Abstract
Single, severe traumatic brain injury (TBI) which elevates CNS amyloid, increases the risk of Alzheimer's disease (AD); while repetitive concussive and subconcussive events as observed in athletes and military personnel, may increase the risk of chronic traumatic encephalopathy (CTE). We describe two clinical cases, one with a history of multiple concussions during a career in the National Football League (NFL) and the second with frontotemporal dementia and a single, severe TBI. Both patients presented with cognitive decline and underwent [18F]-Florbetapir positron emission tomography (PET) imaging for amyloid plaques; the retired NFL player also underwent [18F]-T807 PET imaging, a new ligand binding to tau, the main constituent of neurofibrillary tangles (NFT). Case 1, the former NFL player, was 71 years old when he presented with memory impairment and a clinical profile highly similar to AD. [18F]-Florbetapir PET imaging was negative, essentially excluding AD as a diagnosis. CTE was suspected clinically, and [18F]-T807 PET imaging revealed striatal and nigral [18F]-T807 retention consistent with the presence of tauopathy. Case 2 was a 56-year-old man with personality changes and cognitive decline who had sustained a fall complicated by a subdural hematoma. At 1 year post injury, [18F]-Florbetapir PET imaging was negative for an AD pattern of amyloid accumulation in this subject. Focal [18F]-Florbetapir retention was noted at the site of impact. In case 1, amyloid imaging provided improved diagnostic accuracy where standard clinical and laboratory criteria were inadequate. In that same case, tau imaging with [18F]-T807 revealed a subcortical tauopathy that we interpret as a novel form of CTE with a distribution of tauopathy that mimics, to some extent, that of progressive supranuclear palsy (PSP), despite a clinical presentation of amnesia without any movement disorder complaints or signs. A key distinguishing feature is that our patient presented with hippocampal involvement, which is more frequently seen in CTE than in PSP. In case 2, focal [18F]-Florbetapir retention at the site of injury in an otherwise negative scan suggests focal amyloid aggregation. In each of these complex cases, a combination of [18F]-fluorodeoxyglucose, [18F]-Florbetapir and/or [18F]-T807 PET molecular imaging improved the accuracy of diagnosis and prevented inappropriate interventions.
Introduction
Recent attention has been focused on the cognitive risks involved in contact sports. These concerns have been associated historically with chronic and repetitive concussions in boxers, but recently professional football players,1,2 hockey players,3 and wrestlers4 have become the focus of expanded attention. In addition, the sometimes-fatal consequence of a single, significant traumatic brain injury (TBI) has been highlighted recently by the media due to high profile cases (for example, http://abcnews.go.com/Entertainment/Movies/story?id=7119825 and http://www.express.co.uk/comment/columnists/richard-and-judy/451938/F1-racing-driver-Michael-Schumacher-ski-crash-highlights-sport-secret-on-head-injuries). On the basis of clinical and postmortem studies, the conventional wisdom has been that a single severe TBI increases risk for Alzheimer's disease (AD),5,6 whereas the consequences of chronic repetitive TBI, first identified in boxers, increases the risk for a tangle-predominant disease, or tauopathy, known as dementia pugilistica.7 More recently, the term ‘chronic traumatic encephalopathy (CTE)' has been applied when dementia pugilistica-like neuropathology was observed in retired National Football League (NFL) players, as well as in entertainment wrestlers, victims of domestic violence and in military veterans exposed to blast and concussive injuries from improvised explosive devices.1,2,4,8, 9, 10 Neuropathologically, CTE is characterized by prominent tauopathy, variable degrees of diffuse amyloid deposition, and degeneration of the neocortex, hippocampus, amygdala, basal forebrain and mammillary bodies. The nature, distribution and patterns of neurofibrillary degeneration in CTE are distinctive from AD,11,12 and neuropsychological, mood and neurobehavioral dysfunction in CTE typically presents in midlife after a latency period, usually years or decades after exposure to the repetitive trauma.13 The cognitive and behavioral symptoms of CTE begin insidiously, followed by progressive deterioration. Mood symptoms typically include depression, apathy, irritability and suicidality. Behavioral symptoms include poor impulse control, disinhibition and aggression, as well as frequent comorbid substance abuse. Nevertheless, CTE remains a controversial diagnosis, as it is a pathological diagnosis, and no consensus on the clinical diagnosis has yet been developed.14,15 Therefore, diagnosis may be difficult to determine on the basis of a standard clinical and laboratory evaluation criteria.
