Abstract
Genetic polymorphisms in the APOE ɛ and TOMM40 ‘523' poly-T repeat gene loci have been associated with significantly increased risk of Alzheimer's disease. This study investigated the independent effects of these polymorphisms on human cognitive ageing, and the extent to which nominally significant associations with cognitive ageing were mediated by previously reported genetic associations with brain white matter tract integrity in this sample. Most participants in the Lothian Birth Cohort 1936 completed a reasoning-type intelligence test at age 11 years, and detailed cognitive/physical assessments and structural diffusion tensor brain magnetic resonance imaging at a mean age of 72.70 years (s.d.=0.74). Participants were genotyped for APOE ɛ2/ɛ3/ɛ4 status and TOMM40 523 poly-T repeat length. Data were available from 758–814 subjects for cognitive analysis, and 522–543 for mediation analysis with brain imaging data. APOE genotype was significantly associated with performance on several different tests of cognitive ability, including general factors of intelligence, information processing speed and memory (raw P-values all<0.05), independently of childhood IQ and vascular disease history. Formal tests of mediation showed that several significant APOE-cognitive ageing associations—particularly those related to tests of information processing speed—were partially mediated by white matter tract integrity. TOMM40 523 genotype was not associated with cognitive ageing. A range of brain phenotypes are likely to form the anatomical basis for significant associations between APOE genotype and cognitive ageing, including white matter tract microstructural integrity.
Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disease characterized by cognitive impairment. Two genetic risk factors for late onset Alzheimer's disease are in the apolipoprotein-e (APOE; http://www.ncbi.nlm.nih.gov/gene/348) and translocase of outer mitochondrial membrane 40 (TOMM40) gene poly-T repeat loci (http://www.ncbi.nlm.nih.gov/gene/10452).1,2
Previous studies have investigated associations between APOE genotype and cognitive ability in non-demented older adults. Wisdom et al.3 conducted a meta-analysis of 77 studies, excluding samples with any disorders that may affect cognitive ability such as dementia or Parkinson's disease (final N=40 942; weighted estimate mean age=63.14 years, s.d.=13.10). For analytic purposes, they then constructed a ‘d' effect size metric that weighted standardized differences between the groups in terms of sample sizes. Significant deleterious effects of the ɛ4 allele were found for cognitive domains of episodic memory, global cognitive function, executive function and perceptual speed (all P<0.05). There were no significant effects on verbal ability, primary memory, attention or visuospatial functioning.
The association between APOE ɛ4 and cognitive ability may be modified by other genetic variables that exert independent effects.4 The TOMM40 523 poly-T repeat locus has recently been significantly associated with brain-related phenotypes (for example, cognitive decline), independent of APOE genotype.5 The independent effects of this locus on older-age cognitive ability have been examined in three studies. The largest of these was by Caselli et al.6 who reported a deleterious effect of the Very-long/Very-long genotype (vs Short/Short) on the amount of longitudinal change in the auditory verbal learning test (N=639; genotype mean ages=57.8–60.9 years; mean duration of follow-up=6.1 years±3.1; P=0.04 in APOE ɛ3/ɛ3 subgroup). Two other smaller reports by Hayden et al.7 (N=127) and Johnson et al.8 (N=117) have also reported some specific significant effects of TOMM40 523 genotype on cognitive ability in older adults.
One of the main aims of this paper is to investigate brain imaging variables that might mediate previously established genetic-cognitive associations; it is important to understand the anatomical brain substrates of cognitive ageing. Penke et al.9 investigated the role of white matter integrity using different metrics obtained from diffusion tensor magnetic resonance imaging (DT-MRI), one of which was fractional anisotropy (FA; where lower FA reflects reduced brain white matter integrity). They reported that a general factor of FA constructed with principal components analysis (PCA) was significantly associated with general factors of processing speed (gspeed; standardized β=−0.19) and general cognitive ability (g; standardized β=0.13) in the Lothian Birth Cohort 1936 (LBC1936), explaining around 10% of the variance in general cognitive ability. Specifically, lower FA scores were associated with worse general cognitive ability, and slower information processing speed.
A previous report by Lyall et al.10 tested for associations between APOE/TOMM40 523 genotypes and tract-averaged FA values in several brain white matter tracts in the 73-year-old LBC1936 (the same sample whose data are analysed in the present study), assessed by DT-MRI. We found significant and independent deleterious effects of the APOE ɛ4 and TOMM40 523 ‘Short' (vs Long/Very-long) alleles on specific tracts, independent of the covariates of age, gender, vascular disease history and childhood intelligence. For APOE genotype, these tracts were the left ventral cingulum and the left inferior longitudinal fasciculus, and for TOMM40 523, the left uncinate fasciculus, left rostral cingulum, left ventral cingulum and GFA. The statistically significant association with GFA—which reflects shared variance among the examined tracts—may indicate a general effect of TOMM40 523 on white matter tract integrity in the brain. It is unclear why tract-specific measures show significant deleterious effects of genetic variation at the APOE/TOMM40 gene loci, compared with other white matter tracts.10 These specific tracts may be particularly sensitive to injury or pathology.11 (Note however that group differences in white matter FA may be due to differences in, for example, axon diameter, packing density or membrane permeability, and in this sense lower FA may not necessarily reflect lower microstructural integrity per se.12 ) In that report (Lyall et al.10), we did not assess the effects of APOE/TOMM40 523 genotypes on cognitive ability, and we are aware of no studies that formally test the extent to which deleterious effects of APOE ɛ4 on cognitive ageing are mediated by brain white matter tract integrity (although see Ryan et al.13).
