Abstract
Objectives. We evaluated cardiovascular disease (CVD) risk factors in American Indians/Alaska Natives (AI/ANs) with diabetes in the Special Diabetes Program for Indians Healthy Heart (SDPI-HH) Demonstration Project.
Methods. Multidisciplinary teams implemented an intensive case management intervention among 30 health care programs serving 138 tribes. The project recruited 3373 participants, with and without current CVD, between 2006 and 2009. We examined data collected at baseline and 1 year later to determine whether improvements occurred in CVD risk factors and in Framingham coronary heart disease (CHD) risk scores, aspirin use, and smoking status.
Results. A1c levels decreased an average of 0.2% (P < .001). Systolic and diastolic blood pressure, low-density lipoprotein (LDL) cholesterol, and triglyceride levels decreased, with the largest significant reduction in LDL cholesterol (∆ = −5.29 mg/dL; P < .001). Average Framingham CHD risk scores also decreased significantly. Aspirin therapy increased significantly, and smoking decreased. Participants with more case management visits had significantly greater reductions in LDL cholesterol and A1c values.
Conclusions. SDPI-HH successfully translated an intensive case management intervention. Creative retention strategies and an improved understanding of organizational challenges are needed for future Indian health translational efforts.
Cardiovascular disease (CVD), a leading cause of death among American Indian and Alaska Native (AI/AN) adults,1,2 is more common among this population than in the United States generally.1–6 This excessive burden of CVD appears to be attributable to a high prevalence of diabetes, reportedly twice as high as among non-Hispanic Whites and significantly higher than among Asian Americans, Hispanics, and non-Hispanic Blacks.7–9 People with diabetes have 2 to 4 times the risk of heart disease as people without diabetes.1,3 CVD risk reduction, therefore, is critical to reducing morbidity and mortality and improving health and quality of life in AI/ANs, especially among those with diabetes.
In 1986, the Indian Health Service (IHS) Division of Diabetes developed its first IHS Standards of Care for Diabetes. For more than 20 years, these guidelines have helped health care professionals provide excellent diabetes care to AI/ANs. Indeed, rates of adherence to nationally recommended guidelines for AI/AN health programs frequently equal or surpass rates described for the general population.10,11 Yet the continued growth of the diabetes and CVD epidemic among AI/ANs and the rise of other complex chronic conditions in this population render sustained improvements in diabetes management an unrelenting challenge.
Case management is an important approach to enhancing traditional health care delivery for people at elevated risk of adverse outcomes and high utilization of health care resources, such as individuals with diabetes.12,13 The efficacy of case management has been demonstrated, particularly in improving glycemic control among individuals with diabetes14,15 and in preventing subsequent cardiac events among those with existing CVD.16,17 A case manager—typically a nurse, dietitian, or pharmacist—is assigned responsibility for oversight and coordination of care across a spectrum of clinic and community services. Required features of case management are identification of a target population, comprehensive assessment of individual patient needs, development of an individual participant care plan, implementation of the care plan, and monitoring of outcomes.12
Case management has been shown to improve CVD risk factor control, although most studies have concentrated on the control of a single risk factor, such as blood glucose or blood pressure,18,19 and many have been conducted in academic health centers rather than community settings.20 However, the risk of CVD events in individuals with diabetes can be reduced by as much as 50% through intensive control of multiple risk factors, for example, by reducing blood pressure and cholesterol levels and using aspirin.21–26 Glycemic control, smoking cessation, physical activity, and weight management also have been shown to reduce CVD risk.27 We evaluated the effect on multiple CVD disease risk factors among AI/ANs with diabetes who participated in a large-scale, multidisciplinary, intensive case management intervention, the Special Diabetes Program for Indians Healthy Heart (SDPI-HH) Demonstration Project.
METHODS
In recognition of the high prevalence of diabetes and its complications among AI/ANs, in 2002 the US Congress directed the IHS to establish a competitive grant program to address the primary prevention of diabetes and its most prevalent and deadly complication, CVD, in this special population. The SDPI-HH Demonstration Project was 1 of 2 funded initiatives and was designed to reduce CVD risk among AI/ANs with diabetes through translation of a proven intervention into clinical practice by implementing intensive team case management across a geographically and organizationally diverse array of settings.
The SDPI-HH project funded 30 health care programs, serving 138 tribes in 13 states and each of the 12 IHS administrative areas, to implement the intervention. Seven IHS hospitals or clinics, 21 tribal health care programs, and 2 urban IHS-contracted programs participated. Four grantees were located in urban settings, including an IHS hospital in a large metropolitan area; all others were on reservations.
Intervention
The intervention implemented by the grantees consisted of individual case management, disease management, and self-management education. Participants received a baseline medical evaluation of CVD risk. An assigned nurse, pharmacist, registered dietitian, or behavioral health–social services (the latter with clinical backup) case manager saw patients monthly (initially) and then quarterly (once stabilized). The case manager developed an individualized care plan for CVD risk reduction for each participant and periodically updated it in response to participant progress.
