Abstract
There is evidence that the default mode network (DMN) functional connectivity is impaired in Alzheimer's disease (AD) and few studies also reported a decrease in DMN intrinsic activity, measured by the amplitude of low-frequency fluctuations (ALFFs). In this study, we analyzed the relationship between DMN intrinsic activity and functional connectivity, as well as their possible implications on cognition in patients with mild AD and amnestic mild cognitive impairment (aMCI) and healthy controls. In addition, we evaluated the differences both in connectivity and ALFF values between these groups. We recruited 29 controls, 20 aMCI, and 32 mild AD patients. To identify the DMN, functional connectivity was calculated by placing a seed in the posterior cingulate cortex (PCC). Within the DMN mask obtained, we calculated regional average ALFFs. Compared with controls, aMCI patients showed decreased ALFFs in the temporal region; compared with AD, aMCI showed higher values in the PCC but lower in the temporal area. The mild AD group had lower ALFFs in the PCC compared with controls. There was no difference between the connectivity in the aMCI group compared with the other groups, but AD patients showed decreased connectivity in the frontal, parietal, and PCC. Also, PCC ALFFs correlated to functional connectivity in nearly all subregions. Cognitive tests correlated to connectivity values but not to ALFFs. In conclusion, we found that DMN connectivity and ALFFs are correlated in these groups. Decreased PCC ALFFs disrupt the DMN functional organization, leading to cognitive problems in the AD spectrum.
Key words: : functional MRI, episodic memory, dementia, amnestic mild cognitive impairment, Alzheimer's disease
Introduction
Dementia due to Alzheimer's disease (AD) is a complex clinical condition that affects the brain in different levels, such as molecular functioning, neurotransmitter systems, and anatomic and functional organization, leading to problems in cognition, behavior, and social independence. Amnestic mild cognitive impairment (aMCI) is thought to be a possible prodromal AD, in which patients have objective memory problems, with little-to-no impairment in social and occupational independence.
Recently, increasing attention has been given to the use of functional magnetic resonance imaging (fMRI) to explore the normal functional organization of the brain, as well as in neurologic diseases, such as AD. The functional analysis of a brain “at rest,” that is, without a specific external experimental stimulus (rsfMRI), can inform us about intrinsic brain activity, as well as the level of synchronization of different cerebral regions (functional connectivity). Intrinsic brain activity, measured by the amplitude of low-frequency fluctuations (ALFFs) in BOLD signals, which are usually between 0.01 and 0.08 Hz, concerns the fluctuations in neural activity across time, while functional connectivity describes the linkage between the neural activities of different regions across spatially distinct parts of the brain (Northoff, 2013).
Among the rsfMRI approaches used to study the brain, the functional connectivity analysis is the most widely reported (Brier et al., 2012; Song et al., 2013; Xia et al., 2014). Many studies have shown that functional connectivity in the default mode network (DMN) is impaired in AD patients (Agosta et al., 2012; Greicius et al., 2004; Xia et al., 2014), probably contributing to cognitive and clinical symptoms (Balthazar et al., 2014; Weiler et al., 2014). The function of DMN is not completely understood, but is generally considered a distributed system for self-related cognitive activity that is activated when a person is not focused on activities directed to the external environment (Buckner et al., 2008; Menon, 2011). Also, the key anatomical regions of DMN, which include the precuneus, posterior cingulate cortex (PCC), inferior parietal, hippocampus, and medial prefrontal cortex, may have an important role in episodic memory, a cognitive function invariably disrupted in AD.
Although being a relatively new technique, the ALFF analysis throughout the brain has also brought some interesting results (yet controversial). For example, for MCI subjects, parietal regions can have either decreased (Zhao et al., 2014) or increased (Wang et al., 2011) values. Temporal regions are mostly described as having lower values in these patients (Zhao et al., 2014), but are also reported to have the opposite pattern (Liang et al., 2014; Wang et al., 2011), and the same happens to the frontal regions (Han et al., 2011; Wang et al., 2011; Zhao et al., 2014). For AD patients, in turn, the studies bring mainly decreased ALFF values for all analyzed areas (Liu et al., 2014b; Xi et al., 2012). It is interesting to note, also, that the areas that comprise the DMN are reported as having the highest ALFF values in controls at rest (Zuo et al., 2010), and the ones that are mostly affected in AD patients (Liu et al., 2014b).
The single-level evaluation of the DMN, however, does not permit us to conclude from which network subregion the breakdown in connectivity comes from (Zang et al., 2007). Also, one may suppose that as long as the BOLD signal varies similarly for two regions—even when both are decreased or increased—the correlation between those two regions will remain. In other words, those regions may be synchronized but with pathologic intrinsic activation, and it is within this context that the ALFF measures can shed some light in fMRI research.
Many studies have investigated the DMN synchrony (connectivity) and a few others have explored the DMN intrinsic activity (ALFFs) but, as far as we know, none have explored the relationship between them in AD and aMCI. Recent studies, for instance, have reported an overlap between changes in regional ALFFs and functional connectivity in several brain regions in stuttering (Xuan et al., 2012) and seasonal affective disorder subjects (Abou Elseoud et al., 2014), which supports a relationship between ALFFs and functional connectivity. In this study, we aimed to explore the differences in DMN regional spontaneous activity, measured by ALFFs, between aMCI subjects, mild AD patients, and healthy elderly subjects, and to examine its relationship with connectivity. To best evaluate the regional differences, we divided DMN into four subregions: the ventromedial prefrontal cortex, medial parietal cortex (PCC+precuneus), inferior parietal lobe, and medial temporal lobe. We also aimed to analyze the possible correlations between global cognition and episodic memory performance of these groups with DMN intrinsic activity and functional connectivity.
