeling is attenuated by ETA receptor blockade (7). Acute hypoxia-induced vasoconstriction and hypertension is attenuated in mice after Rho kinase inhibition (53), suggesting a potential interaction between elevated ET-1 levels and Rho kinase activation in this model, similar to that in PPHN lambs as discussed in Animal Models of PPHN section. PH develops in newborn piglets exposed to hypoxia for 3 days and worsens after 10 days of hypoxia (61). These findings are associated with a decrease in lung NO levels and eNOS expression (62). Together, these data suggest that hypoxia may induce PH via mechanisms similar to those associated with antenatal ductal ligation in lambs.
Hyperoxic lung injury
The increase in oxygen tension at birth is one of the most important stimuli that facilitates the fetal to newborn transition, hence the use of high oxygen concentrations to treat hypoxemia and reverse pulmonary vasoconstriction in neonates with PH. Healthy newborn lambs ventilated with 100% oxygen for the first 30 min of life show a more rapid decrease in pulmonary vascular resistance than those ventilated with 21% oxygen (104). However, when weaned to 21% oxygen and studied at 4 h later, the high oxygen group display impaired pulmonary vasodilator responses to endogenous and exogenous NO (104). Further studies in a model of perinatal asphyxia confirmed that vasodilator responses were decreased after resuscitation with 100% O2, and suggested that elevated vascular oxidant stress was responsible (105). The use of 100% oxygen in PPHN lambs does not enhance the decrease in pulmonary vascular resistance relative to ventilation with 21% or 50% oxygen, but diminishes the vasodilator response to inhaled NO and increases the activity of cGMP-specific phosphodiesterase (106). These data suggest that short-term pulmonary vascular benefits of ventilation with high levels of oxygen need to be weighed against longer-lasting adverse effects on vascular reactivity.
Chronic exposure to hyperoxia induces additional factors that may impair normal lung development. Mice and rats are born in the saccular stage of lung development, do not begin alveolarization until postnatal day 5 (P5), and provide useful models to study the effects of hyperoxia in the immature lung. Chronic exposure to hyperoxia (60% O2 and greater) induces PH and vascular remodeling in newborn rats (98) and mice (180). Similar to premature infants who die with BPD (1, 19), lung VEGF expression is decreased in various rodent models of neonatal hyperoxic lung injury (87, 123, 166). As previously noted, the disruption of pulmonary VEGF leads to abnormal vascular and alveolar development, lung hypoplasia, and PH (35, 70, 74), and pharmacologic inhibition of VEGF receptors in neonatal rats produces similar impairments in lung alveolarization and vascular growth (89, 112, 163). In animal models of intrauterine growth restriction (IUGR), reduced VEGF and VEGFR-2 expression are associated with reduced pulmonary vascular density and lung alveolarization (152).
The importance of a hypoxic intrauterine environment suggests a potential role for HIFs in the regulation of normal fetal and neonatal lung development, and hyperoxia-mediated degradation of HIFs may contribute to decreased VEGF expression in rodent models of BPD. Additional studies have also implicated alterations in the thioredoxin system by hyperoxia, which could alter HIF and VEGF signaling (54, 167). These data suggest that loss of HIF activity due to hyperoxia may also induce pulmonary vasoconstriction in the immature lung via this pathway.
Similar to human infants who develop BPD (153), markers of oxidative stress are increased in hyperoxia-exposed mice (141) and rats (136). Together, these data suggest that hyperoxia may contribute to BPD and PH by several distinct mechanisms, including oxygen-mediated attenuation of HIF and VEGF signaling and oxygen-derived ROS.
Sources of ROS in Neonatal PH
A common feature of all animal models of neonatal pulmonary vascular remodeling is an increase in pulmonary arterial ROS levels. ROS are small molecules derived from molecular oxygen that can produce vascular injury through their interaction with proteins, DNA, RNA, and lipids (149). ROS contribute to the pathogenesis of multiple cardiovascular diseases, including hypertension, atherosclerosis, cardiac hypertrophy, heart failure, and restenosis (109). Furthermore, lipid and protein oxidation products are elevated in infants who subsequently develop BPD (153), suggesting that oxidant stress may contribute to the pathogenesis of the disease while oxygen therapy may exacerbate the effects.
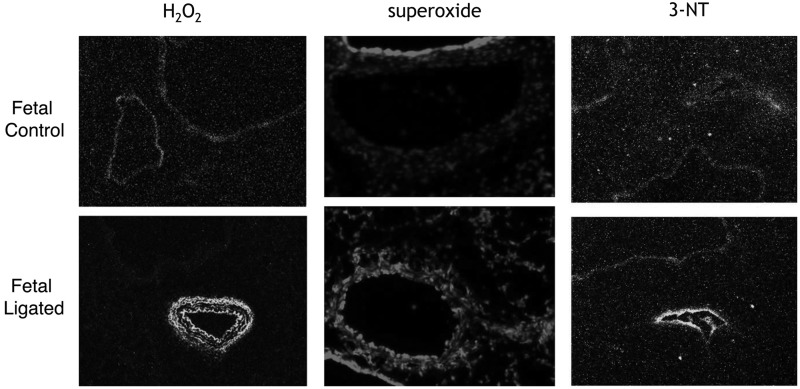
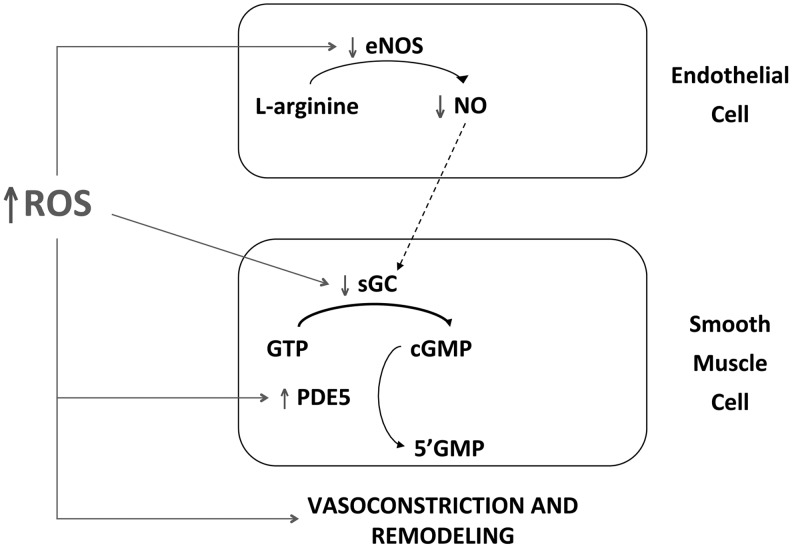
The animal models of PPHN and BPD have also proved valuable in determining underlying mechanisms that contribute to these diseases. Increased superoxide levels have been found in the endothelium and vascular smooth muscle of PPHN pulmonary arteries (22, 27) (Fig. 3). Superoxide reacts rapidly with NO and forms peroxynitrite (ONOO), thus attenuating NO-mediated vasodilation and inactivating other enzymatic pathways via the formation of nitrotyrosine (Fig. 3). In addition, increased production of H2O2 in PPHN pulmonary arteries (187, 191) (Fig. 3) may contribute to decreased eNOS expression (183), impaired cGMP production (57), and elevated PDE5 activity (57). Figure 4 shows impaired NO signaling due to elevated ROS in PPHN. In addition to inhibiting vasodilation, increasing evidence suggests that ROS can stimulate vascular SMC growth and contribute to pulmonary vascular remodeling (146, 162). The next section will discuss the potential sources of ROS generation in animal models of PH.
FIG. 3.
Vascular remodeling is associated with increased ROS in PPHN pulmonary arteries. Frozen sections from control and PPHN lamb lungs were incubated with the H2O2-sensitive dye 2′, 7′-dichlorodihydrofluorescein diacetate [adapted from (187)], with the superoxide-sensitive dye dyhydroethidium [adapted from (21)], or fixed and incubated with an antibody to 3-nitrotyrosine residues (3-NT) [adapted from (103)], and visualized by fluorescence microscopy.
FIG. 4.
Elevated levels of reactive oxygen species (ROS) induces vasoconstriction and pulmonary vascular remodeling in PPHN via multiple mechanisms. In ECs, ROS inhibit eNOS activity, resulting in decreased levels of bioavailable NO. In smooth muscle cells, ROS inactivate sGC and activate PDE5, resulting in decreased levels of cGMP.
NADPH oxidases
Nox enzymes are membrane proteins that transfer electrons from NADPH to molecular oxygen, producing superoxide intracellularly or extracellularly depending on the isoform and subcellular location of the enzyme. Seven Nox enzymatic subtypes have been identified in a wide range of cell types, which include vascular cell expression of the Nox1, Nox2, and Nox4 homologues. Each of these Nox family members is regulated by specific physiological mechanisms, and dysregulation of expression or activity may contribute to pulmonary vascular dysfunction.
Nox1 is expressed in vascular smooth muscle, endothelium, and adventitia (36, 108), and it has been localized to membranes, including plasma membranes, caveolae, and endosomes. Nox1 activity requires the association with other protein subunits, including p22phox, Noxo1, Noxa1, and Rac (23, 36). Overexpression of Nox1 in vascular smooth muscle increases the production of ROS, causes eNOS uncoupling, and decreases nitric oxide bioavailability (47). Nox1 expression is increased in mouse lung cell lines that are exposed to 72h hyperoxia, while hyperoxia-induced ROS generation and lung injury are attenuated in Nox1-deficient mice (24). Increased pulmonary Nox1 expression was reported in neonatal mice with PH after hyperoxia (17) and in neonatal piglets exposed to hypoxia (42), and Nox1 may be involved in pulmonary vascular remodeling in monocrotaline-treated rats (174). Nox1 levels are unchanged in the lungs of fetal lambs with chronic intrauterine PH (187), although alteration of Nox1 activity in this model is currently unknown.
