Abstract
Considerable evidence suggests that depression is related to interhemispheric functional coordination deficits. For depression, electroconvulsive therapy (ECT) is the most rapid and effective therapy, but its underlying mechanism remains unknown. The aim of this study was to explore the impact of ECT on the interhemispheric functional coordination in depression patients. We used resting-state functional magnetic resonance imaging to observe the change of interhemispheric functional coordination with the method of voxel-mirrored homotopic connectivity (VMHC) in 11 depressed patients before and after ECT, compared with 15 healthy controls. The results showed that, compared with depression patients before ECT, VMHC was significantly increased in superior frontal gyri (BA 8), middle frontal gyri (two clusters: BA 8/9 and BA 10) and angular gyri (BA 39) in depression patients after ECT. Compared with healthy controls, VMHC in those areas was significantly lower in the middle frontal gyri (BA 8/9) and angular gyri (BA 39) in depression patients before ECT, but no significant difference was observed in the superior frontal gyri (BA 8) and middle frontal gyri (BA 10). There was no significant correlation between the changes of Hamilton Depression Rating Scale scores and changed VMHC values in those four areas in depression patients. The results suggest that ECT selectively modulated interhemispheric functional coordination in depression patients. Such may play an important mechanistic role in the treatment of depression, and may afford a useful avenue for optimizing treatment.
Introduction
Depression is a chronic psychiatric disorder typically characterized by sadness, despair, insomnia and low self-esteem. More importantly, severe depression can result in death and disability. It has been estimated that depression will be the second most prominent root of disability worldwide by 2020.1
Considerable evidence suggests that depression is related to interhemispheric functional coordination deficits. Electroencephalography studies have shown depression to be associated with relative hypoactivation in the left frontal cortex, as compared with the corresponding region in the right hemisphere.2,3 Reduced coherence of the β and θ frequency bands between two hemispheres has also been found in depression patients.4 Positron-emission tomography, functional magnetic resonance imaging (fMRI) and visual evoked potentials studies have, in addition, demonstrated imbalanced activity in the homologous cortex.5, 6, 7 Furthermore, the neuropsychological studies have documented patients with left side strokes that might be associated with hypoactivity of the left hemisphere experienced depression at much higher rates than the general population.8,9 All these findings of interhemispheric imbalance support the existence of abnormalities of interhemispheric functional coordination in depression patients. In addition, the corpus callosum, the major white-matter tract connecting the two hemispheres, has also been found to show widely abnormal structure in depression patients, which may directly influence the interhemispheric coordination and integration of brain function.10, 11, 12, 13
For severely depressed patients and treatment-resistant depression patients, electroconvulsive therapy (ECT) is the most rapid and effective therapeutic technique, leading to remission in ~50–70% of such patients.14, 15, 16 Studies have consistently demonstrated that ECT is equal or superior to antidepressant medication for curative effect in treatment of depression.17, 18, 19, 20 However, little is known about the underlying therapeutic mechanisms of ECT, even though ECT has been applied in clinical practice for nearly 80 years.21 Our limited knowledge of the mechanisms hinders the optimization of ECT procedures for improving therapeutic efficacy and reducing side effects.
Recently, using the method of resting-state fMRI (rs-fMRI), Perrin et al.22 showed that, in depression patients, bilateral ECT significantly changed the average global functional connectivity of left prefrontal cortical regions, but not bilateral prefrontal cortical regions; that change was accompanied by a significant improvement in depressive symptoms. The bilateral ECT and the asymmetric result are consistent with the findings of interhemispheric imbalance described above. More importantly, that pattern of results implies an interhemispheric rebalancing effect of ECT for depression that occurs by modulating interhemispheric functional coordination.
Despite the implication of rebalanced interhemispheric functional coordination in ECT for depression, few studies have examined the interhemispheric functional coordination in depression patients with ECT. In the work reported here, we evaluated the rs-fMRI data of depression patients before and after ECT with the method of voxel-mirrored homotopic connectivity (VMHC).23 Our aim was to assess changes in interhemispheric functional coordination associated with ECT.
VMHC indexes the resting-state functional connectivity between each voxel in one hemisphere and its mirrored counterpart in the opposite hemisphere. Thus, the degree of connectivity would directly reflect interhemispheric functional coordination. This method has been successfully applied to explore the interhemispheric functional coordination in cocaine addiction, autism, schizophrenia and other disorders,24, 25, 26, 27, 28 and has been demonstrated to be an effective method for evaluating interhemispheric functional coordination.
Materials and methods
Participants
We recruited patients with diagnoses of depression referred for ECT by psychiatrists from the Anhui Mental Health Center. Patients were consecutively included in the study as they became available over the period between 2012 and 2014. The diagnoses of depression were established at the basis of Diagnostic and Statistical Manual of Mental Disorders-IV criteria.29 Patients who showed resistance to drug therapy or a severe suicidal tendency were referred for ECT. We excluded patients with ECT before the current course, substance dependence, pregnancy, life-threatening somatic disease, neurological disorders, other comorbid mental disorders or MRI-related contraindications. Twenty patients met the inclusion criteria. Of those patients, four refused to undergo MRI scanning and three did not complete the second MRI scanning. Thirteen patients completed the procedures of this study but two were later excluded for head movements during MRI scanning. At last, 11 patients remained for this study and all them continued to take antidepression drugs during ECT administration. One of the 11 patients were also receiving lithium therapy. Three of the 11 patients also took antipsychotic medication (quetiapine or aripiprazole).
We also recruited healthy control participants who met the same inclusion criteria as the depression patients, except for the diagnosis of depression. Eventually, we enrolled 15 control participants matched as a group for gender, age and education years. Every control participant completed the procedures of this study and none were excluded for head movements during MRI scanning. All the depression patients and healthy controls were right-handed.
All the patients and healthy controls were undertaken with the understanding and written consents. This study was approved by the Anhui Medical University Ethics Committee.