Methods
Subjects
Participants were recruited in the Neuropsychology and Dementia outpatient clinic of the Universidade Estadual de Campinas (UNICAMP) University Hospital. Eighty-one subjects were evaluated in this study: 29 healthy elderly subjects (controls), 20 aMCI patients, and 32 mild AD patients. Experienced attending doctors and neuropsychologists made the diagnosis of the AD patients according to the National Institute of Aging and Alzheimer's Association criteria for a diagnosis of probable AD (McKhann, 2011). Examination of each subject included medical history, neurological examination, neuropsychological and neuropsychiatric assessment, Clinical Dementia Rating (CDR) (Morris, 1993), a Hachinski ischemic score ≤4, and standard laboratory tests, including B12, folate, and thyroid hormone levels, and syphilis serology. The study included only AD patients who were classified as CDR 1.
aMCI patients were diagnosed using the core criteria of the National Institute of Aging/Alzheimer's Association for MCI (Albert et al., 2011). All aMCI participants had a CDR score of 0.5 (with an obligatory memory score of 0.5). This classification was performed using a semistructured interview.
Controls were identified as cognitively normal: they did not exhibit any neurological or psychiatric disorders or require psychoactive medication; they demonstrated normal Mini Mental State Examination (MMSE) scores, considering age and education (Brucki et al., 2003); and their structural images were without any abnormalities. Memory complaints or neurological deficits were not observed in the control group.
Exclusion criteria for all subjects included the following: a history of other neurological or psychiatric diseases or a head injury with loss of consciousness, use of sedative drugs in the last 24 h before the neuropsychological assessment, drug or alcohol addiction, prior chronic exposure to neurotoxic substances, and a Hachinski ischemic score of >4. This study was approved by the Medical Research Ethics Committee of UNICAMP, and written informed consent (either from the subjects or from their responsible guardians, if incapable) was obtained from all participants before study initiation, according to the Declaration of Helsinki.
Neuropsychological evaluation
An experienced neuropsychologist, who was blinded to MRI data, performed the neuropsychological evaluations. Global cognitive status was measured using the MMSE (Brucki et al., 2003; Folstein et al., 1975) and episodic memory was evaluated by the Rey Auditory Verbal Learning Test (RAVLT) (subitems: encoding, delayed recall, and recognition) (Malloy-Diniz et al., 2007). Visual perception was assessed with subtests of Luria's neuropsychological investigation (Christensen, 1975). Evaluation of language included the Boston naming test (Kaplan et al., 1983), verbal fluency for category (animals), and phonologic fluency (FAS) (Christensen and Guilford, 1959). We assessed constructive praxis with the Rey-Osterrieth complex figure test (Osterrieth, 1944); executive function with the trail making test A and B, the Stroop color-word test (congruent and incongruent) (Stroop, 1935), and the clock drawing test (Sunderland et al., 1989); and working memory with the forward and backward digit span subtest of the Wechsler Adult Intelligence Scale (WAIS) (Wechsler, 1987).
RsfMRI data acquisition
All imaging was performed on a 3.0 T Philips Achieva MRI scanner. Foam padding was provided for comfort and to minimize head motion. Functional MR images were acquired while at rest; subjects were instructed to keep their eyes closed, to avoid initiating goal-directed, attention-demanding activity during the scanning sessions, and to remain awake. The following protocol was applied to each subject: (1) functional images: axial T2*-weighted images (TR/TE=2000/30 ms, FOV=240×240, and isotropic voxels set to 3×3×3 mm3); for each participant, we acquired 6 min of EPI data, which corresponds to 180 volumes with 40 axial slices each. (2) Structural images: (a) sagittal high-resolution T1-weighted (gradient echo images, TR/TE=7/3.2 ms, FOV=240×240 and isotropic voxels of 1 mm3); (b) coronal and axial fluid-attenuated inversion recovery (FLAIR) T2-weighted images, anatomically aligned at the hippocampus (TR/TE/TI=12,000/140/2850 ms, FOV=220×206, voxels reconstructed to 0.45×0.45×4.0 mm3, and the gap between slices set to 1 mm); (c) coronal IR (inversion recovery) T1-weighted images (TR/TE/TI=3550/15/400 ms, FOV=180×180, and voxels reconstructed to 0.42×0.42×3.00 mm3); and (d) coronal multi-echo (five echos) T2-weighted images (TR/TE=3300/30, FOV=180×180, and voxels reconstructed to 0.42×0.42×3.00 mm3).
All subjects underwent MRI scanning in the same week that neuropsychological assessment was performed.