Disease management included treating CVD risk factors to target goals. Participants had individualized weight loss goals developed from targets of either a body mass index (defined as weight in kilograms divided by the square of height in meters) of less than 30 or at least a 7% reduction in body weight. Case managers also recommended regular physical activity, at least 150 minutes per week. Self-management education was based on IHS and American Diabetes Association standards and covered instruction on routine diabetes care, risk of CVD in AI/ANs with diabetes, and CVD risk reduction in people with diabetes. General recommendations for improved nutrition included a lower-fat, lower-calorie diet and salt intake reduction, if indicated. The CVD education component was augmented at the majority of sites by the National Heart, Lung, and Blood Institute’s Honoring the Gift of Heart Health curriculum.28
Data Collection
The project required participants to be AI/AN, to be 18 years of age or older, and to have diabetes. Participants with previous CVD, defined as coronary artery disease, cerebral vascular disease, peripheral vascular disease, or aortic disease, were not excluded. Individuals were excluded if they were pregnant, were receiving dialysis for end-stage renal disease, were undergoing cancer treatment, had active alcohol or substance abuse problems, or had any other condition that would prohibit successful participation, according to provider judgment (e.g., unstable CVD or cognitive impairment). Project staff identified potential participants mainly through electronic medical records or diabetes registries, but also recruited through community and clinic activities (e.g., health fairs, provider referrals).
Project staff completed the baseline assessment of participants. This included measurements of weight, body mass index, and blood pressure; documentation of a physical exam; glucose and lipid laboratory test results; documentation of prescription medications for dyslipidemia, hypertension, and diabetes management; and health behavior questions regarding degree of physical activity and smoking status. Participants also completed a baseline questionnaire covering factors associated with successful project participation and improved health knowledge, attitudes, and behaviors.
Enrollment has been ongoing since January 2006, with each participant scheduled to complete an annual assessment and questionnaire. We examined all baseline and first annual assessment data collected for the 3373 participants enrolled by August 2009.
Measures
Primary outcome variables were blood glucose, blood pressure, and lipid control. We assessed glucose control by examining hemoglobin A1c levels of participants at baseline and first annual assessments. We also obtained systolic and diastolic blood pressure measurements and determined lipid control by high-density and low-density lipoprotein (HDL and LDL) cholesterol and triglyceride values at baseline and 1 year. Local or regional laboratories assessed the A1c and lipid values with standard available assays. The target goal level for A1c was less than 7.0% (53 mmol/mol). Target levels for systolic and diastolic blood pressure were 130/80 millimeters of Mercury. Target levels for HDL cholesterol were more than 40 milligrams per deciliter for men and more than 50 milligrams per deciliter for women. The target for LDL cholesterol was less than 100 milligrams per deciliter and for triglycerides, less than 150 milligrams per deciliter.27
To summarize changes in all CVD risk factors, we calculated the Framingham CHD risk score for each participant, according to 1998 gender-specific Framingham point score algorithms by Wilson et al.29 Although this risk score system was originally developed for prediction of CHD risk among individuals without previous major CVD events, the algorithms combine information on several major CVD risk factors, including age, gender, high blood pressure, smoking, dyslipidemia, and diabetes status. Hence, it can be used as a composite measure of change in modifiable major CVD risk factors—an approach used in our study and previous efficacy evaluations of other CVD multifactor risk reduction interventions.16,30,31 Because age is not a modifiable risk factor, to allow for comparisons we fixed age at 50 years when calculating these risk scores.
Secondary outcome variables were smoking status and aspirin use assessed at baseline and 1 year later. We examined medication use for dyslipidemia, hypertension, and diabetes management for clients who did not meet target goals for lipid, blood pressure, and glycemic control. For dyslipidemia, we classified use of a statin or a cholesterol absorption inhibitor as treatment for high LDL cholesterol, use of a statin or prescription niacin as treatment for low HDL cholesterol, and use of a fibrate, Welchol, Lovaza, or cholestryamine resin as treatment for high triglyceride levels. We also examined aspirin use in all clients not already on antiplatelet therapy, excluding those with a known contraindication to aspirin, such as Coumadin use or a documented drug allergy.
Statistical Analysis
We compared baseline characteristics between participants who completed the first annual assessment and those who did not with the χ2 test for categorical variables and the 2-sample t test for continuous variables. We used multiple logistic regression models to assess the relationships between retention and each participant characteristic after adjustment for the other baseline characteristics we examined.
We used linear mixed-effects models to obtain adjusted mean changes for each primary outcome and the Framingham CHD risk score at the first annual assessment, with baseline age, gender, and a binary time variable indicating the assessment period (baseline vs 1 year later) included in the models. We included a random intercept at the participant level to model participant-level heterogeneity.32 Because triglyceride level is a highly skewed variable, we took a log transformation of it before fitting linear mixed models. We also fit linear mixed models with additional grantee-level random effects to account for the clustering effects of participants from the same grantee site. Results of these models were essentially the same; therefore, we reported only results of the simpler model.
We used the McNemar test to compare the percentage of participants not meeting the treatment target for each CVD risk factor and not on proper medication at the first annual assessment with that of their matched baseline sample. We used multiple linear regression models to investigate the relationship between changes in health outcomes at the first annual assessment (dependent variable) and number of case management visits, after adjustment for age, gender, and baseline level of each outcome (independent variables).