The challenge faced by many clinicians in such cases is making an accurate diagnosis from among the various dementias that share cognitive, mood and behavioral symptoms, particularly when considering the differential diagnosis of AD or frontotemporal dementia (FTD) and CTE. To order the appropriate tests and implement the most effective treatment available, clinicians must determine the underlying neuropathology on the basis of clinical evaluation and history, and will ultimately diagnose an individual with a neurodegenerative disease to be proven at the postmortem examination. Three new positron emission tomography (PET) tracers have been approved recently by the US Food and Drug Administration as clinical tools to estimate brain amyloid burden in patients being evaluated for cognitive impairment (CI) or dementia. Imaging with these tracers, if positive, means AD plaques are present; if the scan is negative, cerebral amyloidosis is absent, and a negative scan, such as the one in this case using [18F]-Florbetapir, provides in vivo confirmation that the dementia is not AD, if negative. Such scans increase the accuracy of diagnosis. Amyloid imaging may improve etiological likelihood in situations where the differential diagnosis cannot be resolved on the basis of standard clinical and laboratory criteria.16 In the absence of brain amyloid it is unlikely that the cause of an individual's cognitive decline is due to AD.17
Early studies suggested that TBI increased the risk for AD at an earlier age of onset; however, these findings were not based entirely on neuropathological evidence and some were methodologically flawed (for review, see ref. 18). The diagnosis of AD requires the presence of amyloid β neuritic plaques and neurofibrillary tangles in brain.19 The clinical distinction of AD from other causes of posttraumatic CI can be challenging, but accurate diagnosis is required to avoid misdiagnosis, incorrect prognosis, incorrect family history, and potentially, initiation of inappropriate treatment.
Several new PET imaging neurotracers have been developed that detect pathological tau in vivo. One such ligand, [18F]-T807, is one of a novel class of 5H-pyrido[4,3-b]indole labeled with [18F] that has a high affinity and selectivity for tau over amyloid β (>25 fold).20 Preliminary imaging studies in AD transgenic mice and patients found that this ligand has favorable imaging kinetics, high target cortical to cerebellum uptake ratios, and an accumulation pattern that followed the characteristic tau deposition pattern seen in AD.19 Here we present two clinical cases in which the in vivo findings utilizing these new PET tracers for amyloid plaques and neurofibrillary tangles clarified the diagnoses.
Case Report
Case 1
A 71-year-old retired professional football player who had sustained multiple concussions during a decade-long NFL career ending approximately 40 years ago presented with a history of progressive CI. During his professional career, he reported experiencing multiple concussions but was unable to estimate their number. He did not recall any episodes of loss of consciousness, but did remember being dazed and confused for up to a full day following some of these injuries; having difficulty finding his way home after a game or, upon awakening on the day after some games, being unable to recall the identity of the team against whom he had played the previous day. After retiring from the sport, he had a successful business career (outside of sports) for approximately 20 years. After retiring from business, he coached high school football. Over the past several years, however, he and his wife noticed impaired memory and thinking, notably short-term memory, and more so in the past year. Agitation emerged after his memory decline, and increased in the year before evaluation. There were no other reported behavioral disturbances; mood was normal. He was evaluated for progressive neurodegeneration by the NFL Neurological Care Program team at Mount Sinai Hospital, a multidisciplinary team consisting of a neurologist, a dual-board-certified neurologist-psychiatrist, a neuropsychologist and two neuroradiologists. All evaluators were TBI and AD experts. At the time of the evaluation, the patient was taking both donepezil and memantine, neither of which had any benefit that was obvious to the patient's wife.
The evaluation included comprehensive neurologic and neuropsychological assessment and, given the potential differential diagnosis, a clinical [18F]-Florbetapir PET scan to determine the presence or absence of amyloid. A research [18F]-T807 PET scan was performed to determine the possible presence of tauopathy. Magnetic resonance imaging (MRI) of the brain was conducted 3 months before PET imaging. The MRI revealed global volume loss, especially in the hippocampus. Arachnoid cysts were present in the middle fossa bilaterally. The mammillary bodies were poorly visualized and therefore suspected to be atrophic. The hypothalamic/infundibular/pituitary system appeared preserved structurally. Neurologic exam was grossly normal. Eye movements were normal in all directions. Muscle tone and strength were intact, reflexes were brisk and symmetrical, and no movement disorder was detected upon initial exam. Retrospectively, mild hypomimia was noted.