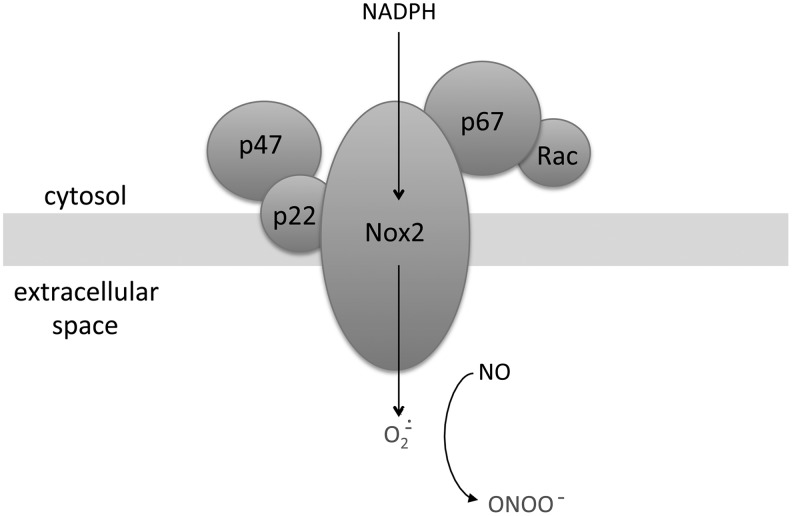
The Nox2 isoform is expressed in phagocytic cells as well as in cells comprising the vascular wall and is activated by pathways very similar to Nox1. It requires the assembly of protein subunits, including p22phox, p47phox, p67phox, and Rac, for activation. When assembled in the plasma membrane, Nox2 secretes superoxide into the extracellular space and potentially inhibits NO signaling (Fig. 5), but when endocytotic vesicles arising from the plasma membrane are formed, the superoxide is secreted into the lysosome (26, 142). Similar to Nox1, Nox2 produces superoxide that is associated with vasoconstriction, and increased Nox2 subunit expression correlates with increased superoxide levels and impaired pulmonary vasorelaxation in both lamb and piglet models of neonatal PH (22, 63). Nox2 knockout mice display attenuated hypoxia-induced ROS, vascular remodeling, and PH (115), and the Nox inhibitor apocynin improves oxygenation and decreases ROS in PPHN lambs (188). In addition, serum-induced PASMC proliferation requires the Nox subunit Rac1 (138).
FIG. 5.
Diagram illustrating subunit organization of NADPH oxidase 2 (Nox2) at the plasma membrane. Nox2 and p22 are present within the membrane, while p47, p67 and Rac are located in the cytosol. The transfer of electrons from NADPH in the cytosol generates superoxide in the extracellular space. Superoxide decreases bioavailable NO in the rapid reaction and generates the vasoconstrictor peroxynitrite (ONOO).
Nox4 is more abundantly expressed in vascular cells relative to Nox1 and Nox2, it and has been shown to localize to the mitochondria, endoplasmic reticulum, and nucleus (30, 101, 172). Nox4 was initially thought to require only p22phox for activity and to be constitutively active (156), although recent data indicate that polymerase delta interacting protein 2 (Poldip2) may interact with Nox4 to enhance its activity (117). Nox4 activity appears to be regulated primarily by expression, and Nox4 has been shown to generate both superoxide and H2O2 depending on the stimulus and cell type (45). Increased Nox4 expression correlates with increased H2O2 in PPHN pulmonary arteries (Fig. 3), while Nox4 knockdown attenuates ROS levels in SMC isolated from PPHN pulmonary arteries relative to controls (187). Nox4-derived H2O2 may contribute to impaired vasodilation in PPHN lambs via multiple mechanisms, including decreased eNOS and sGC expression and increased PDE5 activity (187) (Fig. 4). Nox4 has also been implicated in the regulation of several cellular processes, including migration, growth, and differentiation (109), and increased Nox4 activity may also contribute to pulmonary vascular remodeling in PPHN lambs. Nox4 mediates PASMC proliferation in response to transforming growth factor-β1 (160), urotensin II (49), and hypoxia (43). An understanding of the downstream targets of ROS-induced PASMC proliferation is just emerging. Cyclin D1 regulates the transition from G0/G1 to S phase in the cell cycle, resulting in the activation of genes that are necessary for cell-cycle progression. Cyclin D1 expression is increased in PPHN lungs and PASMC (187) and in PASMC isolated from monocrotaline-treated rats (174). Nox4 small interfering RNA decreases cyclin D1 expression in PPHN PASMC, and intratracheal catalase decreases cyclin D1 expression in the lungs of ventilated PPHN lambs (187). Together, these data indicate a link between increased ROS generation, activation of cell-cycle promoters, and pulmonary vascular remodeling in PH. Further studies are warranted to determine whether cell-cycle proteins are dysregulated in other models of PH. The identification of such proteins may improve the early detection of pulmonary vascular remodeling, and their targeting may attenuate or reverse this process. Nox4 may also contribute to hypoxia-induced PH (127), although data from Nox4 knockout models in mice are just emerging. Novel Nox pharmacologic inhibitors are currently under investigation. Administration of the Nox1/Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary artery (PA) wall thickness and right ventricular hypertrophy in mice (73), while reduced ROS levels have been detected in several animal models treated with the pan-Nox inhibitor VAS 2870 (6).
The membrane protein p22phox is the common subunit to Nox1, Nox2, and Nox4, and p22phox expression is elevated in lungs, PA, and PASMC isolated from PPHN lambs (187). The transcription factor NFκB regulates p22phox and Nox4 transcription in human aortic SMC (121, 122), and NFκB activity is elevated in PA and PASMC of fetal lambs with chronic intrauterine PH (187). NFκB inhibition decreases Nox4 expression in PPHN PASMC (187), and it attenuates monocrotaline-induced pulmonary arterial hypertension in rats (96, 154), suggesting a potential role for NFκB in pulmonary vascular ROS generation. Hyperoxia selectively activates NFκB in fetal, but not adult, lung fibroblasts (195), suggesting that NFκB could also play a role in the development of neonatal PH due to hyperoxic lung injury. NFκB activity is regulated through its interaction with the regulatory protein IκB. NFκB is activated by ROS via the phosphorylation of IκB, which targets IκB for protein degradation (169) and enables NFκB to translocate into the nucleus and regulate the transcription of target genes. Together, these data suggest the possibility of a feed-forward mechanism in PPHN by which ROS generated by Nox isoforms increases the activity of key transcription factors such as NFκB, resulting in sustained Nox subunit expression. NFκB could prove to be an attractive target to reduce the amplification of ROS in hypertensive diseases.
Mitochondrial electron transport chain
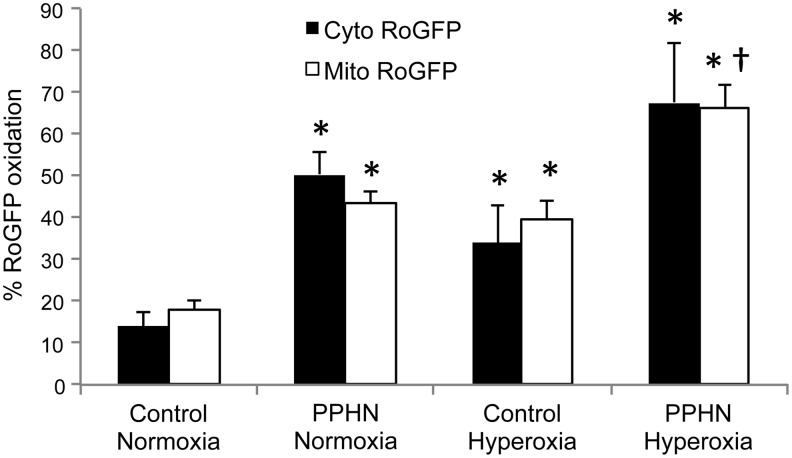
During normal oxidative phosphorylation, electrons are transferred to molecular oxygen at the terminal cytochrome oxidase in the mitochondrial electron transport chain, generating H2O. However, some electrons are captured by O2 at more proximal sites, resulting in the formation of the superoxide radical. Superoxide generated at Complex I, II, or III can result in oxidant stress in the mitochondrial matrix or inter-membrane space (75). In some conditions such as atherosclerosis, the mitochondria become dysfunctional and the leak of electrons is enhanced (118). Mitochondrial ROS appear to trigger increased Nox activity in hypoxic pulmonary arteries (147), and increased oxidative stress in the mitochondrial matrix is associated with increased Nox4 expression in PA SMC isolated from PPHN lambs (55, 187). Emerging evidence indicates cross-talk between the mitochondria and Nox isoforms (44), and further investigation of the underlying mechanisms may produce novel therapies for pulmonary hypertensive diseases. In addition, selective inhibition of mitochondrial oxidant stress reduces vascular oxidant stress and hypertension in systemic vessels (46), suggesting that effective antioxidant therapy will require the targeting of specific subcellular compartments. Indeed, hypoxia decreases ROS levels in the mitochondrial matrix while increasing ROS derived from mitochondrial complex III in the mitochondrial intermembrane space and cytosol (181, 182). Conversely, hyperoxia and PPHN increase oxidant stress in the mitochondrial matrix and cytosol in fetal sheep PASMC (Fig. 6) (55, 189), suggesting that precise antioxidant targeting within organelles may be required to treat hypertensive diseases with different etiologies.
FIG. 6.
Hyperoxia and PPHN increase oxidant stress in the cytosol and mitochondrial matrix of PASMC. PASMC were isolated from control or PPHN lambs and infected with an adenovirus expressing the ROS-sensitive protein RoGFP in the cytosol (Cyto RoGFP) or in the mitochondrial matrix (Mito RoGFP). Cells were exposed to 21% (normoxia) or 95% O2 (hyperoxia) for 24 h and RoGFP oxidation determined by flow cytometry. *p<0.05 versus control normoxia; †p<0.05 versus PPHN normoxia. Adapted from (55, 189).
Endothelial nitric oxide synthase
The activity of eNOS is usually regulated by the availability of a substrate as well as by several cofactors, including calcium-calmodulin, HSP90, and BH4, and mechanisms that inhibit eNOS activity or attenuate downstream NO signaling can induce vasoconstriction. Elevated ROS decrease eNOS expression, reduce available BH4 in PPHN lambs (157, 183), and reduce downstream responses to NO by decreasing sGC expression (191) and increasing cGMP-specific phosphodiesterase activity (57). Impaired eNOS expression and activity may also contribute to abnormal lung and vascular development that produce BPD. Substantial reductions in total NOS activity and expression of all three NOS isoforms have been observed in a baboon and rodent models of hyperoxic lung injury (4, 114). NO not only mediates the downstream effects of VEGF during lung development, but may also up-regulate VEGF expression (116). IUGR increases the risk for BPD (28), and PAECs isolated from a lamb model of IUGR exhibit decreased eNOS expression and phosphorylation, decreased NO production, and attenuated tube formation and migration (152). eNOS-deficient mice display PH and vascular remodeling when exposed to mild hypoxia (52), further highlighting the central role of eNOS in maintaining normal vascular tone and development.