We performed all data analyses in SAS version 9.2 (SAS Institute Inc, Cary, NC). Because we considered multiple outcomes and assessments, we used a P value of .01 for statistical significance to mitigate issues of multiple comparisons.
RESULTS
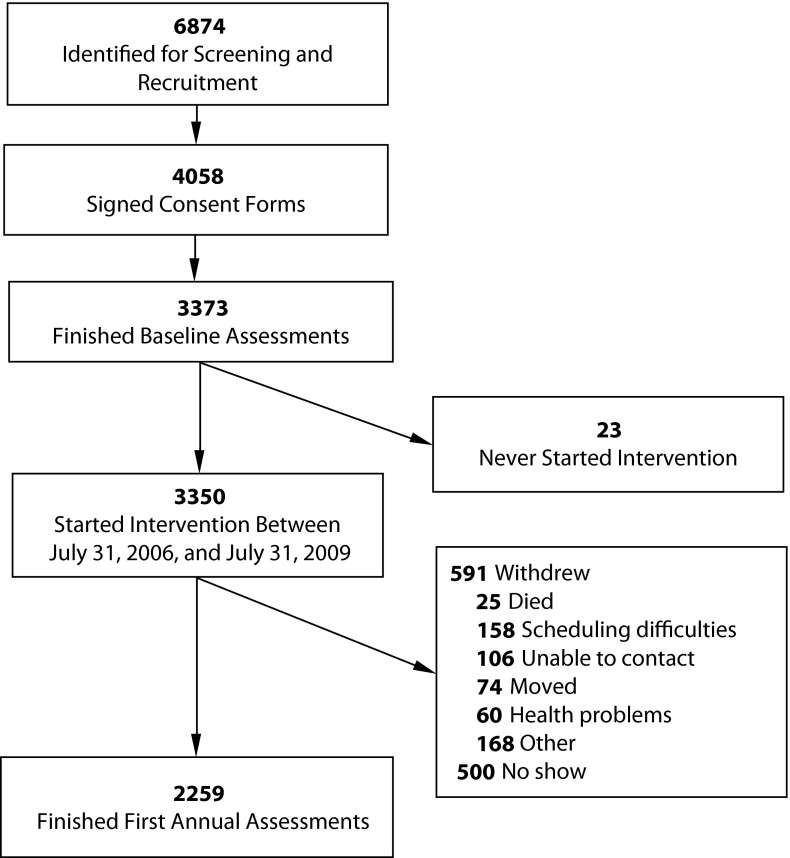
Figure 1 summarizes recruitment and retention. Of 6874 potential participants contacted by SDPI-HH grant programs, 4058 provided informed consent to participate. Approximately 83% of individuals who agreed to participate completed the baseline assessment. Of those who completed the baseline assessment, approximately 67% completed the first annual assessment.
FIGURE 1—
Recruitment and retention flowchart for the Special Diabetes Program for Indians Healthy Heart Demonstration Project, 2006–2009.
Baseline Demographic Characteristics and Risk Factors
Baseline demographic characteristics and CVD risk factors are presented in Table 1. Of the 3373 participants at baseline, 65% were women and 66% were aged 50 years or older. Nineteen percent of participants had less than a high school education; 25% reported that their formal education ended with high school graduation. Fifty-two percent of participants were employed, yet the majority (59%) reported an annual household income less than $ 30 000. On average, participants were obese, with a mean body mass index of 36.5, and had controlled blood pressure (< 130/80 mm Hg). Mean LDL cholesterol (98.4 mg/dL) was less than the target (< 100 mg/dL). Mean HDL cholesterol for women (45.7 mg/dL) was lower than the target (> 50 mg/dL). Mean HDL cholesterol for men (39.6 mg/dL) was slightly lower than the target (> 40 mg/dL; data not shown). Mean triglyceride level (197.0 mg/dL) was higher than the target (< 150 mg/dL).