Case 2
Case 2 was a 59-year-old male physician who had spent nearly 30 years as a member of a group medical practice. He had suffered a head injury while skiing (wearing a helmet) approximately 10 months before the evaluation. Specifically, he fell off a ski lift with head impact on concrete. He later had a fall on the slopes. He denied loss of consciousness (his family noted transient loss of awareness), but reported persistent headache for a few weeks following the head injury. He then began to experience intermittent numbness of his right ear and numbness and weakness in his right hand, for which he sought neurological evaluation approximately 3 months post injury near his residence in another part of the country. MRI revealed recent and chronic bleeding over the left hemispheric convexity, and he underwent a craniotomy to evacuate a left frontal subdural hematoma. The site of the injury was right occipital, thus the subdural hematoma appeared to be a result of a contrecoup injury. Following craniotomy, his headaches persisted as did the intermittent numbness and weakness in his right hand, for which he was begun on levetiracetam.
Detailed history revealed that for many months before the accident his family had noted a change in his personality, episodic increased agitation and altered cognition (word retrieval, short-term memory). They reported that the patient had difficulty recalling recent events but that recall of childhood information remained generally intact. He forgot recent conversations and began having difficulty recognizing the faces of acquaintances, patients and old friends, as well as pictures of famous people. He struggled with abstract reasoning (for example, how people and things related to one another) and had trouble following directions. He would ask the same questions repeatedly and told the same stories multiple times without recalling he had done so. He was unable to maintain attention and concentration when a story became increasingly complicated, and would become agitated and irritable as a result of not being able to follow the story line. When confronted about his cognitive difficulties he would become angry and agitated, and would ‘act out' in the presence of his family. According to the family, the patient would experience ‘manic highs and depressed lows'. When in a good mood, he enjoyed being with others and was largely socially appropriate. His mood could change rapidly, and he would become withdrawn or belligerent. When in a depressed state he expressed suicidal ideation. According to the family, the patient was less emotionally available for things that had been important to him (for example, family relationships). For months before his fall, he had become apathetic and lost interest in hobbies that he previously enjoyed. Approximately 4 years ago (3 years before the fall), the patient had experienced a depressive episode. He had attributed his depression to work-related stress and was treated with sertraline. He had no history of psychiatric hospitalization or psychotherapy. At the time of his evaluation at Mount Sinai Medical Center he was not taking antidepressants.
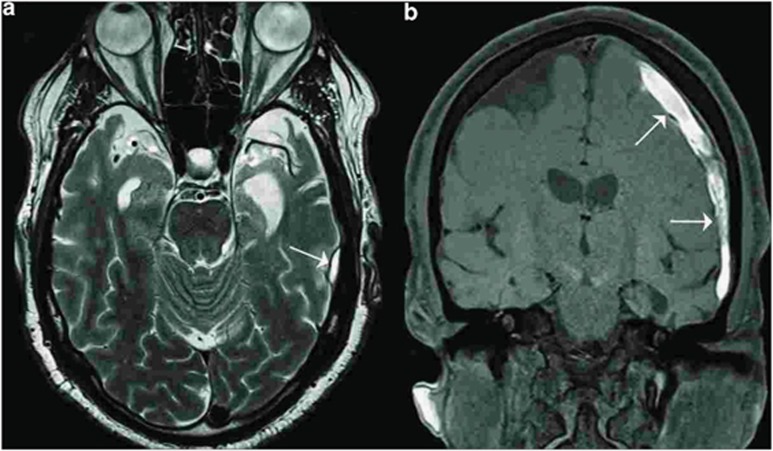
The patient was referred for a comprehensive neuropsychological assessment (JM) and neurological evaluation (SG, AA) at the Mount Sinai Alzheimer's Disease Research Center, as well as appropriate laboratory studies. CT and MRI revealed atrophy of the anterior poles of both temporal and frontal lobes (Figure 1). Molecular imaging included both [18F]-fluorodeoxyglucose (FDG) PET and [18F]-Florbetapir scans. At the time of the evaluation, his medications were atorvastatin 20 mg daily and acetaminophen PRN. He had had a previous neuropsychological evaluation at another facility, 3 months before our assessment. The findings on the prior examination were consistent with the current evaluation.
