Abstract
Understanding how malnutrition contributes to depression is building momentum. In the present study we unravel molecular and cellular mechanisms by which nutritional disturbances lead to impaired emotional behaviour in mice. Here we report that nutritional n-3 polyunsaturated fatty acids (PUFA) deficiency induces a chronic stress state reflected by disrupted glucocorticoid receptor (GR)-mediated signalling pathway along with hypothalamic–pituitary–adrenal (HPA) axis hyperactivity. This hyperactivity in turn resulted in neuronal atrophy in the dorsolateral (dl)- and dorsomedial (dm)- prefrontal cortex (PFC) and subsequent mood-related behaviour alterations, similarly to chronic social defeat stress. Supplementation of n-3 PUFA prevented detrimental chronic social defeat stress-induced emotional and neuronal impairments by impeding HPA axis hyperactivity. These results indicate a role for dietary n-3 PUFA in the prevention of HPA axis dysfunction associated with the development of some neuropsychiatric disorders including depression.
Introduction
Compared with other organs, the brain is highly enriched in long-chain polyunsaturated fatty acid (PUFA) including arachidonic acid (20:4n−6) and docosahexaenoic acid (DHA, 22:6n−3) that are crucial to its normal development and function. In mammals, arachidonic acid and DHA are converted from their precursors, linoleic acid (LA, 18:2n−6) and α-linolenic acid (ALA, 18:3n−3), respectively.1 LA and ALA are not synthesised de novo by mammals and are solely provided through diet to maintain sufficient brain levels of long-chain PUFAs. A compelling body of evidence suggests that anxiety and depressive disorders are linked to dietary lipids, especially the n-3 PUFAs.2, 3, 4 Numerous clinical studies revealed that subjects with depressive symptoms and with social anxiety disorders display significant lower levels of n-3 PUFAs and higher ratio of n-6 to n-3 PUFAs in the blood and in the brain.2, 3, 4 Supporting clinical observations, epidemiological lines of evidence have linked nutritional n-3 PUFA deficiency observed in industrialised countries5,6 and the prevalence of mood disorders.7 In animal models, it has been shown that transient or maternal n-3 PUFA-deficient diet induces depressive- and anxiety-like symptoms8, 9, 10 as well as abnormal social behaviour11 in adult offspring. However, mechanisms underlying the effects of n-3 PUFA-deficient diet on emotional behaviour remain largely unknown. Interestingly, similar behavioural impairments occur in mice after exposure to chronic social defeat stress, a well-characterised preclinical model of anxiety and depression.12, 13, 14 Mechanisms of chronic stress-induced emotional behaviour deficits involve retraction of the apical dendritic tree pyramidal neurons in several brain regions such as the prefrontal cortex (PFC) and the hippocampus.15, 16, 17, 18 Moreover, the hypothalamic–pituitary–adrenal (HPA) axis is directly involved in inducing anxiety- and depressive-like behaviour after chronic social defeat stress as shown by the enhancement in behavioural resiliency in mice adrenalectomised before social defeat.19 This led us to an intriguing question regarding whether dietary n-3 PUFA deficiency-induced emotional impairments are due to HPA axis hyperactivity that induces neuronal atrophy in the PFC, similarly to chronic social defeat stress.
To answer this question, we first compared the effects of dietary n-3 PUFA deficiency to those of social defeat stress on emotional behaviour, morphology of PFC pyramidal neurons and HPA axis function in C57BL6/J mice. We then tested whether HPA axis malfunction is responsible for anxiety- and depressive-like behaviours observed in n-3-deficient mice by clamping HPA axis output through adrenalectomy. Finally, we tested the hypothesis that dietary n-3 PUFA-supplementation can preclude the occurrence of depression-like behaviour induced by chronic social defeat stress by impeding HPA axis hyperactivity. This part directly addresses the existing controversy regarding the benefits of a nutritional intervention in treating/preventing mood disorders.
Materials and methods
Animals
All experiments were performed according to criteria of the European Communities Council Directive (50120103-A). Behavioural and biochemical experiments were performed on C57Bl6/J mice obtained from Charles River (Arbresle, France). Mice were maintained under standard housing conditions on corn cob litter in a temperature- (23±1 °C) and humidity (40%) -controlled animal room with a 12-h light/dark cycle (0700–1900 hours), with ad libitum access to food and water. Retired CD1 breeders used as the aggressors in the social defeat experiments were obtained from Charles River. All tests were conducted during the light period. C57BL6/J male mice were housed two per cage and maintained in a temperature- and humidity-controlled facility on a 12-h light dark cycle with food and water ad libitum. Mice were 3- to 4-month-old when the behavioural and biochemical analysis were conducted.
Diets
C57Bl6/J mice were given water and isocaloric experimental diets ad libitum (pellets prepared by UPAE-INRA, Jouy-en-Josas, France replaced daily) as previously described.11,20,21 Pellets were stored at +4 °C, and fatty acid composition was regularly controlled via gas chromatography analyses of organic extracts from manufactured food pellets. Nutritional n-3 PUFA-deficiency experiments: after mating, C57BL6/J females were fed throughout gestation and lactation with a diet containing 6% of rapeseed oil (rich in α-linolenic acid, 18:3n-3; the control diet) or 6% fat in the form of sunflower oil (rich in LA, 18:2n-6; the n-3-deficient diet). After weaning, male offsprings were fed with the same diet as their dam until the end of the experiments. Nutritional n-3 PUFA-supplementation experiment: C57BL6/J mice dams and their offsprings were kept on a standard diet (A04, 3.1% lipids, SAFE, Augy, France) until weaning of the pups. At the age of 3 weeks, male mice were assigned to the control diet or to a n-3 isocaloric supplemented diet containing 6% of tuna oil (rich in eicosapentaenoic acid 20:5n-3 and docosahexaenoic acid 22:6n-3, the n-3 supplemented diet) until they are 3-month-old (Supplementary Tables S1 and S2). This period of time was chosen on the basis of previous studies, showing that a 2-month supplementation with a diet enriched in tuna oil increases DHA levels in the brain.21
Chronic social defeat stress
Social defeat was performed as previously described.12 Briefly, intruder mice (control diet, n-3-deficient and n-3-supplemented) were exposed individually to an aggressive CD1 mouse for 5 min per day, during which they were attacked and displayed subordinate posturing. Each episode of stress was followed by 3 h of protected sensory contact with their aggressor. Mice were exposed to a different aggressor each day for 10 days in order to prevent any habituation to the resident aggressor. Undefeated mice were placed in pairs within a home cage set-up identical to that of the defeated mice, with one undefeated mice per side separated by a perforated Plexiglas divider for the duration of each sensory contact session. Twenty-four hours after the last episode of social defeat, we conducted social exploration test consisting of two consecutive sessions of 5 min. During the first session, the open field contained an empty wire mesh in the corner of the field. During the second session, a social target animal (unfamiliar CD1 male mouse) was introduced into the cage and active investigatory behaviour was recorded to assess social interaction. Forty-eight hours after the last session of stress, open-field test was performed to assess anxiety-like behaviour. The same animals were used for plasmatic corticosterone levels as well as morphological analyses. Basal condition groups are indicated as ‘undefeated' and social defeat condition groups as ‘defeated'. To avoid maximum suffering, social defeat was carried out with the minimum number of animals.