Functional connectivity analysis
Functional images were preprocessed by applying slice-time and motion correction algorithms and by removing linear trends. Data preprocessing also included smoothing, with a 6-mm full width at half maximum (FWHM), and bandpass filtering (0.01–0.08 Hz). For spatial normalization, structural images were first linearly registered to the MNI152 (standard space) with a 12-parameter affine transformation. The resulting image was again registered to the MNI152 space, now using a nonlinear warping algorithm. Functional data was initially registered to the structural image using a 6-parameter affine transformation, and then warped to standard space using the transformations calculated for the structural image. Six movement parameters (three translational and three rotational) were included as nuisance regressors aiming to directly correct to head motion noises. Also, participants were instructed to keep their eyes closed, relax, and move as little as possible. Foam pads were used to reduce head movements and scanner noise. The global signal time series of the cerebrospinal fluid and white matter were also included in the model, increasing the regression power to confounding signal components that arise from physiological events. All of these steps were performed using AFNI (http://afni.nimh.nih.gov/afni) and FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) software.
To identify the DMN, seed-based functional connectivity was calculated by placing a seed in the posterior cingulate cortex (0, −51, 15; MNI; seed radius=3 mm). The PCC has already been reported as a key node of the DMN (Greicius et al., 2003) and used as a seed region previously (Weiler et al., 2014; Wells et al., 2013). Specifically, for each subject, the average time course of voxels within this seed was extracted to generate a reference time-series. Each time series was then correlated with all the voxels within the brain for each subject. Subsequently, r-scores of each voxel were then transformed using Fisher's r-to-z method, so that these data could be used in parametric statistical analyses to obtain whole cortical statistical z-score maps.
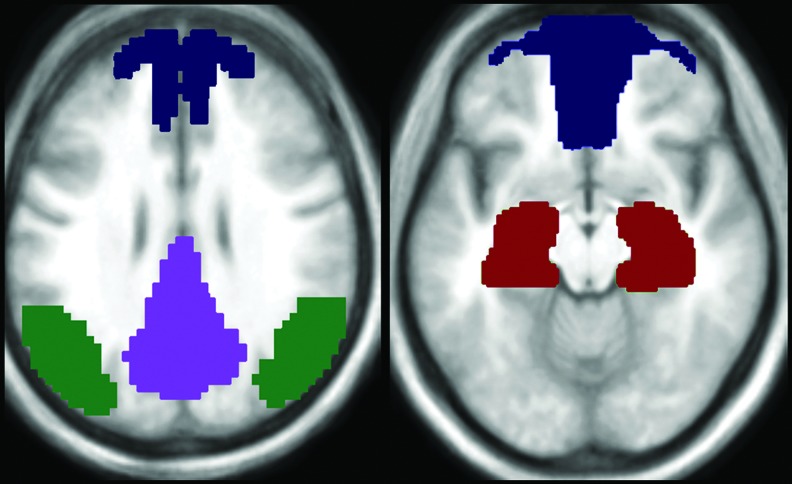
To calculate the DMN z-average, we used a mask of DMN based on our control subjects' statistical maps (z-score). These images were created according to the study methodology, with a seed placed on the PCC. All maps were used to create an average image that was smoothed (FWHM=6×6×6 mm3) and binarized using a minimum threshold of 0.3 (z-score value). This template was used as reference to define the DMN regions of each volunteer and to extract their average connectivity values. The volunteer's connectivity maps were not thresholded and all voxels with positive connectivity scores (higher than zero) that overlapped with the DMN template were included to calculate the average values. Additionally, the binarized DMN template was divided into four distinct subregions: the ventromedial prefrontal cortex, medial parietal cortex (PCC+precuneus), inferior parietal lobe, and medial temporal lobe; for this analysis, these subregions are referred to as “frontal,” “PCC,” “parietal,” and “temporal” subregions, respectively. These DMN subregion masks were used to overlap each statistical map and to calculate the average z-score value to each defined DMN region (Fig. 1).
FIG. 1.
Default mode network (DMN) subregion mask used to overlap each statistical map and to calculate the average z-score amplitude of low-frequency fluctuations (ALFFs) and functional connectivity measures. Dark blue corresponds to the ventromedial prefrontal cortex; purple corresponds to the medial parietal cortex (PCC+precuneus); green corresponds to the inferior parietal lobe; and red corresponds to the medial temporal lobe.
ALFF analysis
The ALFF preprocessing was performed with FSL processing tools, by using the script from the 1000 Functional Connectomes Project (www.nitrc.org/projects/fcon_1000). The preprocessing routine was based initially on the removing of the image spikes, removing of very low and high frequencies (0.01–0.08 Hz), linear-trend removing, spatial smoothing (FWHM=6 mm), and scaling to the grand mean, which aimed to put all time series on a common scale. The time series were transformed to frequency domain using fast Fourier transform (parameters: taper percent=0, fast Fourier transform length=shortest) to obtain the signal power spectrum. Then, this power spectrum was square-rooted and then averaged across 0.01–0.08 Hz at each voxel. ALFFs were calculated as the sum of the amplitudes in the low-frequency band. Subsequently, these data were transformed to z-score maps and normalized to standard space (MNI152) (Zuo et al., 2010). The same DMN mask described previously was used to calculate regional average z-score ALFFs of DMN and its subregions.