TABLE 1—
Baseline Characteristics of Participants Who Completed and Who Did Not Complete First Annual Assessment: Special Diabetes Program for Indians Healthy Heart Demonstration Project, 2006–2009
| Characteristic | Total (n = 3373), No. (%) or Mean ±SD | Completed Assessment (n = 2259), No. (%) or Mean ±SD | Did Not Complete Assessment (n = 1114),a No. (%) or Mean ±SD | Pb |
| Demographic characteristics | ||||
| Gender | .004 | |||
| Female | 2199 (65.2) | 1510 (66.8) | 689 (61.8) | |
| Male | 1174 (34.8) | 749 (33.2) | 425 (38.2) | |
| Age, y | < .001 | |||
| 18–39 | 403 (11.9) | 218 (9.7) | 185 (16.6) | |
| 40–49 | 736 (21.8) | 453 (20.1) | 283 (25.4) | |
| 50–59 | 1105 (32.8) | 758 (33.6) | 347 (31.1) | |
| ≥ 60 | 1129 (33.5) | 830 (36.7) | 299 (26.8) | |
| Education | .15 | |||
| < high school | 546 (19.1) | 372 (19.0) | 174 (19.3) | |
| High school | 725 (25.3) | 505 (25.7) | 220 (24.4) | |
| Some college | 1164 (40.7) | 776 (39.6) | 388 (43.1) | |
| ≥ college | 427 (14.9) | 309 (15.7) | 118 (13.1) | |
| Employment status | .01 | |||
| Employed | 1399 (51.8) | 930 (50.4) | 469 (54.9) | |
| Unemployed | 762 (28.2) | 516 (27.9) | 246 (28.8) | |
| Retired | 499 (18.5) | 372 (20.1) | 127 (14.9) | |
| Student | 41 (1.5) | 29 (1.6) | 12 (1.4) | |
| Annual household income, $ | .18 | |||
| 0–14 999 | 761 (31.1) | 505 (30.1) | 256 (33.5) | |
| 15 000–29 999 | 690 (28.2) | 475 (28.3) | 215 (28.1) | |
| 30 000–49 999 | 601 (24.6) | 432 (25.7) | 169 (22.1) | |
| ≥50 000 | 392 (16.0) | 267 (15.9) | 125 (16.3) | |
| Clinical indicators | ||||
| BMI | 36.5 ±8.1 | 36.7 ±8.1 | 36.1 ±7.9 | .04 |
| A1c, % | 7.9 ±2.0 | 7.7 ±1.9 | 8.1 ±2.1 | < .001 |
| A1c, IFCC mmol/mol | 62.3 ±21.6 | 61.0 ±20.6 | 65.1 ±23.5 | < .001 |
| DBP, mm Hg | 76.2 ±10.0 | 75.8 ±9.9 | 77.0 ±10.3 | .001 |
| SBP, mm Hg | 128.7 ±16.6 | 128.6 ±16.3 | 129.1 ±17.1 | .39 |
| HDL cholesterol, mg/dL | 43.6 ±11.8 | 43.7 ±11.7 | 43.2 ±12.1 | .26 |
| LDL cholesterol, mg/dL | 98.4 ±32.5 | 97.0 ±32.8 | 101.3 ±31.7 | < .001 |
| Triglycerides, mg/dL | 197.0 ±303 | 191.5 ±166 | 208.1 ±472 | .25 |
| Framingham CHD risk score | 8.9 ±3.6 | 8.8 ±3.6 | 9.0 ±3.6 | .11 |
Note. BMI = body mass index; CHD = coronary heart disease; DBP = diastolic blood pressure; HDL = high-density lipoprotein; IFCC = International Federation of Clinical Chemistry; LDL = low-density lipoprotein; SBP = systolic blood pressure.
Including 23 participants who never started the intervention.
For demographic characteristics, the χ2 test, and for clinical indicators, the 2-sample t test compared participants who did and did not complete the first annual assessment.
Table 1 also compares the baseline characteristics of participants who completed and did not complete the first annual assessment. Those who completed the first annual assessment were significantly older than those who did not and were more likely to be female and retired (vs employed). Participants assessed after 1 year also had lower diastolic blood pressure, A1c, and LDL cholesterol values at baseline.
Assessment After 1 Year
Participants who completed the first annual assessment had significant changes from baseline in several risk factors (Table 2). Participants' A1c levels decreased significantly—on average by approximately 0.2% (2.28 mmol/mol; P < .001). Both systolic and diastolic blood pressure were significantly lower (P = .01 and P = .005, respectively). Average HDL cholesterol levels were significantly higher (change = 0.50 mg/dL; P = .02). LDL cholesterol declined by about 5.29 milligrams per deciliter (P < .001), and the mean triglyceride level decreased by about 6% (−0.06 on log scale; P < .001). Smoking also decreased significantly: the percentage of current smokers decreased from 19.5% at baseline to 16.3% at the first annual assessment (P < .001; data not shown).
TABLE 2—
Changes From Baseline to First Annual Assessment in Cardiovascular Risk Scores and Risk Factors: Special Diabetes Program for Indians Healthy Heart Demonstration Project, 2006–2009
| CVD Risk Factor | Baseline,a Mean (SD) | Change,b Mean (95% CI) | P |
| A1c, % | 7.85 (1.97) | −0.21 (−0.29, −0.12) | < .001 |
| A1c, IFCC mmol/mol | 62.33 (21.65) | −2.31 (−3.24, −1.39) | < .001 |
| SBP, mm Hg | 128.75 (16.55) | −1.47 (−2.57, −0.37) | .01 |
| DBP, mm Hg | 76.19 (10.04) | −1.05 (−1.75, −0.35) | .005 |
| HDL cholesterol, mg/dL | 43.58 (11.80) | 0.50 (0.09, 0.90) | .02 |
| LDL cholesterol, mg/dL | 98.38 (32.49) | −5.29 (−6.90, −3.69) | < .001 |
| Log−transformed triglycerides | 5.09 (0.56) | −0.06 (−0.08, −0.04) | < .001 |
| Framingham CHD risk score | 8.87 (3.60) | −0.53 (−0.68, −0.38) | < .001 |
Note. CHD = coronary heart disease; CI = confidence interval; CVD = cardiovascular disease; DBP = diastolic blood pressure; HDL = high-density lipoprotein; IFCC = International Federation of Clinical Chemistry; LDL = low-density lipoprotein; SBP = systolic blood pressure.
