Abstract
Aims: The aims of this study were to determine hyperoxia effects on S-nitrosothiol (SNO) accumulation and L-type amino acid transporter 1 (LAT1) expression/function in alveolar epithelium and to determine whether hyperoxia impairs exogenous nitric oxide (NO) treatment effects in alveolar epithelium through effects on LAT1 expression and/or function. Results: SNO accumulation in vitro and in vivo after NO treatment was dependent on the LAT1 system transport. Hyperoxia (60% or 90%) impaired NO effects on SNO accumulation and soluble guanylyl cyclase activation in proportion to the magnitude of hyperoxia and the duration of exposure, up to 12 h, in type I-like (R3/1) and type II-like (L2) rat and human (A549) alveolar epithelial cells. LAT function, determined by sodium-independent 3H-leucine uptake, was impaired in a parallel manner. Hyperoxia impaired LAT1 expression in alveolar epithelial cells, determined by immunoblots and immunofluorescence, and in newborn rats exposed to 60% O2 for 4 days, determined by immunohistochemistry. Innovation: Despite significant preclinical evidence, inhaled NO has shown disappointing limitations in clinical applications. Our studies suggest an important explanation: oxidative stress, a common feature of diseases in which therapeutic NO would be considered, impairs LAT1 expression and function, blocking a major route for inhaled NO (iNO) action, that is, the uptake of S-nitrosocysteine via LAT1. Conclusions: SNO uptake after NO treatment is dependent on LAT1. Hyperoxia impairs SNO uptake and NO effects during NO exposure and impairs LAT system function and LAT1 expression. Effects on SNO formation and transport must be considered for rational optimization of NO-based therapeutics. Antioxid. Redox Signal. 21, 1823–1836.
Introduction
Inhaled nitric oxide (iNO) therapy is used in the treatment of pulmonary hypertension, particularly persistent pulmonary hypertension of the newborn, because it is able to activate soluble guanylyl cyclase (sGC), which, in turn, mobilizes calcium in pulmonary vascular smooth muscle, lowering pulmonary vascular resistance (14). However, iNO therapy is ineffective for this clinical condition in as many as 20% of cases for reasons that are poorly understood (11, 25), which may include oxidation of NO to peroxynitrite, since cotreatment with superoxide dismutase augmented therapeutic NO effects (24). Clinical trials of iNO for the prevention of bronchopulmonary dysplasia have yielded mixed results, despite very promising preclinical evidence (9).
Innovation.
Despite significant preclinical evidence, inhaled nitric oxide (iNO) has shown disappointing limitations in clinical applications. Our studies suggest an important explanation: oxidative stress, a common feature of diseases in which therapeutic NO would be considered, impairs L-type amino acid transporter 1 (LAT1) expression and function, blocking a major route for iNO action, that is, uptake of S-nitrosocysteine via LAT1. Defects in SNO formation and transport via LAT1 must be considered for rational optimization of NO-based therapeutics.
Although NO gas may readily diffuse across lipid boundaries (18), the path across the alveolus is more complex. Recent studies suggest that a substantial amount of NO-based signaling depends on the transport of S-nitrosothiols, in particular S-nitrosocystiene, via the L-type amino acid transporter (LAT) system (19, 30), also termed solute carrier family 7 member A5 (SLC7A5), which specifies LAT1, or member A6 (SLC7A6), which specifies LAT2. NO gas exposure effects on sGC activation and S-nitrosothiol (NO) accumulation in alveolar epithelial cells in vitro are predominantly LAT-dependent, rather than diffusion-dependent (6, 12). Theoretically, SNO uptake could also take place by importation of S-nitrosocysteinylglycine via dipeptide transporter 2 (PEPT2). Cysteinylglycine could be produced by metabolism of extracellular glutathione by γ-glutamyltranspeptidase, which is expressed in alveolar epithelium, providing substrate for S-nitrosocysteinylglycine formation (10). However, our in vitro studies have shown this route to be relatively minor compared with LAT transport in cells of the pulmonary alveolus (6, 7, 12).
It therefore follows that disease states could theoretically affect iNO efficacy through alterations in SNO uptake through the LAT system. Clinical conditions for which iNO would be prescribed—pulmonary hypertension (1) and prevention of bronchopulmonary dysplasia—are typically accompanied by oxidative stress, which could affect SNO formation and therefore uptake through oxidation of NO to higher nitrogen oxides, or cysteine to cystine. We hypothesized that oxidative stress could also impair SNO uptake through direct effects on LAT expression, structure, or function. To test the hypothesis that oxidative stress can alter SNO uptake through effects on LAT transport during NO administration, we exposed rat type I-like (R3/1) and type II-like (L2) alveolar epithelial cells, and human A549 alveolar epithelial cells to NO gas and tested the effects of hyperoxia exposure on SNO accumulation and activation of sGC, two important signaling pathways for NO. We determined the effects of hyperoxia on LAT1-dependent uptake of 3H-Leu. To determine if the functional defects we observed in vitro were potentially attributable to changes in transporter expression, we measured the effects of hyperoxia exposure on LAT1 expression in vitro and in vivo in newborn rats.
Results
Effects of hyperoxia on oxidative stress measurements after NO treatment of alveolar epithelial cells
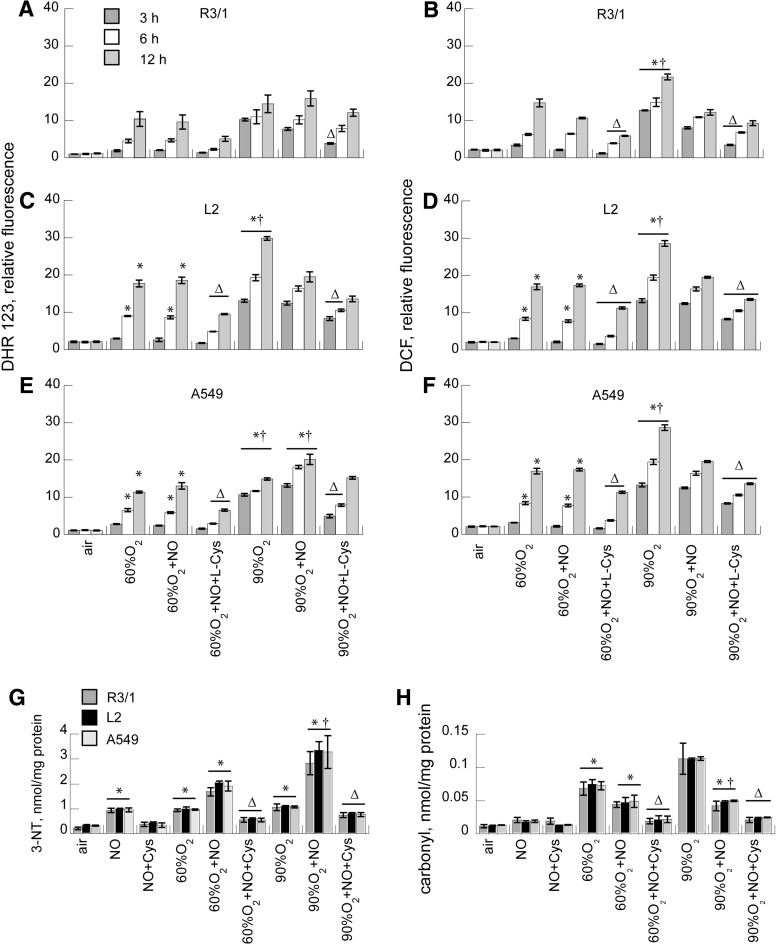
To compare the magnitude of nitrosative stress under basal conditions with those exerted by the differing concentrations of oxygen±NO treatment on R3/1, L2, and A549 cells, we measured DHR fluorescence in air- and oxygen-exposed cells under basal conditions and at 3, 6, and12 h after exposure to air or oxygen followed by treatment with NO 20 ppm±L-Cys×30 min (Fig. 1). As duration of hyperoxia exposure increased, we observed increasing DHR fluorescence (90% >60%) for all three cell types. The addition of NO for 30 min did not affect 60% O2-induced DHR fluorescence but did increase 90% O2-induced fluorescence in A549 cells. R3/1 cells were unaffected, but NO actually reduced DHR fluorescence in L2 cells. The addition of L-Cys (0.1 M) partly prevented the hyperoxia-induced rise in DHR in most cases, presumably because NO contributed to SNO rather than peroxynitrite formation and due to the antioxidant effects of added thiol. Combined hyperoxia and NO increased 3-nitrotyrosine (3-NT) above air control, with the greatest concentrations observed with 90% O2+NO. As in the case of DHR, the addition of L-Cys diminished this effect.