Statistical analysis
Statistical data analysis for functional connectivity and ALFF z-values of DMN subregions between groups, demographic and neuropsychological evaluation, and significance of correlations was performed using SPSS (version 20; SPSS, Inc., Chicago, IL) software. We first performed the Kolmogorov–Smirnov test to determine normality and as our data did not follow a normal distribution, we performed nonparametric tests. To examine the relationship between both functional analyses, we performed simple linear regressions between functional connectivity and ALFF z-values for each of the DMN subregions. At this stage, to increase our data variance, all individuals were combined into one unique group (normal aging, aMCI, and AD groups). In addition, simple linear regressions were performed among the MMSE and RAVLT (subitems: encoding, delayed recall, and recognition) scores, functional connectivity, and ALFF z-values. Results were considered to be statistically significant when p<0.05.
Results
Demographics and neuropsychological evaluation
Table 1 displays demographic and neuropsychological scores/statistics for healthy controls, aMCI patients, and mild AD patients in the sample. There were no differences across groups regarding age, years of education, and gender. aMCI patients performed worse than controls, and mild AD patients performed worse than healthy controls and aMCI patients in all tests.
Table 1.
Demographics and Neuropsychological Evaluation of Healthy Controls, aMCI, and AD Groups
| Controls | aMCI | Mild AD | |
|---|---|---|---|
| Age | 70.5 (6.81) | 67.9 (6.95) | 72.8 (6.67) |
| Sex (female) | 21 (72%) | 13 (65%) | 24 (75%) |
| Education (in years) | 10.6 (5.08) | 10 (5.2) | 7.4 (5.12) |
| MMSE | 28.66 (1.67) | 25.94 (2.62)a* | 19.86 (3.87)a**,b** |
| RAVLT-encoding | 44.9 (7.7) | 30.25 (7.02)a** | 19.9 (6.5)a**,b** |
| RAVLT-A7 | 8.59 (2.23) | 2.75 (2.02)a** | 0.59 (0.78)a**,b** |
| RC-FP | 11.66 (2.39) | 4 (5.93)a** | −3.45 (5.99)a**,b** |
| Forward digit span | 5.03 (1.78) | 4.94 (1.12) | 3.72 (1.51) |
| Backward digit span | 4.11 (1.29) | 3.81 (1.05) | 2.03 (1.50)a**,b** |
| Stroop C time | 56.62 (16.62) | 55.29 (11.57) | 73.21 (29.51)a**,b** |
| Stroop C errors | 0.08 (0.27) | 0.07 (0.27) | 0.42 (0.87) |
| Stroop I time | 106.92 (28.52) | 135.79 (42.46) | 179.10 (74.70)a**,b** |
| Stroop I errors | 2.8 (3.52) | 7.79 (8.39) | 28.79 (20.37)a**,b** |
| Semantic verbal fluency | 17.11 (4.47) | 12.93 (5.32) | 9.17 (4.69)a**,b** |
| Phonological verbal fluency | 33.07 (10.82) | 27.73 (11.79) | 19.24 (10.53)a**,b** |
| Luria's neuropsychological investigation | 18.29 (1.15) | 17.88 (1.41) | 15.31 (3.04)a**,b** |
| Clock drawing | 9.5 (1.35) | 9.53 (1.30) | 6.39 (2.81)a**,b** |
| Rey complex figure copy | 34.96 (3.43) | 32.37 (4.76) | 18.02 (14.18)a**,b** |
| Trail making test-A | 65.14 (17.75) | 81.13 (54.20) | 198.14 (98.50)a**,b** |
| Trail making test-B | 136.79 (93.85) | 161.47 (82.55) | 286.93 (42.38)a**,b** |
| Boston naming test | 52.04 (4.76) | 47.67 (10.42) | 36.45 (10.75)a**,b** |
Data presented as average (SD) except for sex.
Significantly different from controls.
Significantly different from amnestic mild cognitive impairment (aMCI).
p<0.05.
p<0.001.
aMCI, amnestic mild cognitive impairment subjects; mild AD, mild Alzheimer's disease patients; MMSE, Mini-Mental Status Examination; Stroop C, Stroop test congruent; Stroop I, Stroop test incongruent; RAVLT, Rey auditory verbal learning test; RAVLT-A7, delayed recall of Rey auditory verbal learning test; RC-FP, Rey auditory verbal learning test true recognition (i.e., recognition minus false positives).
ALFF z data
Compared with the healthy elderly subjects, the aMCI patients exhibited significantly decreased ALFF z-values in the temporal region (p=0.012). Compared with the AD patients, the aMCI patients exhibited greater values in the PCC region (p=0.007) but lower ALFF z-values in the temporal region (p=0.005). Only the mild AD group PCC exhibited a significantly lower standardized ALFFs when compared with control (p=0.007) (Table 2). Distribution of ALFF z-values across the three groups is demonstrated in Figure 2.
Table 2.
Values of the Amplitude of Low-Frequency Fluctuation Average z Scores in Controls, aMCI, and AD Patients
| Controls | aMCI | Mild AD | |
|---|---|---|---|
| Frontal ALFF z | 0.3798 (0.1907) | 0.5016 (0.3859) | 0.4229 (0.2255) |
| Temporal ALFF z | 0.6052 (0.1692) | 0.4518 (0.2594)a* | 0.6243 (0.2212)b* |
| Parietal ALFF z | 0.3856 (0.1895) | 0.4863 (0.2881) | 0.347 (0.1990) |
| PCC ALFF z | 0.8093 (0.3400) | 0.8025 (0.3241) | 0.5697 (0.2346)a*.b** |
| DMN ALFF z | 0.7093 (0.0877) | 0.8008 (0.2728) | 0.7238 (0.0631) |
Data presented as average (SD).