The present study therefore aims to: (1) assess the effects of APOE/TOMM40 523 genotypes on cognitive abilities in older people, at first unadjusted and then adjusted for age 11 intelligence (that is, reflecting cognitive ageing); and (2) determine the extent to which any such significant associations are mediated by previously reported associations between these gene loci and white matter tract integrity (FA), assessed with DT-MRI in this same sample.10
Materials and Methods
Sample
The LBC1936 is a longitudinal ageing sample of generally healthy, community-dwelling older adults.14,15 Briefly, most of the LBC1936 sample completed the Moray House Test (no.12) of verbal reasoning as part of the Scottish Mental Survey 1947 at a mean age of 11 years, and were recruited for detailed cognitive, medical and demographic assessments at the Wellcome Trust Clinical Research Facility (WTCRF, Edinburgh; http://www.wtcrf.ed.ac.uk) around the ages of ~70 (Wave 1) and ~73 years (Wave 2). It is the Wave 2 data that are examined here. Participants also received detailed brain MRI around the same time (mean interval=65.1 days16). Diagnoses of clinical conditions were elicited via interview. Clinical vascular conditions asked about included high blood pressure, diabetes, stroke, high cholesterol and any vascular pathology. All subjects gave written, informed consent.
Genotyping
Participants were genotyped for APOE ɛ on the basis of DNA isolated from whole blood taken from the Wave 1 (age 70) assessment, and genotyped by TaqMan assay (Applied Biosystems, Carlsbad, CA, USA) at the WTCRF genetics core.15 TOMM40 523 was genotyped by the laboratory of Dr Ornit Chiba-Falek (Duke University, Durham, NC, USA) using a method described previously.17
Cognitive assessment
Moray house test no.12
This was completed in June 1947 at a mean age of 11 years. This assessment has a 45-min time limit, has a maximum score of 76 and includes questions with a range of numerical, visuospatial and (primarily) verbal reasoning items.15 Scores were adjusted for age in days at the time of assessment, and standardized to an IQ score with a mean of 100 and s.d. of 15 for the whole LBC1936 sample.
Assessment of cognitive domains
The tests completed at a mean age of 73 years in Wave 2 are described by Deary et al.14,15 In brief, the cognitive domains assessed were as follows. Working memory was assessed with digit span backwards and letter-number sequencing (both from the WAIS-IIIUK battery18). Processing speed was assessed using: digit symbol coding and symbol search tests from the WAIS-IIIUK; simple reaction time (RT), and four-choice RT via a self-contained device;19 and a visual discrimination task called Inspection Time, which assesses speed of basic visual processing and where higher total scores reflect better performance.15 Verbal declarative memory was assessed with the verbal paired associates I and II, and logical memory I and II tests (both from the WMS-IIIUK battery20). Specifically, we summed the respective I/II test scores to create verbal paired associates total scores, and logical memory total scores for each individual. Visuospatial ability and memory were assessed with block design and spatial span (WAIS-IIIUK and WMS-IIIUK, respectively). Abstract reasoning was assessed using matrix reasoning from the WAIS-IIIUK.
Data reduction was applied to the cognitive test scores using three separate PCAs, which produced the following summary cognitive variables. First, ‘general intelligence' (g) included the six nonverbal Wechsler subtests of digit span backwards, matrix reasoning, letter-number sequencing, block design, symbol search and digit symbol coding. Second, ‘processing speed' (gspeed) included symbol search, digit symbol coding, simple RT, four-choice RT and Inspection time.21 Third, a ‘memory' factor (gmemory) was formed from logical memory, spatial span, verbal paired associates, letter-number sequencing and digit span backwards.22 The first unrotated principal component score in each domain accounted for 50–54% of the respective variance, and all individual variables had high loadings on their respective first unrotated components. Strictly speaking, PCA does not produce latent factors, however, we use the term here because it is a common usage.
In each PCA, the g/gspeed/gmemory scores reflect variance that is shared between their respective lists of included cognitive tests, hence eliminating task-specific variance.23 However because different tasks assess specific aspects of mental ability (for example, logical memory explicitly assesses declarative verbal memory), it is still informative to include tests of association between APOE/TOMM40 genotypes and these individual test-score phenotypes.24 Further, the large battery of tests applied to the LBC1936 is rare, and other research teams are likely to test relationships studied here with individual tests. Therefore, to allow comparisons with others' work, we include the individual test results in addition to the component scores.
Diffusion MRI and tractography
The protocol for DT-MRI processing is described in detail in the MRI protocol paper, including imagine acquisition parameters of the DTI sequence.16 Briefly, participants underwent whole-brain diffusion MRI acquired using a GE Signa Horizon HDxt 1.5T clinical scanner (General Electric, Milwaukee, WI, USA). The diffusion MRI data were preprocessed using FSL tools (FMRIB, Oxford, UK; http://www.fmrib.ox.ac.uk). Underlying tractography connectivity data were generated using BedpostX/ProbTrackX with a two-fibre model.25 Fourteen tracts were identified using probabilistic neighbourhood tractography, an approach for automatic and reproducible tract segmentation.26,27 These tracts were considered to be of relevance to cognitive and brain ageing, on the basis of several lines of evidence.16
To make sure that segmented tracts were anatomically plausible representations of the tract-of-interest, a researcher inspected all masks, blind to all other data and excluded tracts with aberrant or truncated pathways. Generally, the probabilistic neighbourhood tractography method, which had been designed specifically for use in older brains, reliably segmented the twelve tracts of interest; tracts that did not meet quality critera (for example, truncation, aberrant route), ranged from 0.3% for the splenium of the corpus callosum, to 16% for the left anterior thalamic radiation (mean=5% (ref. 16)). The protocol paper by Wardlaw et al.16 is an open access article that details specific methodology of probabilistic neighbourhood tractography for each tract, and displays examples of tracts segmented for use in the LBC1936 sample. Tracts assessed were the genu and splenium of corpus callosum, and bilateral anterior thalamic radiations, ventral and rostral cingulum bundles, arcuate, uncinate and inferior longitudinal fascicule; see Lyall et al.10 for a detailed examination of associations between APOE/TOMM40 523 and average FA scores for these tracts.
Statistical analysis
APOE/TOMM40 analysis
We first tested the effects of APOE ɛ4 allele presence vs absence, that is, pooled ɛ2/ɛ4, ɛ3/ɛ4 and ɛ4/ɛ4 genotypes vs pooled ɛ2/ɛ2, ɛ2/ɛ3 and ɛ3/ɛ3.