Superoxide reacts rapidly with NO and forms peroxynitrite (ONOO). Peroxynitrite is a potent vasoconstrictor of the neonatal pulmonary vasculature (15), and it inhibits NOS activity via mechanisms that include decreased association with HSP90 (164, 190). eNOS becomes a source of ROS when the enzyme becomes “uncoupled,” resulting in an incomplete reduction of molecular oxygen with the formation of superoxide. eNOS uncoupling is evident in hypoxic piglets (60) and can occur via several mechanisms, including degradation or oxidation of cofactors such as BH4 and HSP90, or by inactivation of the enzyme through increased peroxynitrite levels (103, 110). Increased Nox activity may be considered an important trigger for eNOS uncoupling (47, 107), while eNOS uncoupling can promote mitochondrial dysfunction and ROS generation via increased peroxynitrite (161). Together, these data suggest that abnormal regulation of ROS can promote oxidant production from additional sources, thus amplifying and sustaining a pathological state.
Antioxidants
ROS levels in cells are determined by their removal as well as by their generation, and cells employ a wide variety of enzymatic scavengers (12, 33). The expression of these ROS scavengers is specific to certain subcellular compartments, and most antioxidant enzymes are selective for a single type of ROS molecule. SOD degrades superoxide to H2O2, and H2O2 produced by SOD is regulated by its rate of degradation by enzymes such as catalase, glutathione peroxidase (GPx), and peroxiredoxins (PRx) (148).
There are three known forms of SOD: Cu/ZnSOD (SOD1), MnSOD (SOD2), and extracellular SOD (ecSOD or SOD3). Cu/ZnSOD is expressed in the cytosol and intermembrane space of the mitochondria, and ecSOD is secreted to the extracellular space, where it binds to the extracellular matrix. Increased expression of all three SOD isoforms is evident in the lungs of 8 week-old mice relative to 1 week-old mice (17), suggesting that neonates may be more susceptible to increased oxidant stress due to limited antioxidant capacity. MnSOD is localized to the mitochondria, and it is responsible for protecting against excessive mitochondrial superoxide generation. Mice with homozygous deletion of the MnSOD gene die from oxidative stress shortly after birth (67), and mice lacking one allele of MnSOD develop hypertension with aging and in response to a high salt diet (151). MnSOD levels are reduced in PAECs of PPHN lambs (3), but are unexpectedly higher in PPHN PASMC (55, 187), highlighting the complex cell-specific regulatory mechanisms.
ecSOD is predominantly synthesized by the vascular SMC and comprises a significant component of the total SOD activity in the blood vessel wall (66). It is the most highly expressed in the lung (134, 135), and is present in high concentrations between the endothelium and smooth muscle surrounding blood vessels, the same domain that NO should pass through to stimulate smooth muscle relaxation. This suggests that high concentrations of ecSOD in this region are especially important in maintaining low superoxide concentrations and preserving NO function (143). ecSOD activity is decreased in PPHN lungs and PASMC (189), potentially via a mechanism involving Nox4-derived H2O2 that oxidizes copper at the enzyme active site (187). Decreased ecSOD activity is predicted to decrease bioavailable NO via the formation of peroxynitrite, while protein nitration inhibits ecSOD activity (119), indicating a potential feed-forward mechanism of enzyme inhibition. Therapeutic interventions to maintain ecSOD activity are, therefore, predicted to be beneficial in the treatment of cardiovascular disease. The overexpression of ecSOD ameliorates PH in rats (91), protects lung development (5), and attenuates pulmonary vascular remodeling in hypoxic mice (133). The H2O2 scavenger catalase also enhances ecSOD activity and decreases PA superoxide in PPHN lambs (189). The development of novel proteins such as a chimeric SOD2/3 (32) may enable more sustained pulmonary vascular antioxidant activity. Treatment with a peroxynitrite decomposition catalyst attenuates hyperoxia-induced decreases in VEGF expression and enhances alveolar formation in neonatal rats (124), suggesting that this approach may also increase ecSOD activity and improve NO signaling in PPHN.
Catalase functions to catalyze the decomposition of hydrogen peroxide to water and oxygen. However, mice deficient in catalase develop normally, indicating that other complementary antioxidant systems should be present (85). The GPx and PRx systems utilize reduced glutathione to scavenge H2O2 and are critical for minimizing oxidant stress and for regulating redox signaling pathways. GPx levels are decreased in the lungs of patients with IPAH (125), although genetic deletion of GPx-1 does not affect the increase in aortic pressure or vascular hypertrophy induced by angiotensin II, and GPx-1 levels are unchanged in the lungs of PPHN lambs (11, 191). By contrast, the deletion of PRx 1 induces hemolytic anemia and a significant decrease in lifespan (131), while the deletion of PRx 3 leads to oxidant-mediated lung inflammation and an enhanced susceptibility to LPS challenge (113).
Therapeutic Considerations
While the data presented earlier suggest a potential role for antioxidant therapy in the treatment of PH, there has been very limited success in clinical trials of antioxidant therapy for a wide range of diseases, including BPD (37). Antioxidant therapy may be ineffective once the disease has progressed beyond a critical stage, suggesting that early detection strategies may improve treatment outcomes. ROS scavenging may also interfere with normal signaling pathways in the developing lung. Furthermore, oxidant stress may be localized to specific subcellular compartments in different diseases, which may limit the efficacy of nonspecific antioxidants. Thus, highly targeted therapies may be required to maximize the potential of antioxidants in the treatment of hypertensive diseases.
Inhaled NO therapy improves PH resulting from impaired eNOS signaling, but could potentially increase peroxynitrite formation, resulting in the nitration and inhibition of endogenous eNOS activity (190). Intratracheal antioxidants decrease ROS, increase eNOS expression, and normalize BH4 levels in PPHN lambs (59, 188), suggesting that the combination of inhaled NO and antioxidants could be more effective therapeutically than inhaled NO alone. Intratracheal recombinant human SOD also reduces ONOO-mediated protein nitration (103), decreases PDE5 activity, and increases cGMP in the PAs of ventilated PPHN lambs (58), suggesting that antioxidant therapy may improve NO signaling at multiple points in the pathway. The SOD mimetic MnTE-2-PyP attenuates hypoxia-induced pulmonary vascular remodeling and PH in mice (177), and the SOD mimetic M40403 improves NO-mediated relaxation in PAs isolated from hypoxic piglets (60).
The administration of intratracheal catalase to ventilated PPHN lambs improves oxygenation, increases ecSOD activity, and decreases PA superoxide levels (189). Further, intratracheal catalase decreases PDE5 activity and increases cGMP in the PAs of ventilated PPHN lambs (55). These data suggest that H2O2 scavenging improves NO signaling in PPHN, possibly by increasing ecSOD activity. Accordingly, treatment with PEG-catalase improves NO-mediated vasodilation in PAs isolated from PPHN lambs (191) and also from hypoxic piglets (60). Anti-inflammatory glucocorticoids are used to treat neonates with MAS (170), and hydrocortisone improves oxygenation, decreases PA ROS, decreases PDE5 activity, and increases cGMP levels in PPHN lambs (139). Figure 7 illustrates the localized interaction between antioxidants and ROS, and Table 1 summarizes improved arterial to alveolar PO2 ratios in PPHN lambs administered intratracheal antioxidants.
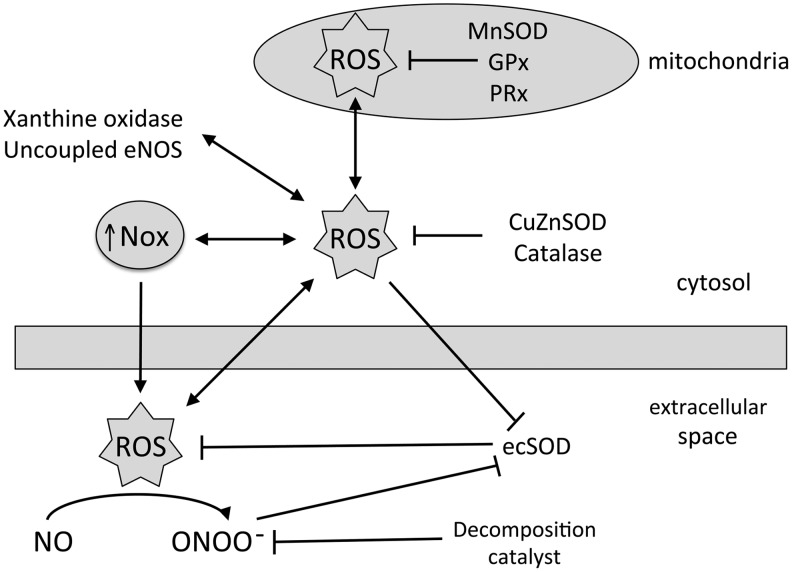
FIG. 7.
Diagram depicting the interactions between cellular ROS generators and scavengers. In mitochondria, ROS levels are regulated by enzymes, including manganese superoxide dismutase (MnSOD), glutathione peroxidase (GPx), and peroxyredoxin (PRx). In the cytosol, NADPH oxidases (Nox), xanthine oxidase, and uncoupled endothelial nitric oxide synthase (eNOS) generate ROS, while copper/zinc superoxide dismutase (CuZnSOD) and catalase scavenge ROS. Nox also contribute to ROS in the extracellular space, while extracellular superoxide dismutase (ecSOD) scavenges extracellular superoxide. Increased extracellular superoxide decreases bioavailable NO in the formation of peroxynitrite (ONOO), and this vasoconstrictor is removed in the presence of a decomposition catalyst.
Table 1.