FIG. 1.
Effect of hyperoxia on ROS/RNS accumulation in NO-treated alveolar epithelial cells. DHR123 (A, C, E) and DCF (B, D, F) fluorescence in response to hyperoxia±NO 20 ppm,±L-Cys 0.1 mM in R3/1 (A, B), L2 (C, D), and A549 (E, F) cells. Data are normalized to cell number estimated using Hoechst 33342. Results are mean of 9±SEM. Effect of hyperoxia×12 h±NO±L-Cys on 3-nitrotyrosine (G), and protein carbonyls (H), normalized to cellular protein. Results are mean of 3±SEM. *p<0.05 versus corresponding air, †p<0.05 versus 60% O2, Δp<0.05 versus corresponding group without L-Cys. DCF, dichlorofluorescein; NO, nitric oxide.
To measure the effects on oxidative stress, dichlorofluorescein (DCF) fluorescence was measured in the same conditions. The effects were essentially parallel to those observed on DHR fluorescence in all three alveolar epithelial cell types. Adding L-Cys partly prevented the effects of hyperoxia on DCF fluorescence. Protein oxidation, estimated by measuring protein carbonyls, was increased by hyperoxia, and the addition of NO gas did not further increase this. Instead, NO diminished the protein oxidation. Adding L-Cys further diminished protein oxidation.
NO gas effects on SNO uptake and sGC activation are LAT1-dependent
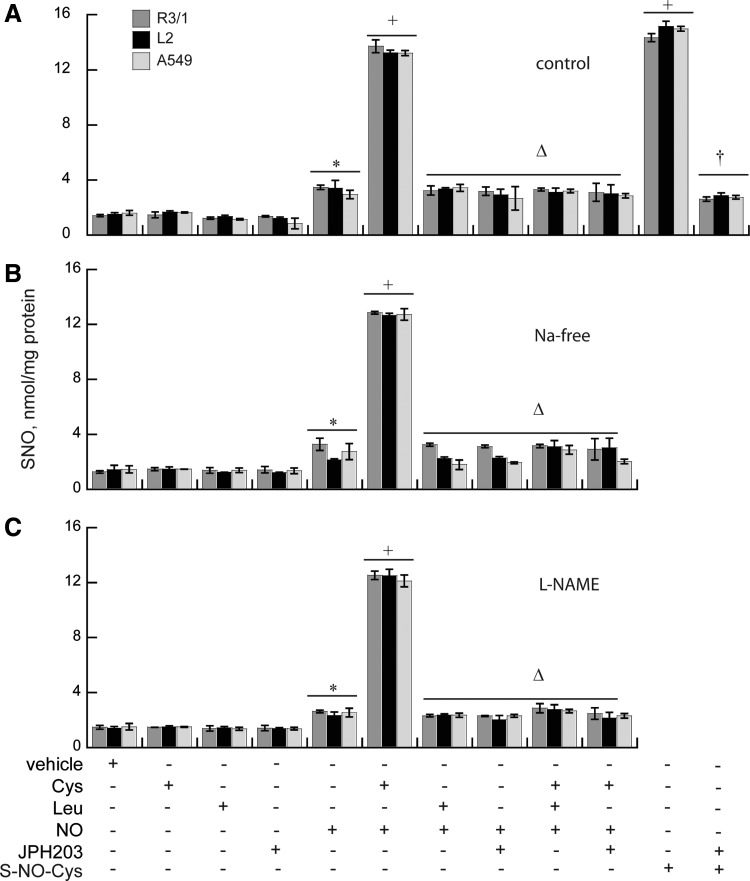
NO gas treatment at air–liquid interface roughly doubled the basal SNO accumulation found in untreated cells R3/1, L2, and A549 cells under basal conditions, but the addition of L-Cys increased SNO ∼12-fold (Fig. 2). NO gas treatment effects on SNO uptake were abolished in the presence of LAT1 inhibitor JPH203 10 nM (28) and comparably inhibited by competition with Leu 5 mM. These effects were entirely system L-dependent since identical results were found when choline chloride was substituted for NaCl in the incubation medium. Endogenously produced NO did not appear to contribute: there were no significant differences observed in cells preincubated with L-NAME. SNO concentrations were unaffected by the addition of Leu, JPH203, or Cys alone.
FIG. 2.
LAT1-dependent SNO uptake in vitro. (A) Effects of NO or S-NO-Cys on basal SNO uptake by R3/1, L2, and A549 cells±0.1 mM L-Cys, 5 mM Leu, or 10 nM JPH203. (B) Experiments repeated in the absence of sodium in the buffer or (C) in the presence of L-NAME. Results are mean of 3–6/group±SEM. *p<0.05 versus air, +p<0.05 versus NO, Δp<0.05 versus NO+L-Cys, †p<0.05 versus S-NO-Cys. LAT1, L-type amino acid transporter 1; SNO, S-nitrosothiol.
JPH203 did not exhibit off-target effects when screened using a battery of radioligand binding assays. In no case did the use of JPH203 at 10 mM achieve 50% inhibition (or stimulation) of activity (Table 1). Since we used JPH203 at 50 nM, which is 200-fold less than that used in the screening battery, it is very unlikely there was off-target signaling that would confound interpretation of the results.
Table 1.
JPH203 Radioligand Binding Screening Results
| Target | % inhibition | % stimulation | Species |
|---|---|---|---|
| Adenosine A1 | 37 | Human | |
| Adenosine A2A | 15 | Human | |
| α2A adrenergic | 2 | Human | |
| β1 adrenergic | 10 | Human | |
| β2 adrenergic | 2 | Human | |
| α1A adrenergic | 3 | Rat | |
| α1B adrenergic | 3 | Rat | |
| L-type Ca channel | 2 | Human | |
| Cannabinoid CB1 | 5 | Human | |
| Dopamine D1 | 9 | Human | |
| Dopamine D25 | 8 | Human | |
| GABAA flunitrazepam | 35 | Rat | |
| GABAA muscimol | 1 | Rat | |
| NMDA | 3 | Rat | |
| Histamine H1 | 18 | Human | |
| Imidazoline I2 | 13 | Rat | |
| Opiate, μ | 6 | Human | |
| Phorbol ester | 3 | Mouse | |
| Potassium channel [KATP] | 11 | Human | |
| Potassium channel hERG | 3 | Human | |
| Prostanoid EP4 | 6 | Human | |
| Rolipram | 27 | Rat | |
| Serotonin (5-HT2B) | 1 | Human | |
| Sigma | 19 | Human | |
| Sodium channel, site 2 | 25 | Rat | |
| Norepinephrine transporter | 20 | Human |
Inhaled NO effects on SNO uptake in vivo±LAT1 inhibition
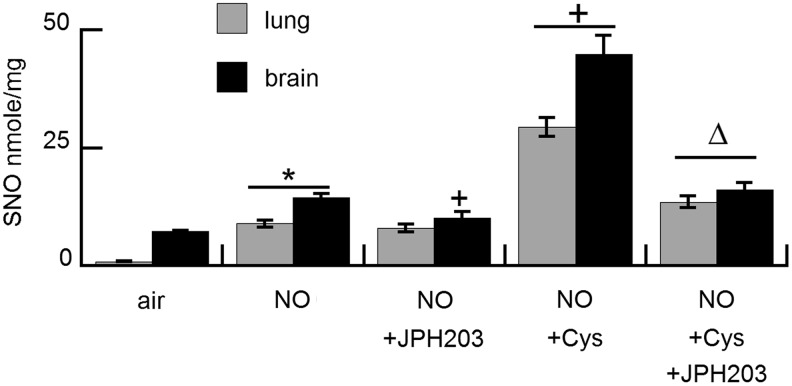
These in vitro findings were confirmed in vivo. Treatment with NO gas 20 ppm×2 h increased SNO accumulation in both the lungs and brain (Fig. 3). SNO accumulation in the brain but not in the lungs was partly inhibited by pretreatment with LAT1 inhibitor JPH203. Pretreatment with nebulized L-cysteine significantly augmented SNO accumulation in both the lungs and brain. This effect was significantly decreased by pretreatment with intravenous JPH203 just before the treatment with nebulized cysteine.
FIG. 3.
LAT1-dependent SNO uptake in vivo. Effect of inhaled NO on SNO uptake in the lungs and brain in adult rats±pretreatment with nebulized 10 mM L-Cys±pretreatment with JPH203. Results are mean of four animals/treatment group±SEM. *p<0.05 versus air, +p<0.05 versus NO, Δp<0.05 versus NO+Cys.