ECT procedures
Patients underwent modified bifrontal ECT, which was the standard at the Anhui Mental Health Center. We used a Thymatron System IV Integrated ECT Instrument (Somatics, Lake Bluff, IL, USA). All the ECT administrations were conducted in the Anhui Mental Health Center. The first three ECT administrations occurred on consecutive days, and the remaining ECT administrations were conducted every other day with a break of weekends until patients' symptoms remitted. If the patient was older than 50 years, the initial percent energy dial setting was to the patient's age (for example, 53% for a 53-year-old patient), and if not, the initial percent energy dial was setted as patient's age minus five (for example, 40% for a 45-year-old patient). If no seizure activity resulted, the percent energy would increase until a therapeutically satisfactory seizure was obtained. During each ECT procedure, patients were under propofol anesthesia. We administered succinylcholine and atropine to relax muscles and suppress the secretion of glands, and monitored seizure activity with electroencephalography.
Clinical measure
We administered the 17-item Hamilton Depression Rating Scale (HAMD)30 to assess depressive symptoms. Patients completed the scale 12–24 h before the first ECT and 24–72 h after the last ECT.
MRI data acquisition
All depression patients and healthy controls underwent the fMRI scans at the First Affiliated Hospital of Anhui Medical University. Two scans were perspectively performed at 12–24 h before ECT and 24–72 h after the last ECT for depression patients. Healthy controls just underwent one fMRI scan. We instructed all healthy controls and depression patients during the scan to keep their eyes closed and to relax, to remain awake and not to think of anything in particular. All scans used a clinical 3.0 T whole-body MRI scanner (Signa HDxt 3.0 T, GE Healthcare, Buckinghamshire, UK) with a standard echo planar imaging sequence. The resting-state functional images were recorded with the following parameters: repetition time/echo time ratio=2000/22.5 ms, flip angle=30 degrees, 33 slices, thickness/gap ratio=4.0/0.6 mm, voxel size=3.4 × 3.4 × 4.6 mm3, matrix size=64 × 64, field of view=220 × 220 mm2. T1-weighted anatomic images were acquired in sagittal orientation with three-dimensional inversion recovery prepared fast spoiled gradient recalled sequence (repetition time/echo time ratio=8.676/3.184 ms, inversion time=800 ms, flip angle=8 degrees, field of view=256 × 256 mm2, matrix size=256 × 256, slice thickness=1 mm, voxel size=1 × 1 × 1 mm3, sections=188).
Data processing
Functional image preprocessing
We used the Data Processing Assistant for Resting-State Functional MR Imaging toolkit (DPARSF),31 which is based on the Resting State Functional MR Imaging Toolkit (REST; http://www.restfmri.net)32 and statistical parametric mapping software package (SPM8; www.fil.ion.ucl.ac.uk/spm). To achieve stable longitudinal magnetization and allow for the participants to adapt to the scanning environment, we discarded the first 10 volumes of data. The remaining 230 volumes were processed (including slice timing correction, realignment, and head-motion correction), normalized to the standard Montreal Neurological Institute space and resampled at a resolution of 3 × 3 × 3 mm3. We excluded from further analysis subjects with head motion >2.0 mm of maximal displacement (in any direction: x, y or z) or 2.0 degrees of maximal rotation in any angular dimension. We then co-registered the individual T1 images to functional images. For the nominalization, T1 images were segmented (gray matter, white matter and cerebrospinal fluid) and normalized to the Montreal Neurological Institute space by using a 12-parameter nonlinear transformation. These transformation parameters were applied to the functional images. The functional images were smoothed with a Gaussian kernel of 6 mm at full-width at half-maximum. After linear detrending, data was band-pass filtered (0.01–0.08 Hz) to reduce low-frequency drift and high-frequency noise. Then several sources of spurious covariance were removed, including the six head-motion parameters obtained by rigid body correction, and signals from the white matter and cerebrospinal fluid.33
For the VMHC computation, we transformed the normalized functional images to a symmetric space with the following steps: (a) all the normalized gray matter images were averaged to create a mean image; (b) the generated mean image was then averaged with its left–right mirrored version to get a symmetrical template and mask for VMHC; and (c) individual normalized gray matter images were registered again to the generated symmetric template and applied to the normalized functional images with the nonlinear transformation.
Voxel-mirrored homotopic connectivity
The VMHC was also computed with the DPARSF software. For each subject, the Pearson correlations between the preprocessed time series of every pair of symmetrical interhemispheric voxels were calculated. The resulting correlation maps were then Fisher z-transformed for analysis. The unilateral hemispheric gray matter from the generated symmetric template was used as the mask for the statistical analyses of VMHC.
Statistical analysis
We analyzed clinical and demographic data using SPSS 19.0 (SPSS, Chicago, IL, USA). Paired two-sample t-test evaluated changes of VMHC before and after ECT using REST software. As the resting-state functional connectivity could be affected by micromovements from one time point to the next,34 the framewise displacement values were calculated for each subject with the method proposed by Jenkinson et al.35 And the mean framewise displacement was used as a nuisance covariant in the group comparisons of VMHC.36 We corrected for multiple comparisons of the connectivity results by Monte–Carlo simulation (see AlphaSim in Analysis of Functional NeuroImages (AFNI), http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf) within the unilateral hemisphere of the symmetric template. The VMHC data of the changed regions before and after ECT were extracted and compared with the healthy controls with independent two-sample t-test, and Pearson correlation analysis was used to explore the associations between the changed values of VMHC in changed regions and the reduction in the HAMD score before and after ECT, with P<0.05 set as the significance level. The metering data were presented as mean±s.d.