Improved Arterial to Alveolar PO2 Ratios (A/A Ratio) After 24 H in PPHN Lambs Ventilated with 100% O2 in Combination with Nitric Oxide and/or Intratracheal Antioxidants
| Lamb | Treatment | a/A ratio±SEM | Reference |
|---|---|---|---|
| Control | O2 | 0.58±0.06 | (87) |
| PPHN | O2 | 0.19±0.08 | (87) |
| PPHN | O2+iNO | 0.51±0.1 | (87) |
| PPHN | O2+rhSOD | 0.48±0.12 | (87) |
| PPHN | O2+catalase | 0.50±0.25 | (162) |
| PPHN | O2+apocynin | 0.30±0.12 | (161) |
| PPHN | O2+hydrocortisone | 0.54±0.07 | (119) |
iNO, inhaled nitric oxide (20ppm); rhSOD, recombinant human superoxide dismutase (5 mg/kg), PEG-catalase (20,000 U/kg), apocynin (3 mg/kg), hydrocortisone (3×5 mg/kg).
Overexpression of GTP-cyclohydrolase, the enzyme catalyzing the rate-limiting step in BH4 synthesis, attenuates hypoxic PH (94), which could be due to improved NOS function. GTP-cyclohydrolase expression is diminished in PPHN lambs, and it is restored by treatment with antioxidants (59). Clinical trials with oral BH4 have been conducted in adults with PH (150), and future studies may determine the efficacy of this approach in the treatment of children. Other approaches to increase NOS activity include supplementation with L-citrulline. eNOS generates NO from the oxidation of L-arginine, and L-citrulline is formed as a byproduct. L-citrulline is converted back to L-arginine by enzymes that colocalize with eNOS in the endothelium, and L-citrulline may be a more potent activator of eNOS by providing a supply of L-arginine in close proximity to eNOS. Oral supplementation with L-citrulline attenuates PH and increases NO production in newborn piglets exposed to hypoxia (9). Moreover, hyperoxia decreases plasma L-arginine and L-citrulline levels in a rat model of BPD; while supplementation with L-citrulline preserves lung alveolar and vascular development, and attenuates PH and vascular remodeling (171). L-arginine can also be converted to urea and L-ornithine by arginase enzymes expressed in the lung, and increased arginase activity decreases NO production from eNOS by competing for the same substrate. Hypoxia induces human PASMC proliferation via arginase (29), while hypoxic mice deficient in MAP kinase signaling display elevated arginase expression, exaggerated PH and vascular remodeling, and decreased eNOS expression relative to wild-type mice (90). Decreased L-arginine promotes eNOS uncoupling (155), suggesting that therapies including L-citrulline supplementation and arginase inhibition may attenuate eNOS uncoupling and stimulate NO production in hypertensive diseases.
Augmenting cGMP concentrations through other routes may also prevent or reverse pulmonary vascular remodeling due to oxidant stress. Novel activators of sGC such as cinaciguat (BAY 58-2667) are functional in the enzyme's oxidized, NO-resistant state (31, 34), and phosphodiesterase inhibitors such as sildenafil may represent a logical approach to overcome increased PDE5 activity. Sildenafil treatment of neonatal rats exposed to hyperoxia normalizes lung alveolar and vascular development and attenuates PH and vascular remodeling (102). Furthermore, sildenafil treatment at 6 days after the initiation of hyperoxia significantly reduces medial wall thickness and right ventricular hypertrophy, suggesting that hyperoxia-induced vascular dysfunction is reversible (39). Iron is an important regulator of ROS levels, and an iron chelator prevents hypoxia-induced PH and pulmonary vascular remodeling in rats (194). Future studies are needed to determine whether similar approaches are effective in the neonatal pulmonary vasculature.
Other studies using pharmacologic inhibition of constrictor pathways have also demonstrated encouraging results in animal models of PH. The Rho kinase inhibitor fasudil attenuates increased pulmonary vascular resistance in response to compression of the ductus arteriosus in fetal lambs (168), and the Rho kinase inhibitor Y-27632 attenuates acute hypoxia-induced vasoconstriction and hypertension in adult mice (53). In the late-gestation ovine fetus, 5-HT-induced pulmonary vasoconstriction is attenuated by ketanserin, a 5-HT 2A receptor antagonist, while antagonists to other 5-HT receptor subtypes are ineffective (40). Selective ETA antagonists have also proved successful in several different models of neonatal PH. BQ-123 lowers pulmonary arterial pressure in a piglet model of meconium aspiration (100), TBC3711 attenuates hypoxia-induced increases in pulmonary arterial pressure in neonatal piglets (140), and ambrisentan reduces pulmonary arterial hypertension in neonatal rats exposed to hyperoxia (178).
Summary and Conclusions
Oxidant stress potentially plays multiple roles in the susceptibility and pathogenesis of PH in preterm infants and newborns. Increased oxidant stress can arise due to exposure to a variety of injurious stimuli, including hyperoxia and hypoxia, that activate ROS generators, and/or inactivate endogenous antioxidants. The mechanisms involved may differ between different types of PH, thus having implications for the most effective therapy to treat a specific disease. Further investigation into the specific mechanisms involved, along with the development of novel antioxidant and nonantioxidant therapies, may improve the outcomes for infants with PH.
Abbreviations Used
- 5-HT
serotonin
- BH4
tetrahydrobiopterin
- BPD
bronchopulmonary dysplasia
- CDH
congenital diaphragmatic hernia
- cGMP
cyclic guanosine monophosphate
- CuZnSOD
copper zinc superoxide dismutase
- EC
endothelial cells
- ecSOD
extracellular superoxide dismutase
- eNOS
endothelial nitric oxide synthase
- ET-1
endothelin-1
- ETA
endothelin receptor subtype A
- GPx
glutathione peroxidase
- HIF
hypoxia-inducible factor
- iNO
inhaled nitric oxide
- IUGR
intrauterine growth restriction
- MAS
meconium aspiration syndrome
- MnSOD
manganese superoxide dismutase
- NO
nitric oxide
- Nox
NADPH oxidase
- ONOO
peroxynitrite
- PA
pulmonary artery
- PASMC
pulmonary artery smooth muscle cells
- PDE5
phosphodiesterase type 5
- PH
pulmonary hypertension
- PPHN
persistent pulmonary hypertension of the newborn
- PRx
peroxiredoxin
- rhSOD
recombinant human superoxide dismutase
- ROS
reactive oxygen species
- sGC
soluble guanylate cyclase
- SMC
smooth muscle cells
- SOD
superoxide dismutase
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
References
- 1.Abman SH. Impaired vascular endothelial growth factor signaling in the pathogenesis of neonatal pulmonary vascular disease. Adv Exp Med Biol 661: 323–335, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Abman SH, Shanley PF, and Accurso FJ. Failure of postnatal adaptation of the pulmonary circulation after chronic intrauterine pulmonary hypertension in fetal lambs. J Clin Invest 83: 1849–1858, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afolayan AJ, Eis A, Teng RJ, Bakhutashvili I, Kaul S, Davis JM, and Konduri GG. Decreases in manganese superoxide dismutase expression and activity contribute to oxidative stress in persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 303: L870–L879, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afshar S, Gibson LL, Yuhanna IS, Sherman TS, Kerecman JD, Grubb PH, Yoder BA, McCurnin DC, and Shaul PW. Pulmonary NO synthase expression is attenuated in a fetal baboon model of chronic lung disease. Am J Physiol Lung Cell Mol Physiol 284: L749–L758, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Ahmed MN, Suliman HB, Folz RJ, Nozik-Grayck E, Golson ML, Mason SN, and Auten RL. Extracellular superoxide dismutase protects lung development in hyperoxia-exposed newborn mice. Am J Respir Crit Care Med 167: 400–405, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Altenhofer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, and Schmidt HH. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci 69: 2327–2343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambalavanan N, Bulger A, Murphy-Ullrich J, Oparil S, and Chen Y. Endothelin-A receptor blockade prevents and partially reverses neonatal hypoxic pulmonary vascular remodeling. Pediatr Res 57: 631–636, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.An HS, Bae EJ, Kim GB, Kwon BS, Beak JS, Kim EK, Kim HS, Choi JH, Noh CI, and Yun YS. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J 40: 131–136, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ananthakrishnan M, Barr FE, Summar ML, Smith HA, Kaplowitz M, Cunningham G, Magarik J, Zhang Y, and Fike CD. L-Citrulline ameliorates chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol 297: L506–L511, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angus DC, Linde-Swirble WT, Clermont G, Griffin MF, and Clark RH. Epidemiology of neonatal respiratory failure in the United States. Am J Resp Crit Care Med 164: 1154–1160, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Ardanaz N, Yang XP, Cifuentes ME, Haurani MJ, Jackson KW, Liao TD, Carretero OA, and Pagano PJ. Lack of glutathione peroxidase 1 accelerates cardiac-specific hypertrophy and dysfunction in angiotensin II hypertension. Hypertension 55: 116–123, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asikainen TM. and White CW. Pulmonary antioxidant defenses in the preterm newborn with respiratory distress and bronchopulmonary dysplasia in evolution: implications for antioxidant therapy. Antioxid Redox Signal 6: 155–167, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Balasubramaniam V, Maxey AM, Morgan DB, Markham NE, and Abman SH. Inhaled NO restores lung structure in eNOS-deficient mice recovering from neonatal hypoxia. Am J Physiol Lung Cell Mol Physiol 291: L119–L127, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Balasubramaniam V, Tang JR, Maxey A, Plopper CG, and Abman SH. Mild hypoxia impairs alveolarization in the endothelial nitric oxide synthase-deficient mouse. Am J Physiol Lung Cell Mol Physiol 284: L964–L971, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Belik J, Pan J, Jankov R, and Tanswell A. Peroxynitrite inhibits relaxation and induces pulmonary artery muscle contraction in the newborn rat. Free Radic Biol Med 37: 1384–1392, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Berkelhamer S, Mestan KK, and Steinhorn RH. Pulmonary hypertension in bronchopulmonary dysplasia. Semin Perinatol 37: 