Figure 1.
Imaging from a 59-year-old, physician with a sports-related injury (case 2). Magnetic resonance imaging showing atrophy of the frontal poles of the frontal and temporal lobes bilaterally. (a) and (b) Arrows indicate a subdural hematoma (SDH).
Results
Case 1
Neuropsychological function
The patient had 16 years of education and premorbid intellectual function was estimated to be in the average range. Neuropsychological tests revealed impaired information processing speed, fine motor function, verbal comprehension and fluency, confrontational naming, and immediate and delayed verbal recall. Intellectual function was preserved. On self-report, he acknowledged minimal depression and mild anxiety (Table 1). Norms were corrected for age and years of education.
Table 1. Neuropsychological scores and percentiles for case 1 (multiple concussions).
|
Case 1–age 71 years | ||
|---|---|---|
| Score | Percentile | |
| WAIS-IV indices | ||
| Full scale | 103 | 58 |
| Verbal comprehension | 83 | 13 |
| Perceptual reasoning | 105 | 63 |
| Working memory | 128 | 97 |
| Processing speed | 102 | 55 |
| General ability | 94 | 34 |
| WMIS-IV indices | ||
| Auditory memory | 69 | 2 |
| Visual memory | 58 | <1 |
| Visual working memory | 85 | 16 |
| Immediate memory | 69 | 2 |
| Delayed memory | 58 | <1 |
| Other neuropsychological testsa | ||
| Memory | ||
| CVLT-LDFR | 0 | <1 |
| Rey-O delay | <1 | |
| Language | ||
| FAS | 19 | |
| Animals | 6 | |
| BNT | <1 | |
| Motor function | ||
| Perdue dom/non-dom | 3rd/8th | |
| General dom/non-dom | 21st/2nd | |
| Visual perception | ||
| REY-O copy | WNL | |
| Attention | ||
| CPT | WNL | |
| Executive function | ||
| SCT | 96 | |
| DK-FST | 91 | |
| Tower task | 63 | |
| Trails B | 70 | |
Abbreviations: BNT, Boston Naming Test; CPT, Connors' Continuous Performance Test II; CVLT-LDFR, California Verbal Learning Test long delay free recall; DK-FST, Delis Kaplan Free Sort Test (norms corrected for age and years of education); FAS, Controlled Oral Word Association Test; GPB, grooved pegboard; SCT, Short Category Test; WAIS-IV, Wechsler Adult Intelligence Scale, 4th edn; WNL, within normal limits.
Scores under other neuropsychological tests reported only as percentiles. Raw scores were not available.
Following this comprehensive evaluation, the experts disagreed as to whether AD was present, in addition to likely posttraumatic encephalopathy. Two independent neuropsychologists from the Mount Sinai Alzheimer's Disease Research Center (ADRC) reviewed the neuropsychological test results and supported the inclusion of possible AD on the basis of the neuropsychological phenotype, although the primary examining team (with the exception of one of the neurologists) opposed the inclusion of possible AD as a diagnosis. Thus the patient was referred for [18F]-Florbetapir PET imaging for diagnostic clarification.
Molecular imaging
[18F]-Florbetapir imaging was conducted with a GE Discovery STE 16-slice PET/CT camera. The patient was injected with 370 MBq (10 mCi) of [18F]-Florbetapir. Image acquisition began approximately 60 min post injection, for 10 min. Images were acquired in three dimensions, using a one-frame and one-bed position. Reconstruction was performed with a 120 × 120 matrix utilizing iterative reconstruction, with 35 subsets and two iterations. The z axis filter was standard, and a 2.57-mm full width/half maximum filter was used. The field of view was 30 cm in diameter, with 47 total slices.