Results
Subject characterization and effects of ECT on depression patients
Eleven of the patients met the criteria were enrolled in the study. Compared with the nine patients who were excluded form the study, the 11 patients showed no significant differences in term of gender (patients in the study: seven males and four females; patients out of the study: four males and five females; P=0.653; Fisher's exact probability), age (patients in the study: 35.45±10.18 years; patients out of the study: 41.67±11.77 years; t=−1.266; P=0.222) and years of education (patients: 10.18±3.06 years; controls: 8.67±4.42 years; t=0.905; P=0.377). For the 11 patients enrolled in the study, they received ECT until the depressive symptoms remitted (mean number of treatments =6.82±2.40). There were no significant differences in term of gender (patients: seven males and four females; controls: ten males and five females; P>0.50; Fisher's exact probability), age (patients: 35.45±10.18 years, controls: 31.53±10.56 years; t=0.950; P=0.352) and years of education (patients: 10.18±3.06 years; controls: 11.60±2.75 years; t=−1.240; P=0.227) between depression patients and healthy controls in the study. Patients' mean HAMD score before treatment was 21.91±4.15, indicating severe depression, and the mean score after treatment was 3.91±2.39. This reduction in mean HAMD score of depressive symptoms (18.00±4.67) was statistically significant (t=12.786; P<0.001) and indicated excellent therapeutic effects of ECT.
ECT effects on VMHC in depression patients
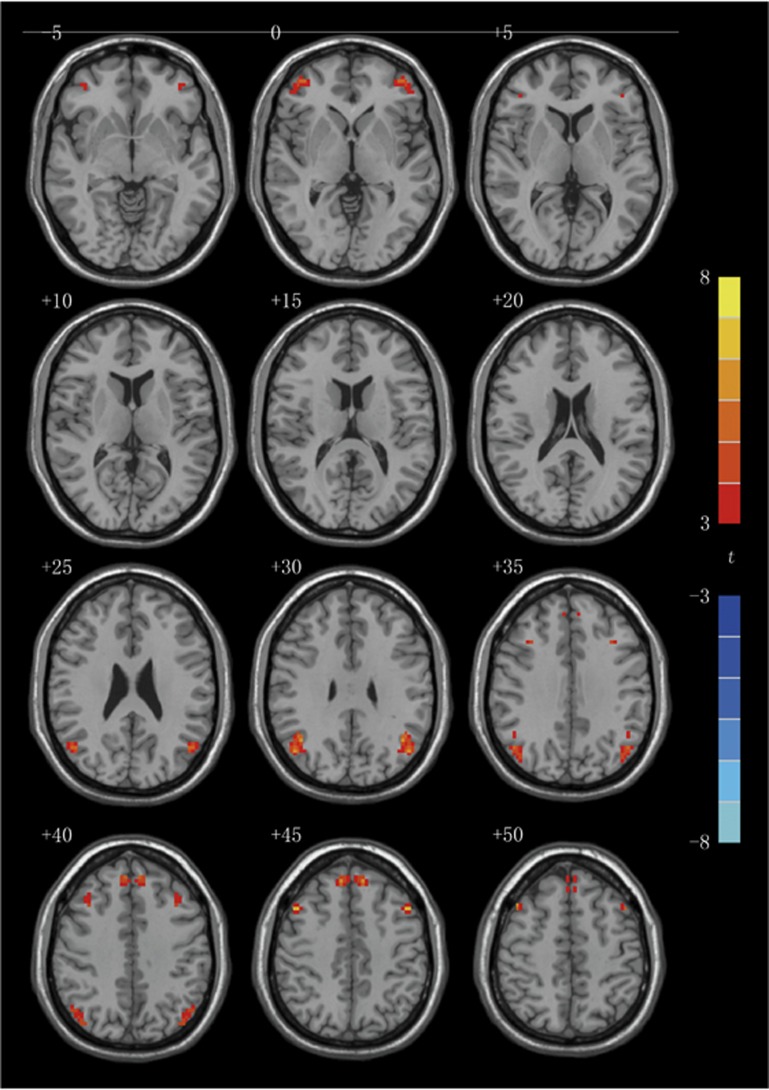
To identify potentially significant changes in VMHC between depression patients before and after ECT, the analysis of gray matter was performed correcting thresholds by the Monte–Carlo simulation with the Alphasim program in AFNI (P<0.05, corrected with P<0.01 for each voxel and a cluster volume ⩾32 voxels). As shown in Table 1 and Figure 1, VMHC was significantly increased in superior frontal gyri (BA 8), middle frontal gyri (two clusters: BA 8/9 and BA 10) and angular gyri (BA 39) in patients after ECT, compared with patients before ECT.
Table 1. Regions showing significant changes in VMHC between depression patients before and after ECT.
| Brain regions | BA | Voxel number | t-score | MNI coordinates (x, y, z) |
|---|---|---|---|---|
| Superior frontal gyri | 8 | 42 | 5.6490 | ±9, 42, 45 |
| Middle frontal gyri | 8/9 | 35 | 6.0747 | ±48, 15, 45 |
| 10 | 33 | 7.2623 | ±42, 51, -3 | |
| Angular gyri | 39 | 97 | 6.1551 | ±48, −66, 27 |
Abbreviations: BA, Brodmann area; ECT, electroconvulsive therapy; VHMC, voxel-mirrored homotopic connectivity; MNI coordinates, Montreal Neurological Institute coordinates of the peak voxel; t, statistical value of the peak voxel; 1 voxel=3 × 3 × 3 mm3. VMHC computation bases on a symmetrical template, so the MNI coordinates of the peak voxel is also symmetrical.
Figure 1.
Statistical maps of voxel t-values of voxel-mirrored homotopic connectivity comparisons of depression patients before and after electroconvulsive therapy. The numbers at the top left of images refer to the z-coordinates in Montreal Neurological Institute space. The threshold has been set at a corrected P<0.05 (corrected with P<0.01 for each voxel and a cluster volume ⩾32 voxels) and the t-score bar is shown at the right of the map. The left side of the each image corresponds to the right hemisphere of the brain and vice versa.