Raw mean and SD of baseline values.
Estimates from linear mixed-effects models with control for baseline age and gender.
Participants' average Framingham CHD risk score decreased significantly from baseline to the first annual assessment, by about 0.5 (P < .001). In other words, the average risk for developing CHD in the next 10 years among these participants decreased from 11% at baseline to 10% at the first annual assessment.29
Among participants who completed both the baseline and first annual assessments, the percentages who were not at therapeutic goals for hypertension and dyslipidemia and who were not prescribed the appropriate medications for these conditions decreased significantly: from 9.8% to 6.7% for hypertension and from 8.6% to 5.4% for dyslipidemia (P < .001; data not shown). In addition, prescriptions for aspirin and other antiplatelet therapy increased significantly: from 79.5% at baseline to 88.1% after 1 year (P < .001).
On average, participants attended approximately 7 case management visits in the initial year following enrollment. The number of visits each participant attended ranged from 1 to 12, fairly uniformly distributed, with about 8% of participants in each category of attendance. After controlling for age, gender, and baseline level of the corresponding CVD risk factor, those who had more case management visits had significantly greater reductions in A1c and LDL cholesterol values at the first annual assessment (P < .001; Table 3). Each additional case management visit was associated with a 0.05% (0.5 mmol/mol) further decrease in A1c and a 0.74 milligrams per deciliter greater reduction in LDL cholesterol. The association between an increase in case management visits and a reduction in Framingham CVD risk score was marginally significant (P = .03).
TABLE 3—
Associations Between Changes in Cardiovascular Risk Factors From Baseline to First Annual Assessment and Number of Case Management Visits: Special Diabetes Program for Indians Healthy Heart Demonstration Project, 2006–2009
| CVD Risk Factor | Number of Visits, b (95% CI)b | P |
| A1c, % | –0.05 (–0.07, –0.02) | < .001 |
| A1c, IFCC mmol/mol | –0.50 (–0.74, –0.26) | < .001 |
| SBP, mm Hg | –0.12 (–0.36, 0.12) | .34 |
| DBP, mm Hg | 0.04 (–0.10, 0.18) | .56 |
| LDL cholesterol, mg/dL | –0.74 (–1.16, –0.32) | < .001 |
| HDL cholesterol, mg/dL | 0.09 (–0.03, 0.22) | .14 |
| Triglycerides, mg/dl | –0.72 (–4.41, 2.98) | .7 |
| Framingham CHD risk score | –0.04 (–0.08, 0.00) | .03 |
Note. CHD = coronary heart disease; CI = confidence interval; CVD = cardiovascular disease; DBP = diastolic blood pressure; HDL = high-density lipoprotein; IFCC = International Federation of Clinical Chemistry; LDL = low-density lipoprotein; SBP = systolic blood pressure. Values were adjusted by age, gender, and baseline level of each outcome for each outcome change.
DISCUSSION
Across the diverse array of settings in the SDPI-HH Demonstration Project, we observed improvements in the primary outcome variables of blood sugar, blood pressure, and lipid control, with the largest improvement seen in lipid control. Participants’ average Framingham CHD risk score declined significantly between baseline and 1 year, decreasing the average risk of developing CHD in the next 10 years from 11% at baseline to 10%. Similar findings have been reported in previous efficacy trials. We also found improvements in smoking status, aspirin and other antiplatelet therapy, and prescribed pharmacotherapy for CVD risk factors, further demonstrating success in reduction of multiple CVD risk factors.
Changes in some primary outcomes were moderate, likely because baseline levels of most of these primary outcomes were already at or below target. Nonetheless, the longitudinal findings from the Strong Heart Study demonstrate that hypertension increases with age among AIs.33 Thus, this intervention may have prevented worsening of blood pressure control and may have allowed motivated participants to stay within target goals for the other outcomes.
The largest measurable effect of the intervention was reduction in LDL cholesterol from baseline to 1 year. Changes in LDL cholesterol of this magnitude have been reported infrequently in recent diabetes case management literature.18 Our finding may have been driven by a lower optimal LDL cholesterol (< 70 mg/dL), a national guideline for very high–risk individuals with diabetes, such as those with existing heart disease, who were not excluded from participation in this project. Lifestyle change, augmented by a curriculum specific for AI/ANs, Honoring the Gift of Heart Health, and individualized nutrition education, as well as more intensive pharmaceutical management, also may account for sustained improvements in LDL cholesterol.
We observed significant improvements in our secondary outcomes of smoking status and aspirin use. Few, if any, studies have shown improvements in smoking status with case management.34–36 An AI/AN-specific curriculum, frequent contact, and lifestyle modification support by case managers may have encouraged smoking cessation. Although success in increasing aspirin use has been noted in other high-risk populations,37 a previous nurse case management intervention among AI/ANs with diabetes resulted in lower aspirin use.38 Nurse case managers in that study did not meet regularly with providers but did need approval from the patient’s provider before initiating aspirin therapy. The multidisciplinary team approach of the SDPI-HH project required initial monthly and then periodic meetings of the lead case manager with a licensed primary care provider and a pharmacist. These regular team meetings may have accounted for higher aspirin use among SDPI-HH participants.