The variable-length poly-T repeat rs10524523 (‘523') was split into three categories:28 ‘Short' (<20T residues; S), ‘Long' (≥20; L) and ‘Very-long' (≥30; VL) of which the S allele may or may not be protective in terms of neurodegenerative pathology.5 In the first analytic step applied to the whole sample (‘Step 1'), a general linear model tested for a significant effect of the TOMM40 523 genotype (that is, S/S; S/L; L/L; L/VL; VL/VL). To investigate the effects of TOMM40 523 repeat length independent of biological variation in APOE genotype, analysis then focussed separately on two different APOE ɛ genotype subgroups. First, participants with the ɛ3/ɛ4 genotype were analysed (‘Step 2'); the S allele may offset or interact biologically with the ɛ3/ɛ4 ‘risk' genotype.29 Finally, analysis focussed on participants with the ‘neutral' APOE genotype (ɛ3/ɛ3; ‘Step 3'), because this eliminates variance associated with protective and risk APOE alleles.8 This subgroup analysis means that any statistically significant effects of TOMM40 523 genotype cannot be attributed to variation in APOE ɛ status. In steps 2 and 3 (i.e., analysis of TOMM40 523 in APOE ɛ3/ɛ4 and ɛ3/ɛ3 genotype subgroups, respectively), the L and VL alleles were pooled into an “L*” group, as is relatively common;10 participants with the S/S genotype were compared with those carrying only one S allele (pooled S/L and S/VL; hereinafter S/L*), and also against participants carrying no S alleles (pooled L/L, L/VL, and VL/VL; hereinafter L*/L*).6
Covariate models
All covariate models controlled for age and gender (‘Model 1'). Cognitive scores were then corrected for age 11 IQ, to investigate ‘cognitive ageing' (‘Model 2'). Significant associations were then were re-tested controlling for the following covariates in addition to those in Model 2; self-reported history of hypertension, stroke, type 2 diabetes, hypercholesterolaemia and all-inclusive vascular disease (‘Model 3').30 This reduces the chance that any significant associations occur as secondary to genetic associations with vascular pathology.
An online calculator was used to perform tests of Hardy–Weinberg equilibrium and determine minor allele frequencies (http://www.had2know.com/academics/hardy-weinberg-equilibrium-calculator-3-alleles.html). Data were otherwise analysed with IBM Statistical Package for the Social Sciences, Version 19.0 (SPSS, Chicago, IL, USA). Specifically, univariate general linear models tested the fixed effects of separate APOE and TOMM40 genotypes upon the outcome variables. Outliers of more than 3.30 s.d. from mean values were removed from all cognitive variables to exclude outlying data; this did not affect any final results. To protect against Type 1 errors, false discovery rate (FDR) was used to estimate the number of truly significant findings in the context of testing multiple associations.31 A Microsoft Excel program32 was used to conduct classical one-stage FDR based on associations with cognitive abilities. P-values <0.05 were considered to be nominally significant. All P-values are raw unless stated as being FDR-adjusted.
Mediation analysis
Mediation analysis was used to test the indirect effect of the predictor variable (APOE/TOMM40) on the outcome (cognitive ageing), through the hypothesized mediator (white matter integrity). Mediation analysis was run using the INDIRECT bootstrapping macro.33 Briefly, variable X's effects (APOE/TOMM40) on variable Y (cognitive ageing) can be either direct, or indirect via variable M (white matter integrity). In Figure 1, path a represents the effect of X on M, while path b represents the effect of M on Y, partialling out the effect of X. The direct effect of X on Y is represented by path c. The indirect effect can then be quantified as the combined product of paths a and b. The bias-corrected bootstrapping point estimate coefficients that are reported here each reflect this indirect product.33
Figure 1.
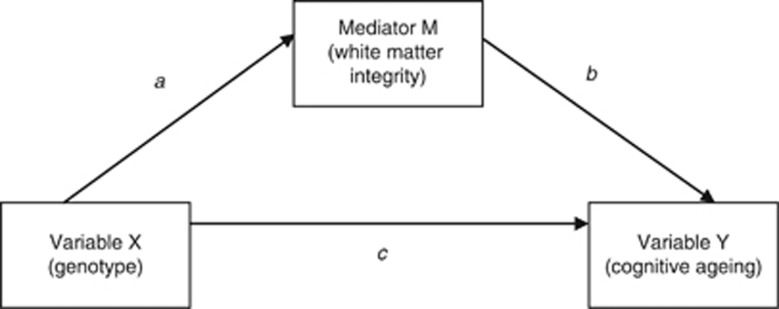
An example mediation model, where variable X's effects (APOE/TOMM40) on variable Y (cognitive ageing) can be either direct, or indirect via variable M (white matter integrity). Path a represents the effect of X on M, while path b represents the effect of M on Y, partialling out the effect of X. The direct effect of X on Y is represented by path c (adapted from Preacher and Hayes33).
Bootstrapping point estimate coefficients were unstandardized and averaged over 5000 bootstrap estimates.33 The indirect point estimate coefficients (commonly simply ‘effects') were considered statistically significant if the 95% confidence intervals (CIs) did not cross 0.00.33 This method has been used previously to investigate the brain substrates of genetic-cognitive ageing associations.34
Results
Descriptive statistics
Of the 1091 LBC1936 participants originally enrolled in the study, 866 attended Waves 1 and 2. Individuals who had Mini-Mental State Examination scores <24, a cutoff commonly used to indicate dementia,35 did not complete the Mini-Mental State Examination at Wave 2, or had a reported history of dementia were excluded from analysis. Overall, this left 859 participants, of which 811 and 823 participants had successful genotyping for APOE and TOMM40, respectively.