Behavioural testing
For social exploration measurement, mice were transferred to a new cage (40 × 40 cm). A social exploration session comprised 5 min without target followed by 5-min exposure of an unfamiliar adult CD1 male enclosed in a wire mesh placed in the corner of the field. Number of active investigatory behaviour (mainly sniffing the anogenital region, mouth, ears, trunk and tail) was manually counted by an experimenter blind to the conditions.
Open-field test was performed as previously published.20 Each animal was transferred to the open-field apparatus (40 × 40 cm) and was allowed to freely explore for 10 min the open field. A video tracking system (Smart, Panlab, Barcelona, Spain) recorded the exact track of each mouse as well as total distance travelled (cm) and time spent in the inner region (%).
For the forced swimming test, each mouse was individually placed into a dark-grey polyvinylchloride cylinder (15 cm in diameter, 30 cm high) half filled with water (25±1 °C), so that it would neither reach the base nor the edge of the cylinder. The water was changed between subjects. The amount of swimming during the 6-min test was used as an index of despair-like behaviour. Videotaped behaviours of mice in the forced swimming test were scored by using a time sampling technique to rate the predominant behaviour over a 10-s interval.20 An animal was considered to be immobile when it made only minimal movements to keep its head above water.
Pharmacological experiments
To examine the role of endogenous corticosterone in the behavioural, morphological and biochemical changes measured in n-3-deficient mice, corticosterone deficiency was created by adrenalectomy and low corticosterone replacement or metyrapone pretreatment.
Surgery and chronic low corticosterone replacement
For corticosterone replacement experiment, bilateral adrenalectomy (Adx) was performed under isoflurane anaesthesia on 8-week-old control and n-3-deficient mice (n=6–8 mice per group). Sham-operated animals were subjected to anesthesia and bilateral laparotomy. Immediately after the surgery, adrenalectomised mice received corticosterone (25 μg ml−1 in 0.9% saline and 2% absolute ethanol) through drinking water for 4 weeks. This intervention has previously been used to both lower and normalise corticosterone levels as well as to mimic the normal circadian pattern of corticosterone secretion.19,22,23 Emotional behaviour, neuronal dendritic arborisation within the PFC as well as plasma corticosterone levels were then analysed as described above.
Metyrapone treatment
Metyrapone (Enzo Life Sciences, Villeurbanne, France) is a corticosterone synthesis inhibitor24 that incompletely blocks glucocorticoid synthesis. It was administered as an acute treatment to avoid HPA axis adaptation to the drug over the course of a chronic treatment. N-3-deficient mice received a single intraperitoneal injection of drug dissolved in saline (0.9% NaCI, 75 mg per kg; 0.1 mI per 10 g) or 0.9% NaCl in control condition, 90 min before the forced swimming test, which is known to be sensitive to acute treatment.
Plasmatic corticosterone analysis
Trunk blood collection in ethylenediaminetetraacetic acid-lined tubes (EDTA) was performed during diurnal rise period, previously determined to occur 60 min before lights off.25 Corticosterone was measured with an in-house radioimmunosorbent assay in the plasma as previously described.25 Briefly, after steroid extraction with absolute ethanol, total corticosterone was measured by competition between cold corticosterone (B) and 3H-B (B*) by a specific anticorticosterone antibody provided by Dr H Vaudry (University of Rouen, France). We conducted corticosterone analysis 2 weeks after the last session of social defeat in order to validate our model of social defeat previously reported to produce long-lasting effects on several physiological parameters and behaviours.
Morphological analysis
Brains were quickly removed, washed in phosphate-buffered saline and processed for staining of individual neurons following the manufacter's instructions for the rapid Golgi kit (FD Neurotech, Columbia, MD, USA). Golgi-stained brain slices of 100 μm containing the dorsal PFC were used for morphological analysis. Pyramidal neurons of PFC II/III layers have been repeatedly characterized as those exhibiting remodelling in response to chronic stress16 and, therefore, were chosen for morphological analysis in our study. The dorsal PFC can be divided into dorsolateral PFC including frontal association (FrA) cortex (from 2.58 to 3.08 mm anterior to bregma); and dorsomedial PFC including prelimbic cortex (from 1.5 to 2.3 mm anterior to bregma). Three to six neurons per mouse and per region were reconstructed by a trained experimenter blind to the conditions using a Leica (Westar, Germany) and a Zeiss microscope Axio Imager 2 (x100) (Lena, Germany) and analysed using the Neurolucida software (Micro Bright Field Europe, Magdeburg, Germany). Morphological analyses were conducted 2 weeks after the last session of social defeat.
Western blot analysis
Another batch of n-3-deficient and control diet mice was used for western blot measurement performed as previously described.11,20 PFC and hippocampus were homogenised in lysis buffer (TRIS 20 mM pH 7.5, anti-protease cocktail, 5 mM MgCl2, 1 mM dithiothreitol, 0.5 M EDTA, 1 mM NaOV, 1 mM NaF). After centrifugation, protein concentration was determined using a BCA assay kit (Uptima, Montlucon, France). Equal amounts of proteins (50 μg) were loaded onto SDS-PAGE gel (10%) and transferred onto polyvinyl difluoride membrane (Millipore, Billerica, MA, USA). Membranes were incubated overnight (4 °C) with anti-GR (glucocorticoid receptor; M-20; 1:5000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-MR (mineralocorticoid receptor; H-300; 1:500, Santa Cruz Biotechnology), anti-BDNF (1:5000, Abcam, Cambridge, UK), anti-FKBP51 (1:500, Santa Cruz Biotechnology) and anti-actin (1:2500, Sigma, Saint Louis, MA, USA). After washing, membranes were incubated 1h with rabbit peroxidase-conjugated secondary antibody (1:5000, Jackson Immuno Research laboratories, Westgrove, PA, USA). Between each revelation, membranes were incubated 15 min in stripping buffer (Reblot plus, Millipore) to remove the previous antibody. Staining was revealed with ECL- Plus western blotting system (Perkin Elmer, Forest City, CA, USA). Chemiluminescence was captured and quantified using the gene Tools software (Syngene, Cambridge, UK).