LAT1 siRNA knockdown effect on LAT1 function and SNO uptake
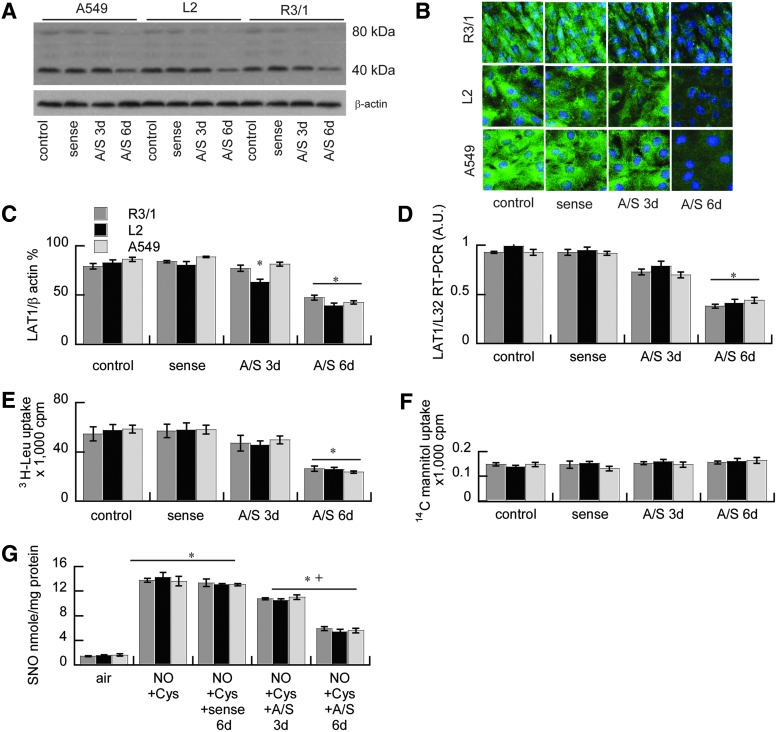
The LAT1 dependence of SNO uptake and Na-independent 3H-Leu uptake in alveolar epithelium was confirmed using adenoviral-mediated LAT1 siRNA knockdown. This suppressed LAT1 mRNA expression in R3/1, L2, and A549 cells 3 and 6 days after infection, determined by real-time quantitative polymerase chain reaction (qRT-PCR), immunoblot, and immunocytochemistry compared with cells infected with the adenoviral expressing the sense sequence (Fig. 4). Cells in which LAT1 was knocked down showed suppression of Na-independent 3H-Leu uptake and SNO uptake after the treatment with NO in the presence of L-Cys, confirming that NO effects on SNO uptake are LAT1-dependent. There were no effects of adenovirus on 14C-mannitol uptake (Fig. 4) or viability determined by trypan blue exclusion (data not shown).
FIG. 4.
LAT1 siRNA knockdown effect on LAT1 function and SNO uptake. LAT1 expression in A549, L2, and R3/1 alveolar epithelial cells 3 or 6 days after adenoviral siRNA (anti-sense, A/S) knockdown versus sense control, determined by (A) immunoblot and (B) immunofluorescence. (C) Immunoblot quantified by densitometry. Data are the mean of four independent experiments±SEM. *p<0.05 versus sense. (D) Effects of LAT1 siRNA knockdown on LAT1 mRNA normalized to L32. Data are mean of three independent experiments±SEM, *p<0.05 versus sense. (E) Effects of knockdown on Na-independent 3H-Leu uptake, and (F)14C mannitol control, mean of six independent experiments±SEM. *p<0.05 versus sense. (G) Effects of knockdown on SNO uptake in R3/1, L2, and A549 cells after NO exposure. The air-exposed controls are the same data depicted in Figure 2. Data are mean of five independent experiments±SEM. *p<0.05 versus air, +p<0.05 versus sense. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
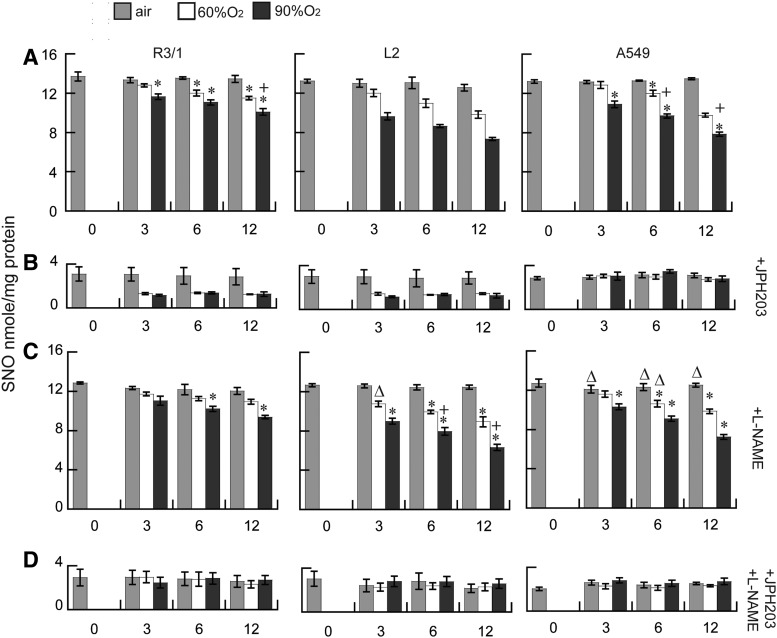
Effect of hyperoxia on NO-induced SNO uptake via LAT1
Exposure to hyperoxia decreased SNO accumulation after NO treatment in the presence of L-Cys, in all three alveolar epithelial cell types (Fig. 5). This effect was greater after longer durations (6 and 12 h) and the higher concentration (90%) of hyperoxia exposure. Under all conditions, SNO accumulation was largely prevented in the presence of 10 nM JPH203 (or 5 mM Leu, data not shown). To ensure that observed decreases of intracellular SNO accumulation could not be attributable to depletion of extracellular SNO, we compared the SNO concentrations in the apical buffer and the cell lysates after hyperoxia. As expected, extracellular SNO concentrations were ∼1000-fold higher than intracellular concentration. The extracellular SNO concentrations under basal conditions were between 39.9±1.5 and 41.0+2.5 μM, whereas the intracellular basal SNO concentrations were 58.0±1.3 to 60±2.2 nM (n=6/group±SEM). The maximum hyperoxia (90%×12 h) exposure produced the most severe decrement in extracellular SNO, but this was still 49% of the baseline air-exposed concentration, so hyperoxia is unlikely to have limited SNO uptake in these conditions.
FIG. 5.
Effects of hyperoxia on NO-induced SNO uptake via LAT1. (A) Effects of 60% or 90% O2 exposure up to 12 h on NO treatment induced SNO accumulation in R3/1, L2, and A549 cells, (B) with the addition of 10 nM JPH203, (C) L-NAME, or (D) both. Results are mean of 6/group±SEM. *p<0.05 versus air, +p<0.05 versus corresponding 60% O2, Δp<0.05 added L-NAME versus corresponding treatment.
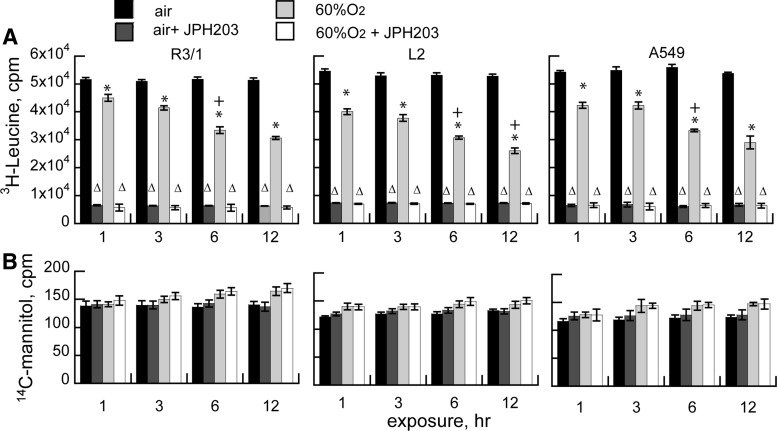
Effects of hyperoxia on LAT1 function in vitro
Effects of hyperoxia on LAT transporter-dependent amino acid uptake were assessed by culturing R3/1, L2, and A549 cells in the presence of 3H-leucine, substituting choline for sodium in the incubation buffers to verify the LAT dependence (8). We found that 3H-leucine uptake was inhibited by hyperoxia in a time- and concentration-dependent manner (Fig. 6). Uptake was largely via LAT1, rather than LAT2, since it was almost completely inhibited by JPH203, a highly selective potent LAT1 competitor (28). There were no significant effects of any of the treatment conditions on cellular permeability assessed by 14C-mannitol exclusion.