APOE had allele frequencies of ɛ2=7.3%, ɛ3=76.9% and ɛ4=15.8%, with genotype frequencies of: ɛ2/ɛ2=3 (0.4%), ɛ2/ɛ3=95 (11.7%), ɛ2/ɛ4=18 (2.2%), ɛ3/ɛ3=472 (58.2%), ɛ3/ɛ4=208 (25.6%), and ɛ4/ɛ4=15 (1.8%) (total n=811). TOMM40 523 had allele frequencies of S=41.0%, L=15.4% and VL=43.6%, with genotype frequencies of S/S=125 (15.2%), S/L=123 (14.9%), S/VL=302 (36.7%), L/L=18 (2.2%), L/VL=95 (11.5%) and VL/VL=160 (19.4%) (total n=823). Exact tests confirmed that APOE and TOMM40 were in Hardy–Weinberg equilibrium (P-values=0.44 and 0.06, respectively).
APOE, TOMM40 and cognitive ability—not adjusted for childhood intelligence (Model 1)
Significant deleterious/negative effects of the APOE ɛ4 allele (vs absence) were found on three tasks: specifically symbol search (P=0.048), inspection time (P=0.004) and spatial span (P=0.033; see Supplementary Table 1). For TOMM40 523, no significant effects were found in the whole sample (‘Step 1') or ɛ3/ɛ3 genotype subgroup (‘Step 3'). A single significant protective effect of the S allele was found in APOE ɛ3/ɛ4 genotype subgroup only, for letter-number sequencing (P=0.035; ‘Step 2' see Supplementary Table 2). Neither APOE ɛ nor TOMM40 523 genotypes were associated with age 11 IQ scores themselves (P>0.05; see Supplementary Tables 1 and 2).
APOE, TOMM40 and cognitive ability—adjusted for childhood intelligence (Models 2 and 3)
Significant deleterious effects of APOE ɛ4 allele presence (vs absence) were found for nine out of a possible fifteen age 73 ‘cognitive ageing' variables (see Table 1). Specifically these were g (P=0.005), matrix reasoning (P=0.041), gspeed(P=0.016), digit symbol coding (P=0.048), symbol search (P=0.014), inspection time (P=0.001), gmemory (P=0.020), logical memory Total (P=0.013) and spatial span (P=0.008; ‘Step 1'). Each significant association survived correction for vascular disease history (P<0.05; see Supplementary Table 3).
Table 1. APOE ɛ and cognitive ability at age 73 years, adjusted for age 11 IQ.
| Cognitive test |
ɛ4 allele presence (vs absence) |
||
|---|---|---|---|
| (d.f.) F statistics | P | Partial η2 | |
| General factor: intelligence (g) | (1, 746)=8.04 | 0.005 | 0.011 |
| Digit span backwards | (1, 755)=0.53 | 0.468 | 0.001 |
| Matrix reasoning | (1, 754)=4.19 | 0.041 | 0.006 |
| Block design | (1, 753)=3.70 | 0.055 | 0.005 |
| Letter-number sequencing | (1, 754)=0.70 | 0.403 | 0.001 |
| General factor: processing speed (gspeed) | (1, 716)=5.80 | 0.016 | 0.008 |
| Digit symbol coding | (1, 753)=3.92 | 0.048 | 0.005 |
| Symbol search | (1, 750)=6.04 | 0.014 | 0.008 |
| Simple reaction time (seconds) | (1, 747)=0.04 | 0.845 | 0.000 |
| Four-choice reaction time (seconds) | (1, 751)=0.30 | 0.587 | 0.000 |
| Inspection time | (1, 729)=11.10 | 0.001 | 0.015 |
| General factor: memory (gmemory) | (1, 739)=5.43 | 0.020 | 0.007 |
| Logical memory | (1, 753)=6.23 | 0.013 | 0.008 |
| Verbal paired associates | (1, 741)=1.03 | 0.311 | 0.001 |
| Spatial span | (1, 751)=7.10 | 0.008 | 0.009 |
Age in days at the time of testing, gender and age 11 IQ statistically controlled. Associations significant at P<0.05 are in bold.
As shown in Table 2, two significant protective associations with S allele possession were found for TOMM40 523: in the whole sample for spatial span (P=0.043; ‘Step 1'), and for letter-number sequencing in the APOE ɛ3/ɛ4 genotype subgroup only (P=0.035; ‘Step 2'). The significant association with spatial span scores did not remain significant when corrected for possession of the APOE ɛ4 allele (P>0.05). The significant association with letter-number sequencing attenuated to (marginal) nonsignificance when corrected for vascular disease history (P=0.050, rounded down). Because of this, associations between TOMM40 523 and cognitive ageing were not examined further.
Table 2. TOMM40 ‘523' poly-T repeat length genotype and cognitive ability—adjusted for age 11 IQ.