Statistical analyses
All values are given as mean±s.e.m. Results obtained in social defeat experiments (behavioural tests, neuronal arborisation and corticosterone analysis) were all analysed by a two-way analysis of variance (ANOVA), with social defeat stress and diet as fixed factors. In Metyrapone experiment (locomotor activity and forced swimming test), treatment and diet were established as fixed factors. Analyses were followed by the Bonferroni post hoc test when appropriate. Pearson correlation coefficients (r) were calculated to establish relationships between neuronal arborisation in the dlPFC, dmPFC and respective corticosterone plasma levels. Results obtained in Adx experiments (behavioural tests, neuronal arborisation and corticosterone analysis) were all analysed by an unpaired t-test. Results obtained in western blot experiments (GR, MR, BDNF and FKBP51 expression) were all analysed by an unpaired t-test. All statistical tests were performed with GraphPad Prism (GraphPad software, San Diego, CA, USA) using a critical probability of P<0.05. Statistical analyses performed for each experiment are summarised in each legends of figures with the chosen statistical test, n and P-values, as well as degree of freedom and F/t values.
Results
Experiment 1: dietary n-3 PUFA-deficiency induces chronic stress phenotype in mice
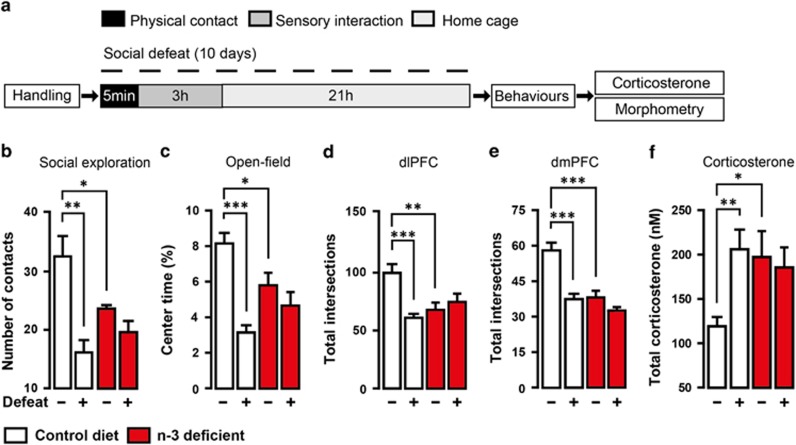
We first compared the effects of dietary n-3 PUFA deficiency to those of social defeat stress on emotional behaviour, morphology of PFC pyramidal neurons and HPA axis function in C57BL6/J mice (Figure 1a). Undefeated n-3-deficient mice displayed a decrease in the number of active exploration of an unfamiliar mouse and in the time spent exploring the centre of an open-field compared with undefeated control diet mice (Figures 1b and c). This is consistent with previous reports from our group11,20 showing that undefeated n-3-deficient mice do not engage socially with unfamiliar mice and exhibit anxiety-like behaviour. Dietary n-3 PUFA deficiency alone induced emotional impairment that was similar to the one observed following social defeat stress. We next used Golgi Cox staining to evaluate dendritic arborisation of pyramidal neurons within the layer II/III of the dlPFC and dmPFC (Supplementary Figure S1). Apical dendritic trees of both dlPFC and dmPFC pyramidal neurons displayed decreased number of intersections in undefeated n-3-deficient mice compared with undefeated control diet mice (Figures 1d and e). Interestingly, neuronal atrophy in undefeated n-3-deficient mice was similar to that observed in defeated control diet mice. These effects were specific to apical dendrites as complexity of basal dendrites was not altered in any of the experimental groups (Supplementary Figure S2). We also measured HPA axis activity and found that n-3 PUFA deficiency induced a significant increase in plasma corticosterone levels in undefeated n-3-deficient mice compared with undefeated control diet mice (Figure 1f). Similar increase in basal corticosterone levels was found in undefeated n-3-deficient mice and defeated control diet mice. In addition, we observed an inverse correlation between plasma corticosterone levels and the number of apical dendritic intersections in the dlPFC and dmPFC (Supplementary Figure S3). Importantly, social defeat did not induce further modifications of emotional behaviour, neuronal morphology or corticosterone levels in n-3-deficient mice (Figures 1b–f). This suggests that dietary n-3 PUFA deficiency induces a state of chronic stress that is identical to chronic social defeat stress.
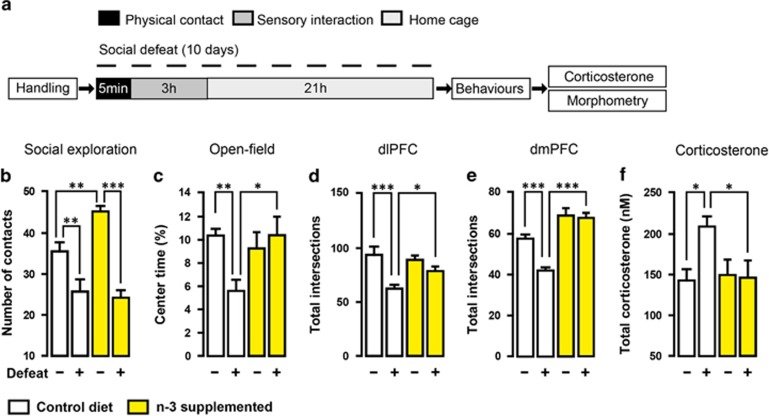
Figure 1.
Dietary n-3 PUFA deficiency induces chronic stress phenotype in mice. (a) Experimental timeline. Control diet (white) and n-3-deficient (red) mice were repeatedly (10 days) submitted to bouts of social defeat before behavioural tests (social exploration and open-field test). Undefeated n-3-deficient mice showed (b) reduced number of contact in social exploration (interaction: F1,17=7.413, P=0.01, two-way ANOVA; *P<0.05; **P<0.01, Bonferroni's test, n=5–6 per group), (c) reduced percentage of time spent in the centre of an open-field (interaction: F1,17=9.268, P<0.01, two-way ANOVA; *P<0.05; ***P<0.001, Bonferroni's test, n=5–6 per group), (d, e) simplification of apical dendritic tree on pyramidal neurons of the dlPFC (interaction: F1,59=11.57, P<0.001, two-way ANOVA; **P<0.01; ***P<0.001, Bonferroni's test, n=15 neurons per group) and dmPFC (interaction: F1,78=12.03, P<0.01, Two-way ANOVA; ***P<0.001, Bonferroni's test, n=20 neurons per group) and (f) total corticosterone elevation (interaction: F1,17=5.592, P<0.05, two- way ANOVA; *P<0.05; **P<0.01, Bonferroni's test, n=5–6 per group). Data are displayed as mean±s.e.m.