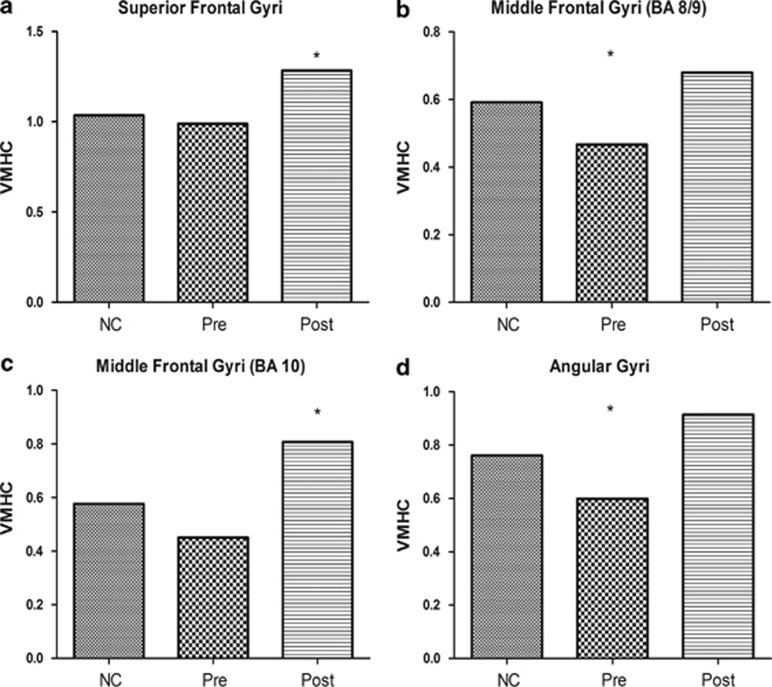
As stated above, we found the VMHC increased in four symmetrical clusters, but we did not know the VMHC of these clusters in health controls and the comparison with patients before and after ECT. So we saved the changed clusters (superior frontal gyri, middle frontal gyri and angular gyri) as a mask, and the VMHC data of the these clusters were extracted and compared (healthy controls vs patients before ECT and healthy controls vs patients after ECT). Compared with healthy controls, VMHC was significantly lower in the middle frontal gyri (BA 8/9) (t=−2.286; P=0.031) and the angular gyri (BA 39) (t=2.632; P=0.015) in patients before ECT, but no significant difference was observed in the superior frontal gyri (BA 8) (t=−0.540; P=0.594) and the middle frontal gyri (BA 10) (t=−1.650; P=0.112). VMHC in patients after ECT was significantly higher than in healthy controls in the superior frontal gyri (BA 8) (t=2.292; P=0.031) and middle frontal gyri (BA 10) (t=2.549; P=0.018). There was no significant difference in the middle frontal gyri (BA 8/9) (t=1.156; P=0.265) and angular gyri (BA 39) (t=1.740; P=0.095) between healthy controls and patients after ECT. Figure 2 shows these outcomes.
Figure 2.
The voxel-mirrored homotopic connectivity (VMHC) value of superior frontal gyri (BA 8), middle frontal gyri (two clusters: BA 8/9 and BA 10) and angular gyri (BA 39) in the three groups (healthy controls, patients before and after electroconvulsive therapy (ECT)). HC, healthy controls; Pre, depression patients before ECT; Post, depression patients after ECT. *There is a significant difference between the corresponding group and healthy controls (P<0.05). (a) Compared with healthy controls, VMHC of the superior frontal gyri (BA 8) was significantly higher in depression patients after ECT (t=2.292; P=0.031), but no significant difference was observed between healthy controls and depression patients before ECT (t=−0.540; P=0.594). (b) Compared with healthy controls, VMHC of the middle frontal gyri (BA 8/9) was significantly lower in depression patients before ECT (t=−2.286; P=0.031), but no significant difference was observed between healthy controls and depression patients after ECT (t=1.156; P=0.265). (c) Compared with healthy controls, VMHC of the middle frontal gyri (BA 10) was significantly higher in depression patients after ECT (t=2.549; P=0.018), but no significant difference was observed between healthy controls and depression patients before ECT (t=−1.650; P=0.112). (d) Compared with healthy controls, VMHC of the angular gyri (BA 39) was significantly lower in depression patients before ECT (t=2.632; P=0.015). No significant difference was observed between healthy controls and depression patients after ECT (t=1.740; P=0.095).
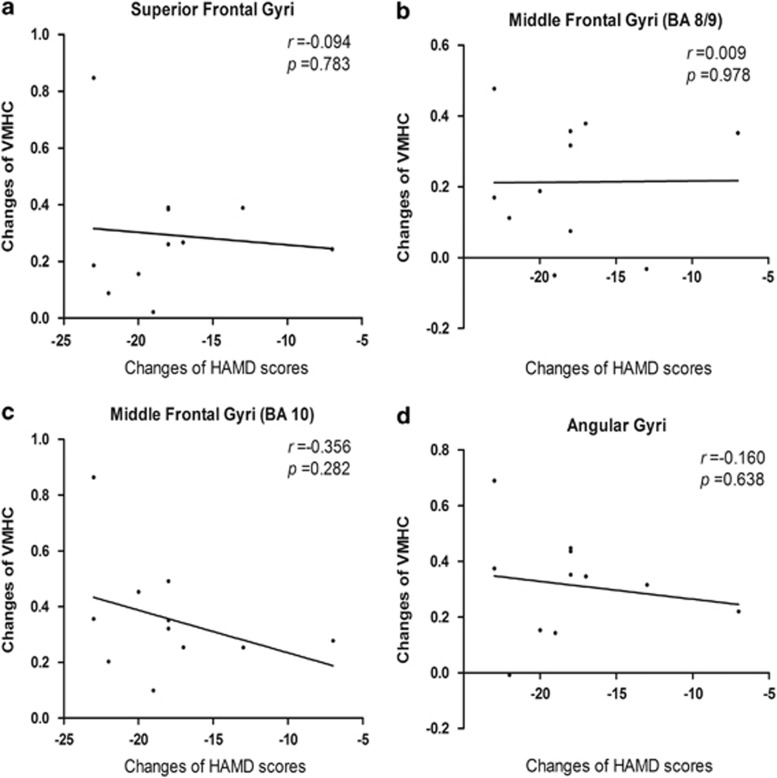
The correlation between changes of HAMD scores and changes of VMHC
We calculated changed VMHC values (VMHC after ECT minus VMHC before ECT) in the four significant clusters (superior frontal gyri (BA 8), middle frontal gyri (two clusters: BA 8/9 and BA 10) and angular gyri (BA 39)). Similarly, post-ECT scores minus pre-ECT scores indicated HAMD changes. No significant correlation was observed between the changes of HAMD scores and the changed VMHC values in the superior frontal gyri (BA 8) (r=−0.094; P=0.783), middle frontal gyri (BA 8/9) (r=0.009; P=0.978), middle frontal gyri (BA 10) (r=−0.356; P=0.282) or in the angular gyri (BA 39) (r=−0.160; P=0.638). The results were shown in Figure 3.