124–131, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berkelhamer SK, Kim GA, Radder JE, Wedgwood S, Czech L, Steinhorn RH, and Schumacker PT. Developmental differences in hyperoxia-induced oxidative stress and cellular responses in the murine lung. Free Radic Biol Med 61C: 51–60, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhat R, Salas AA, Foster C, Carlo WA, and Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics 129: e682–e689, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, and Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med 164: 1971–1980, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Black S, DeVol J, and Wedgwood S. Regulation of fibroblast growth factor-2 expression in pulmonary arterial smooth muscle cells involves increased reactive oxygen species generation. Am J Physiol Cell Physiol 294: C345–C354, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Black SM, Johengen MJ, and Soifer SJ. Coordinated regulation of genes of the nitric oxide and endothelin pathways during the development of pulmonary hypertension in fetal lambs. Pediatr Res 44: 821–830, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, and Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res 92: 683–691, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Brown DI. and Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med 47: 1239–1253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carnesecchi S, Deffert C, Pagano A, Garrido-Urbani S, Metrailler-Ruchonnet I, Schappi M, Donati Y, Matthay MA, Krause KH, and Barazzone Argiroffo C. NADPH oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice. Am J Respir Crit Care Med 180: 972–981, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chambers CD, Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Jones KL, and Mitchell AA. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 354: 579–587, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Chamseddine AH. and Miller FJ., Jr.Gp91phox contributes to NADPH oxidase activity in aortic fibroblasts but not smooth muscle cells. Am J Physiol Heart Circ Physiol 285: H2284–H2289, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Chandrasekar I, Eis A, and Konduri GG. Betamethasone attenuates oxidant stress in endothelial cells from fetal lambs with persistent pulmonary hypertension. Pediatr Res 63: 67–72, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Check J, Gotteiner N, Liu X, Su E, Porta N, Steinhorn R, and Mestan KK. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. J Perinatol 33: 553–557, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen B, Calvert AE, Cui H, and Nelin LD. Hypoxia promotes human pulmonary artery smooth muscle cell proliferation through induction of arginase. Am J Physiol Lung Cell Mol Physiol 297: L1151–L1159, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Chen K, Kirber MT, Xiao H, Yang Y, and Keaney JF., Jr.Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol 181: 1129–1139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chester M, Tourneux P, Seedorf G, Grover TR, Gien J, and Abman SH. Cinaciguat, a soluble guanylate cyclase activator, causes potent and sustained pulmonary vasodilation in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 297: L318–L325, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke MB, Wright R, Irwin D, Bose S, Van Rheen Z, Birari R, Stenmark KR, McCord JM, and Nozik-Grayck E. Sustained lung activity of a novel chimeric protein, SOD2/3, after intratracheal administration. Free Radic Biol Med 49: 2032–2039, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clerch LB. and Massaro D. Rat lung antioxidant enzymes: differences in perinatal gene expression and regulation. Am J Physiol 263: L466–L470, 1992 [DOI] [PubMed] [Google Scholar]
- 34.Coggins MP. and Bloch KD. Nitric oxide in the pulmonary vasculature. Arterioscler Thromb Vasc Biol 27: 1877–1885, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, and Carmeliet P. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med 8: 702–710, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Csanyi G, Taylor WR, and Pagano PJ. NOX and inflammation in the vascular adventitia. Free Radic Biol Med 47: 1254–1266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis JM, Parad RB, Michele T, Allred E, Price A, Rosenfeld W, and North American Recombinant Human CuZnSOD Study Group. Pulmonary outcome at 1 year corrected age in premature infants treated at birth with recombinant human CuZn superoxide dismutase. Pediatrics 111: 469–476, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Dawes GS, Mott JC, Widdicombe JG, and Wyatt DG. Changes in the lungs of the newborn lamb. J Physiol 121: 141–162, 1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Visser YP, Walther FJ, Laghmani el H, Boersma H, van der Laarse A, and Wagenaar GT. Sildenafil attenuates pulmonary inflammation and fibrin deposition, mortality and right ventricular hypertrophy in neonatal hyperoxic lung injury. Respir Res 10: 30, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delaney C, Gien J, Grover TR, Roe G, and Abman SH. Pulmonary vascular effects of serotonin and selective serotonin reuptake inhibitors in the late-gestation ovine fetus. Am J Physiol Lung Cell Mol Physiol 301: L937–L944, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delaney C, Gien J, Roe G, Isenberg N, Kailey J, and Abman SH. Serotonin contributes to high pulmonary vascular tone in a sheep model of persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 304: L894–L901, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dennis KE, Aschner JL, Milatovic D, Schmidt JW, Aschner M, Kaplowitz MR, Zhang Y, and Fike CD. NADPH oxidases and reactive oxygen species at different stages of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol 297: L596–L607, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diebold I, Petry A, Hess J, and Görlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell 21: 2087–2096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 51: 1289–1301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dikalov S, Dikalova A, Bikineyeva A, Schmidt H, Harrison D, and Griendling K. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med 45: 1340–1351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, and Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107: 106–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dikalova AE, Gongora MC, Harrison DG, Lambeth JD, Dikalov S, and Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol 299: H673–H679, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Djonov V, Schmid M, Tschanz SA, and Burri PH. Intussusceptive angiogenesis: its role in embryonic vascular network formation. Circ Res 86: 286–292, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Djordjevic T, BelAiba RS, Bonello S, Pfeilschifter J, Hess J, and Gorlach A. Human urotensin II is a novel activator of NADPH oxidase in human pulmonary artery smooth muscle cells. Arterioscler Thromb Vasc Biol 25: 519–525, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Dunn JA, Lorch V, and Sinha SN. Responses of small intrapulmonary arteries to vasoactive compounds in the fetal and neonatal lamb: norepinephrine, epinephrine, serotonin, and potassium chloride. Pediatr Res 25: 360–363, 1989 [DOI] [PubMed] [Google Scholar]
- 51.Eddahibi S, Raffestin B, Pham I, Launay JM, Aegerter P, Sitbon M, and Adnot S. Treatment with 5-HT potentiates development of pulmonary hypertension in chronically hypoxic rats. Am J Physiol 272: H1173–H1181, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Fagan KA, Fouty BW, Tyler RC, Morris KG, Jr., Hepler LK, Sato K, LeCras TD, Abman SH, Weinberger HD, Huang PL, McMurtry IF, and Rodman DM. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Invest 103: 291–299, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fagan KA, Oka M, Bauer NR, Gebb SA, Ivy DD, Morris KG, and McMurtry IF. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol 287: L656–L664, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Farrell MR, Rogers LK, Liu Y, Welty SE, and Tipple TE. Thioredoxin-interacting protein inhibits hypoxia-inducible factor transcriptional activity. Free Radic Biol Med 49: 1361–1367, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farrow K, Wedgwood S, Lee K, Czech L, Gugino S, Lakshminrusimha S, Schumacker P, and Steinhorn R. Mitochondrial oxidant stress increases PDE5 activity in persistent pulmonary hypertension of the newborn. Respir Physiol Neurobiol 174: 272–281, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farrow KN, Fliman P, and Steinhorn RH. The diseases treated with ECMO: focus on PPHN. Semin Perinatol 29: 8–14, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Farrow KN, Groh BS, Schumacker PT, Lakshminrusimha S, Czech L, Gugino SF, Russell JA, and Steinhorn RH. Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells. Circ Res 102: 226–233, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farrow KN, Lakshminrusimha S, Czech L, Groh BS, Gugino S, Davis JM, Russell JA, and Steinhorn RH. SOD and inhaled nitric oxide normalize phosphodiesterase 5 expression and activity in neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 299: L109–L116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farrow KN, Lakshminrusimha S, Reda WJ, Wedgwood S, Czech L, Gugino SF, Davis JM, Russell JA, and Steinhorn RH. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L979–L987, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fike CD, Dikalova A, Slaughter JC, Kaplowitz MR, Zhang Y, and Aschner JL. Reactive oxygen species-reducing strategies improve pulmonary arterial responses to nitric oxide in piglets with chronic hypoxia-induced pulmonary hypertension. Antioxid Redox Signal 18: 1727–1738, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fike CD, Kaplowitz MR, Rehorst-Paea LA, and Nelin LD. L-Arginine increases nitric oxide production in isolated lungs of chronically hypoxic newborn pigs. J Appl Physiol 88: 1797–1803, 2000 [DOI] [PubMed] [Google Scholar]
- 62.Fike CD, Kaplowitz MR, Thomas CJ, and Nelin LD. Chronic hypoxia decreases nitric oxide production and endothelial nitric oxide synthase in newborn pig lungs. Am J Physiol 274: L517–L526, 1998 [DOI] [PubMed] [Google Scholar]