FIG. 6.
Effects of hyperoxia on LAT function in vitro. (A) System-L transport-dependent 3H-Leu uptake±JPH203 by R3/1, L2, and A549 cells after exposure to air versus 60% O2, 1–12 h. Results are mean of 7 for R3/1 and L2 cells and mean of 4 for A549 cells±SEM. *p<0.05 versus air, +p<0.05 versus 3 h, Δp<0.05 versus corresponding “no inhibitor” group. (B) 14C mannitol uptake in the same conditions.
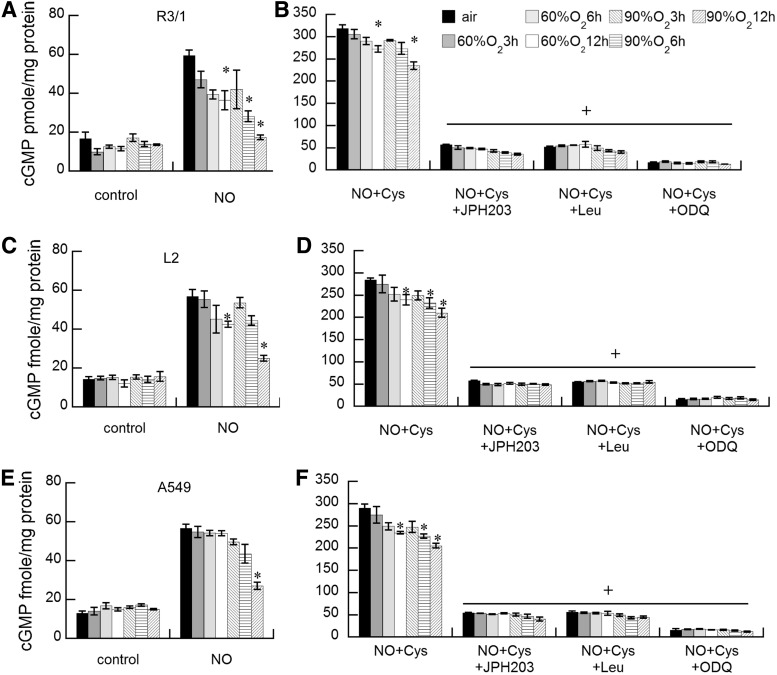
Effects of hyperoxia on LAT1-dependent NO-induced sGC
Effects of NO gas±hyperoxia on sGC activation estimated by cGMP quantification in R3/1, L2, andA549 cells were essentially parallel to the effects on SNO accumulation (Fig. 7). Under basal conditions, NO stimulated sGC in all three cell types, producing a three-fold rise in cGMP, but this was suppressed in the presence of hyperoxia. With the addition of L-Cys, NO-induced sGC activity increased ∼15-fold. Hyperoxia exposure suppressed NO-induced cGMP in all three cell types to a similar extent in a time- and concentration-dependent manner. In all cases, the accumulation was inhibited by 1-H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and therefore sGC-dependent. The addition of JPH203 suppressed the NO-induced sGC activity by >80% in all three cell types, consistent with dependence on LAT1 for the NO effect on sGC.
FIG. 7.
Effects of hyperoxia on LAT1-dependent NO-induced soluble guanylyl cyclase. NO induction of cGMP accumulation after air or hyperoxia exposure in R3/1 (A, B) L2 (C, D), and A549 (E, F) cells in the absence (A, C, E) or presence (B, D, F) of L-Cys. Note different ordinate scales. Results are mean of 3–5±SEM, *p<0.05 versus air, +p<0.05 versus NO+Cys. cGMP, cyclic guanosine monophosphate.
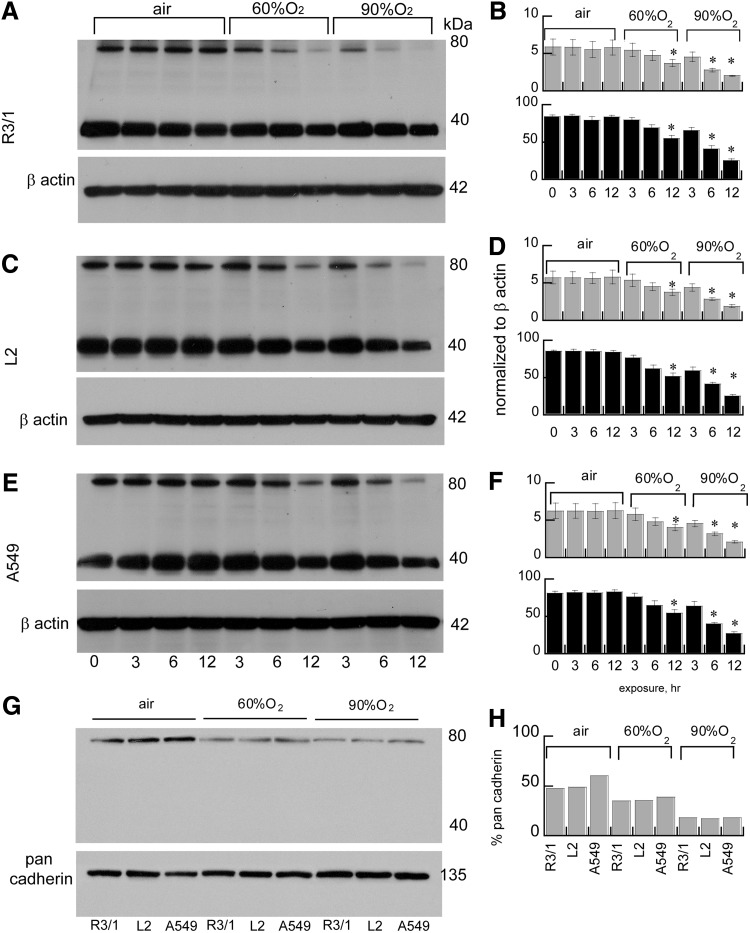
Effects of hyperoxia on LAT1 expression in alveolar epithelium
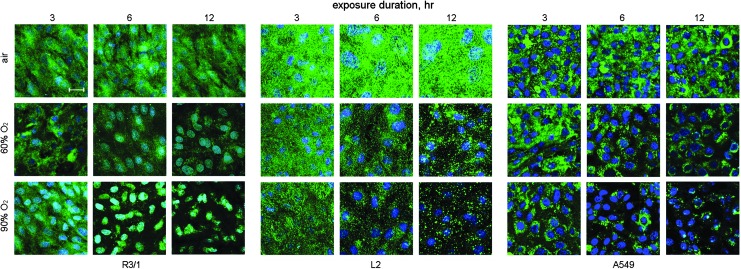
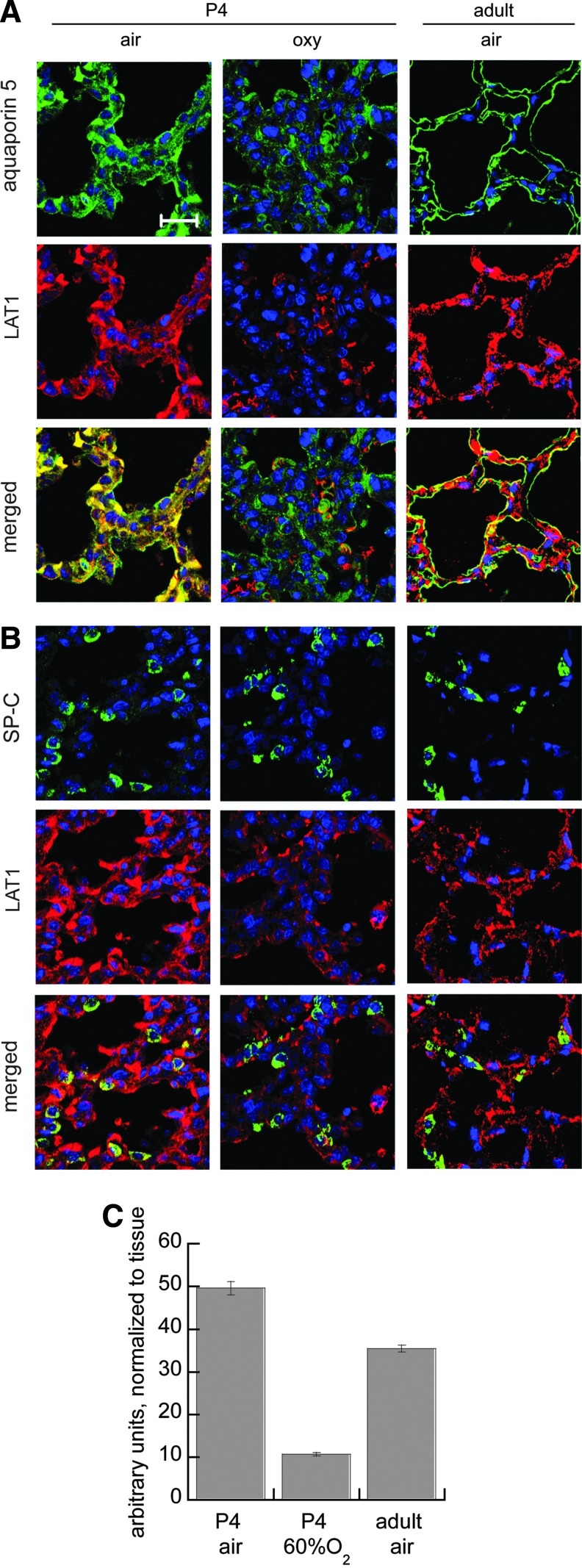
Hyperoxia (60% or 90% up to 12 h) suppressed LAT1 expression in rat type I-like (R3/1 cells), type II-like (L2 cells), and human type II-like A549 alveolar epithelial cell lysates detected by immunoblot. LAT1 was detected in whole cell lysates predominantly as the reduced light chain ∼40 kDa, but we also observed a form at 80 kDa. In the membrane preparations, only this heavier form was observed (Fig. 8). The effects on abundance detected by immunoblot are consistent with the immunocytochemistry and immunohistochemistry results (Figs. 9 and 10), both of which show diminished and ectopic LAT1 expression. Loss of expression was not attributable to cell death since there were no acute effects on viability determined by trypan blue exclusion (data not shown). In vivo, we found that LAT1 expression colocalized to both type I (aquaporin-5-positive) and type II (pro-SP-C-positive) cells in neonatal and adult rats under basal conditions. Similar to the effects in vitro, we found that LAT1 expression in alveolar epithelium was also suppressed in newborn rat lungs obtained from pups exposed to 60% O2 for 4 days compared with air-exposed pups. Loss of colocalization of LAT1 with aquaporin 5 in apical membrane was apparent at postnatal day 4. Studies were performed in four animals/treatment group, and representative confocal images are shown in Figure 10. Quantification of random images confirmed that hyperoxia exposure suppressed LAT1 expression in P4 rat alveolar septal tissue. LAT1 fluorescence was normalized to DAPI fluorescence as an estimate of tissue area, shown in Figure 10.
FIG. 8.
Effects of hyperoxia on LAT1 expression in alveolar epithelial cells in vitro. Effects on LAT1 accumulation detected by immunoblot in whole cell lysates (A–F, 90 μg/lane) and plasma membrane fractions (G, H, 30 μg/lane) in R3/1, L2, and A549 cells. Representative immunoblots and densitometry for R3/1 (A, B) L2 (C, D), and A549 (E, F) cells after exposure to air or hyperoxia (60% or 90%). Densitometry analysis of LAT1 expression (80 kDa bands, gray bars; 40 kDa bands, black bars) normalized to β actin or pan-cadherin, mean of 4–5±SEM for whole cell lysates. Data for plasma membrane fractions are pooled from 4 plates. *p<0.05 versus corresponding air control.
FIG. 9.
Hyperoxia effects on spatial LAT1 expression. LAT1 immunofluorescence in R3/1, L2, and A549 cells, scale bar=10 μm, counterstained with DAPI. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 10.