| Cognitive test |
Step 1 Whole sample |
Step 2 ɛ3/ɛ4 genotype subgroup only |
Step 3 ɛ3/ɛ3 genotype subgroup only |
||||||
|---|---|---|---|---|---|---|---|---|---|
| (d.f.) F statistics | P | Partial η2 | (d.f.) F statistics | P | Partial η2 | (d.f.) F statistics | P | Partial η2 | |
| General factor: intelligence (g) | 5, 753=1.52 | 0.182 | 0.010 | (1, 186)=1.04 | 0.309 | 0.006 | (2, 418)=0.92 | 0.401 | 0.004 |
| Digit span backwards | 5, 762=0.08 | 0.995 | 0.001 | (1, 187)=0.07 | 0.786 | 0.000 | (2, 426)=0.74 | 0.476 | 0.003 |
| Matrix reasoning | 5, 761=0.73 | 0.602 | 0.005 | (1, 187)=1.02 | 0.314 | 0.005 | (2, 425)=0.56 | 0.572 | 0.003 |
| Block design | 5, 760=1.39 | 0.228 | 0.009 | (1, 187)=0.25 | 0.618 | 0.001 | (2, 424)=1.31 | 0.277 | 0.006 |
| Letter-number sequencing | 5, 761=1.19 | 0.312 | 0.008 | (1, 187)=4.51 | 0.035 | 0.024 | (2, 425)=1.39 | 0.250 | 0.007 |
| General factor: processing speed (gspeed) | 5, 723=0.93 | 0.463 | 0.006 | (1, 173)=0.31 | 0.578 | 0.002 | (2, 407)=0.30 | 0.739 | 0.001 |
| Digit symbol coding | 5, 760=0.93 | 0.459 | 0.006 | (1, 187)=0.31 | 0.578 | 0.002 | (2, 424)=0.89 | 0.410 | 0.004 |
| Symbol search | 5, 757=1.18 | 0.316 | 0.008 | (1, 186)=0.00 | 0.951 | 0.000 | (2, 422)=0.37 | 0.692 | 0.002 |
| Simple reaction time (seconds) | 5, 754=0.55 | 0.741 | 0.004 | (1, 185)=0.42 | 0.518 | 0.002 | (2, 421)=1.25 | 0.287 | 0.006 |
| Four-choice reaction time (seconds) | 5, 758=0.35 | 0.880 | 0.002 | (1, 186)=0.84 | 0.462 | 0.004 | (2, 424)=0.44 | 0.646 | 0.002 |
| Inspection time | 5, 736=2.13 | 0.060 | 0.014 | (1, 177)=2.26 | 0.135 | 0.013 | (2, 414)=0.20 | 0.820 | 0.001 |
| General factor: memory (gmemory) | 5, 746=2.11 | 0.063 | 0.014 | (1, 183)=0.17 | 0.685 | 0.001 | (2, 416)=0.79 | 0.457 | 0.004 |
| Logical memory | 5, 760=2.13 | 0.060 | 0.014 | (1, 186)=0.27 | 0.601 | 0.001 | (2, 425)=2.33 | 0.099 | 0.011 |
| Verbal paired associates | 5, 748=0.95 | 0.449 | 0.006 | (1, 184)=0.02 | 0.891 | 0.000 | (2, 417)=1.09 | 0.337 | 0.005 |
| Spatial span | 5, 758=2.31 | 0.043 | 0.015 | (1, 187)=3.09 | 0.081 | 0.016 | (2, 422)=1.25 | 0.288 | 0.006 |
Age in days at the time of testing, gender and age 11 IQ statistically controlled. Associations significant at P<0.05 are in bold.
Correction for multiple testing with FDR
When the above tests of associations were corrected for multiple testing with FDR, all significant associations attenuated to nonsignificance (all FDR-adjusted P-values >0.05). Further exploratory analyses were conducted on the basis that they could provide directions for future studies.
Mediation: intercorrelations between white matter tract integrity and cognitive ageing
Analyses next examined the mediation of genetic-cognitive ageing associations via white matter tract integrity metrics. We examined correlations between white matter tract variables, which showed significant raw deleterious association with APOE ɛ4 possession as reported by Lyall et al.10—namely left inferior longitudinal fasciculus FA and right ventral cingulum FA—and cognitive ageing variables, which were significantly associated with APOE ɛ4 in the present report after correction for vascular disease history, that is, those at P<0.05 in Table 1 which survived this correction (see Supplementary Table 3 for the exact data).
As can be seen in Table 3, several semi-partial correlations between white matter tract FA metrics and cognitive scores were statistically significant, controlling for age, gender and age 11 IQ, where both the cognitive and imaging variables were also associated with APOE ɛ4 (detailed below).
Table 3. Intercorrelations between cognitive ageing and white matter tract variables that are each significantly associated with APOE ɛ genotype.
| r (P-value) | Right ventral cingulum FA | Left inferior longitudinal fasciculus FA |
|---|---|---|
| General factor: intelligence (g) | 0.02 (0.347) | 0.20 (<0.001) |
| Matrix reasoning | −0.01 (0.411) | 0.10 (0.008) |
| General factor: processing speed (gspeed) | 0.04 (0.196) | 0.17 (<0.001) |
| Digit symbol coding | 0.03 (0.276) | 0.18 (<0.001) |
| Symbol search | −0.03 (0.240) | 0.11 (0.004) |
| Inspection time | 0.07 (0.042) | 0.14 (<0.001) |
| General factor: memory (gmemory) | 0.01 (0.441) | 0.08 (0.024) |
| Logical memory | 0.02 (0.328) | 0.05 (0.131) |
| Spatial span | −0.02 (0.334) | 0.09 (0.015) |
Abbreviation: FA, fractional anisotropy.
Pearson semi-partial correlations controlling for age, gender and age 11 IQ. Figures reflect ‘r' correlations. FA, fractional anisotropy. Significant correlations in bold reflect a significant correlation (P<0.05) where both variables are also associated with APOE ɛ4 independent of vascular disease history (Supplementary Table 3).