- 63.Fike CD, Slaughter JC, Kaplowitz MR, Zhang Y, and Aschner JL. Reactive oxygen species from NADPH oxidase contribute to altered pulmonary vascular responses in piglets with chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L881–L888, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fineman JR, Soifer SJ, and Heymann MA. Regulation of pulmonary vascular tone in the perinatal period. Ann Rev Physiol 57: 115–134, 1995 [DOI] [PubMed] [Google Scholar]
- 65.Fornaro E, Li D, Pan J, and Belik J. Prenatal exposure to fluoxetine induces fetal pulmonary hypertension in the rat. Am J Respir Crit Care Med 176: 1035–1040, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Fukai T, Folz RJ, Landmesser U, and Harrison DG. Extracellular superoxide dismutase and cardiovascular disease. Cardiovasc Res 55: 239–249, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Fukui T, Lassegue B, Kai H, Alexander RW, and Griendling KK. Cytochrome b-558 alpha-subunit cloning and expression in rat aortic smooth muscle cells. Biochim Biophys Acta 1231: 215–219, 1995 [DOI] [PubMed] [Google Scholar]
- 68.Furchgott RF. and Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J 3: 2007–2018, 1989 [PubMed] [Google Scholar]
- 69.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, and Ferrara N. VEGF is required for growth and survival in neonatal mice. Development 126: 1149–1159, 1999 [DOI] [PubMed] [Google Scholar]
- 70.Gien J, Seedorf GJ, Balasubramaniam V, Markham N, and Abman SH. Intrauterine pulmonary hypertension impairs angiogenesis in vitro: role of vascular endothelial growth factor nitric oxide signaling. Am J Respir Crit Care Med 176: 1146–1153, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gien J, Seedorf GJ, Balasubramaniam V, Tseng N, Markham N, and Abman SH. Chronic intrauterine pulmonary hypertension increases endothelial cell Rho kinase activity and impairs angiogenesis in vitro. Am J Physiol Lung Cell Mol Physiol 295: L680–L687, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gien J, Tseng N, Seedorf G, Roe G, and Abman SH. Endothelin-1 impairs angiogenesis in vitro through Rho-kinase activation after chronic intrauterine pulmonary hypertension in fetal sheep. Pediatr Res 73: 252–262, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Green DE, Murphy TC, Kang BY, Kleinhenz JM, Szyndralewiez C, Page P, Sutliff RL, and Hart CM. The Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation. Am J Respir Cell Mol Biol 47: 718–726, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grover TR, Parker TA, Zenge JP, Markham NE, Kinsella JP, and Abman SH. Intrauterine hypertension decreases lung VEGF expression and VEGF inhibition causes pulmonary hypertension in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 284: L508–L517, 2003 [DOI] [PubMed] [Google Scholar]
- 75.Guzy RD. and Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol 91: 807–819, 2006 [DOI] [PubMed] [Google Scholar]
- 76.Hall SM, Hislop AA, Pierce CM, and Haworth SG. Prenatal origins of human intrapulmonary arteries: formation and smooth muscle maturation. Am J Respir Cell Mol Biol 23: 194–203, 2000 [DOI] [PubMed] [Google Scholar]
- 77.Han RN, Babaei S, Robb M, Lee T, Ridsdale R, Ackerley C, Post M, and Stewart DJ. Defective lung vascular development and fatal respiratory distress in endothelial NO synthase-deficient mice: a model of alveolar capillary dysplasia? Circ Res 94: 1115–1123, 2004 [DOI] [PubMed] [Google Scholar]
- 78.Hassoun PM, Thappa V, Landman MJ, and Fanburg BL. Endothelin 1: mitogenic activity on pulmonary artery smooth muscle cells and release from hypoxic endothelial cells. Proc Exp Bio Med 199: 165–170, 1992 [DOI] [PubMed] [Google Scholar]
- 79.Haworth S. and Reid L. Persistent fetal circulation: newly recognized structural features. J Pediatr 88: 614–620, 1976 [DOI] [PubMed] [Google Scholar]
- 80.Haworth SG. Pulmonary vascular disease in different types of congenital heart disease:implications for interpretation of lung biopsy findings in early childhood. Br Heart J 52: 557–571, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heath D. and Edwards JE. The pathology of hypertensive pulmonary vascular disease: a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation 18: 533–547, 1958 [DOI] [PubMed] [Google Scholar]
- 82.Herrera EA, Pulgar VM, Riquelme RA, Sanhueza EM, Reyes RV, Ebensperger G, Parer JT, Valdez EA, Giussani DA, Blanco CE, Hanson MA, and Llanos AJ. High-altitude chronic hypoxia during gestation and after birth modifies cardiovascular responses in newborn sheep. Am J Physiol Regul Integr Comp Physiol 292: R2234–R2240, 2007 [DOI] [PubMed] [Google Scholar]
- 83.Hislop A, Haworth SG, and Reid L. Quantitative structural analysis of pulmonary vessels in isolated ventricular septal defects in infancy. Br Heart J 37: 1014–1021, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hislop AA. and Haworth SG. Pulmonary vascular damage and the development of cor pulmonale following hyaline membrane disease. Pediatr Pulmonol 9: 152–161, 1990 [DOI] [PubMed] [Google Scholar]
- 85.Ho YS, Xiong Y, Ma W, Spector A, and Ho DS. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J Biol Chem 279: 32804–32812, 2004 [DOI] [PubMed] [Google Scholar]
- 86.Hoffman JIE, Rudolph AM, and Heymann MA. Pulmonary vascular disease with congenital heart lesions: pathologic features and causes. Circulation 64: 873–877, 1981 [DOI] [PubMed] [Google Scholar]
- 87.Hosford GE. and Olson DM. Effects of hyperoxia on VEGF, its receptors, and HIF-2alpha in the newborn rat lung. Am J Physiol Lung Cell Mol Physiol 285: L161–L168, 2003 [DOI] [PubMed] [Google Scholar]
- 88.Ivy DD, Parker TA, Ziegler JW, Galan HL, Kinsella JP, Tuder RM, and Abman SH. Prolonged endothelin A receptor blockade attenuates chronic pulmonary hypertension in the ovine fetus. J Clin Invest 99: 1179–1186, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, and Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 279: L600–L607, 2000 [DOI] [PubMed] [Google Scholar]
- 90.Jin Y, Calvert TJ, Chen B, Chicoine LG, Joshi M, Bauer JA, Liu Y, and Nelin LD. Mice deficient in Mkp-1 develop more severe pulmonary hypertension and greater lung protein levels of arginase in response to chronic hypoxia. Am J Physiol Heart Circ Physiol 298: H1518–H1528, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kamezaki F, Tasaki H, Yamashita K, Tsutsui M, Koide S, Nakata S, Tanimoto A, Okazaki M, Sasaguri Y, Adachi T, and Otsuji Y. Gene transfer of extracellular superoxide dismutase ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med 177: 219–226, 2008 [DOI] [PubMed] [Google Scholar]
- 92.Keller RL, Tacy TA, Hendricks-Munoz K, Xu J, Moon-Grady AJ, Neuhaus J, Moore P, Nobuhara KK, Hawgood S, and Fineman JR. Congenital diaphragmatic hernia: endothelin-1, pulmonary hypertension, and disease severity. Am J Respir Crit Care Med 182: 555–561, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, and Mullen MP. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 120: 1260–1269, 2007 [DOI] [PubMed] [Google Scholar]
- 94.Khoo JP, Zhao L, Alp NJ, Bendall JK, Nicoli T, Rockett K, Wilkins MR, and Channon KM. Pivotal role for endothelial tetrahydrobiopterin in pulmonary hypertension. Circulation 111: 2126–2133, 2005 [DOI] [PubMed] [Google Scholar]
- 95.Kim YM, Barnes EA, Alvira CM, Ying L, Reddy S, and Cornfield DN. Hypoxia-inducible factor-1alpha in pulmonary artery smooth muscle cells lowers vascular tone by decreasing Myosin light chain phosphorylation. Circ Res 112: 1230–1233, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kimura S, Egashira K, Chen L, Nakano K, Iwata E, Miyagawa M, Tsujimoto H, Hara K, Morishita R, Sueishi K, Tominaga R, and Sunagawa K. Nanoparticle-mediated delivery of nuclear factor kappaB decoy into lungs ameliorates monocrotaline-induced pulmonary arterial hypertension. Hypertension 53: 877–883, 2009 [DOI] [PubMed] [Google Scholar]
- 97.Konduri G, Solimano A, Sokol G, Singer J, Ehrenkranz R, Singhal N, Wright L, Van Meurs K, Stork E, Kirpalani H, and Peliowski A. A randomized trial of early vs. standard inhaled nitric oxide therapy in term and near-term newborn infants with hypoxic respiratory failure. Pediatrics 113: 559–564, 2004 [DOI] [PubMed] [Google Scholar]
- 98.Koppel R, Han RN, Cox D, Tanswell AK, and Rabinovitch M. Alpha 1-antitrypsin protects neonatal rats from pulmonary vascular and parenchymal effects of oxygen toxicity. Pediatr Res 36: 763–770, 1994 [DOI] [PubMed] [Google Scholar]
- 99.Kumar P, Kazzi NJ, and Shankaran S. Plasma immunoreactive endothelin-1 concentrations in infants with persistent pulmonary hypertension of the newborn. Am J Perinatol 13: 335–341, 1996 [DOI] [PubMed] [Google Scholar]
- 100.Kuo CY. Endothelin-A receptor antagonist prevents neonatal pulmonary hypertension in meconium aspiration in piglets. J Formos Med Assoc 100: 420–423, 2001 [PubMed] [Google Scholar]
- 101.Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y, Sueishi K, and Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells 10: 1139–1151, 2005 [DOI] [PubMed] [Google Scholar]
- 102.Ladha F, Bonnet S, Eaton F, Hashimoto K, Korbutt G, and Thebaud B. Sildenafil improves alveolar growth and pulmonary hypertension in hyperoxia-induced lung injury. Am J Respir Crit Care Med 172: 750–756, 2005 [DOI] [PubMed] [Google Scholar]
- 103.Lakshminrusimha S, Russell J, Wedgwood S, Gugino S, Kazzaz J, Davis J, and Steinhorn RH. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med 174: 1370–1377, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lakshminrusimha S, Russell JA, Steinhorn RH, Swartz DD, Ryan RM, Gugino SF, Wynn KA, Kumar VH, Mathew B, Kirmani K, and Morin FC., 3rd.Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatr Res 62: 313–318, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lakshminrusimha S, Steinhorn RH, Wedgwood S, Savorgnan F, Nair J, Mathew B, Gugino SF, Russell JA, and Swartz DD. Pulmonary hemodynamics and vascular reactivity in asphyxiated term lambs resuscitated with 21 and 100% oxygen. J Appl Physiol 111: 1441–1447, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lakshminrusimha S, Swartz DD, Gugino SF, Ma CX, Wynn KA, Ryan RM, Russell JA, and Steinhorn RH. Oxygen concentration and pulmonary hemodynamics in newborn lambs with pulmonary hypertension. Pediatr Res 66: 539–544, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, and Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209., 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lassegue B. and Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285: R277–R297, 2003 [DOI] [PubMed] [Google Scholar]