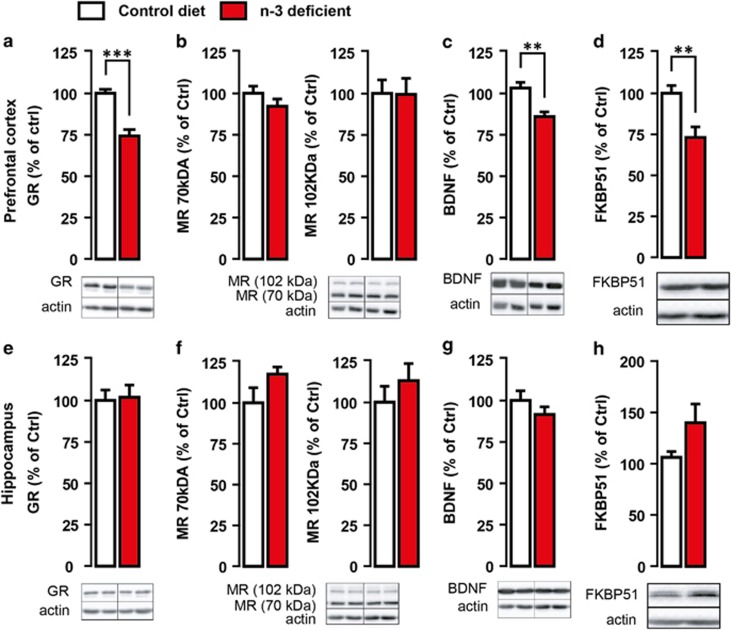
High corticosterone levels in n-3-deficient mice prompted us to further investigatethe expression of its receptors (GR and MR) as well as the GR-dependent target BDNF and FKBP51. We observed that exposure to dietary n-3 PUFA deficiency not only affected corticosterone secretion but also disturbed GR-mediated signalling pathway in PFC (Figure 2). Precisely, expressions of GR (Figure 2a) and GR-responsive genes, that is, BDNF (Figure 2c) and FKBP51 (Figure 2d), were downregulated specifically in PFC but not in the hippocampus (Figures 2g and h) of n-3-deficient versus control diet mice. No significant change was revealed in both 70- and 102-kDa MR expressions in the PFC (Figure 2b) or HC (Figure 2f) whatever the group. Collectively, these results show that dietary n-3 PUFA deficiency alone induces neuronal atrophy in the PFC and HPA axis hyperactivity similarly to social defeat stress.
Figure 2.
Glucocorticoid receptors (GR) and GR targets expression is impaired in the PFC of n-3-deficient mice. GR, MR, FKB51 and BDNF proteins expression was measured with western blot analysis in the PFC and the hippocampus of control diet (white) and n-3-deficient mice (red). n-3-deficient mice showed (a) a marked downregulation of GR expression within the PFC as compared with control diet mice (t11=5.659, ***P<0.0001, unpaired t-test, n=6–7 per group). (b) There was no significant effect of n-3 PUFA-deficient diet on either the 70-kDa MR (t12=1.274, P>0.05, unpaired t-test, n=7 per group) or the 102-kDa MR (t12=0.05060, P>0.05, unpaired t-test, n=7 per group) expression within the PFC. GR-dependent target (c) BDNF and (d) FKBP51 expression was significantly reduced in the PFC of n-3-deficient mice as compared with control diet mice (t10=3.671, **P<0.01, unpaired t-test, n=6 per group and t10=3.351, **P<0.01, unpaired t-test, n=6 per group, respectively). There was no effect of dietary n-3 PUFA deficiency on the expression of (e) GR (t11=0.1953, P>0.05, unpaired t-test, n=6–7 per group), (f) 70-kDa MR (t12=1.599, P>0.05, unpaired t-test, n=7 per group), 120-kDa MR (t10=0.8858, P>0.05, unpaired t-test, n=6 per group), (g) GR-dependent target BDNF (t11=1.097, P>0.05, unpaired t-test, n=6–7 per group) and (h) FKBP51 (t8=1.730, P>0.05, unpaired t-test, n=5 per group) in the hippocampus as compared with control diet. Data are displayed as mean±s.e.m.
Experiment 2: Dietary n-3 PUFA deficiency induces chronic stress phenotype through HPA axis hyperactivity
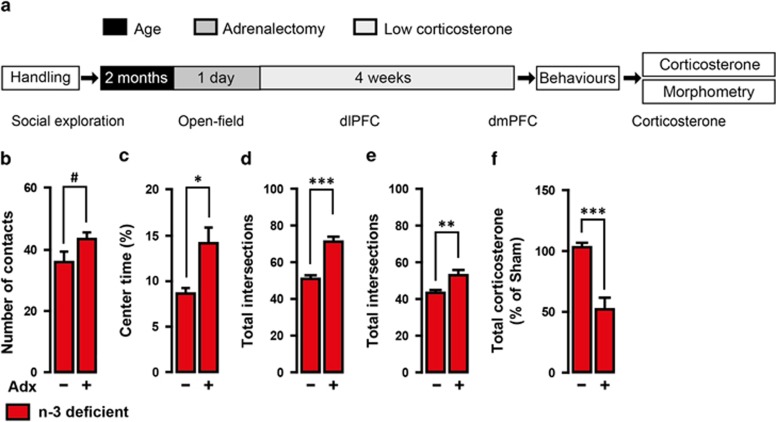
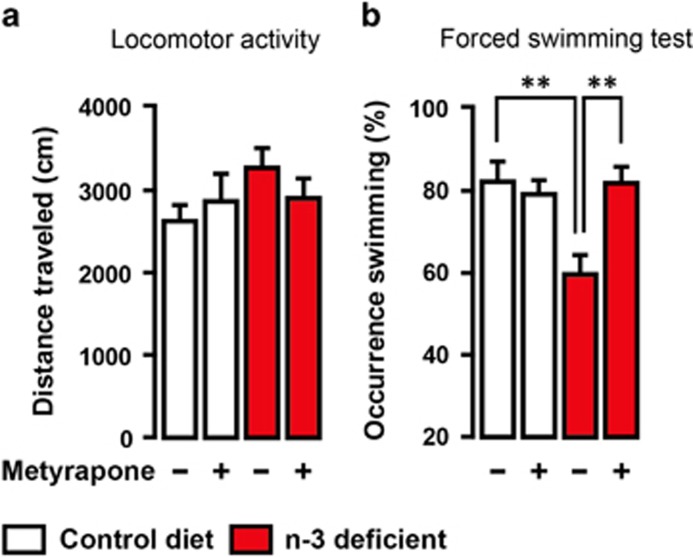
To test whether HPA axis hyperactivity is responsible for dietary n-3 PUFA deficiency-induced emotional impairment, we clamped the HPA axis output by adrenalectomy followed by low corticosterone replacement19 (Adx groups; Figure 3a). In the absence of high corticosterone levels, Adx n-3-deficient mice exhibited an increase in the number of social exploration and in the time spent exploring the centre of an open field compared with sham-operated n-3-deficient mice. In addition, complexity of neuronal arborisation in the dlPFC and dmPFC was higher in Adx n-3-deficient mice than sham-operated n-3-deficient mice (Figures 3b–e). Adrenalectomy and low corticosterone replacement maintained plasma corticosterone levels in Adx n-3-deficient mice at 50% lower than those measured in sham-operated n-3-deficient mice (Figure 3f). No effect of Adx and low corticosterone replacement was found in control diet mice on all the parameters measured (Supplementary Figure S4). Furthermore, acute administration of a corticosterone synthesis blocker, Metyrapone, reversed despair-like behaviour previously reported in n-3-deficient mice using the forced swimming test11,20 (Figure 4). These data demonstrate that dietary n-3 PUFA deficiency leads to emotional and neuronal impairments through chronic adrenal activation.