Figure 3.
The correlation between changes of Hamilton Depression Rating Scale (HAMD) scores and changes of voxel-mirrored homotopic connectivity (VMHC). (a) There was no significant correlation between the changes of HAMD scores and the changed VMHC values in superior frontal gyri (BA 8) (r=−0.094; P=0.783). (b) There was no significant correlation between the changes of HAMD scores and the changed VMHC values in the middle frontal gyri (BA 8/9) (r=0.009; P=0.978). (c) There was no significant correlation between the changes of HAMD scores and the changed VMHC values in the middle frontal gyri (BA 10) (r=−0.356; P=0.282). (d) There was no significant correlation between the changes of HAMD scores and the changed VMHC values in angular gyri (BA 39) (r=−0.160; P=0.638).
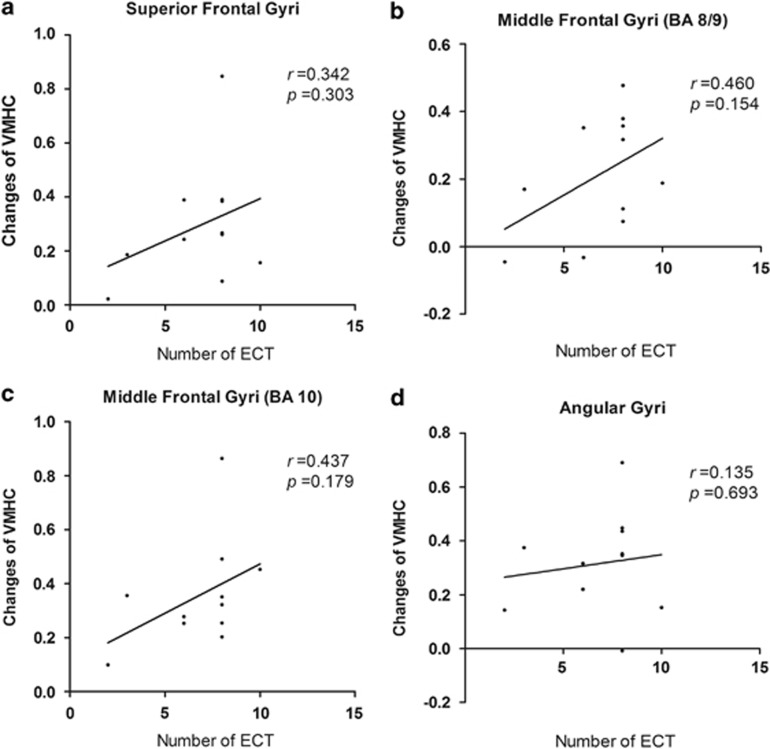
The correlation between number of ECT and changes of VMHC
To determine whether there is a dose effect of ECT, the Pearson correlation analysis was performed between the number of ECT and changes of VMHC (VMHC after ECT minus VMHC before ECT). The results showed that there were no significant correlation between the number of ECT and the changed VMHC values in the superior frontal gyri (BA 8) (r=0.342; P=0.303), middle frontal gyri (BA 8/9) (r=0.460; P=0.154), middle frontal gyri (BA 10) (r=0.437; P=0.179) or in the angular gyri (BA 39)(r=0.135; P=0.693). The results were shown in Figure 4.
Figure 4.
The correlation between number of electroconvulsive therapy (ECT) and changes of voxel-mirrored homotopic connectivity (VMHC). (a) There was no significant correlation between the number of ECT and the changed VMHC values in superior frontal gyri (BA 8) (r=0.342; P=0.303). (b) There was no significant correlation between the number of ECT and the changed VMHC values in the middle frontal gyri (BA 8/9) (r=0.460; P=0.154). (c) There was no significant correlation between the number of ECT and the changed VMHC values in the middle frontal gyri (BA 10) (r=0.437; P=0.179). (d) There was no significant correlation between the number of ECT and the changed VMHC values in angular gyri (BA 39) (r=0.135; P=0.693).
Discussion
The results demonstrate that the correlated activity between special homologous brain regions increased after ECT in patients with depression. We found significant increases in VMHC in the superior frontal gyri, middle frontal gyri and angular gyri, accompanied by significant reduction in HAMD scores in depression patients after ECT, compared with patient before ECT. Further, we showed that VMHC of middle frontal gyri (BA 8/9) and angular gyri (BA 10) was significantly lower in depression patients before ECT than in healthy controls. Collectively, these findings confirm both the existence of interhemispheric functional coordination deficits in depression patients, and that ECT can remediate to some degree those deficits. Taken together, this set of results demonstrates that modulation of interhemispheric functional coordination is a component of the therapeutic mechanisms of ECT for depression. In addition, these findings reinforce our understanding of depression psychopathology and have implications for the more focused and optimized electrical therapy on depression patients.