- 109.Lassegue B. and Griendling KK. NADPH Oxidases: Functions and Pathologies in the Vasculature. Arterioscler Thromb Vasc Biol 30: 653–661, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lassegue B. and Griendling KK. Reactive oxygen species in hypertension; An update. Am J Hypertens 17: 852–860, 2004 [DOI] [PubMed] [Google Scholar]
- 111.Lassus P, Turanlahti M, Heikkila P, Andersson LC, Nupponen I, Sarnesto A, and Andersson S. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med 164: 1981–1987, 2001 [DOI] [PubMed] [Google Scholar]
- 112.Le Cras TD, Markham NE, Tuder RM, Voelkel NF, and Abman SH. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am J Physiol Lung Cell Mol Physiol 283: L555–L562, 2002 [DOI] [PubMed] [Google Scholar]
- 113.Li L, Shoji W, Takano H, Nishimura N, Aoki Y, Takahashi R, Goto S, Kaifu T, Takai T, and Obinata M. Increased susceptibility of MER5 (peroxiredoxin III) knockout mice to LPS-induced oxidative stress. Biochem Biophys Res Commun 355: 715–721, 2007 [DOI] [PubMed] [Google Scholar]
- 114.Lin YJ, Markham NE, Balasubramaniam V, Tang JR, Maxey A, Kinsella JP, and Abman SH. Inhaled nitric oxide enhances distal lung growth after exposure to hyperoxia in neonatal rats. Pediatr Res 58: 22–29, 2005 [DOI] [PubMed] [Google Scholar]
- 115.Liu J, Zelko I, Erbynn E, Sham J, and Folz R. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 290: L2–L10, 2006 [DOI] [PubMed] [Google Scholar]
- 116.Lopez E, Boucherat O, Franco-Montoya ML, Bourbon JR, Delacourt C, and Jarreau PH. Nitric oxide donor restores lung growth factor and receptor expression in hyperoxia-exposed rat pups. Am J Respir Cell Mol Biol 34: 738–745, 2006 [DOI] [PubMed] [Google Scholar]
- 117.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, and Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res 105: 249–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Madamanchi NR. and Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res 100: 460–473, 2007 [DOI] [PubMed] [Google Scholar]
- 119.Mamo LB, Suliman HB, Giles BL, Auten RL, Piantadosi CA, and Nozik-Grayck E. Discordant extracellular superoxide dismutase expression and activity in neonatal hyperoxic lung. Am J Respir Crit Care Med 170: 313–318, 2004 [DOI] [PubMed] [Google Scholar]
- 120.Manchester D, Margolis HS, and Sheldon RE. Possible association between maternal indomethacin therapy and primary pulmonary hypertension of the newborn. Am J Obstet Gynecol 126: 467–469, 1976 [DOI] [PubMed] [Google Scholar]
- 121.Manea A, Manea S, Gafencu A, and Raicu M. Regulation of NADPH oxidase subunit p22(phox) by NF-kB in human aortic smooth muscle cells. Arch Physiol Biochem 113: 163–172, 2007 [DOI] [PubMed] [Google Scholar]
- 122.Manea A, Tanase L, Raicu M, and Simionescu M. Transcriptional regulation of NADPH oxidase isoforms, Nox1 and Nox4, by nuclear factor-kappaB in human aortic smooth muscle cells. Biochem Biophy Res Commun 396: 901–907, 2010 [DOI] [PubMed] [Google Scholar]
- 123.Maniscalco WM, Watkins RH, D'Angio CT, and Ryan RM. Hyperoxic injury decreases alveolar epithelial cell expression of vascular endothelial growth factor (VEGF) in neonatal rabbit lung. Am J Respir Cell Mol Biol 16: 557–567, 1997 [DOI] [PubMed] [Google Scholar]
- 124.Masood A, Belcastro R, Li J, Kantores C, Jankov RP, and Tanswell AK. A peroxynitrite decomposition catalyst prevents 60% O2-mediated rat chronic neonatal lung injury. Free Radic Biol Med 49: 1182–1191, 2010 [DOI] [PubMed] [Google Scholar]
- 125.Masri F, Comhair S, Dostanic-Larson I, Kaneko F, Dweik R, Arroliga A, and Erzurum S. Deficiency of lung antioxidants in idiopathic pulmonary arterial hypertension. Clin Transl Sci 1: 99–106, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Meyrick B. and Reid L. Ultrastructural findings in lung biopsy material from children with congenital heart defects. Am J Pathol 101: 527–537, 1980 [PMC free article] [PubMed] [Google Scholar]
- 127.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, and Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 101: 258–267, 2007 [DOI] [PubMed] [Google Scholar]
- 128.Morin FC, III. Ligating the ductus arteriosus before birth causes persistent pulmonary hypertension in the newborn lamb. Pediatr Res 25: 245–250, 1989 [DOI] [PubMed] [Google Scholar]
- 129.Murphy JD, Vawter GF, and Reid LM. Pulmonary vascular disease in fatal meconium aspiration. J Pediatr 104: 758–762, 1984 [DOI] [PubMed] [Google Scholar]
- 130.Nakamura K, Koga Y, Sakai H, Homma K, and Ikebe M. cGMP-dependent relaxation of smooth muscle is coupled with the change in the phosphorylation of myosin phosphatase. Circ Res 101: 712–722, 2007 [DOI] [PubMed] [Google Scholar]
- 131.Neumann CA, Krause DS, Carman CV, Das S, Dubey DP, Abraham JL, Bronson RT, Fujiwara Y, Orkin SH, and Van Etten RA. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature 424: 561–565, 2003 [DOI] [PubMed] [Google Scholar]
- 132.Niermeyer S. Cardiopulmonary transition in the high altitude infant. High Alt Med Biol 4: 225–239, 2003 [DOI] [PubMed] [Google Scholar]
- 133.Nozik-Grayck E, Suliman H, Majka S, Albietz J, Van Rheen Z, Roush K, and Stenmark K. Lung EC-SOD overexpression attenuates hypoxic induction of Egr-1 and chronic hypoxic pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol 295: L422–L430, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Oury TD, Chang L, Marklund S, Day BJ, and Crapo JD. Immunocytochemical localization of extracellular superoxide dismutase in human lung. Lab Invest 70: 889–898, 1994 [PubMed] [Google Scholar]
- 135.Oury TD, Day BJ, and Crapo JD. Extracellular superoxide dismutase in vessels and airways of humans and baboons. Free Radic Biol Med 20: 957–965, 1996 [DOI] [PubMed] [Google Scholar]
- 136.Pan L, Fu JH, Xue XD, Xu W, Zhou P, and Wei B. Melatonin protects against oxidative damage in a neonatal rat model of bronchopulmonary dysplasia. World J Pediatr 5: 216–221, 2009 [DOI] [PubMed] [Google Scholar]
- 137.Parker TA, Roe G, Grover TR, and Abman SH. Rho kinase activation maintains high pulmonary vascular resistance in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 291: L976–L982, 2006 [DOI] [PubMed] [Google Scholar]
- 138.Patil S, Bunderson M, Wilham J, and Black SM. Important role for Rac1 in regulating reactive oxygen species generation and pulmonary arterial smooth muscle cell growth. Am J Physiol Lung Cell Mol Physiol 287: L1314–L1322, 2004 [DOI] [PubMed] [Google Scholar]
- 139.Perez M, Lakshminrusimha S, Wedgwood S, Czech L, Gugino SF, Russell JA, Farrow KN, and Steinhorn RH. Hydrocortisone normalizes oxygenation and cGMP regulation in lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 302: L595–L603, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Perreault T, Berkenbosch JW, Barrington KJ, Decker ER, Wu C, Brock TA, and Baribeau J. TBC3711, an ET(A) receptor antagonist, reduces neonatal hypoxia-induced pulmonary hypertension in piglets. Pediatr Res 50: 374–383, 2001 [DOI] [PubMed] [Google Scholar]
- 141.Perveen S, Patel H, Arif A, Younis S, Codipilly CN, and Ahmed M. Role of EC-SOD overexpression in preserving pulmonary angiogenesis inhibited by oxidative stress. PLoS One 7: e51945, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, and Gorlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal 8: 1473–1484, 2006 [DOI] [PubMed] [Google Scholar]
- 143.Qin Z, Reszka KJ, Fukai T, and Weintraub NL. Extracellular superoxide dismutase (ecSOD) in vascular biology: an update on exogenous gene transfer and endogenous regulators of ecSOD. Transl Res 151: 68–78, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rabinovitch M, Haworth SG, Castaneda AR, Nadas AS, and Reid LM. Lung biopsy in congenital heart disease: a morphometric approach to pulmonary vascular disease. Circulation 58: 1107–1122, 1978 [DOI] [PubMed] [Google Scholar]
- 145.Rajatapiti P, van der Horst IW, de Rooij JD, Tran MG, Maxwell PH, Tibboel D, Rottier R, and de Krijger RR. Expression of hypoxia-inducible factors in normal human lung development. Pediatr Dev Pathol 11: 193–199, 2008 [DOI] [PubMed] [Google Scholar]
- 146.Rao G. and Berk B. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res 18: 775–794, 1992 [DOI] [PubMed] [Google Scholar]
- 147.Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, and Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med 45: 1223–1231, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rhee SG, Chae HZ, and Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med 38: 1543–1552, 2005 [DOI] [PubMed] [Google Scholar]
- 149.Rhoades R, Packer C, Roepke D, Jin N, and Meiss R. Reactive oxygen species alter contractile properties of pulmonary arterial smooth muscle. Can J Physiol Pharmacol 68: 1581–1589, 1990 [DOI] [PubMed] [Google Scholar]
- 150.Robbins IM, Hemnes AR, Gibbs JS, Christman BW, Howard L, Meehan S, Cabrita I, Gonzalez R, Oyler T, Zhao L, Du RH, Mendes LA, and Wilkins MR. Safety of sapropterin dihydrochloride (6r-bh4) in patients with pulmonary hypertension. Exp Lung Res 37: 26–34, 2011 [DOI] [PubMed] [Google Scholar]
- 151.Rodriguez-Iturbe B, Sepassi L, Quiroz Y, Ni Z, Wallace DC, and Vaziri ND. Association of mitochondrial SOD deficiency with salt-sensitive hypertension and accelerated renal senescence. J Appl Physiol 102: 255–260, 2007 [DOI] [PubMed] [Google Scholar]
- 152.Rozance PJ, Seedorf GJ, Brown A, Roe G, O'Meara MC, Gien J, Tang JR, and Abman SH. Intrauterine growth restriction decreases pulmonary alveolar and vessel growth and causes pulmonary artery endothelial cell dysfunction in vitro in fetal sheep. Am J Physiol Lung Cell Mol Physiol 301: L860–L871, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Saugstad OD. Oxygen and oxidative stress in bronchopulmonary dysplasia. J Perinat Med 38: 571–577, 2010 [DOI] [PubMed] [Google Scholar]
- 154.Sawada H, Mitani Y, Maruyama J, Jiang B, Ikeyama Y, Dida F, Yamamoto H, Imanka-Yoshida K, Shimpo H, Mizoguchi A, Maruyama K, and Komada Y. A nuclear factor-kappaB inhibitor pyrrolidine dithiocarbamate ameliorates pulmonary hypertension in rats. Chest 132: 1265–1274, 2007 [DOI] [PubMed] [Google Scholar]