Figure 3.
Dietary n-3 PUFA deficiency induces chronic stress phenotype through HPA axis hyperactivity. (a) Experimental timeline. n-3-deficient mice (2-month-old) were bilaterally adrenalectomised (Adx) or sham-operated. Adx mice were provided low dose (25 μg ml−1of corticosterone for 4 weeks before behavioural measurements. (b) Adx n-3-deficient mice increased the number of contacts in social exploration (t13=1.855, #P<0.09, unpaired t-test, n=7–8 per group) and (c) the percentage of time spent in the centre of an open field (t13=2.319, *P<0.05, unpaired t-test, n=7–8 per group). Total apical dendritic intersections of (d) dlPFC (t52=5.287, ***P<0.001, unpaired t-test, n=27 neurons per group) and (e) dmPFC (t43=2.983, **P<0.01, unpaired t-test, n=22 neurons per group) pyramidal neurons were increased in Adx n-3-deficient mice. (f) Adx n-3-deficient mice with corticosterone replacement showed an expected reduction of plasma corticosterone levels (t8=5.586, ***P<0.001, unpaired t-test, n=5 per group). Data are displayed as mean±s.e.m.
Figure 4.
Corticosterone synthesis inhibitor blunts depressive-like behaviour in forced swimming test in n-3-deficient mice. n-3-deficient mice displayed (b) reduced swimming occurrence as compared with control diet mice. Metyrapone administration (75 mg kg−1) 90 min before forced swimming test prevented depressive-like behaviour in n-3-deficient mice (interaction: F1,27=7.486, P<0.05, two-way ANOVA; **P<0.01, Bonferroni's test, n=7–8 per group) that was not due to (a) locomotor activity changes (interaction: F1,27=1.407, P>0.05, diet effect: F1,27=1.795, P>0.05, treatment effect: F1,27=0.06567, P>0.05, two-way ANOVA, n=7–8 per group). Data are displayed as mean±s.e.m.
Experiment 3: dietary n-3 PUFA supplementation prevents chronic stress phenotype in mice
As we show that both social defeat stress and dietary n-3 PUFA deficiency induce HPA axis hyperactivity, we wondered whether n-3 PUFA supplementation that normalises HPA axis activity in chronically stressed rats26 could attenuate the effects of chronic social defeat stress on emotional behaviour (Figure 5a). Increase in corticosterone levels after defeat in control diet mice was completely prevented by chronic dietary n-3 PUFA supplementation (Figure 5f). Although chronic dietary n-3 PUFA supplementation failed to reduce social avoidance after defeat (Figure 5b), it was sufficient to attenuate social defeat-induced anxiety-like behaviour (Figure 5c). Moreover, complexity of neuronal dendrites in both dlPFC and dmPFC of defeated n-3-supplemented mice was similar to that observed in undefeated n-3-supplemented mice (Figures 5d and e). Collectively, after social defeat stress, n-3-supplemented mice showed significant resilience to the effects of social defeat on behaviour, dendritic arborisation in the PFC and HPA axis activity. Hence, it is possible to attenuate chronic stress-induced emotional impairment through n-3 PUFA supplementation that prevents HPA axis hyperactivity and neuronal atrophy in the PFC.
Figure 5.
Dietary n-3 PUFA supplementation prevents chronic stress phenotype in mice. (a) Experimental timeline. Social defeat was conducted once a day for 10 days before behaviours on control diet (white) and n-3-supplemented (yellow) mice. n-3 PUFA supplementation (b) failed to reduce number of social exploration (interaction: F1,30=6.305, P=0.0177, two-way ANOVA; **P<0.01; ***P<0.001, Bonferroni's test, n=8–9 per group) but prevented (c) anxiety-like behaviour induced by social defeat (interaction: F1,30=6.012, P<0.05, Two-way ANOVA; *P<0.05; **P<0.01, Bonferroni's test, n=8–9 per group), (d, e) simplification of apical dendritic tree on pyramidal neurons of the dlPFC (interaction: F1,54=4.201, P<0.05, two-way ANOVA; *P<0.05; ***P<0.001, Bonferroni's test, n=14 neurons per group) and dmPFC (interaction: F1,60=7.260, P<0.01, two-way ANOVA; ***P<0.001, Bonferroni's test, n=16 neurons per group) and (f) total corticosterone elevation (interaction: F1,24=4.144, P=0.05, two-way ANOVA; *P<0.05, Bonferroni's test, n=7 per group). Data are displayed as mean±s.e.m.