The middle frontal gyrus is associated with emotion regulation, while negative emotional bias is a typical characteristic of depression.37,38 The relationship between the middle frontal gyrus and depression is confirmed by many studies.39, 40, 41, 42, 43 These studies have reported reduced gray matter volume and altered functional activity of the middle frontal gyrus in depression patients. In addition, rs-fMRI studies have found decreased fractional amplitude of low-frequency fluctuations in the right middle frontal gyrus44 and increased fractional amplitude of low-frequency fluctuations in the left middle frontal gyrus45 in patients with depression, which indicates the imbalance of left and right middle frontal gyrus activity and the functional coordination deficit in the bilateral middle frontal gyri in depression patients. In our study, the results indicated that VMHC of the middle frontal gyri (BA 8/9) is significantly lower in depression patients before treatment than in healthy controls. The lower value for VMHC in the middle frontal gyri (BA 8/9) suggests lack of coordination and imbalance between the left and right middle frontal gyrus, a result consistent with the studies mentioned above. The result provides further evidence that depression is closely related with the functional coordination deficit of the bilateral middle frontal gyri. After ECT, the VMHC of the middle frontal gyri (two clusters: BA 8/9 and BA 10) significantly increased in depression patients and was accompanied by remission of depressive symptoms. The results presumably reflects that rebalancing of the left and right middle frontal gyri is vital for the therapeutic mechanisms of ECT.
Along with the middle frontal gyrus finding, we also observed increased VMHC in the superior frontal gyrus and angular gyrus after ECT. The abnormality of the superior frontal gyrus in depression has been consistently demonstrated by functional and structural imaging studies. Voxel-based morphometry studies46,47 report reduced gray matter volume of the superior frontal gyrus in depression patients. Also, by means of fMRI, the lower activation of the superior frontal gyrus has been observed in depression patients, especially in treatment-nonresponder or treatment-resistant patients.48, 49, 50 In our study, the VMHC of the superior frontal gyri was lower in depression patients than in healthy controls, but the difference did not reach statistical significance, which did not support the earlier studies. However, the significantly increased VMHC of the superior frontal gyri after ECT accompanied by remission of depressive symptoms is observed in our study. Incorporating our findings and the above-mentioned lower activation of the superior frontal gyrus that related to treatment nonresponse or treatment resistance in earlier studies,48, 49, 50 it suggests that the increased VMHC of the superior frontal gyri may contribute to the therapeutic effect of ECT for treatment-resistant depression.
As an important component of the default mode network, angular gyrus is involved in a variety of cognitive processes. It has also been found that the angular gyrus is involved in depression. Bench et al.51 have reported that the regional cerebral blood flow in the left angular gyrus decreased in depression patients. One rs-fMRI52 study has shown that the amplitude of low-frequency fluctuation in the left angular gyrus decreased in depression patients, and another study53 has reported that depression patients showed significantly decreased coherence-based regional homogeneity in the right angular gyrus. The observed changes in angular gyrus in depression patients present the characteristic of lateralization, which means that functional coordination between the left and right angular gyri is impaired in depression patients. Our results showed that the VMHC of the angular gyrus was significantly lower in depression patients before ECT than in healthy controls. Thus, this result in our study confirmed the impaired functional coordination of the angular gyri. We have also found that the VMHC of angular gyri increased significantly after ECT, which demonstrated that ECT treatment modulated the functional coordination of the angular gyrus in depression patients. Consequently, these findings indicate that the rebalance of left and right angular gyrus activity may also contribute to the therapeutic mechanism of ECT for depression.
Also, our study have shown that the the VMHC of superior frontal gyri (BA 8) and middle frontal gyri (BA 10) are significantly higher in patients after ECT than normal controls. For this result, it should be noticed that depression patients after ECT not only accompany by remitted depressive symptoms but also can appear cognitive side effects.54,55 Cognitive side effects associated with ECT are mainly limited to the first 3 days after the last ECT,55 when the second fMRI scan have been preformed for the depression patients in our study. That is to mean that patients after ECT is in an special status mixed with remitted depressive symptoms and cognitive abnormalities, and they are different form the normal controls. The special status may contribute to the higher VMHC of superior frontal gyri and middle frontal gyri (BA 10) in patients after ECT. And we should note that the cognitive abnormalities related to ECT is reversible, which improve in 15 days after the last ECT.55 For further study, the third fMRI scan could be performed at 15 days after the last ECT to clarify the change of VMHC.
In addition, our results showed that there was no significant correlation between the number of ECT and the changed VMHC values in the superior frontal gyri (BA 8), middle frontal gyri (two clusters: BA 8/9 and BA 10) or in the angular gyri (BA 39). It suggested that there was no dose effect between the number of ECT and the changed VMHC values. As the changes of VMHC are related to the therapeutic effect of ECT for depression, it is worth to explore the factors correlated with the VMHC values in further studies.
For our study, several practical constraints should be considered for our findings. First, only bifrontal ECT was used in our study. Results conceivably might have been altered under a different protocol (bilateral ECT, right unilateral ECT and focal electrically administered seizure therapy). Our hypothesis needs to be validated in various ECT protocols prospectively. Of course, the study of Perrin et al.22 supports our hypothesis in which bilateral ECT was used. Second, the calculation of VMHC was based on a symmetric template, but the brain is not absolutely symmetric. The bias caused by the symmetric template should be considered. Third, the sample size is small in our study and it limited the statistical power of our results. To further unravel the mechanism of ECT, a larger sample size is needed.
In summary, the results support the suggestion that the psychopathology of depression arises, at least in part, from interhemispheric functional coordination deficits. The findings reported here confirm our hypothesis that the interhemispheric rebalance and modulation of interhemispheric function coordination are involved in the therapeutic effect of ECT for depression. Our results, therefore, offer a powerful explanation for the mode of ECT action. That explanation may inform future efforts to optimize ECT protocols and enhance the ECT therapeutic effect.
Acknowledgments
This work was supported by the Key Project of the National Natural Science Foundation of China (91232717), the National Basic Research Program of China (2011CB707805) and the National Natural Science Foundation of China (81171273, 81100806 and 81471117).