Mediation: APOE/TOMM40→white matter tract integrity→cognitive ageing
Brain imaging and cognitive variables that showed nominally significant raw associations with APOE ɛ4, and that were themselves significantly correlated, were examined further for mediation. Analyses tested exploratory mediation on the basis of the above correlations, with the bootstrapping technique.33
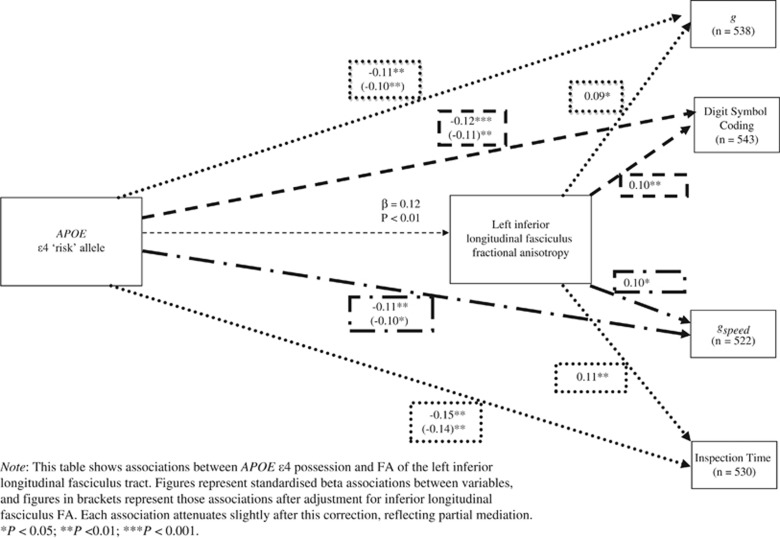
Bootstrapping statistics indicated that left inferior longitudinal fasciculus FA significantly mediated the association between APOE and g (indirect effect=−0.02, 95% CIs=−0.05 to −0.00), gspeed (indirect effect=−0.03, 95% CIs=−0.07 to −0.01), digit symbol coding (indirect effect=−0.30, 95% CIs=−0.79 to −0.05) and inspection time (indirect effect=−0.30, 95% CIs=−0.76 to −0.06), but not matrix reasoning (indirect effect=−0.06, 95% CIs=−0.20 to 0.17), symbol search (indirect effect=−0.08, 95% CIs=−0.30 to 0.21), gmemory (indirect effect=<−0.00, 95% CIs=−0.22 to 0.21) or spatial span (indirect effect=−0.04, 95% CIs=−0.12 to 0.01). Cases of statistically significant mediation are displayed in Figure 2. Bootstrapping statistics indicated that right ventral cingulum FA did not significantly mediate the association between APOE and inspection time total scores.
Figure 2.
Three-way associations between APOE ɛ, left inferior longitudinal fasciculus tract integrity and cognitive ability adjusted for age 11 IQ.
Discussion
Overview
This report found significant deleterious effects of APOE ɛ4 allele possession on several cognitive tasks, independent of age, gender, childhood IQ and vascular disease history; namely tests of nonverbal reasoning (matrix reasoning), visuospatial ability (spatial span), processing speed (digit symbol coding, symbol search, inspection time), and memory (logical memory total). We also found significant nominal effects of APOE genotype on general factors of intelligence, processing speed and memory, constructed with PCA, consistent with recent large studies.3,36 The current study did not find significant effects of TOMM40 523 genotype (that remained significant when we added the covariates of APOE ɛ4 or vascular disease history). Formal tests of mediation with bootstrapping showed that the left inferior longitudinal fasciculus significantly mediated associations between APOE ɛ4 and g, digit symbol coding, gspeed and inspection time total scores. This tract did not completely mediate these significant associations; the gene-cognitive associations did not attenuate markedly.
All associations with cognitive ability attenuated to nonsignificance when corrected for type 1 error with FDR. Cognitive variables are strongly intercorrelated, as reflected by the general factors that were constructed with PCA. Although FDR is less affected by test statistics that are based on intercorrelated variables when compared with other type 1 error adjustment procedures such as Bonferroni,31 this can still make correction for multiple testing overly conservative.37 We interpret all significant raw gene-cognitive associations cautiously, and emphasize that they require independent replications. These intercorrelations also highlight the fact that the genetic associations with cognitive variables are not independent and may partly reflect a degree of shared variance between these tasks (as indicated by g).
Interpretation: APOE ɛ genotype
The demonstration that some significant associations between APOE and aspects of ‘cognitive ageing' (cognitive test scores in older age adjusted for childhood intelligence) are mediated by white matter tract integrity is novel, possibly reflecting the fact that relatively few studies have the requisite genetic, imaging and cognitive data.38 This is important because it helps to elucidate the neural substrates underpinning the association between APOE and cognitive ageing.
It is plausible that the left inferior longitudinal fasciculus at least partly mediates specific APOE-cognitive ageing associations. The inferior longitudinal fasciculus is an occipito-temporal tract.39 Correlations between white matter integrity and cognitive ability may reflect a ‘computational bottleneck' whereby lowered integrity limits the amount of information that can be transmitted between brain structures (Westlye et al.,40 pp. 514). This would theoretically strengthen the FA-cognitive correlation:40 for example, Ryan et al.13 reported that associations between FA and cognitive ability in relatively healthy older adults (N=126) differed significantly according to APOE genotype (for example, frontal lobe FA correlated with an 'Executive function' score at r=0.46 for ɛ4 carriers, vs. r=0.04 in non-carriers); we indirectly add to this finding with a relatively large study that includes formal tests of mediation.33 This theory, overall, provides a reasonable biological explanation for the mediating role of the inferior longitudinal fasciculus and performance on specific cognitive tasks. It is also possible that the integrity of this tract correlates significantly with the actual mechanistic brain phenotype, which was perhaps not examined here.38
Interpretation: TOMM40 ‘523' poly-T repeat genotype
We are aware of three previous independent studies of TOMM40 523 genotype and cognitive ability in older adults.6, 7, 8 The current findings in the LBC1936 sample contrast with previous studies in showing no significant effects of TOMM40 523 genotype, independent of APOE or vascular disease history. The relatively small samples reported by Johnson et al.8 (N=117) and Hayden et al.7 (N=127), and the marginal significance reported by Caselli et al.6 (P=0.04) caution that previous significant findings may to an extent reflect type 1 error.
Limitations and future research
This report examined variables that were statistically significantly intercorrelated at P<0.05, and where mediation would therefore be most likely (‘Causal Steps' approach41). A limitation of this ‘Causal Steps' approach is that each hypothesis test carries a possibility of type 1 or type 2 error. Rather, Hayes41 suggests hypothesis-driven testing of indirect effects, regardless of the statistical significance of the mediator's association with the independent and dependent variables; it is possible that significant APOE-cognitive ageing associations occur via very small effects on a large, distributed range of brain phenotypes. Future studies should consider a large-scale examination of APOE genotype, different brain imaging phenotypes and cognitive ageing variables, possibly using a structural equation modelling/path analytic framework formally to test the network of associations and for the presence of mediation.