Another major strength of the SDPI-HH Demonstration Project was the flexibility exercised by grantees. Many, for instance, implemented local, culturally adapted motivational group activities involving family members in addition to the formal intervention. Activities ranged from fun walks, community gardening, and talking circles to traditional games and other activities rooted in the tribal culture of the local communities. Other strengths were the geographic and tribal diversity of the program settings, large-scale implementation, participant pool size, and application of a case management intervention to a population in urgent need of interventions to reduce daunting diabetes disparities.
Limitations
The SDPI-HH project did not have a control group, which compromised our ability to determine CVD outcomes among participants had they not received the intervention. However, this design was appropriate for translational projects39; furthermore, input received from participating sites during the initial collaborative planning year of the demonstration project strongly influenced the choice of this design. Participants recruited were likely particularly motivated and willing to comply with program visits, telephone follow-up, and required laboratory testing. These participants might not have fully reflected those seeking services from IHS, tribal, and urban programs.
Case management is a complex intervention, and we could not confidently identify which components of the intervention resulted in specific benefits. Our outcomes also were proximal measures for cardiac events that the SDPI-HH project is intended to prevent.
Competing priorities and high mobility of participants compromised retention rates. About 30% of participants did not complete the first annual assessment. As shown in Figure 1, the most common withdrawal reasons were scheduling difficulties, inability to contact participants, and participant relocation. We used linear mixed models, which give unbiased estimation for model parameters when the missing-at-random assumption is met, to help address this retention problem.40 However, this assumption is difficult to evaluate, leaving open the possibility that bias might exist that could limit the generalizability of our results. Many translational projects face similar challenges in retaining participants, and our results suggest that future translational initiatives need additional creative retention strategies.41
Conclusions
A multisite evidence-based case management intervention targeting multiple CVD risk factors in individuals with diabetes was successfully translated in AI/AN communities participating in the SDPI-HH Demonstration Project. CVD is a leading cause of mortality and a major source of morbidity for AI/AN people with diabetes; thus it is crucial for the IHS to disseminate this intervention to other AI/AN communities. Rapid translation of newer integrated and transformative care strategies also should be encouraged to further reduce CVD and other diabetes-related disparities.42,43
Further study is needed to improve our understanding of organizational challenges in adoption and implementation of this case management intervention. This knowledge will be important for the long-term sustainability of not only this intervention but also other evidence-based applications newly translated within the SDPI and the IHS.
Acknowledgments
Grants to to Spero M. Manson were funded by the IHS (HHSI242200400049C), the National Institute of Diabetes and Digestive and Kidney Diseases (P30 DK092923), the National Institute on Aging (P30 AG15297), and the National Institute of Minority Health and Health Disparities (P60 MD000507).
Grant programs participating in the SDPI-HH project were the Absentee Shawnee Tribe of Oklahoma; Albuquerque Service Unit; Bad River Band of Lake Superior Chippewa; Blackfeet Tribe; Choctaw Nation of Oklahoma; Confederated Salish and Kootenai Tribes; Montana/Wyoming Tribal Consortium, with Assiniboine & Gros-Ventre, Chippewa Cree Tribe, and Crow Nation; Hualapai Tribe; Indian Health Care Resource Center of Tulsa, Inc, in consortium with Northeastern Tribal Health System Miami Service Unit; Indian Health Council, Inc; Leech Lake Reservation Tribal Council; Mille Lacs Band of Ojibwe, in consortium with St. Croix Chippewa Indians of Wisconsin; Muscogee Creek Nation Health System; Navajo Area Indian Health Service, with Northern Navajo Medical Center and Inscription House Clinic; Northwest Washington Indian Health Board, with Lummi Indian Nation, Nooksack Tribe of Indians, Swinomish Tribal Community, and Upper Skagit Indian Tribe; Ramah Navajo School Board, Inc; Redding Rancheria Indian Health Clinic; Hoopa Valley Tribe; Riverside–San Bernardino County Indian Health, Inc; Santo Domingo Tribe; Sault Ste Marie Tribe Chippewa; Seattle Indian Health Board; St. Regis Mohawk Health Services; Taos–Picuris Service Unit; Tohono O’Odham Healthy Heart Project; Toiyabe Indian Health Project, Inc.; Uintah & Ouray IHS Clinic; Wagner Health Care Center IHS; Whiteriver IHS Service Unit; Yakama Indian Health Center–IHS; Yukon–Kuskokwim Health Corporation.
We thank the Indian Health Service as well as tribal and urban Indian health programs and participants involved in the SDPI-HH project.
Note. Kelly Moore was a member of the National Advisory Board of the Merck Company Foundation Alliance to Reduce Disparities in Diabetes and received an honorarium for this activity. The opinions expressed in this article are the authors' and do not necessarily reflect the views of the Indian Health Service or the Department of Health and Human Services.