Effects of 60% O2×4 days on LAT1 expression in newborn rat lungs. LAT1 (red) colocalizes (merged) with (A) aquaporin 5 (green), which identifies type I alveolar epithelium and (B) SP-C (green), which identifies type II alveolar epithelium. Nuclei are counterstained with DAPI (blue). LAT1 expression in alveolar epithelium is readily identified in air exposed P4 and adult rat lungs but is decreased in hyperoxia-exposed (oxy) P4 rat lungs, scale bar=20 μm. (C) Image analysis comparison of pulmonary alveolar LAT1 expression normalized to alveolar tissue from P4 rats exposed to air versus 60% O2, and air-exposed adult controls. Data are mean of four to six animals/treatment group±SEM, *p<0.05 versus air.
Discussion
Impaired efficacy of iNO treatment has been linked with the formation of higher-order nitrogen oxides in a number of experimental models, but our present studies suggest a previously unexplored hurdle: impaired transport of SNO across LAT1. Cellular uptake of S-nitrosocysteine is dependent on the LAT system (19, 21, 23, 30). We have previously shown that the effects of NO gas on pulmonary vascular resistance depend on S-nitrosocysteine transport via the LAT system ex vivo, an effect previously thought to be largely dependent on NO diffusion (26). Our earlier studies showed that NO gas pharmacologic effects on pulmonary alveolar cells were dependent on SNO uptake via the LAT system (6, 7) rather than simply via diffusion. Using the newly developed LAT1 inhibitor JPH203 and LAT1 siRNA, we now show that LAT1 is the member of the system-L transporter family, which conveys SNO into alveolar epithelium during NO treatment in vitro. We confirmed this in vivo showing augmentation of SNO uptake in the lungs and brain after inhaled NO treatment in animals pretreated with L-Cys that is LAT1-dependent. We show that oxidative stress can alter the LAT function in vitro and LAT1 expression in vitro and in vivo, which likely contributes to impaired NO pharmacologic effects.
Members of the system-L amino acid transporter family (SLC7A5, 6, 7, and 8) are high-affinity heterodimeric transmembrane transporters of neutral amino acids, which can convey cysteine (27). Transporters in this group differ in amino acid affinity/specificity depending on the structure of the lighter subunit. We have previously shown that LAT1 (SLC7A5) is expressed in rat alveolar epithelium (12) and mouse alveolar epithelium as well as pulmonary vascular (smooth muscle and endothelial) cells (26). LAT2 (SLC7A6) expression in the pulmonary alveolus appeared to be confined to type II epithelium in mice, although it was also found to be expressed in vascular cells (28). To determine the relative contribution of LAT1 to SNO uptake, we used a recently developed, highly potent (half-maximal inhibitory concentration [IC50] ∼60 nM), highly selective antagonist to LAT1, JPH-203 (28). We found that SNO uptake, Na-independent 3H-Leu uptake, and NO-mediated sGC activation were completely blocked in alveolar epithelial cells, suggesting the primacy of LAT1 (SLC7A5) over other system L (SLC7A6-8) family members for S-nitrosocysteine uptake in alveolar epithelium. The dependence of NO on LAT1 for effects on SNO uptake in alveolar epithelial cells was confirmed using siRNA knockdown of LAT1. Assessments of sGC activity were limited to the ODQ inhibitable cGMP accumulation. While ODQ may have off-target effects, it would be unlikely at the 0.5 μm concentration we used.
Because the effects of NO gas treatment on SNO uptake and sGC activation were dependent on LAT1-mediated transport, we focused our studies on LAT1 expression. Although we observed PEPT2-dependent SNO uptake by alveolar epithelial cells (data not shown), the magnitude was relatively small compared with uptake via LAT1, consistent with our earlier studies that showed the LAT system to predominate over PEPT2 for SNO uptake (7, 12).
Since hyperoxia exposure had duration and concentration-dependent inhibitory effects on LAT1-dependent SNO uptake and sGC activation during NO exposure, we evaluated LAT1 expression in vitro and in vivo, finding that hyperoxia exposure suppressed LAT1 expression in rat alveolar type I and type II-like (R3/1 and L2) cells, and in human A549 cells. Effects on expression in whole cell lysates and in the plasma membrane fraction were essentially parallel to effects on function. Alveolar epithelial expression of LAT1 was similarly impaired in 60% O2-exposed rats at postnatal day 4. We chose this in vivo oxygen exposure regimen to more closely mimic the degree of oxidative stress in clinical BPD (29), for which iNO may be prescribed, although it must be acknowledged that full-term newborn babies often receive higher FIO2, sometimes for days, for the treatment of persistent pulmonary hypertension.