There is evidence that APOE genotype may have a significant effect on brain white matter throughout the lifecourse, including early development.42, 43, 44 This could imply that ageing effects may be superimposed on underlying vulnerabilities in the way that brain white matter develops in APOE ɛ4 carriers (vs non-carriers). The current sample does not have empirical brain imaging at younger ages to address this possibility further.
Although APOE ɛ4 is an established risk factor for late onset Alzheimer's disease and for worse cognitive ageing, this polymorphism accounts for only a small fraction of phenotypic variance. Other genetic loci or environmental variables are likely to have significant interactive or independent roles. The approach described here will be useful in testing similar genetic/brain imaging/cognitive associations, however future studies will require large, well-phenotyped cohorts.
Acknowledgments
The participation of LBC1936 members is gratefully acknowledged. We thank the study secretary Paula Davies, Janie Corley, Catherine Murray, Alison Pattie, Caroline Brett and Ross Henderson for data collection and data entry; the nurses, radiographers and other staff at the Wellcome Trust Clinical Research Facility (http://www.wtcrf.ed.ac.uk) and the Brain Research Imaging Centre (http://www.bric.ed.ac.uk) where the medical and cognitive assessments, genotyping and brain MRI data collection were performed; and the staff at Lothian Health Board and at the SCRE Centre, University of Glasgow. LBC1936 data collection was supported by the Disconnected Mind project (http://disconnectedmind.ed.ac.uk) funded by Age UK. JMW is part-funded by the Scottish Funding Council as part of the SINAPSE Collaboration (http://www.sinapse.ac.uk). The work was undertaken within the University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology (http://www.ccace.ed.ac.uk), part of the cross council Lifelong Health and Wellbeing Initiative (G0700704/84698). Funding from the Biotechnology and Biological Sciences Research Council (BBSRC), Engineering and Physical Sciences Research Council (EPSRC), Economic and Social Research Council (ESRC) and MRC is gratefully acknowledged.
ADR is the CEO and only stock holder of Zinfandel Pharmaceuticals, a company in an Alliance with Takeda Pharmaceuticals, to perform the prospective qualification of the TOMM40 marker for age of onset distribution of Alzheimer's disease. For this study, Zinfandel Pharmaceuticals paid for the TOMM40 assays to be performed for medical research, not as a clinical diagnostic. AMS is the spouse of ADR, and AMS and MWL are consultants to Zinfandel Pharmaceuticals. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Roses AD, Lutz MW, Amrine-Madsen H, Saunders AM, Crenshaw DG, Sundseth SS, et al. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer's disease. Pharmacogenomics J. 2010;10:375–384. doi: 10.1038/tpj.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roses AD, Lutz MW, Crenshaw DG, Grossman I, Saunders AM, Gottschalk WK, et al. TOMM40 and APOE: Requirements for replication studies of association with age of disease onset and enrichment of a clinical trial. Alzheimer's Dement. 2013;9:132–136. doi: 10.1016/j.jalz.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol Ageing. 2011;32:63–74. doi: 10.1016/j.neurobiolaging.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Reinvang I, Espeseth T, Westlye LT. APOE-related biomarker profiles in non-pathological ageing and early phases of Alzheimer's disease. Neurosci Biobehav Rev. 2013;37:1322–1335. doi: 10.1016/j.neubiorev.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Crenshaw DG, Gottschalk WK, Lutz MW, Grossman I, Saunders AM, Burke R, et al. Using genetics to enable studies on the prevention of Alzheimer's disease. Clin Pharmacol Ther. 2013;93:177–185. doi: 10.1038/clpt.2012.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli R, Dueck AC, Huentelman MJ, Lutz MW, Saunders AM, Reiman EM, et al. Longitudinal modelling of cognitive aging and the TOMM40 effect. Alzheimer's Dement. 2012;8:490–495. doi: 10.1016/j.jalz.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden KM, McEvoy JM, Linnertz C, Attix D, Kuchibhatla M, Saunders AM, et al. A homopolymer polymorphism in the TOMM40 gene contributes to cognitive performance in aging. Alzheimer's Dement. 2012;8:381–388. doi: 10.1016/j.jalz.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Rue A, Hermann BP, Xu G, Koscik RL, Jonaitis EM, et al. The effect of TOMM40 poly-T length on gray matter volume and cognition in middle-aged persons with APOE ɛ3/ɛ3 genotype. Alzheimer's Dement. 2011;7:456–465. doi: 10.1016/j.jalz.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penke L, Maniega SM, Bastin ME, Valdés Hernández MC, Murray C, Royle NA, et al. Brain white matter tract integrity as a neural foundation for general intelligence. Mol Psychiatry. 2012;17:1026–1030. doi: 10.1038/mp.2012.66. [DOI] [PubMed] [Google Scholar]
- Lyall DM, Harris SE, Bastin ME, Muñoz Maniega S, Murray C, Lutz MW, et al. Alzheimer's disease susceptibility genes APOE and TOMM40, and brain white matter integrity in the Lothian Birth Cohort 1936. Neurobiol Aging. 2014;35:1513.e25–33. doi: 10.1016/j.neurobiolaging.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez A, Fieremans E, Jensen JH, Falangola MF, Tabesh A, Ferris SH, et al. White matter tract integrity metrics reflect the vulnerability of late-myelinating tracts in Alzheimer's disease. Neuroimage Clin. 2014;4:64–71. doi: 10.1016/j.nicl.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: The do's and don'ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Ryan L, Walther K, Bendlin B, Lue L, Walker DG, Glisky EL, et al. Age-related differences in white matter integrity and cognitive function are related to APOE status. Neuroimage. 2011;54:1565–1577. doi: 10.1016/j.neuroimage.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Gow AJ, Pattie A, Starr JM. Cohort profile: the Lothian Birth Cohorts of 1921 and 1936. Int J Epidemiol. 2012;41:1576–1584. doi: 10.1093/ije/dyr197. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Gow AJ, Taylor MD, Corley J, Brett C, Wilson V, et al. Lothian Birth Cohort 1936: a study to examine influences on cognitive ageing from age 11 to age 70 and beyond. BMC Geriatr. 2007;7:1–12. doi: 10.1186/1471-2318-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Bastin ME, Hernandez MV, Munoz Maniega S, Royle N, Morris Z, et al. Brain ageing, cognition in youth and old age, and vascular disease in the Lothian Birth Cohort 1936: rationale, design and methodology of the imaging protocol. Int J Stroke. 2011;6:547–559. doi: 10.1111/j.1747-4949.2011.00683.x. [DOI] [PubMed] [Google Scholar]