The authors declare no conflict of interest.
References
- Michaud CM, Murray CJ, Bloom BR. Burden of disease — implications for future research. JAMA. 2001;285:535–539. doi: 10.1001/jama.285.5.535. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. J Abnorm Psychol. 1991;100:535–545. doi: 10.1037//0021-843x.100.4.535. [DOI] [PubMed] [Google Scholar]
- Flor-Henry P, Lind JC, Koles ZJ. A source-imaging (low-resolution electromagnetic tomography) study of the EEGs from unmedicated males with depression. Psychiatry Res. 2004;130:191–207. doi: 10.1016/j.pscychresns.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Armitage R, Roffwarg HP, Rush AJ, Calhoun JS, Purdy DG, Giles DE. Digital period analysis of sleep EEG in depression. Biol Psychiatry. 1992;31:52–68. doi: 10.1016/0006-3223(92)90006-l. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Phelps ME, Mazziotta JC, Schwartz JM, Grener RH, Selin CE, et al. Cerebral metabolic rates for glucose in mood disorders: studies with positron emission tomography and fluorodeoxyglucose F18. Arch Gen Psychiatry. 1985;42:441–447. doi: 10.1001/archpsyc.1985.01790280019002. [DOI] [PubMed] [Google Scholar]
- Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, et al. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiatry. 2008;63:369–376. doi: 10.1016/j.biopsych.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Janocha A, Pilecki W, Bolanowski M, Małyszczak K, Salomon E, Laszki-Szczachor K, et al. Interhemispheric cerebral asymmetry detected by VEPS in diabetic patients with recognized depression. Neuro Endocrinol Lett. 2009;30:119–124. [PubMed] [Google Scholar]
- Starkstein SE, Robinson RG. Affective disorders and cerebral vascular disease. Br J Psychiatry. 1989;154:170–182. doi: 10.1192/bjp.154.2.170. [DOI] [PubMed] [Google Scholar]
- Wexler BE. Cerebral laterality and psychiatry: a review of the literature. Am J Psychiatry. 1980;137:279–291. doi: 10.1176/ajp.137.3.279. [DOI] [PubMed] [Google Scholar]
- Ma N, Li L, Shu N, Liu J, Gong G, He Z, et al. White matter abnormalities in first-episode, treatment-naive young adults with major depressive disorder. Am J Psychiatry. 2007;164:823–826. doi: 10.1176/ajp.2007.164.5.823. [DOI] [PubMed] [Google Scholar]
- Aghajani M, Veer IM, van Lang ND, Meens PH, van den Bulk BG, Rombouts SA, et al. Altered white-matter architecture in treatment-naive adolescents with clinical depression. Psychol Med. 2013;16:1–12. doi: 10.1017/S0033291713003000. [DOI] [PubMed] [Google Scholar]
- Cole J, Chaddock CA, Farmer AE, Aitchison KJ, Simmons A, McGuffin P, et al. White matter abnormalities and illness severity in major depressive disorder. Br J Psychiatry. 2012;201:33–39. doi: 10.1192/bjp.bp.111.100594. [DOI] [PubMed] [Google Scholar]
- Xu K, Jiang W, Ren L, Ouyang X, Jiang Y, Wu F, et al. Impaired interhemispheric connectivity in medication-naive patients with major depressive disorder. J Psychiatry Neurosci. 2013;38:43–48. doi: 10.1503/jpn.110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Task Force on Electroconvulsive Therapy The practice of ECT: recommendations for treatment, training and privileging. Convuls Ther. 1990;6:85–120. [PubMed] [Google Scholar]
- Roose SP, Nobler M. ECT and onset of action. J Clin Psychiatry. 2001;62 (Suppl 4:24–26. [PubMed] [Google Scholar]
- Husain SS, Kevan IM, Linnell R, Scott AI. Electroconvulsive therapy in depressive illness that has not responded to drug treatment. J Affect Disord. 2004;83:121–126. doi: 10.1016/j.jad.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Merkl A, Heuser I, Bajbouj M. Antidepressant electroconvulsive therapy: mechanism of action, recent advances and limitations. Exp Neurol. 2009;219:20–26. doi: 10.1016/j.expneurol.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Janicak PG, Davis JM, Gibbons RD, Ericksen S, Chang S, Gallagher P. Efficacy of ECT: a meta-analysis. Am J Psychiatry. 1985;142:297–302. doi: 10.1176/ajp.142.3.297. [DOI] [PubMed] [Google Scholar]
- Pagnin D, de Queiroz V, Pini S, Cassano GB. Efficacy of ECT in depression: a meta-analytic review. J ECT. 2004;20:13–20. doi: 10.1097/00124509-200403000-00004. [DOI] [PubMed] [Google Scholar]
- Prudic J, Haskett RF, Mulsant B, Malone KM, Pettinati HM, Stephens S, et al. Resistance to antidepressant medications and short-term clinical response to ECT. Am J Psychiatry. 1996;153:985–992. doi: 10.1176/ajp.153.8.985. [DOI] [PubMed] [Google Scholar]
- Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357:1939–1945. doi: 10.1056/NEJMct075234. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Merz S, Bennett DM, Currie J, Steele DJ, Reid IC, et al. Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proc Natl Acad Sci USA. 2012;109:5464–5468. doi: 10.1073/pnas.1117206109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, et al. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. 2010;30:15034–15043. doi: 10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Zuo XN, Gotimer K, Cox CL, Lynch L, Brock D, et al. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol Psychiatry. 2011;69:684–692. doi: 10.1016/j.biopsych.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Druzgal TJ, Froehlich A, DuBray MB, Lange N, Alexander AL, et al. Decreased interhemispheric functional connectivity in autism. Cereb Cortex. 2011;21:1134–1146. doi: 10.1093/cercor/bhq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, Zuo XN, D'Angelo D, Mauro CJ, Butler PD, Milham MP, et al. Decreased interhemispheric coordination in schizophrenia: a resting state fMRI study. Schizophr Res. 2012;141:1–7. doi: 10.1016/j.schres.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Milham M, Zuo XN, Kelly C, Jaggi H, Herbert J, et al. Functional homotopic changes in multiple sclerosis with resting-state functional MR imaging. AJNR Am J Neuroradiol. 