The ectopic expression of LAT1 following hyperoxia exposure in vitro and in vivo may indicate abnormalities of cellular processing due to modifications of subunit that confers amino acid specificity. Site-directed mutagenesis of two specific Cys sites, C88S and C439S in rabbit LAT1, impaired the transporter activity (5). In those studies, LAT1 was not visualized in plasma membranes of frog oocytes injected with cloned cRNA corresponding to the C439S mutant, which would be predicted to impair the assembly of the smaller subunit with the larger membrane-spanning subunit. We are not aware of any reported loss of LAT1 function among humans with mutations in SLC7A5. Reported polymorphisms in the SLC7A5 gene do not appear to affect pharmacokinetics of the phenylalanine analog melphalan (17). Complete loss of LAT1 is embryonically lethal, and targeted deletion in double mutant mice carrying a floxed Slc7a5 allele confirms significant loss of large neutral amino acid transport, although cysteine transport was not directly measured (22).
LAT1 was readily detected in the type II-like L2 alveolar epithelial cells; however, the degree of LAT1 colocalization with pro-SP-C positive cells in air and hyperoxia-exposed newborns, and in adult rat, was nonuniform. Relatively lower LAT1 expression in type II alveolar epithelium could potentially contribute to greater vulnerability to the hyperoxia-impaired iNO pharmacologic effects in those cells. In vitro, we found that L2 and A549 cells displayed greater hyperoxia-induced suppression of LAT1 expression by immunofluorescence than that observed in R3/1 cells, but measurements of function (3H-Leu uptake, sGC activation) were similarly affected among all three cell types. Our previous studies in adult mice under basal conditions showed minimal expression of LAT1 in type II alveolar epithelium detected by immunofluorescence (26). In addition to the effects on sGC activation, we would also anticipate that other NO- (or SNO-) dependent effects would be similarly affected. For example, our previous studies showed that enhanced SNO uptake in alveolar macrophages was correlated with enhanced suppression of NF-κB and augmentation of phagocytosis (7).
Although we attributed the decreased SNO accumulation after NO treatment under these experimental conditions to effects on LAT1-dependent transport, it is possible under some conditions that extracellular SNO formation could be impaired, limiting uptake. Under our experimental conditions, we found that maximum hyperoxia (90% O2×12 h) reduced extracellular SNO after NO administration by ∼50% compared with air exposure, but extracellular SNO concentrations were ∼1000-fold higher than cell lysate SNO levels and therefore unlikely to be limiting. However, in vivo, prolonged oxidative stress may take place over days, not hours, and could have the potential to deplete thiol, cysteine in particular, by potentially limiting transportable SNO formed in the alveolar lining fluid during inhaled NO therapy. Treatment with nebulized cysteine augmented SNO accumulation in the lungs and brain after NO treatment, but we did not test this approach under conditions of severe oxidative stress.
Extension of these studies in vivo will be required to determine the contributions of LAT1 to NO physiologic and pharmacologic effects. We have shown that we can augment SNO uptake in the lungs and brain—in an LAT1-dependent manner—with inhaled cysteine treatment before inhaled NO, but it is not clear whether the magnitude of increase would be adaptive under conditions for which inhaled NO would be considered. As suggested by our in vivo expression studies, and others, the expression and function of the LAT1 transport system may vary by cell and organ type. Quantification of LAT1 expression in whole organs is unlikely to accurately predict SNO transport. Determining the effects of loss of LAT1 function on SNO transport in particular cell types in vivo will likely require temporospatial control of LAT1 expression using an inducible Cre-Lox system as recently described (22). Another approach would be augmentation of LAT1 expression through gene transfer, potentially improving SNO uptake during NO treatment if sufficient cysteine were present.
Although we observed that hyperoxia decreased LAT1 expression in vitro and in vivo, we did not determine if the decreased LAT1 is transcriptionally regulated under our experimental conditions. Nearly all studies of LAT1 transcriptional regulation have been in the context of neoplastic disease where functional overexpression of SLC7A5 in tumors appears to be a neoplastic survival factor [see Ref. (15) for review]. However, recent studies suggest that the regulation of LAT1 may be induced by shifts in amino acid concentrations in muscle cells (20) or by activation of the T-cell receptor in T lymphocytes (13). To the best of our knowledge, LAT transporter expression has not been evaluated in the context of redox stress.
In conclusion, our studies suggest that LAT1 is the member of the LAT family of amino acid transporters, which is the predominant route for NO gas exposure effects on NO signaling in alveolar epithelium in vitro and on pulmonary SNO uptake in vivo. Hyperoxia impairs NO gas effects on SNO uptake and sGC activation in type I-like and type II-like rat alveolar epithelium and in human A549 alveolar epithelial cells substantially through its effects on LAT1 function. Hyperoxia-impaired LAT1 function is accompanied by decreased and ectopic LAT1 expression in alveolar epithelial cells in vitro and in vivo. We speculate that the loss of LAT1 function at a critical window of vulnerability will diminish the ability of inhaled NO to achieve desired pharmacologic effects. Augmentation of SNO formation, possibly with supplementation of L-Cys, may help to overcome hyperoxia-impaired SNO formation and uptake during iNO treatment.
Materials and Methods
Reagents were obtained from Sigma-Aldrich (St. Louis, MO) except where noted. Rat type I-like pulmonary alveolar epithelial cells (R3/1) were a gift from R. Koslowski (Dresden, Germany) (16). Rat type II-like (L2) pulmonary alveolar epithelial cells were obtained from the American Type Culture Collection (Manassas, VA). The culture conditions, gas exposure methods, and air–liquid interface model were as previously described in detail (6). Medical gases were obtained from the National Specialty Gases (Durham, NC). LAT1 inhibitor compound JPH-203 was synthesized and diluted as described (28). JPH203 was screened against a battery of 27 receptors and ion channels using radioligand binding assays performed by a commercial drug screening laboratory (HitProfiling Screen, MDS Pharma Services-Taiwan, Ltd., Taipei, Taiwan R.O.C., http://discovery.mdsps.com/Catalog/). Tests were performed in duplicate.
NO±hyperoxia exposure: in vitro
Cells cultured in 6.5-mm transwell inserts were exposed to test gases at air–liquid interface as we have previously described in detail (6) in the presence of incubation buffer, consisting of Hank's balanced salt solution (HBSS), 0.1 mM glutathione, 25 mM HEPES, pH 7.4, plus the indicated amino acids and transporter competitors. Na+-independence of transporter effects was verified by substituting choline chloride for NaCl, and the contribution of endogenous NOS-dependent NO was evaluated by adding L-NAME 100 mM. When cells achieved ∼75% confluence, media were removed from the apical surface of the transwell to produce the air–liquid interface. Cells were then exposed to air or oxygen 60% or 90% (balance nitrogen) for up to 12 h in the Billups-Rothenberg chamber at air–liquid interface. During the last 30 min of exposure, the indicated amino acids, competitors, and inhibitors in incubation buffer were added to the apical surface, followed by treatment with air or NO 20 ppm. Immediately after the final exposures, cells were processed as described previously (5), and apical media were snap frozen for SNO analysis. For all cell culture studies, cells in the test conditions were studied in at least four, separate independent experiments. For the principal comparisons, a minimum of six experiments per condition were performed, as noted in the Results section. For the siRNA experiments, SNO measurements were made from five independent experiments and isotope uptake measurements were made from six independent experiments for each cell type.
Dihydrorhodamine123 and DCF fluorescence
After exposure, buffer was removed and cells were washed briefly with HBSS and incubated with DHR 10 μM (Cayman Chemical, Ann Arbor, MI) or DCF (Invitrogen, Carlsbad, CA) in HBSS+diethylenetriaminepenta-acetate 0.1 mM, pH 7, for 15 min to estimate peroxynitrite, followed by the addition of Hoechst 33342, 1 μg/ml (Life Technologies, Grand Island NY), which labels DNA, for 5 min to estimate cell number. Fluorescence was detected in a monochromator plate reader (SAFIRE, Tecan, Durham, NC) at the following wavelengths: DHR Ex: 500 nm, Em: 536 nm; DCF Ex: 488 nm, Em: 510 nm; Hoechst 33342 Ex: 350 nm, Em: 460 nm. Data are expressed as arbitrary fluorescence units normalized to the Hoechst fluorescence±SEM.
Protein carbonyl and 3-NT
Protein carbonyls were measured from cell homogenates using 2,4-dinitrophenylhydrazine derivatization according to the manufacturer's directions (Protein Carbonyl Assay Kit, cat#ab126287; Abcam, Cambridge MA). 3-NT was measured by ELISA according to the manufacturer's directions (Nitrotyrosine ELISA Kit, cat#ab113848; Abcam).