- Linnertz C, Saunders AM, Lutz MW, Crenshaw DM, Grossman I, Burns DK, et al. Characterization of the Poly-T Variant in the TOMM40 gene in diverse populations. PLoS One. 2012;7:e30994. doi: 10.1371/journal.pone.0030994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-III UK Administration and Scoring Manual. Psychological Corporation: London UK; 1998. [Google Scholar]
- Cox BD, Huppert FA, Whichelow MJ. The Health and Lifestyle Survey: Seven Years on. Dartmouth Publications: Aldershot, UK; 1993. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-III UK Administration and Scoring Manual. Psychological Corporation: London UK; 1998. [Google Scholar]
- Luciano M, Gow AJ, Harris SE, Hayward C, Allerhand M, Starr JM, et al. Cognitive ability at age 11 and 70 years, information processing speed, and APOE variation: the Lothian Birth Cohort 1936 study. Psychol Aging. 2009;24:129–138. doi: 10.1037/a0014780. [DOI] [PubMed] [Google Scholar]
- Houlihan LM, Wyatt ND, Harris SE, Hayward C, Gow AJ, Marioni RE, et al. Variation in the uric acid transporter gene (SLC2A9) and memory performance. Hum Mol Genet. 2010;19:2321–2330. doi: 10.1093/hmg/ddq097. [DOI] [PubMed] [Google Scholar]
- Carroll JB. Human Cognitive Abilities: A Survey of Factor-Analytic Studies. Cambridge University Press: New York, NY, USA; 1993. [Google Scholar]
- Lezak MD, Howieson DB, Loring DW.Neuropsychological Assessment4th edn. Oxford University Press: Oxford, UK; 2004 [Google Scholar]
- Behrens TEJ, Berg HJ, Jbabdi R, Rushworth MFS, Woolrich WM. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain. Neuroimage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayden JD, Storkey AJ, Bastin ME. A probabilistic model-based approach to consistent white matter tract segmentation. IEEE Trans Med Imaging. 2007;26:1555–1561. doi: 10.1109/TMI.2007.905826. [DOI] [PubMed] [Google Scholar]
- Clayden JD, Munoz Maniega S, Storkey AJ, King MD, Bastin ME, Clark CA, et al. TractoR: magnetic resonance imaging and tractography with R. J Stat Softw. 2011;44:1–18. [Google Scholar]
- Lutz MW, Crenshaw DG, Saunders AM, Roses AD. Genetic variation at a single locus and age of onset for Alzheimer's Disease. Alzheimers Dement. 2010;6:125–131. doi: 10.1016/j.jalz.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno D, Pomara N, Nierenberg JJ, Ritchie JC, Lutz MW, Zetterberg H, et al. Levels of cerebrospinal fluid neurofilament light protein in healthy elderly vary as a function of TOMM40 variants. Exp Gerontol. 2012;47:347–352. doi: 10.1016/j.exger.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiepers OJG, Harris SE, Gow AJ, Pattie A, Brett CE, Starr JM, et al. APOE e4 status predicts age-related cognitive decline in the ninth decade: longitudinal follow-up of the Lothian Birth Cohort 1921. Mol Psychiatry. 2012;17:315–324. doi: 10.1038/mp.2010.137. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29:1165–1188. [Google Scholar]
- Pike N. Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol Evol. 2011;2:278–282. [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;30:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Lyall DM, Lopez LM, Bastin ME, Munoz-Maniega S, Penke L, Hernandez M, et al. ADRB2, brain white matter integrity and cognitive ageing in the Lothian Birth Cohort 1936. Behav Genet. 2013;43:13–23. doi: 10.1007/s10519-012-9570-x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state'. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Davies G, Harris SE, Reynolds CA, Payton A, Knight HM, Liewald DC, et al. A genome-wide association study implicates the APOE locus in nonpathological cognitive ageing. Mol Psychiatry. 2012;19:76–87. doi: 10.1038/mp.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SC, Haines JL. Correcting away the hidden heritability. Ann Hum Genet. 2011;75:348–350. doi: 10.1111/j.1469-1809.2011.00640.x. [DOI] [PubMed] [Google Scholar]
- Salthouse T. Neuroanatomical substrates of age-related cognitive decline. Psychol Bull. 2011;137:753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten TM. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Westlye ET, Hodneland E, Haasz J, Espeseth T, Lundervold A, Lundervold AJ, et al. Episodic memory of APOE ɛ4 carriers is correlated with fractional anisotropy, but not cortical thickness, in the medial temporal lobe. Neuroimage. 2012;63:507–516. doi: 10.1016/j.neuroimage.2012.06.072. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monogr. 2009;76:408–420. [Google Scholar]
- Knickmeyer RC, Wang J, Zhu H, Geng G, Woolson S, Hamer RM, et al. Common variants in psychiatric risk genes predict brain structure at birth. Cereb Cortex. 2013;24:1230–1246. doi: 10.1093/cercor/bhs401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J, Krainc D. Alzheimer gene APOE ɛ4 linked to brain development in infants. JAMA. 2014;311:298–299. doi: 10.1001/jama.2013.285400. [DOI] [PubMed] [Google Scholar]
- Dean DC, Jerskey BA, Chen K, Protas H, Thiyyagura P, Roontiva A, et al. Brain differences in infants at differential genetic risk for late-onset alzheimer disease. JAMA Neurol. 2014;71:11–22. doi: 10.1001/jamaneurol.2013.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.