Significantly different from controls.
Significantly different from aMCI.
p<0.05.
p<0.001.
ALFFs, amplitude of low-frequency fluctuations; DMN, default mode network; PCC, posterior cingulate cortex.
FIG. 2.
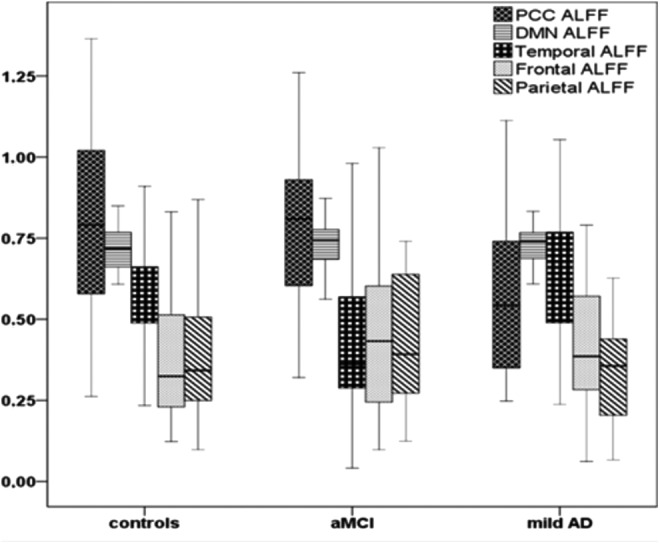
Box plot displaying medians, minimum, and maximum values of ALFF z data for the DMN and its subareas across controls, amnestic mild cognitive impairment (aMCI), and mild Alzheimer's disease (mild AD) patients.
Functional connectivity data
There was no significant difference between the connectivity in the aMCI group compared with controls, nor in the aMCI group compared with mild AD patients. The frontal (p<0.001), the PCC (p=0.004), and parietal (p=0.014) regions in mild AD patients showed decreased connectivity with the seed region when compared with the control group (Table 3). Distribution of functional connectivity data across the three groups is demonstrated in Figure 3.
Table 3.
Values of Functional Connectivity in Controls, aMCI, and AD Patients (Average z Scores)
| Controls | aMCI | mild AD | |
|---|---|---|---|
| Frontal connectivity | 0.348 (0.093) | 0.299 (0.108) | 0.259 (0.107)a* |
| Temporal connectivity | 0.212 (0.078) | 0.182 (0.069) | 0.176 (0.054) |
| Parietal connectivity | 0.368 (0.107) | 0.350 (0.103) | 0.306 (0.123)a* |
| PCC connectivity | 0.508 (0.120) | 0.472 (0.124) | 0.411 (0.105)a* |
| DMN connectivity | 0.249 (0.045) | 0.244 (0.063) | 0.221 (0.060) |
Data presented as average (SD).
Significantly different from controls.
p<0.05.
FIG. 3.
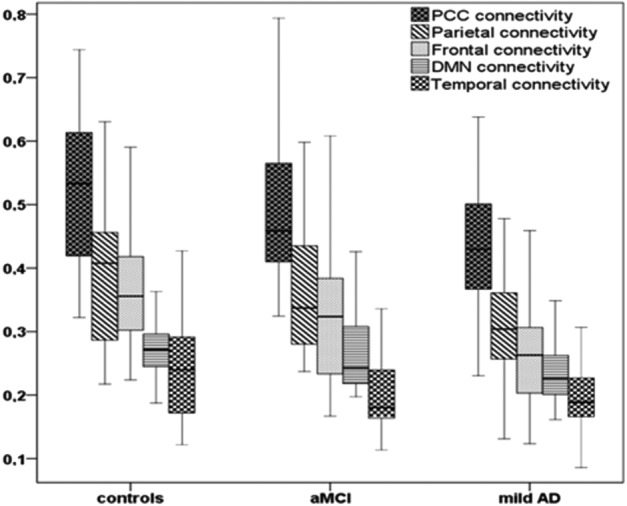
Box plot displaying medians, minimum, and maximum values of connectivity data for the DMN and its subareas across controls, amnestic mild cognitive impairment (aMCI), and mild Alzheimer's disease (mild AD) patients.
Relationship between functional connectivity and ALFF values
Significant results were observed mainly in the PCC subarea. For example, PCC ALFF z-value correlated to the frontal connectivity (r=0.289, p<0.05), to the parietal connectivity (r=0.272, p<0.05), and, as expected, to the PCC connectivity (r=0.487, p<0.001). Among the other subregions, also the parietal region showed a correlation between its ALFF z-value and the PCC (r=0.297, p<0.01) and parietal connectivities (r=0.221, p<0.05). No other subregion ALFF z-value correlated to any other subregion functional connectivity.
Relationship between cognitive performance and ALFF values
Simple linear regressions failed to show any statistically significant association between any score of the cognitive tests (MMSE and RALVT subitems: encoding, delayed recall, and recognition) and ALFF values.