3H-leucine uptake
To determine whether the effects of hyperoxia exposure on SNO uptake were attributable to the effects on LAT transporter function, R3/1, L2, and A549 cells were cultured at the air–liquid interface in the presence of 3H-4,5-Leu 10 μCi, 120 Ci/mmol (Moravek Biochemicals, Brea, CA)±JPH203 10 nM. To ensure that effects on Leu uptake were system L-dependent, choline chloride was substituted for sodium chloride in the incubation buffers. Effects on cell permeability were assessed by the exclusion of 14C mannitol as previously described (6). After incubation with isotopes and washing in buffer, cells were lysed and isotope uptake was detected by scintillation counting (LKB-Wallac Rackbeta 1214; Waverly, Victoria, Australia).
SNO uptake and LAT1 function±LAT1 siRNA knockdown
R3/1, L2, and A549 cells were infected with the adenoviral shuttle vector containing the siRNA directed against human LAT1 as previously described in detail (19). The sequences used had >95% homology with the rat LAT1 cDNA. Suppression of LAT1 expression was determined by qRT-PCR, immunoblotting, and immunocytochemistry. For qRT-PCR, total RNA was isolated using TRIsure (Bioline, Taunton, MA) immediately after exposure and LAT1 gene expression (normalized to ribosomal protein L32 RNA) was analyzed by qRT-PCR using the SensiFAST SYBR Hi-ROX one step RT-PCR kit (Bioline). Triplicates of each RNA sample (150 ng/well) were loaded in 96-well plates with the master mix according to the manufacturer's protocol. Primers were synthesized by the Integrated DNA Technologies.
Rat LAT1:
Forward: 5′-TTA ATG GCG TGT GCC TGA TA-3′
Reverse: 5′-AGG CCA AAA GAG GCA CTG TA-3′
Rat L32:
Forward: 5′-TGG TGA AGC CCA AGA TCG TC-3′
Reverse: 5′-TTC ACA TAT CGG TCC GAC TGG 3′
Human LAT1 primers:
Forward: 5′-CAA CCT GGC CTA CTT CAC CA-3′
Reverse: 5′-TGA CGC CCA GGT GAT AGT TC-3′
Human L32:
Forward: 5′-GCA TTG ACA ACA GGG TTC GT-3′
Reverse: 5′-GGC AGC ATG TGC TTT GTT TT-3′
For assay controls, RT-PCR was also performed without RT and without RNA template in the reaction mix. Relative LAT1 mRNA values were calculated after normalizing the respective threshold cycle (Ct) values with the respective L32 Ct values. For immunoblot, cells were lysed from six-well plates immediately after exposures and treatments. The protein concentration was determined using dye absorption (Bio-Rad, Hercules, CA), and 40 μg of protein was loaded for each lane. Electrophoresis, immunoblotting, and transfer were conducted as previously described (3). LAT1 was detected with goat-anti-SLC7A5 1:2000 (Sigma). Secondary donkey anti-goat peroxidase conjugate was used at 1:5000 for detection (Vector Laboratories, Burlingame, CA). Blots were detected using enhanced chemiluminescence (SuperSignal; Thermo Scientific, Rockford, IL) as we have previously described (6). Loading and transfer were estimated by detection with anti-β-actin. (cat#ab8227, 1:10,000; Abcam, Cambridge, MA). Expression was quantified by densitometry. Cells cultured in ALI plates after exposures were fixed and detected by anti-SLC7A5 immunofluorescence and counterstained with DAPI as described previously (6). Effects on LAT system function were determined by Na-independent 3H-Leu uptake as described above and on SNO uptake by ozone chemiluminescence (12).
Hyperoxia exposure in vivo
In vivo procedures were approved by the Institutional Animal Care and Use Committee. Rats were obtained from Charles River (Raleigh, NC). Newborn Sprague-Dawley rat litters and their dams were exposed to air or hyperoxia (60%) administered as previously described (29). Animals, which included male rats (250–300 g) as adult controls, were killed with sodium pentobarbital 250 mg/kg i.p. and lungs were inflation fixed with paraformaldehyde as previously described (2).
NO exposure in vivo
Adult Sprague-Dawley rats were anesthetized with 3% isoflurane and injected with 3 mg/kg (2.5 ml/kg) of JPH203 or vehicle (3% DMSO in sterile water) via external jugular vein using a 30G needle. Following the injections, rats were placed in a plastic chamber (18×20×12 cm) and then exposed to nebulized L-Cys 10 mM in 5 ml of sterile 0.9% NaCl, using an AeroTech II nebulizer (median mass aerodynamic diameter ∼1 μm) driven by a compressor (Pulmo-Aide, DeVilbiss Home Health, Somerset, PA). Rats were then treated with NO 20 ppm, 21% O2, balance N2 as previously described (3). After 2 h, animals were euthanized with ketamine 250 mg/kg i.p, and organs were rapidly removed, blotted free of blood, and snap frozen. Control rats received air exposure.
S-nitrosothiol measurements
SNO in cell lysates and apical media was measured by mercury-coupled UV photolysis–chemiluminescence using freshly prepared S-nitrosoglutathione standards, as previously described in detail (4, 6). SNO in the lung and brain homogenates were measured using ozone chemiluminescence (12). This second method of SNO analysis was used for tissue homogenate lysates due to flow limitations in the mercury-coupled UV photolysis–chemiluminescence apparatus.
sGC activation
Effects of NO gas treatment±hyperoxia on sGC activation were estimated by effects on cyclic guanosine monophosphate (cGMP). cGMP was measured using the Direct Biotrak EIA (GE Healthcare, Piscataway, NJ) according to the manufacturer's directions in the presence of isobutylmethylxanthine (1 mM). Dependence of cGMP effects on sGC was confirmed by incubation with 1 H-[17]oxaldizo[18]quinoxalin-1-one 0.5 μM as previously described (6). Data were normalized to protein concentration of the cell lysates
Hyperoxia effects on LAT1 expression
Immunoblots to detect LAT1 (1:3000) were performed using whole cell lysates (90 μg of protein/lane) and plasma membrane fractions (30 μg/lane) (12). Plasma membrane fractions of R3/1, L2, and A549 cells were prepared using a membrane preparation kit (Membrane 1 Ready Prep, cat#163-2088; Bio-Rad, Hercules, CA). Anti-β-actin was used as a loading/transfer control for the whole cell lysate immunoblots, and pan-cadherin (cat#C3678, 1:2000; Sigma) was used as loading/transfer control for the membrane fraction immunoblots.
Cells cultured in ALI plates after exposures were fixed and detected by immunofluorescence as described previously (6). For immunohistochemistry studies, random paraffin sections were obtained from the left lung as previously described. After dewaxing, antigen retrieval was performed using Tris HCl 10 mM, EDTA 1 mM, Tween-20™ 0.05%, pH 9, heated to boiling using a rice cooker. Sections were heated for 10 min, after which the vessel was allowed to cool without the lid for another 10 min, followed by washing in phosphate-buffered saline and proceeding with sequential blocking in the Image iT™ FX signal enhancer (Life Technologies), 3% donkey serum, each for 30 min. Sections were incubated overnight at 4°C with polyclonal goat anti-human SLC7A5 (cat#SAB2501232, 1:1000; Sigma-Aldrich) and rabbit anti-human aquaporin 5 (cat#A4979, 1:200; Sigma-Aldrich) or rabbit anti-human pro-SP-C (cat#AB3428, 1:800; Millipore, Billerica MA) to identify type I and type II alveolar epithelial cells, respectively. Secondary antibodies donkey anti-rabbit AlexaFluor 488 and anti-goat AlexaFluor 546 (1:500; Life Technologies) were used for detection. Sections were mounted with the DAPI-containing medium (Dako, Carpinteria, CA) and sealed with clear nail polish. Micrographic images were obtained by laser confocal microscopy (LSM 780; Carl Zeiss AG, Jena, Germany) and analyzed using ZEN software version 2010 (Carl Zeiss). Confocal scanning for each condition was performed under identical illumination conditions as previously described (26).
For analysis of the immunohistochemical expression of LAT1, three random high-power fields from two random sections from four animals per treatment group were used (air, 60% O2, postnatal day 4). Images were scanned by confocal microscopy under identical conditions using lasers appropriate to the fluorescence signal. Only alveolar septal tissue was imaged. If the random field included large airways or blood vessels, the microscope stage was moved laterally. Images were obtained by a microscopist masked to treatment condition. The total fluorescence intensity for both LAT1 and DAPI was recorded for each random field of view at the same magnification used for the photomicrographs, and the LAT1 intensity was normalized to the corresponding DAPI intensity as an estimate of tissue area.