Human Participant Protection
The protocol was approved by the institutional review boards of the University of Colorado Denver and the IHS. When required, grantees obtained approval from other entities charged with overseeing research in their programs (e.g., tribal review boards). All participants provided written informed consent and Health Insurance Portability and Accountability Act authorization.
References
- 1.Howard BV, Lee ET, Cowan LD et al. Rising tide of cardiovascular disease in American Indians. The Strong Heart Study. Circulation. 1999;99(18):2389–2395. doi: 10.1161/01.cir.99.18.2389. [DOI] [PubMed] [Google Scholar]
- 2.Rhoades DA. Racial misclassification and disparities in cardiovascular disease among American Indians and Alaska Natives. Circulation. 2005;111(10):1250–1256. doi: 10.1161/01.CIR.0000157735.25005.3F. [DOI] [PubMed] [Google Scholar]
- 3.Jolly S, Kao C, Bindman AB, Korenbrot C. Cardiac procedures among American Indians and Alaska Natives compared to non-Hispanic Whites hospitalized with ischemic heart disease in California. J Gen Intern Med. 2010;25(5):430–434. doi: 10.1007/s11606-009-1235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Prevalence of coronary heart disease—United States, 2006–2010. MMWR Morb Mortal Wkly Rep. 2011;60(40):1377–1381. [PubMed] [Google Scholar]
- 5.Zhang Y, Galloway JM, Welty TK et al. Incidence and risk factors for stroke in American Indians: the Strong Heart Study. Circulation. 2008;118(15):1577–1584. doi: 10.1161/CIRCULATIONAHA.108.772285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Prevalence of heart disease—United States, 2005. MMWR Morb Mortal Wkly Rep. 2007;56(6):113–118. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA: US Dept of Health and Human Services; 2011. [Google Scholar]
- 8.Centers for Disease Control and Prevention. Diabetes prevalence among American Indians and Alaska Natives and the overall population—United States, 1994–2002. MMWR Morb Mortal Wkly Rep. 2003;52(30):702–704. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) Prevalence of diagnosed diabetes among American Indians/Alaskan Natives: United States, 1996. MMWR Morb Mortal Wkly Rep. 1998;47:901–904. [PubMed] [Google Scholar]
- 10.Acton KJ, Shields R, Rith-Najarian S et al. Applying the Diabetes Quality Improvement Project indicators in the Indian Health Service primary care setting. Diabetes Care. 2001;24(1):22–26. doi: 10.2337/diacare.24.1.22. [DOI] [PubMed] [Google Scholar]
- 11.Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Narayan KM. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136(8):565–574. doi: 10.7326/0003-4819-136-8-200204160-00005. [DOI] [PubMed] [Google Scholar]
- 12.Norris SL, Nichols PJ, Caspersen CJ et al. The effectiveness of disease and case management for people with diabetes. A systematic review. Am J Prev Med. 2002;22(4 suppl):15–38. doi: 10.1016/s0749-3797(02)00423-3. [DOI] [PubMed] [Google Scholar]
- 13.Technology Assessment: Care Management for Chronic Illness, the Frail Elderly, and Acute Myocardial Infarction. Bloomington, MN: Institute for Clinical Systems Integration; 1998. [Google Scholar]
- 14.Krein SL, Klamerus ML, Vijan S et al. Case management for patients with poorly controlled diabetes: a randomized trial. Am J Med. 2004;116(11):732–739. doi: 10.1016/j.amjmed.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 15.Shojania KG, Ranji SR, McDonald KM et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296(4):427–440. doi: 10.1001/jama.296.4.427. [DOI] [PubMed] [Google Scholar]
- 16.Ma J, Berra K, Haskell WL et al. Case management to reduce risk of cardiovascular disease in a county health care system. Arch Intern Med. 2009;169(21):1988–1995. doi: 10.1001/archinternmed.2009.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haskell WL, Alderman EL, Fair JM et al. Effects of intensive multiple risk factor reduction on coronary atherosclerosis and clinical cardiac events in men and women with coronary artery disease. The Stanford Coronary Risk Intervention Project (SCRIP) Circulation. 1994;89(3):975–990. doi: 10.1161/01.cir.89.3.975. [DOI] [PubMed] [Google Scholar]
- 18.Ishani A, Greer N, Taylor BC et al. Effect of nurse case management compared with usual care on controlling cardiovascular risk factors in patients with diabetes. Diabetes Care. 2011;34(8):1689–1694. doi: 10.2337/dc10-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai AC, Morton SC, Mangione CM, Keeler EB. A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag Care. 2005;11(8):478–488. [PMC free article] [PubMed] [Google Scholar]