Relationship between cognitive performance and functional connectivity values
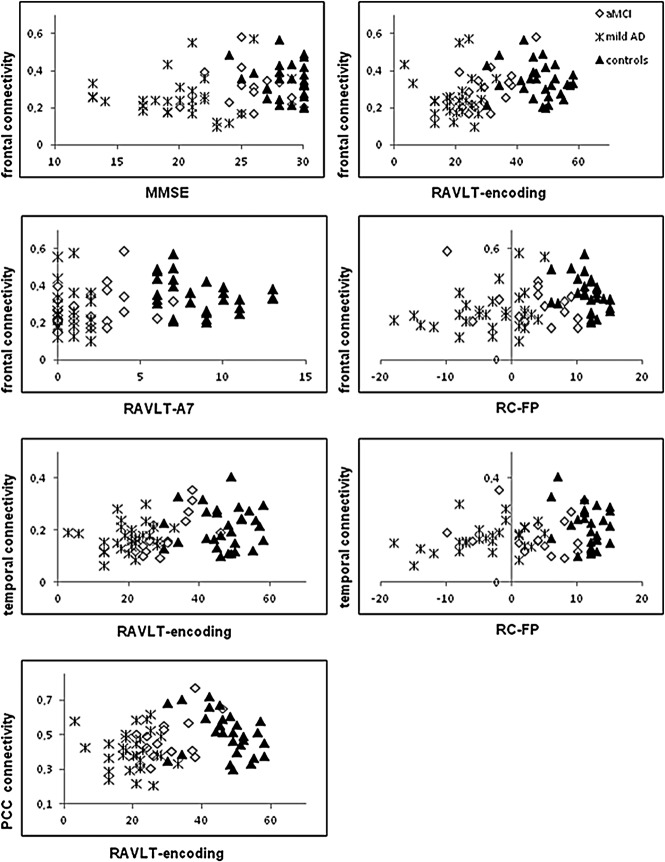
Simple linear regressions showed a statistically significant association between the scores of the MMSE (r=0.281, p<0.05) and RALVT subitems encoding (r=0.323, p<0.001), delayed recall (r=0.256, p<0.05), recognition (r=0.278, p<0.05), and frontal connectivity values. Temporal region connectivity was correlated to RAVLT subitems encoding (r=0.320, p<0.001) and recognition (r=0.237, p<0.05). PCC connectivity was associated with RAVLT encoding subitem (r=0.266, p<0.05). Please see Figure 4 for individual-group behavior.
FIG. 4.
Graphs showing the relationship between cognitive scores and subregions connectivity values. MMSE, Mini-Mental State Examination; RAVLT-encoding, Rey auditory verbal learning test subitem encoding; RAVLT-A7, delayed recall of Rey auditory verbal learning test; RC-FP, Rey auditory verbal learning test true recognition (i.e., recognition minus false positives).
Discussion
This study aimed to explore the differences in regional intrinsic activity (ALFFs) throughout DMN subregions between aMCI patients, mild AD patients, and controls, and to examine its relationship to the functional connectivity. We also aimed to analyze whether the memory performance and cognitive global status of our subjects were more related to DMN ALFFs or with functional connectivity values. Regarding the ALFF analysis, our results can be described as follows: (1) aMCI patients had lower values than controls in the temporal region and, surprisingly, lower than mild AD patients as well; (2) mild AD patients presented lower values in the PCC region compared with both controls and aMCI. Regarding functional connectivity data, (3) the aMCI group showed no significant difference compared with controls, nor with AD patients; (4) mild AD patients exhibited decreased connectivity in the frontal, parietal, and PCC regions compared with controls; and (5) MMSE and memory scores were not related to ALFF values, but only to the level of connectivity between regions (in which the connectivity between the frontal region with the PCC showed association with memory and MMSE tests). Based on these results, we can state that DMN connectivity problems in AD are associated with decreased functional activity in the medial parietal (“PCC”) region.
In the present study, the aMCI group showed significantly decreased ALFF values in the temporal region compared with the healthy elderly group, which likely reflects the neurophysiological alterations widely known to occur in this region, such as neurofibrillary tangles (Thangavel et al., 2009). Compared with the AD group, the aMCI patients presented higher ALFF values in the PCC region, and unexpected lower values in the temporal region. We could speculate that mild AD patients present a compensatory mechanism that increases the activity of the remaining neurons in the medial temporal region, which does not happen in the aMCI phase. Though not statistically significant, it is also interesting to note that aMCI patients exhibited greater ALFF values in relation to controls in the frontal and parietal regions, as well as the whole network, suggesting an incipient imbalance in the DMN activity even in the aMCI phase.
Using rsfMRI ALFF analysis, we found abnormal functional activity in mild AD patients in the PCC region, a region known to be key in the DMN. The DMN is already known as a brain system much like the motor or the visual systems, which contains a set of interacting brain regions that are functionally tightly connected and distinct from other systems within the brain. This network is more active during passive tasks than during goal-directed tasks, and is highly associated with reminiscence of past experiences, planning, and autobiographical episodes (Mazoyer et al., 2001). In particular, regions within the DMN show reduction of metabolic activity and atrophy in AD patients (Zhu et al., 2013), and are among the earliest to show abnormal amyloid deposition (Mintun et al., 2006).