- 155.Schneider R, Raff U, Vornberger N, Schmidt M, Freund R, Reber M, Schramm L, Gambaryan S, Wanner C, Schmidt HH, and Galle J. L-Arginine counteracts nitric oxide deficiency and improves the recovery phase of ischemic acute renal failure in rats. Kidney Int 64: 216–225, 2003 [DOI] [PubMed] [Google Scholar]
- 156.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, and Krause K. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406: 105–114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shaul PW, Yuhanna IS, German Z, Chen Z, Steinhorn RH, and Morin FC, III. Pulmonary endothelial NO synthase gene expression is decreased in fetal lambs with pulmonary hypertension. Am J Physiol 272: L1005–L1012, 1997 [DOI] [PubMed] [Google Scholar]
- 158.Steinhorn RH. Neonatal pulmonary hypertension. Pediatr Crit Care Med 11: S79–S84, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stenmark KR, Yeager ME, El Kasmi KC, Nozik-Grayck E, Gerasimovskaya EV, Li M, Riddle SR, and Frid MG. The adventitia: essential regulator of vascular wall structure and function. Annu Rev Physiol 75: 23–47, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, and Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 290: L661–L673, 2006 [DOI] [PubMed] [Google Scholar]
- 161.Sud N, Wells SM, Sharma S, Wiseman DA, Wilham J, and Black SM. Asymmetric dimethylarginine inhibits HSP90 activity in pulmonary arterial endothelial cells: role of mitochondrial dysfunction. Am J Physiol Cell Physiol 294: C1407–C1418, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, and Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270: 296–299, 1995 [DOI] [PubMed] [Google Scholar]
- 163.Tang JR, Markham NE, Lin YJ, McMurtry IF, Maxey A, Kinsella JP, and Abman SH. Inhaled nitric oxide attenuates pulmonary hypertension and improves lung growth in infant rats after neonatal treatment with a VEGF receptor inhibitor. Am J Physiol Lung Cell Mol Physiol 287: L344–L351, 2004 [DOI] [PubMed] [Google Scholar]
- 164.Teng RJ, Wu TJ, Bisig CG, Eis A, Pritchard KA, and Konduri GG. Nitrotyrosine impairs angiogenesis and uncouples eNOS activity of pulmonary artery endothelial cells isolated from developing sheep lungs. Pediatr Res 69: 112–117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Thebaud B. and Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med 175: 978–985, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, and Archer SL. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation 112: 2477–2486, 2005 [DOI] [PubMed] [Google Scholar]
- 167.Tipple TE, Welty SE, Nelin LD, Hansen JM, and Rogers LK. Alterations of the thioredoxin system by hyperoxia: implications for alveolar development. Am J Respir Cell Mol Biol 41: 612–619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tourneux P, Chester M, Grover T, and Abman SH. Fasudil inhibits the myogenic response in the fetal pulmonary circulation. Am J Physiol Heart Circ Physiol 295: H1505–H1513, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Traenckner E, Wilk S, and Baeuerle P. A proteasome inhibitor prevents activation of NF-{kappa}B and stabilizes a newly phosphorylated form of I{kappa}B-{alpha} that is still bound to NF-{kappa}B. EMBO J 15: 5433–5441, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tripathi S. and Saili A. The effect of steroids on the clinical course and outcome of neonates with meconium aspiration syndrome. J Trop Pediatr 53: 8–12, 2007 [DOI] [PubMed] [Google Scholar]
- 171.Vadivel A, Aschner JL, Rey-Parra GJ, Magarik J, Zeng H, Summar M, Eaton F, and Thebaud B. L-citrulline attenuates arrested alveolar growth and pulmonary hypertension in oxygen-induced lung injury in newborn rats. Pediatr Res 68: 519–525, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Van Buul JD, Fernandez-Borja M, Anthony EC, and Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal 7: 308–317, 2005 [DOI] [PubMed] [Google Scholar]
- 173.Van Marter LJ, Leviton A, Allred EN, Pagano M, Sullivan KF, Cohen A, and Epstein MF. Persistent pulmonary hypertension of the newborn and smoking and aspirin and nonsteroidal antiinflammatory drug consumption during pregnancy. Pediatrics 97: 658–663, 1996 [PubMed] [Google Scholar]
- 174.Veit F, Pak O, Egemnazarov B, Roth M, Kosanovic D, Sommer N, Seimetz M, Ghofrani H, Seeger W, Grimminger F, Brandes RP, Schermuly R, and Weissmann N. Function of NADPH oxidase 1 in pulmonary arterial smooth muscle cells after monocrotaline-induced pulmonary vascular remodeling. Antioxid Redox Signal 19: 2213–2231, 2013 [DOI] [PubMed] [Google Scholar]
- 175.Villaneuva ME, Zaher FM, Svinarich DM, and Konduri GG. Decreased gene expression of endothelial nitric oxide synthase in newborns with persistent pulmonary hypertension. Pediatr Res 44: 338–343, 1998 [DOI] [PubMed] [Google Scholar]
- 176.Villanueva ME, Zaher FM, Svinarich DM, and Konduri GG. Decreased gene expression of endothelial nitric oxide synthase in newborns with persistent pulmonary hypertension. Pediatr Res 44: 338–343, 1998 [DOI] [PubMed] [Google Scholar]
- 177.Villegas LR, Kluck D, Field C, Oberley-Deegan RE, Woods C, Yeager ME, El Kasmi KC, Savani RC, Bowler RP, and Nozik-Grayck E. Superoxide dismutase mimetic, MnTE-2-PyP, attenuates chronic hypoxia-induced pulmonary hypertension, pulmonary vascular remodeling, and activation of the NALP3 inflammasome. Antioxid Redox Signal 18: 1753–1764, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wagenaar GT, Laghmani el H, de Visser YP, Sengers RM, Steendijk P, Baelde HJ, and Walther FJ. Ambrisentan reduces pulmonary arterial hypertension but does not stimulate alveolar and vascular development in neonatal rats with hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol 304: L264–L275, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang Z. and Chesler NC. Pulmonary vascular wall stiffness: an important contributor to the increased right ventricular afterload with pulmonary hypertension. Pulm Circ 1: 212–223, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Warner BB, Stuart LA, Papes RA, and Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol 275: L110–L117, 1998 [DOI] [PubMed] [Google Scholar]
- 181.Waypa G, Marks J, Guzy R, Mungai P, Schriewer J, Dokic D, and Schumacker P. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res 106: 526–535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Waypa GB, Marks JD, Guzy RD, Mungai PT, Schriewer JM, Dokic D, Ball MK, and Schumacker PT. Superoxide generated at mitochondrial complex III triggers acute responses to hypoxia in the pulmonary circulation. Am J Respir Crit Care Med 187: 424–432, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wedgwood S. and Black SM. Endothelin-1 decreases endothelial NOS expression and activity through ETA receptor-mediated generation of hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol 288: L480–L487, 2005 [DOI] [PubMed] [Google Scholar]
- 184.Wedgwood S. and Black SM. Induction of apoptosis in fetal pulmonary arterial smooth muscle cells by a combined superoxide dismutase/catalase mimetic. Am J Physiol Lung Cell Mol Physiol 285: L305–L312, 2003 [DOI] [PubMed] [Google Scholar]
- 185.Wedgwood S, Dettman RW, and Black SM. ET-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am J Physiol Lung Cell Mol Physiol 281: L1058–L1067, 2001 [DOI] [PubMed] [Google Scholar]
- 186.Wedgwood S, DeVol J, Grobe A, Benavidez E, Azakie A, Fineman J, and Black S. Fibroblast growth factor-2 expression is altered in lambs with increased pulmonary blood flow and pulmonary hypertension. Pediatr Res 61: 32–36, 2007 [DOI] [PubMed] [Google Scholar]
- 187.Wedgwood S, Lakshminrusimha S, Czech L, Schumacker PT, and Steinhorn RH. Increased p22phox/Nox4 expression triggers remodeling through hydrogen peroxide signaling in persistent pulmonary hypertension of the newborn. Antioxid Redox Signal 18: 1765–1776, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wedgwood S, Lakshminrusimha S, Farrow KN, Czech L, Gugino SF, Soares F, Russell JA, and Steinhorn RH. Apocynin improves oxygenation and increases eNOS in persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 302: L616–L626, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wedgwood S, Lakshminrusimha S, Fukai T, Russell J, Schumacker P, and Steinhorn R. Hydrogen peroxide regulates extracellular superoxide dismutase activity and expression in neonatal pulmonary hypertension. Antioxid Redox Signal 15: 1497–1506, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wedgwood S, McMullan DM, Bekker JM, Fineman JR, and Black SM. Role for endothelin-1-induced superoxide and peroxynitrite production in rebound pulmonary hypertension associated with inhaled nitric oxide therapy. Circ Res 89: 357–364, 2001 [DOI] [PubMed] [Google Scholar]
- 191.Wedgwood S, Steinhorn RH, Bunderson M, Wilham J, Lakshminrusimha S, Brennan LA, and Black SM. Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 289: L660–L666, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wiklund NP, Persson MG, Gustafsson LE, Moncada S, and Hedqvist P. Modulatory role of endogenous nitric oxide in pulmonary circulation in vivo. Eur J Pharmacol 185: 123–124, 1990 [DOI] [PubMed] [Google Scholar]
- 193.Wild LM, Nickerson PA, and Morin FC, III. Ligating the ductus arteriosus before birth remodels the pulmonary vasculature of the lamb. Pediatr Res 25: 251–257, 1989 [DOI] [PubMed] [Google Scholar]
- 194.Wong CM, Preston IR, Hill NS, and Suzuki YJ. Iron chelation inhibits the development of pulmonary vascular remodeling. Free Radic Biol Med 53: 1738–1747, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wright CJ, Zhuang T, La P, Yang G, and Dennery PA. Hyperoxia-induced NF-kappaB activation occurs via a maturationally sensitive atypical pathway. Am J Physiol Lung Cell Mol Physiol 296: L296–L306, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, and Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415, 1988 [DOI] [PubMed] [Google Scholar]
- 197.Yeh JL, Hsu JH, Dai ZK, Liou SF, Chen IJ, and Wu JR. Increased circulating big endothelin-1, endothelin-1 and atrial natriuretic peptide in infants and children with heart failure secondary to congenital heart disease. Int J Cardiol 104: 15–20, 2005 [DOI] [PubMed] [Google Scholar]