Discussion
During the industrial era, the rapid expansion of Western countries has been associated with a huge shift in the ratio of dietary PUFAs leading to n-3 PUFA deficiency and a high ratio of n-6 to n-3 PUFAs. Nutritional n-3 PUFA deficiency has been associated with many diseases, including mood disorders in Western countries. In the present study we unravel molecular and cellular mechanisms by which nutritional disturbances lead to impaired emotional behaviour in mice. First, we report that nutritional n-3 PUFA deficiency, such as chronic social defeat stress, induces detrimental morphofunctional changes inducing a chronic stress state. Second, our data provide clear evidence that n-3 PUFA deficiency induces HPA hyperactivity, reflected by plasma corticosterone elevation that leads to neuronal atrophy in PFC and emotional alterations. Third, we showed that dietary n-3 PUFA supplementation induces resilience to the effects of chronic social defeat stress on emotional behaviours by preventing HPA axis hyperactivity and neuronal atrophy in PFC. This finding provides new arguments in favour of the hypothesis suggesting that increased dietary intake of n-3 PUFAs may be of benefit in treating mood and anxiety disorders.27
By submitting mice to a long-term dietary n-3 PUFA deficiency, we were able to reproduce several behavioural and cellular phenotypes that have been frequently described after exposure to chronic social defeat stress.12,18,28 We found in experiment 1 that n-3 PUFA-deficient diet alone disturbed social behaviour as well as increased anxiety-related behaviour in an open-field test. Our data agree with previous reports showing that transient or maternal dietary n-3 PUFA deficiency induces the development of anxiety- and depressive-like behaviours along with abnormal social interaction in both rats and mice.8, 9, 10, 11,20,29 Interestingly, undefeated n-3-deficient mice exhibited a behavioural phenotype comparable to that of mice exposed to chronic social defeat stress. To investigate cellular mechanisms, we focused our attention on the dendritic arborisation of pyramidal neurons within the layer II/III of the dlPFC and dmPFC for analysis of the effects of n-3 PUFA deficiency on neuronal morphology. The PFC is normally implicated in executive tasks and reward and has recently been proposed to be involved in emotional behaviour and the pathophysiology of depression.30,31 To our knowledge, this is the first study reporting a drastic decrease in the apical but not basal dendritic complexity, reflected by a decreased number of total intersections in the PFC, following long-term n-3 PUFA deficiency in mice. This finding is in line with previous reports in which neurite growth and synaptogenesis are decreased in the brain of n-3 PUFA-deprived mice,32 although promoted by DHA in vitro.33 Moreover, previous studies have shown that chronic stress reduces apical but not basal dendritic arborisation complexity of pyramidal neurons in layer II/III of the PFC.15, 16, 17,34
The mechanism by which chronic stress leads to dendritic simplification has been demonstrated to involve the action of corticosterone.35,37 In this study, we showed that HPA axis activity was severely disrupted after exposure to dietary n-3 PUFA deficiency, in a similar way to chronic stress. We reported an increase in plasma corticosterone release and downregulation of GR and their downstream targets: BDNF and FKBP51 following n-3 PUFA deficiency. Such a decrease in GR expression in the brain could be linked to high corticosterone levels observed in n-3-deficient mice as previous studies have shown that corticosterone regulates GR expression in the PFC. Accordingly, Adx-induced upregulation of GR expression in the PFC of rats is downregulated by 1 week of corticosterone administration.38 In addition, defective GR signalling increases anxiety-like behaviour and reduces FKBP51 expression in the cortex,39 supporting our results. In addition, a recent study in a clinical cohort has demonstrated a link between FKBP51 and stress-related psychiatric disorders.40 Our results further support previous data showing decreased expression of BDNF in the PFC of rats deprived of n-3 PUFAs for 15 weeks.41 Recent data have elegantly linked BDNF and HPA axis activity by using knock-in mice for BDNF(met) polymorphism originally identified in depressed patients.42,43 Indeed, heterozygous BDNF(+/Met) mice display HPA axis hyper-reactivity as well as increased anxiety- and depressive-like behaviours. GR signalling and BDNF have a crucial role in neuronal plasticity including axonal growth neuritis maturation and neuronal activity. They have been postulated to be key mediators of stress-induced neural atrophy in the PFC.44, 45, 46 Finally, we aim in future studies to examine neuronal morphology in a control structure, the hippocampus, in which no change was observed in GR and GR-target genes expression in n-3-deficient mice. If no change in dendritic arborisation is found in hippocampal pyramidal neurons, this would strongly suggest that GR sensitivity is closely associated with neuronal atrophy in n-3-deficient mice.
In our study, we observed that chronic social defeat stress did not induce further modifications of emotional behaviour, neuronal morphology or corticosterone levels in n-3-deficient mice, supporting the idea that these mice are under a state of chronic stress. Taken together, these data suggest that n-3 PUFA brain contents modulate neuronal morphology in PFC along with depressive- and anxiety-related behaviours similarly to chronic social defeat stress through corticosterone secretion. In support of this hypothesis, we observed an inverse correlation between plasma corticosterone levels and the number of apical dendritic intersections in the dlPFC and dmPFC. In addition, repeated administration of corticosterone in rodents is known to reduce the dendritic arborisation of apical pyramidal neurons within the PFC along with altered emotional behaviour.37,47 We also established a causal link between n-3 deficiency-induced morphofunctional changes and HPA axis hyperactivity by clamping corticosterone secretion in n-3-deficient mice. We demonstrated that Adx and low corticosterone replacement in n-3-deficient mice improved both social interaction and anxiety-like behaviour as compared with Sham-operated n-3-deficient mice. These findings are consistent with previous studies showing that Adx improves behavioural impairment in mice exposed to chronic social defeat stress.19 Moreover, we showed that corticosterone synthesis inhibition following treatment with Metyrapone prevented depressive-like behaviour by increasing the occurrence of swimming in n-3-deficient mice evaluated in the FST as compared with saline n-3-deficient mice. These findings demonstrate that normalising HPA activity either by Adx or Metyrapone is associated with significant behavioural enhancement in various tests assessing anxiety- and depressive-like behaviours. We further revealed that elevated corticosterone in n-3-deficient mice is responsible for pyramidal neuron arborisation atrophy within the PFC as Adx n-3-deficient mice displayed enhancement of apical dendritic arborisation. Altogether, data in experiment 2 provide clear evidence that nutritional n-3 PUFA deficiency induces chronic adrenal activation that leads to neuronal atrophy in the dlPFC and dmPFC and mood-related behaviour alteration.
Although mechanisms by which n-3 PUFA deficiency induces HPA axis hyperactivity remain largely unexplored, the relationship between n-3 PUFA deficiency and the endocannabinoid (eCB) system is indicated. Molecularly, the two principal eCBs, anandamide and 2-arachidonoylglycerol, are signalling lipids produced from membrane long-chain PUFAs.48 The eCB system has emerged as a fundamental regulator of HPA axis negative feedback and an important modulator of emotional behaviour.49 In our recent studies, we found that nutritional n-3 PUFA deficiency ablates long-term synaptic depression mediated by eCB in the mPFC via a desensitisation of the cannabinoid type 1 receptor (CB1).11 Moreover, the effect of the CB1 agonist WIN55,212-2 in anxiety-like behaviour is abolished and the CB receptor signalling pathways are altered in the PFC of n-3-deficient mice.20 One hypothesis is that HPA axis hyperactivity observed in n-3-deficient mice in this study is due to the reduction of CB1 receptor function in the PFC of these mice.