Within this context, it is not surprising that the PCC of AD patients showed decreased ALFF values relative to controls, similarly to previous studies (Xi et al., 2012), and may be a reasonable explanation for the cognitive deficits presented in the disease. Interestingly, however, the cognitive scores did not correlate with ALFF values at all, contradicting previous studies (Liang et al., 2014; Liu et al., 2014a; Wang et al., 2011), but only with the connectivity values instead. One possible explanation for our results is that PCC ALFF values are altered in mild AD patients, either due to abnormal amyloid deposition, synaptic dysfunction, or metabolic changes, which leads to a disconnection with the parietal and frontal regions (and the latter causes cognitive problems). Therefore, our results indicate that the cognitive decline in mild AD patients is associated with disrupted functional connectivity between the two main hubs of the DMN, the frontal and the PCC regions, as previous studies have reported (Zhang 2009, 2010). Therefore, rather than the amplitude of DMN regions, the temporal synchrony of them—especially the frontal/PCC connection—is critical for normal cognitive functioning.
Another interesting finding of our study was the association between functional connectivity and ALFF scores, especially in the PCC. Very little is known about the influence of the ALFFs on functional connectivity measures, but a recent study with the healthy elderly population showed that they are in fact related to each other (Di et al., 2013). Likewise, our results showed that the functional connectivity is associated with values of local fluctuation amplitude also in AD. For instance, demented patients exhibited lower ALFF values in the PCC, which in turn was associated with the connectivity with the frontal, parietal, and PCC subareas. In other words, we could suppose that a diminished intrinsic activity in the PCC could lead to a lower connectivity of this area with the frontal and parietal subareas. Also, the parietal ALFF value (although not being statistically lower in AD) had a relationship with the diminished connectivity of the PCC and parietal subareas. Again, we could suppose that the PCC is a core region, important for maintaining the connectivity with many regions, and intrinsic abnormalities in this region may cause disconnection to many others.
There are limitations in the current work that must be highlighted. Because we did not evaluate AD biomarkers (beta-amyloid or total and phosphorylated tau), our aMCI patients may not necessarily be AD converters. This may explain our failure to detect an altered functional connectivity in this group. The replication of our findings longitudinally and the evaluation of AD biomarkers may produce better results.
Conclusions
In the present work, we found that aMCI subjects are characterized by a decrease in intrinsic functional activity in the medial temporal lobe, whereas the dementia stage is characterized by decreased activity in the PCC. Also, the regional BOLD signal amplitude is related to the functional connectivity of some areas, and alteration in the ALFF values of specific regions (such as the PCC) is related to disruption of the synchrony with the frontal and parietal areas. Finally, the cognitive decline observed in mild AD patients is modestly associated with disrupted functional connectivity of some regions (especially the frontal), rather than the intrinsic activity of them. These findings give us some evidence that AD is, among other physiopathological features, a disconnection syndrome.
Acknowledgment
The study was supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo). Grant No. 2011/17092-0 and No. 2013/10431-9.
Author Disclosure Statement
No competing financial interests exist.
References
- Abou Elseoud A, Nissila J, Liettu A, Remes J, Jokelainen J, Takala T, et al. 2014. Altered resting-state activity in seasonal affective disorder. Hum Brain Mapp 35:161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F, Pievani M, Geroldi C, Copetti M, Frisoni GB, Filippi M. 2012. Resting state fMRI in Alzheimer's disease: beyond the default mode network. Neurobiol Aging 33:1564–1578 [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. 2011. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. The Alzheimer's Association. Alzheimers Dement 7:270-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazar ML, Pereira FR, Lopes TM, da Silva EL, Coan AC, Campos BM, et al. 2014. Neuropsychiatric symptoms in Alzheimer's disease are related to functional connectivity alterations in the salience network. Hum Brain Mapp 35:1237–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier MR, Thomas JB, Snyder AZ, Benzinger TL, Zhang D, Raichle ME, et al. 2012. Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. J Neurosci 32:8890–8899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucki SM, Nitrini R, Caramelli P, Bertolucci PH, Okamoto IH. 2003. [Suggestions for utilization of the mini-mental state examination in Brazil]. Arq Neuropsiquiatr Brazil 61:777–781 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. 2008. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38 [DOI] [PubMed] [Google Scholar]
- Christensen A-L. 1975. Luria's Neuropsychological Investigation, Manual and Test Material. 4th ed. Copenhagen: Munksgaard [Google Scholar]
- Christensen P, Guilford J. 1959. Manual for the Christensen Guilford Fluency Tests. 2nd ed. Beverly Hills, CA: Sheridan Supply [Google Scholar]