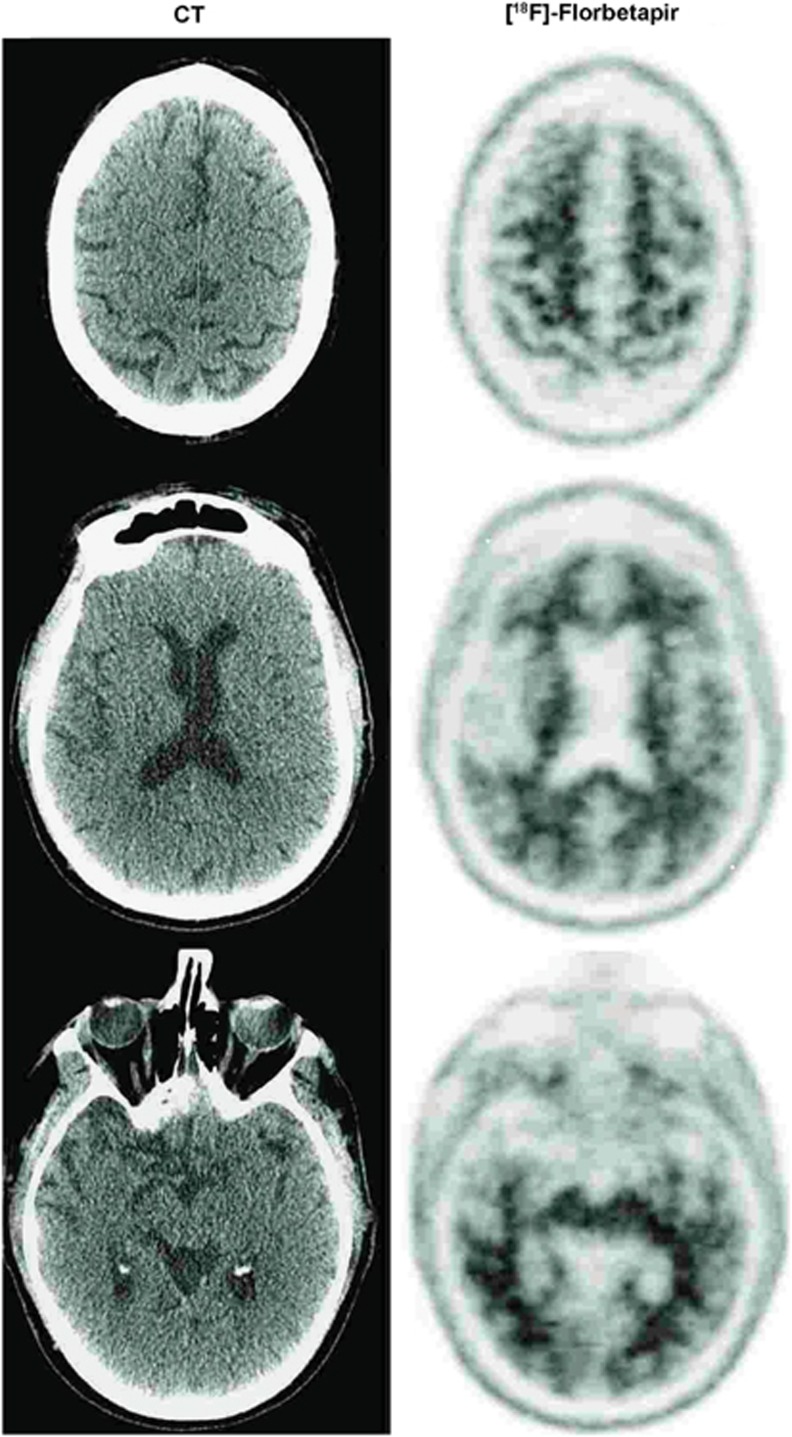
The clinical [18F]-Florbetapir scan results were rated by nuclear medicine physicians (JM or LK) trained to interpret [18F]-Florbetapir scan results using a binary (positive or negative) visual approach.20 Transaxial, coronal and sagittal images were examined. The scan results were considered positive if uptake in the cerebral gray matter equaled or exceeded the uptake in the white matter in at least two major areas of the brain. A positive [18F]-Florbetapir scan indicates moderate-to-frequent fibrillar amyloid plaques; a negative [18F]-Florbetapir scan indicates sparse-to-no fibrillar amyloid plaques, which is inconsistent with a diagnosis of AD. A negative scan thus implied that the cognitive decline was not due to AD. In the retired NFL player under consideration here [18F]-Florbetapir PET scanning was negative for cerebral amyloidosis (Figure 2), thereby excluding AD. This case illustrates the potential for brain amyloid imaging to clarify diagnosis and to prevent inappropriate treatment. Ironically, this patient had sought out this evaluation to assess his eligibility for a clinical trial for AD. The [18F]-Florbetapir results ruled out his inclusion in trials of Aβ-reducing agents.