- 20.Crowley MJ, Bosworth HB, Coffman CJ et al. Tailored Case Management for Diabetes and Hypertension (TEACH-DM) in a community population: study design and baseline sample characteristics. Contemp Clin Trials. 2013;36(1):298–306. doi: 10.1016/j.cct.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothman RL, Malone R, Bryant B et al. A randomized trial of a primary care–based disease management program to improve cardiovascular risk factors and glycated hemoglobin levels in patients with diabetes. Am J Med. 2005;118(3):276–284. doi: 10.1016/j.amjmed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Snow V, Weiss KB, Mottur-Pilson C Clinical Efficacy Assessment Subcommittee of the American College of Physicians. The evidence base for tight blood pressure control in the management of type 2 diabetes mellitus. Ann Intern Med. 2003;138(7):587–592. doi: 10.7326/0003-4819-138-7-200304010-00017. [DOI] [PubMed] [Google Scholar]
- 23.Vijan S, Hayward RA. Treatment of hypertension in type 2 diabetes mellitus: blood pressure goals, choice of agents, and setting priorities in diabetes care. Ann Intern Med. 2003;138(7):593–602. doi: 10.7326/0003-4819-138-7-200304010-00018. [DOI] [PubMed] [Google Scholar]
- 24.White WB, Prisant LM, Wright JT., Jr Management of patients with hypertension and diabetes mellitus: advances in the evidence for intensive treatment. Am J Med. 2000;108(3):238–245. doi: 10.1016/s0002-9343(99)00444-1. [DOI] [PubMed] [Google Scholar]
- 25.Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136(2):161–172. doi: 10.7326/0003-4819-136-2-200201150-00016. [DOI] [PubMed] [Google Scholar]
- 26.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 27.American Diabetes Association. Standards in medical care—2006. Diabetes Care. 2006;29(suppl 1):S4–S42. [PubMed] [Google Scholar]
- 28.National Heart, Lung, and Blood Institute, Indian Health Service. Honoring the Gift of Heart Health: A Heart Health Educator’s Manual. Bethesda, MD: US Department of Health and Human Services; 2006. [Google Scholar]
- 29.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 30.Lear SA, Ignaszewski A, Linden W et al. The Extensive Lifestyle Management Intervention (ELMI) following cardiac rehabilitation trial. Eur Heart J. 2003;24(21):1920–1927. doi: 10.1016/j.ehj.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Wister A, Loewen N, Kennedy-Symonds H, McGowan B, McCoy B, Singer J. One-year follow-up of a therapeutic lifestyle intervention targeting cardiovascular disease risk. CMAJ. 2007;177(8):859–865. doi: 10.1503/cmaj.061059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York, NY: Springer Verlag; 2000. [Google Scholar]
- 33.Wang W, Lee ET, Fabsitz RR et al. A longitudinal study of hypertension risk factors and their relation to cardiovascular disease: the Strong Heart Study. Hypertension. 2006;47(3):403–409. doi: 10.1161/01.HYP.0000200710.29498.80. [DOI] [PubMed] [Google Scholar]
- 34.Wubben DP, Vivian EM. Effects of pharmacist outpatient interventions on adults with diabetes mellitus. Pharmacotherapy. 2008;28(4):421–436. doi: 10.1592/phco.28.4.421. [DOI] [PubMed] [Google Scholar]
- 35.Scott DM, Boyd ST, Stephan M, Augustine SC, Reardon TP. Outcomes of pharmacist-managed diabetes care services in a community health center. Am J Health Syst Pharm. 2006;63(21):2116–2122. doi: 10.2146/ajhp060040. [DOI] [PubMed] [Google Scholar]
- 36.Kelly C, Rodgers PT. Implementation and evaluation of a pharmacist-managed diabetes service. J Manag Care Pharm. 2000;6(6):488–493. [Google Scholar]
- 37.Haskell WL, Berra K, Arias E et al. Multifactor cardiovascular disease risk reduction in medically underserved, high-risk patients. Am J Cardiol. 2006;98(11):1472–1479. doi: 10.1016/j.amjcard.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 38.Wilson C, Curtis J, Lipke S, Bochenski C, Gilliland S. Nurse case manager effectiveness and case load in a large clinical practice: implications for workforce development. Diabet Med. 2005;22(8):1116–1120. doi: 10.1111/j.1464-5491.2005.01604.x. [DOI] [PubMed] [Google Scholar]
- 39.Garfield SA, Malozowski S, Chin MH et al. Considerations for diabetes translational research in real-world settings. Diabetes Care. 2003;26(9):2670–2674. doi: 10.2337/diacare.26.9.2670. [DOI] [PubMed] [Google Scholar]
- 40.Fairclough DL. Design and Analysis of Quality of Life Studies in Clinical Trials. 1st ed. Boca Raton, FL: Chapman and Hall/CRC; 2002. [Google Scholar]
- 41.Manson SM, Jiang L, Zhang L, Beals J, Acton KJ, Roubideaux Y. Special diabetes program for Indians: retention in cardiovascular risk reduction. Gerontologist. 2011;51(suppl 1):S21–S32. doi: 10.1093/geront/gnq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peek ME, Cargill A, Huang ES. Diabetes health disparities: a systematic review of health care interventions. Med Care Res Rev. 2007;64(5 suppl):101S–156S. doi: 10.1177/1077558707305409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Betancourt JR, Duong JV, Bondaryk MR. Strategies to reduce diabetes disparities: an update. Curr Diab Rep. 2012;12(6):762–768. doi: 10.1007/s11892-012-0324-1. [DOI] [PubMed] [Google Scholar]