2013;34:1180–1187. doi: 10.3174/ajnr.A3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr C, Morioka R, Behan B, Datwani S, Doucet M, Ivanovic J, et al. Altered resting-state connectivity in adolescent cannabis users. Am J Drug Alcohol Abuse. 2013;39:372–381. doi: 10.3109/00952990.2013.848213. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders4th edn., American Psychiatric Press: Washington, DC; USA, 1994 [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for "Pipeline" Data Analysis of Resting-State fMRI. Front Syst Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, et al. a comprehensive assessment of regional variation in the impact of head micro movements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl P, Meindl T, Meister F, Born C, Engel RR, Reiser M, et al. Neurobiological underpinnings of shame and guilt: a pilot fMRI study. Soc Cogn Affect Neurosci. 2014;9:150–157. doi: 10.1093/scan/nss114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SW, Norbury R, Goodwin GM, Harmer CJ. Risk for depression and neural responses to fearful facial expressions of emotion. Br J Psychiatry. 2009;194:139–145. doi: 10.1192/bjp.bp.107.047993. [DOI] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, et al. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord. 2009;118:69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe O, Yamasue H, Kasai K, Yamada H, Aoki S, Inoue H, et al. Voxel-based analyses of gray/white matter volume and diffusion tensor data in major depression. Psychiatry Res. 2010;181:64–70. doi: 10.1016/j.pscychresns.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Kong L, Wu F, Tang Y, Ren L, Kong D, Liu Y, et al. Frontal-subcortical volumetric deficits in single episode, medication-naive depressed patients and the effects of 8 weeks fluoxetine treatment: a VBM-DARTEL study. PLoS One. 2009;9:e79055. doi: 10.1371/journal.pone.0079055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CH, Wu YT. Frontal-insula gray matter deficits in first-episode medication-naïve patients with major depressive disorder. J Affect Disord. 2014;160:74–79. doi: 10.1016/j.jad.2013.12.036. [DOI] [PubMed] [Google Scholar]
- Wang L, LaBar KS, Smoski M, Rosenthal MZ, Dolcos F, Lynch TR, et al. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Res. 2008;163:143–155. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Ma X, Wu X, Fan TT, Zhang Y, Zhou FC, et al. Resting-state brain activity in major depressive disorder patients and their siblings. J Affect Disord. 2013;149:299–306. doi: 10.1016/j.jad.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Liu F, Guo W, Liu L, Long Z, Ma C, Xue Z, et al. Abnormal amplitude low-frequency oscillations in medication-naive, first-episode patients with major depressive disorder: a resting-state fMRI study. J Affect Disord. 2013;146:401–406. doi: 10.1016/j.jad.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Li CT, Lin CP, Chou KH, Chen IY, Hsieh JC, Wu CL, et al. Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. Neuroimage. 2010;50:347–356. doi: 10.1016/j.neuroimage.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Serra-Blasco M, Portella MJ, Gómez-Ansón B, de Diego-Adeliño J, Vives-Gilabert Y, Puigdemont D, et al. Effects of illness duration and treatment resistance on grey matter abnormalities in major depression. Br J Psychiatry. 2013;202:434–440. doi: 10.1192/bjp.bp.112.116228. [DOI] [PubMed] [Google Scholar]
- Guo WB, Liu F, Chen JD, Gao K, Xue ZM, Xu XJ, et al. Abnormal neural activity of brain regions in treatment-resistant and treatment-sensitive major depressivedisorder: a resting-state fMRI study. J Psychiatr Res. 2012;46:1366–1373. doi: 10.1016/j.jpsychires.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Wang L, Li K, Zhang Q, Zeng Y, Dai W, Su Y, et al. Short-term effects of escitalopram on regional brain function in first-episode drug-naive patients with major depressive disorder assessed by resting-state functional magnetic resonance imaging. Psychol Med. 2013;2013 13:1–10. doi: 10.1017/S0033291713002031. [DOI] [PubMed] [Google Scholar]
- Samson AC, Meisenzahl E, Scheuerecker J, Rose E, Schoepf V, Wiesmann M, et al. Brain activation predicts treatment improvement in patients with major depressive disorder. J Psychiatr Res. 2011;45:1214–1222. doi: 10.1016/j.jpsychires.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Friston KJ, Brown RG, Frackowiak RS, Dolan RJ. Regional cerebral blood flow in depression measured by positron emission tomography: the relationship with clinical dimensions. Psychol Med. 1993;23:579–590. doi: 10.1017/s0033291700025368. [DOI] [PubMed] [Google Scholar]
- Fan T, Wu X, Yao L, Dong J. Abnormal baseline brain activity in suicidal and non-suicidal patients with major depressive disorder. Neurosci Lett. 2013;534:35–40. doi: 10.1016/j.neulet.2012.11.032. [DOI] [PubMed] [Google Scholar]
- Liu F, Hu M, Wang S, Guo W, Zhao J, Li J, et al. Abnormal regional spontaneous neural activity in first-episode, treatment-naive patients with late-life depression: a resting-state fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:326–331. doi: 10.1016/j.pnpbp.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Crowley K, Pickle J, Dale R, Fattal O. A critical examination of bifrontal electroconvulsive therapy: clinical efficacy, cognitive side effects, and directions for future research. J ECT. 2008;24:268–271. doi: 10.1097/YCT.0b013e318168e72c. [DOI] [PubMed] [Google Scholar]
- Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68:568–577. doi: 10.1016/j.biopsych.2010.06.009. [DOI] [PubMed] [Google Scholar]