Figure 2.
Imaging from a 71-year-old retired NFL player (case 1). Left panel is CT image and right panel is [18F]-Florbetapir PET imaging, which was negative for amyloid accumulation. CT, computed tomography; NFL, National Football League; PET, positron emission tomography.
[18F]-T807 imaging was acquired with a Siemens ECAT EXACT HR+ PET Camera. The patient was injected with 370 MBq (10 mCi) of [18F]-T807. Image acquisition began ~110 min post injection, for 20 min. Images were acquired in three dimensions, using one-frame and one-bed position. Reconstruction was performed with a 128 × 128 matrix utilizing iterative reconstruction, with 16 subsets and 4 iterations. A three-dimensional post hoc Gaussian filter (5 mm) was applied to the image volume. A total of 63 axial slices of 2.42 mm thickness were displayed for visual interrogation by a nuclear medicine physician expert in brain imaging (JS). In addition, quantification of the scan was performed by spatially normalizing the PET image to a T807 template image and applying a modified Hammers volume of interest template21 for extraction on regional standard uptake values (SUV). Brain SUV were divided by the cerebellar cortex SUV to calculate SUV ratios in multiple cortical and subcortical regions.
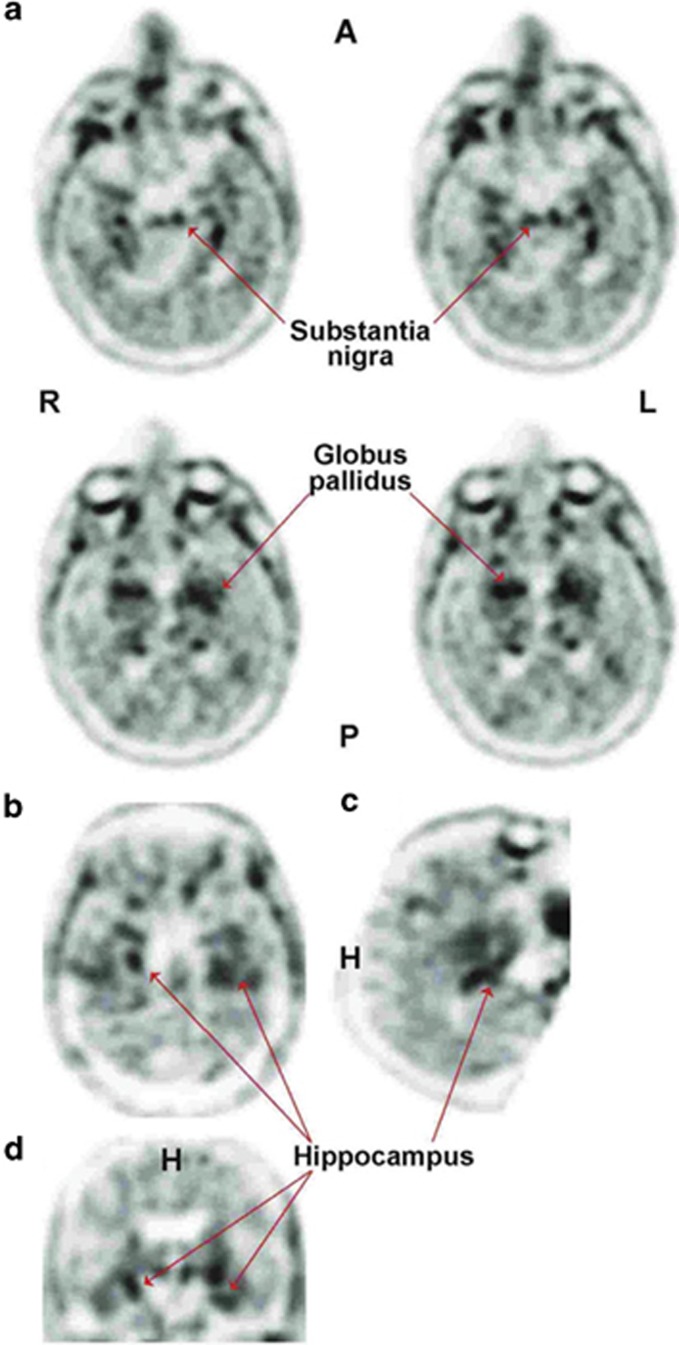
Visually, the [18F]-T807 PET scan revealed [18F] signal in some temporal areas. However, the preponderance of the signal arose bilaterally from the regions of the globus pallidus and the substantia nigra (Figure 3). Consistent with this visual assessment, the region with highest SUV ratios were globus pallidus (1.85), putamen (1.57) and hippocampus (1.45), while the substantia nigra SUV ratio was 1.40.