Statistical analysis
Results were compared among treatment groups by one-way ANOVA, assuming a type I error of 0.05 and type II error of 0.10. Significant differences between treatments were identified by the Tukey's highly significant difference post hoc tests. Pair-wise comparisons were performed using the Student's t-test. Significance was accepted at p<0.05.
Abbreviations Used
- 3-NT
3-nitrotyrosine
- cGMP
cyclic guanosine monophosphate
- Ct
threshold cycle
- DCF
dichlorofluorescein
- DHR
dihydrorhodamine 123
- HBSS
Hank's balanced salt solution
- IC50
half-maximal inhibitory concentration
- iNO
inhaled nitric oxide
- LAT1
L-type amino acid transporter 1
- NO
nitric oxide
- ODQ
1-H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- PEPT2
dipeptide transporter 2
- sGC
soluble guanylyl cyclase
- SNO
S-nitrosothiol
Acknowledgments
This work was supported by the March of Dimes and the Children's Miracle Network. The authors acknowledge the technical assistance of Mr. S. Nicholas Mason and are grateful for the gift of the JPH 203 compound from J-Pharma Company, Ltd.
Author Disclosure Statement
R.L. Auten is co-inventor for U.S. Patent 7,943,667 “Potentiating the Effect of Compound Comprising Nitric Oxide,” which is licensed to Duke University. H. Endou is co-inventor for U.S. Patent 7,345,068, which includes the JPH203 compound. M.V. Brahmajothi, M.F. Wempe, and B.T. Tinch have no potential conflicts of interest to disclose.
References
- 1.Afolayan AJ, Eis A, Teng RJ, Bakhutashvili I, Kaul S, Davis JM, and Konduri GG. Decreases in manganese superoxide dismutase expression and activity contribute to oxidative stress in persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 303: L870–L879, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auten RL, Jr., Mason SN, Tanaka DT, Welty-Wolf K, and Whorton MH. Anti-neutrophil chemokine preserves alveolar development in hyperoxia-exposed newborn rats. Am J Physiol Lung Cell Mol Physiol 281: L336–L344, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Auten RL, Mason SN, Whorton MH, Lampe WR, Foster WM, Goldberg RN, Li B, Stamler JS, and Auten KM. Inhaled ethyl nitrite prevents hyperoxia-impaired postnatal alveolar development in newborn rats. Am J Respir Crit Care Med 176: 291–299, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, and McMahon TJ. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A 104: 17063–17068, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boado RJ, Li JY, Chu C, Ogoshi F, Wise P, and Pardridge WM. Site-directed mutagenesis of cysteine residues of large neutral amino acid transporter LAT1. Biochim Biophys Acta 1715: 104–110, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Brahmajothi MV, Mason SN, Whorton AR, McMahon TJ, and Auten RL. Transport rather than diffusion-dependent route for nitric oxide gas activity in alveolar epithelium. Free Radic Biol Med 49: 294–300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmajothi MV, Sun NZ, and Auten RL. S-nitrosothiol transport via PEPT2 mediates biological effects of nitric oxide gas exposure in macrophages. Am J Respir Cell Mol Biol 48: 230–239, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Broer S. Apical transporters for neutral amino acids: physiology and pathophysiology. Physiology (Bethesda) 23: 95–103, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Cole FS, Alleyne C, Barks JD, Boyle RJ, Carroll JL, Dokken D, Edwards WH, Georgieff M, Gregory K, Johnston MV, Kramer M, Mitchell C, Neu J, Pursley DM, Robinson W, and Rowitch DH. NIH consensus development conference statement: inhaled nitric oxide therapy for premature infants. Pediatrics 127: 363–369, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Dinsdale D, Green JA, Manson MM, and Lee MJ. The ultrastructural immunolocalization of gamma-glutamyltranspeptidase in rat lung: correlation with the histochemical demonstration of enzyme activity. Histochem J 24: 144–152, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Finer NN. and Barrington KJ. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev CD000399, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Granillo OM, Brahmajothi MV, Li S, Whorton AR, Mason SN, McMahon TJ, and Auten RL. Pulmonary alveolar epithelial uptake of S-nitrosothiols is regulated by L-type amino acid transporter. Am J Physiol Lung Cell Mol Physiol 295: L38–L43, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi K, Jutabha P, Endou H, Sagara H, and Anzai N. LAT1 is a critical transporter of essential amino acids for immune reactions in activated human T cells. J Immunol 191: 4080–4085, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Ignarro LJ. Introduction and Overview. Nitric Oxide Biology and Pathobiology, edited by Ignarro LJ. San Diego, CA: Academic Press, 2000 [Google Scholar]
- 15.Kanai Y. and Endou H. Heterodimeric amino acid transporters: molecular biology and pathological and pharmacological relevance. Curr Drug Metab 2: 339–354, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Koslowski R, Barth K, Augstein A, Tschernig T, Bargsten G, Aufderheide M, and Kasper M. A new rat type I-like alveolar epithelial cell line R3/1: bleomycin effects on caveolin expression. Histochem Cell Biol 121: 509–519, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Kuhne A, Kaiser R, Schirmer M, Heider U, Muhlke S, Niere W, Overbeck T, Hohloch K, Trumper L, Sezer O, and Brockmoller J. Genetic polymorphisms in the amino acid transporters LAT1 and LAT2 in relation to the pharmacokinetics and side effects of melphalan. Pharmacogenet Genomics 17: 505–517, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Lancaster JR. The physical properties of nitric oxide. In: Nitric Oxide, edited by Ignarro LJ. San Diego: Academic Press, 2000, pp. 209–224 [Google Scholar]
- 19.Li S. and Whorton AR. Identification of stereoselective transporters for S-nitroso-L-cysteine: role of LAT1 and LAT2 in biological activity of S-nitrosothiols. J Biol Chem 280: 20102–20110, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Luo JQ, Chen DW, and Yu B. Upregulation of amino acid transporter expression induced by L-leucine availability in L6 myotubes is associated with ATF4 signaling through mTORC1-dependent mechanism. Nutrition 29: 284–290, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Nemoto T, Horie S, Okuma Y, Nomura Y, and Murayama T. Possible involvement of amino acid transporters on S-nitroso-cysteine-induced inhibition of arachidonic acid release in PC12 cells. Neurosci Lett 311: 117–120, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Poncet N, Mitchell FE, Ibrahim AF, McGuire VA, English G, Arthur JS, Shi YB, and Taylor PM. The catalytic subunit of the system L1 amino acid transporter (slc7a5) facilitates nutrient signalling in mouse skeletal muscle. PLoS One 9: e89547, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satoh S, Kimura T, Toda M, Maekawa M, Ono S, Narita H, Miyazaki H, Murayama T, and Nomura Y. Involvement of L-type-like amino acid transporters in S-nitrosocysteine-stimulated noradrenaline release in the rat hippocampus. J Neurochem 69: 2197–2205, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Steinhorn RH, Albert G, Swartz DD, Russell JA, Levine CR, and Davis JM. Recombinant human superoxide dismutase enhances the effect of inhaled nitric oxide in persistent pulmonary hypertension. Am J Respir Crit Care Med 164: 834–839, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Steinhorn RH, Kinsella JP, Pierce C, Butrous G, Dilleen M, Oakes M, and Wessel DL. Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension. J Pediatr 155: 841–847.e1, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Torok JA, Brahmajothi MV, Zhu H, Tinch BT, Auten RL, and McMahon TJ. Transpulmonary flux of S-nitrosothiols and pulmonary vasodilation during nitric oxide inhalation: role of transport. Am J Respir Cell Mol Biol 47: 37–43, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, and Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch 447: 532–542, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Wempe MF, Rice PJ, Lightner JW, Jutabha P, Hayashi M, Anzai N, Wakui S, Kusuhara H, Sugiyama Y, and Endou H. Metabolism and pharmacokinetic studies of JPH203, an L-amino acid transporter 1 (LAT1) selective compound. Drug Metab Pharmacokinet 27: 155–161, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Yi M, Jankov RP, Belcastro R, Humes D, Copland I, Shek S, Sweezey NB, Post M, Albertine KH, Auten RL, and Tanswell AK. Opposing effects of 60% oxygen and neutrophil influx on alveologenesis in the neonatal rat. Am J Respir Crit Care Med 170: 1188–1196, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y. and Hogg N. S-Nitrosothiols: cellular formation and transport. Free Radic Biol Med 38: 831–838, 2005 [DOI] [PubMed] [Google Scholar]