Experiment 3 shows that chronic n-3 PUFA supplementation prevents detrimental chronic stress-induced emotional and neuronal impairments by impeding HPA axis hyperactivity. This experiment provides a stronger causal link between dietary n-3 PUFAs and a chronic stress mechanism (HPA axis hyperactivity) regarding the occurrence of mood-related behaviours. These findings support data suggesting that n-3 PUFA supplementation can preclude the occurrence of depression-like behaviour induced by chronic stress by acting on HPA axis.26 Numerous clinical studies revealed that subjects with depressive symptoms and/or with social anxiety disorders display significantly lower levels of n-3 PUFAs (mainly ALA, EPA and DHA) in the blood and/or in the brain 2, 3, 4,50 as compared to non-depressed subjects or subjects with mild symptoms.51 This part of our work addresses an important question regarding the prevention of depression through dietary n-3 PUFA intake. Indeed, our data create a very strong argument for a prophylactic effect of n-3 supplementation52,53 by acting on HPA axis and neuronal morphology. In line with our results, chronic n-3 PUFA supplementation with fish oil rich in EPA and DHA in healthy humans prevents adrenal activation elicited by a mental stress54 or by inflammatory stimuli55 as compared with control. In addition, n-3 supplementation reduces anxiety symptoms among healthy young adults.56 Taken together, these data reinforce the idea that n-3 PUFA supplementation can be efficient as an early prevention for subjects at high risk for mood disorders, including anxiety and depression.57
Protective effect of long-chain n-3 PUFAs could be linked to hippocampal neurogenesis (recently reviewed in Zainuddin et al.58). N-3 PUFAs have been associated with changes in hippocampal neurogenesis in several rodent models.32,59, 60, 61 In addition, changes in hippocampal neurogenesis and cell survival in the dentate gyrus have been correlated with depressive-like behaviour. Interestingly, a recent study demonstrated that clamping glucocorticoid levels prevent chronic social defeat-induced decreases in neurogenesis and depressive-like behaviour in WT mice, but not in mice with a genetic ablation of neurogenesis.19 This is particularly relevant to our results showing that long-chain n-3 PUFA supplementation prevents chronic social defeat-induced HPA axis deregulation. However, whether the beneficial effect of n-3 PUFAs on glucocorticoids and mood is dependent on neurogenesis remains to be evaluated.
In conclusion, this is the first report providing evidence that long-term dietary n-3 PUFA deficiency induces a chronic stress state marked by emotional alterations that are due to HPA axis hyperactivity and cortical neuronal atrophy. Moreover, these data demonstrate that chronic dietary n-3 PUFA supplementation prevents detrimental chronic stress-induced emotional and neuronal impairments by impeding HPA axis hyperactivity. Our study presents dietary n-3 PUFAs as a potential tool in the prevention of neuropsychiatric disorders associated with HPA axis dysfunction such as depression and anxiety.
Acknowledgments
This research was financially supported by INRA, FRM, ANR MoodFood and the Region Aquitaine. TL was the recipient of a postdoctoral fellowship from the ‘société française de nutrition (SFN)' and the Labex BRAIN. The authors declare no competing financial interests. We thank P Birac, C Tridon and M Cadet of the animal facility, the Dr N Abrous and Dr M Koehl for the Neurolucida software as well as the Dr N Arvy for adrenalectomy surgery. We also thank the ‘Biochemistry Platform' of the SFR Neuroscience funded by the Labex BRAIN. We thank Dr G Ferreira, Professor M Darnaudéry and Dr MP Moisan for helpful and fruitful discussions. We Dr A Panatier for her valuable suggestion and advice and Dr C Murphy for correcting the English text of the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Sprecher H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim Biophys Acta. 2000;1486:219–231. doi: 10.1016/s1388-1981(00)00077-9. [DOI] [PubMed] [Google Scholar]
- Adams PB, Lawson S, Sanigorski A, Sinclair AJ. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31:S157–S161. doi: 10.1007/BF02637069. [DOI] [PubMed] [Google Scholar]
- Green P, Hermesh H, Monselise A, Marom S, Presburger G, Weizman A. Red cell membrane omega-3 fatty acids are decreased in nondepressed patients with social anxiety disorder. Eur Neuropsychopharmacol. 2006;16:107–113. doi: 10.1016/j.euroneuro.2005.07.005. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Liu Y. Reduced expression of fatty acid biosynthesis genes in the prefrontal cortex of patients with major depressive disorder. J Affect Disord. 2011;129:359–363. doi: 10.1016/j.jad.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Evolutionary aspects of the dietary omega-6:omega-3 fatty acid ratio: medical implications. World Rev Nutr Diet. 2009;100:1–21. doi: 10.1159/000235706. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- Carrié I, Clément M, de Javel D, Francès H, Bourre JM. Phospholipid supplementation reverses behavioral and biochemical alterations induced by n-3 polyunsaturated fatty acid deficiency in mice. J Lipid Res. 2000;41:473–480. [PubMed] [Google Scholar]
- DeMar JC, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J Lipid Res. 2006;47:172–180. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Taha AY, Tock JL, Totah NKB, Cheon Y, Torres GE, et al. Adolescent behavior and dopamine availability are uniquely sensitive to dietary omega-3 fatty acid deficiency. Biol Psychiatry. 2014;75:38–46. doi: 10.1016/j.biopsych.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafourcade M, Larrieu T, Mato S, Duffaud A, Sepers M, Matias I, et al. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat Neurosci. 2011;14:345–350. doi: 10.1038/nn.2736. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, et al. IκB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Hill MN, Hillard CJ, McEwen BS. Alterations in corticolimbic dendritic morphology and emotional behavior in cannabinoid CB1 receptor-deficient mice parallel the effects of chronic stress. Cereb Cortex. 2011;21:2056–2064. doi: 10.1093/cercor/bhq280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Blugeot A, Rivat C, Bouvier E, Molet J, Mouchard A, Zeau B, et al. Vulnerability to depression: from brain neuroplasticity to identification of biomarkers. J Neurosci. 2011;31:12889–12899. doi: 10.1523/JNEUROSCI.1309-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Brachman RA, Martinowich K, Schloesser RJ, Herkenham M. Glucocorticoids orchestrate divergent effects on mood through adult neurogenesis. J Neurosci. 2013;33:2961–2972. doi: 10.1523/JNEUROSCI.3878-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrieu T, Madore C, Joffre C, Layé S. Nutritional n-3 polyunsaturated fatty acids deficiency alters cannabinoid receptor signaling pathway in the brain and associated anxiety-like behavior in mice. J. Physiol Biochem. 2012;68:671–681. doi: 10.1007/s13105-012-0179-6. [DOI] [PubMed] [Google Scholar]