- Di X, Kim EH, Huang CC, Tsai SJ, Lin CP, Biswal BB. 2013. The influence of the amplitude of low-frequency fluctuations on resting-state functional connectivity. Front Hum Neurosci 7:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. 1975. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198 [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. 2004. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A 101:4637–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Wang J, Zhao Z, Min B, Lu J, Li K, et al. 2011. Frequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: a resting-state fMRI study. Neuroimage 55:287–295 [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. 1983. The Boston Naming Test. 2nd ed. Philadelphia, PA: Febiger La [Google Scholar]
- Liang P, Xiang J, Liang H, Qi Z, Zhong N, Li K. 2014. Altered Amplitude of Low-Frequency Fluctuations in Early and Late Mild Cognitive Impairment and Alzheimer's Disease. Curr Alzheimer Res [Epub ahead of print]; DOI: 10.2174/1567205011666140331225335 [DOI] [PubMed] [Google Scholar]
- Liu X, Wang S, Zhang X, Wang Z, Tian X, He Y. 2014a. Abnormal amplitude of low-frequency fluctuations of intrinsic brain activity in Alzheimer's disease. J Alzheimers Dis 40:387–397 [DOI] [PubMed] [Google Scholar]
- Liu Y, Yu C, Zhang X, Liu J, Duan Y, Alexander-Bloch AF, et al. 2014b. Impaired long distance functional connectivity and weighted network architecture in Alzheimer's disease. Cereb Cortex 24:1422–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy-Diniz LF, Lasmar VA, Gazinelli Lde S, Fuentes D, Salgado JV. 2007. The Rey Auditory-Verbal Learning Test: applicability for the Brazilian elderly. Rev Bras Psiquiatr 29:324–329 [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, et al. 2001. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull 54:287–298 [DOI] [PubMed] [Google Scholar]
- McKhann GM. 2011. Changing concepts of Alzheimer disease. JAMA 305:2458–2459 [DOI] [PubMed] [Google Scholar]
- Menon V. 2011. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15:483–506 [DOI] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, et al. 2006. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 67:446–452 [DOI] [PubMed] [Google Scholar]
- Morris JC. 1993. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43:2412–2414 [DOI] [PubMed] [Google Scholar]
- Northoff G. 2013. What the brain's intrinsic activity can tell us about consciousness? A tri-dimensional view. Neurosci Biobehav Rev 37:726–738 [DOI] [PubMed] [Google Scholar]
- Osterrieth P. 1944. The test of copying a complex figure: a contribution to the study of perception and memory. Arch Psychol 30:206–356 [Google Scholar]
- Song J, Qin W, Liu Y, Duan Y, Liu J, He X, et al. 2013. Aberrant functional organization within and between resting-state networks in AD. PLoS One 8:e63727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop J. 1935. Studies of interference in serial verbal reactions. J Exp Psychol 18:643–662 [Google Scholar]
- Sunderland T, Hill JL, Mellow AM, Lawlor BA, Gundersheimer J, Newhouse PA, et al. 1989. Clock drawing in Alzheimer's disease. A novel measure of dementia severity. J Am Geriatr Soc 37:725–729 [DOI] [PubMed] [Google Scholar]
- Thangavel R, Van Hoesen GW, Zaheer A. 2009. The abnormally phosphorylated tau lesion of early Alzheimer's disease. Neurochem Res 34:118–123 [DOI] [PubMed] [Google Scholar]
- Wang Z, Yan C, Zhao C, Qi Z, Zhou W, Lu J, et al. 2011. Spatial patterns of intrinsic brain activity in mild cognitive impairment and Alzheimer's disease: a resting-state functional MRI study. Hum Brain Mapp 32:1720–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 1987. Manual for the Wechsler Memory Scale-Revised (WMS-R). San Antonio: The Psychological Corporation [Google Scholar]
- Weiler M, Fukuda A, Massabki LH, Lopes TM, Franco AR, Damasceno BP, et al. 2014. Default mode, executive function, and language functional connectivity networks are compromised in mild Alzheimer's disease. Curr Alzheimer Res 11:274–282 [DOI] [PubMed] [Google Scholar]
- Wells RE, Yeh GY, Kerr CE, Wolkin J, Davis RB, Tan Y, et al. 2013. Meditation's impact on default mode network and hippocampus in mild cognitive impairment: a pilot study. Neurosci Lett 556:15–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Q, Zhao XH, Wang PJ, Guo QH, Yan CG, He Y. 2012. Functional MRI study of mild Alzheimer's disease using amplitude of low frequency fluctuation analysis. Chin Med J 125:858–862 [PubMed] [Google Scholar]
- Xia M, Wang Z, Dai Z, Liang X, Song H, Shu N, et al. 2014. Differentially disrupted functional connectivity in posteromedial cortical subregions in Alzheimer's disease. J Alzheimers Dis 39:527–543 [DOI] [PubMed] [Google Scholar]
- Xuan Y, Meng C, Yang Y, Zhu C, Wang L, Yan Q, et al. 2012. Resting-state brain activity in adult males who stutter. PLoS One 7:e30570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, et al. 2007. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev 29:83–91 [DOI] [PubMed] [Google Scholar]
- Zhang HY, Wang SJ, Liu B, Ma ZL, Yang M, Zhang ZJ, et al. 2010. Resting brain connectivity: changes during the progress of Alzheimer disease. Radiology 256:598–606 [DOI] [PubMed] [Google Scholar]
- Zhang HY, Wang SJ, Xing J, Liu B, Ma ZL, Yang M, et al. 2009. Detection of PCC functional connectivity characteristics in resting-state fMRI in mild Alzheimer's disease. Behav Brain Res 197:103–108 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Lu J, Jia X, Chao W, Han Y, Jia J, et al. 2014. Selective changes of resting-state brain oscillations in aMCI: an fMRI Study using ALFF. Biomed Res Int 2014:920902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu DC, Majumdar S, Korolev IO, Berger KL, Bozoki AC. 2013. Alzheimer's disease and amnestic mild cognitive impairment weaken connections within the default-mode network: a multi-modal imaging study. J Alzheimers Dis 34:969–984 [DOI] [PubMed] [Google Scholar]
- Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, et al. 2010. The oscillating brain: complex and reliable. Neuroimage 49:1432–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]