- Labrousse VF, Nadjar A, Joffre C, Costes L, Aubert A, Grégoire S, et al. Short-term long chain omega3 diet protects from neuroinflammatory processes and memory impairment in aged mice. PLoS ONE. 2012;7:e36861. doi: 10.1371/journal.pone.0036861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L, Akana SF, Cascio CS, Shinsako J, Dallman MF. Circadian variations in plasma corticosterone permit normal termination of adrenocorticotropin responses to stress. Endocrinology. 1988;122:1343–1348. doi: 10.1210/endo-122-4-1343. [DOI] [PubMed] [Google Scholar]
- Child DF, Burke CW, Burley DM, Rees LH, Fraser TR. Drug controlled of Cushing's syndrome. Combined aminoglutethimide and metyrapone therapy. Acta Endocrinol (Copenh) 1976;82:330–341. [PubMed] [Google Scholar]
- Richard EM, Helbling J-C, Tridon C, Desmedt A, Minni AM, Cador M, et al. Plasma transcortin influences endocrine and behavioral stress responses in mice. Endocrinology. 2010;151:649–659. doi: 10.1210/en.2009-0862. [DOI] [PubMed] [Google Scholar]
- Ferraz AC, Delattre AM, Almendra RG, Sonagli M, Borges C, Araujo P, et al. Chronic ω-3 fatty acids supplementation promotes beneficial effects on anxiety, cognitive and depressive-like behaviors in rats subjected to a restraint stress protocol. Behav Brain Res. 2011;219:116–122. doi: 10.1016/j.bbr.2010.12.028. [DOI] [PubMed] [Google Scholar]
- Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry. 2011;72:1577–1584. doi: 10.4088/JCP.10m06634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J, Wagner KV, Liebl C, Scharf SH, Wang X-D, Wolf M, et al. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology. 2012;62:332–339. doi: 10.1016/j.neuropharm.2011.07.041. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Iwanaga M, Harada E. Possible regulatory mechanism of DHA-induced anti-stress reaction in rats. Brain Res. 2003;964:136–143. doi: 10.1016/s0006-8993(02)04113-6. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Cao D, Kevala K, Kim J, Moon H-S, Jun SB, Lovinger D, et al. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem. 2009;111:510–521. doi: 10.1111/j.1471-4159.2009.06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon F, Kim H-Y. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem. 2004;90:979–988. doi: 10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37:39–47. doi: 10.1016/j.psyneuen.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R-J, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci USA. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH. [3H]Dexamethasone binding in rat frontal cortex. Brain Res. 1985;328:176–180. doi: 10.1016/0006-8993(85)91340-x. [DOI] [PubMed] [Google Scholar]
- Godavarthi SK, Dey P, Maheshwari M, Jana NR. Defective glucocorticoid hormone receptor signaling leads to increased stress and anxiety in a mouse model of Angelman syndrome. Hum Mol Genet. 2012;21:1824–1834. doi: 10.1093/hmg/ddr614. [DOI] [PubMed] [Google Scholar]
- Fani N, Gutman D, Tone EB, Almli L, Mercer KB, Davis J, et al. FKBP5 and attention bias for threat: associations with hippocampal function and shape. JAMA Psychiatry. 2013;70:392–400. doi: 10.1001/2013.jamapsychiatry.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JS, Ertley RN, Lee H-J, DeMar JC, Arnold JT, Rapoport SI, et al. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2007;12:36–46. doi: 10.1038/sj.mp.4001888. [DOI] [PubMed] [Google Scholar]
- Wichers M, Kenis G, Jacobs N, Myin-Germeys I, Schruers K, Mengelers R, et al. The psychology of psychiatric genetics: evidence that positive emotions in females moderate genetic sensitivity to social stress associated with the BDNF Val-sup-6-sup-6Met polymorphism. J Abnorm Psychol. 2008;117:699–704. doi: 10.1037/a0012909. [DOI] [PubMed] [Google Scholar]
- Yu H, Wang D-D, Wang Y, Liu T, Lee FS, Chen Z-Y. Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J Neurosci. 2012;32:4092–4101. doi: 10.1523/JNEUROSCI.5048-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri D, Vaidya VA. Glucocorticoid regulation of brain-derived neurotrophic factor: relevance to hippocampal structural and functional plasticity. Neuroscience. 2013;239:196–213. doi: 10.1016/j.neuroscience.2012.08.065. [DOI] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol. 2010;70:304–322. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Wood JT, Williams JS, Pandarinathan L, Janero DR, Lammi-Keefe CJ, Makriyannis A. Dietary docosahexaenoic acid supplementation alters select physiological endocannabinoid-system metabolites in brain and plasma. J Lipid Res. 2010;51:1416–1423. doi: 10.1194/jlr.M002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin RJ, Hill MN, Gorzalka BB. A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci Biobehav Rev. 2014;42C:116–131. doi: 10.1016/j.neubiorev.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59:913–919. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- Parker G, Gibson NA, Brotchie H, Heruc G, Rees A-M, Hadzi-Pavlovic D. Omega-3 fatty acids and mood disorders. Am J Psychiatry. 2006;163:969–978. doi: 10.1176/ajp.2006.163.6.969. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Mague SD, Parow AM, Stoll AL, Cohen BM, Renshaw PF. Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats. Biol Psychiatry. 2005;57:343–350. doi: 10.1016/j.biopsych.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Mizunoya W, Ohnuki K, Baba K, Miyahara H, Shimizu N, Tabata K, et al. Effect of dietary fat type on anxiety-like and depression-like behavior in mice. Springerplus. 2013;2:165. doi: 10.1186/2193-1801-2-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarue J, Matzinger O, Binnert C, Schneiter P, Chioléro R, Tappy L. Fish oil prevents the adrenal activation elicited by mental stress in healthy men. Diabetes Metab. 2003;29:289–295. doi: 10.1016/s1262-3636(07)70039-3. [DOI] [PubMed] [Google Scholar]
- Michaeli B, Berger MM, Revelly J-P, Tappy L, Chioléro R. Effects of fish oil on the neuro-endocrine responses to an endotoxin challenge in healthy volunteers. Clin Nutr. 2007;26:70–77. doi: 10.1016/j.clnu.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Glaser R. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav Immun. 2011;25:1725–1734. doi: 10.1016/j.bbi.2011.07.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Strawn JR. Role of long-chain omega-3 fatty acids in psychiatric practice. PharmaNutrition. 2013;1:41–49. doi: 10.1016/j.phanu.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainuddin MSA, Thuret S. Nutrition, adult hippocampal neurogenesis and mental health. Br Med Bull. 2012;103:89–114. doi: 10.1093/bmb/lds021. [DOI] [PubMed] [Google Scholar]
- Kawakita E, Hashimoto M, Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience. 2006;139:991–997. doi: 10.1016/j.neuroscience.2006.01.021. [DOI] [PubMed] [Google Scholar]
- He C, Qu X, Cui L, Wang J, Kang JX. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc Natl Acad Sci USA. 2009;106:11370–11375. doi: 10.1073/pnas.0904835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall SC, Michael GJ, Michael-Titus AT. Omega-3 fatty acids reverse age-related decreases in nuclear receptors and increase neurogenesis in old rats. J Neurosci Res. 2010;88:2091–2102. doi: 10.1002/jnr.22390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.