Figure 3.
Imaging from a 71-year-old retired NFL player (case 1). [18F]-T807 signals (arrows) originate from the globus pallidus (GP), substantia nigra (SN) and hippocampus. Images depict axial (a) sagittal (b) and coronal (c and d) orientation of the brain. A, anterior; H, head; L, left; NFL, National Football League; P, posterior; R, right.
Case 2
Neuropsychological function
His premorbid intellectual function was estimated to be in the high average range. He manifested significant deficits in memory, language (verbal fluency and confrontation naming), and executive functioning; performance on most cognitive measures was far below levels expected for his age, education and estimated premorbid abilities; they ranged from significantly impaired to high average (Table 2). Results were consistent with previous testing, which was suggestive of FTD. On self-report, he endorsed minimal depression that fell within the normal range (Table 2). Norms were corrected for age and years of education.
Table 2. Neuropsychological scores and percentiles for case 2 (single TBI).
|
Case 2—age 59 years | ||
|---|---|---|
| |
Score |
Percentile |
| WAIS-IV indices | ||
| Full scale | NC | NC |
| Verbal comprehension | 63 | 1 |
| Perceptual reasoning | 105 | 63 |
| Processing speed | 92 | 30 |
| Estimated premorbid IQ and orientation | ||
| AMNART estimated premorbid IQ | 107 | 68 |
| MMSE | 23/30 | 0/3 recall |
| WMIS-IV subsets | Scaled score | |
| Logical memory I | 6 | 9 |
| Logical memory II | 6 | 9 |
| Recognition | 51–75 | |
| Other neuropsychological testsa | Z-score/T-score | |
| Memory | ||
| CVLT-II trial 1–5 | T=34 | 5 |
| CVLT short delay free recall | −2 | 2 |
| CVLT long delay free recall | −1.5 | 6 |
| Language | Raw score | |
| FAS | 31 | 11 |
| Animals | 10 | <2 |
| BNT | 3 out of 60 | <1 |
| Rey-O delay | 9 | 3 |
| Visual perception | ||
| REY-O copy | 34 | WNL |
| WAIS-IV block design | 33 | 37 |
| Attention | ||
| WAIS-IV digit span | 28 | 63 |
| WAIS-IV arithmetic | 9 | 9 |
| CVLT-proactive interference | −25 | 50 |
| CVLT-retroactive interference | −71.4 | 30 |
| Executive function | ||
| WCST | ||
| Trails B | 74′ | 56 |
| Mood | ||
| BDI-II | 3 | WNL |
Abbreviations: AMNART, American National Adult Reading Test; BDI-II, Beck Depression Inventory (norms corrected for age and years of education); BNT, Boston Naming Test; CVLT, California Verbal Learning Test; FAS, Controlled Oral Word Association Test (letters FAS); MMSE, Mini Mental Status Examination; TBI, traumatic brain injury; WAIS-IV, Wechsler Adult Intelligence Scale, 4th edn; WCST, Wisconsin Sorting Test; WMS-IV, Wechsler Memory Scale, 4th edn; WNL, within normal limits.
Full scale IQ NC is not calculated due to a 42-point discrepancy between verbal and nonverbal abilities; nonverbal abilities stronger.
Molecular imaging
[18F]-FDG PET imaging: [18F]-FDG PET and [18F]-Florbetapir PET imaging were performed on consecutive days. All imaging procedure details were identical to those described for case 1. The patient was injected with 440.3 MBq (11.9 mCi) of [18F]-FDG. Forty minutes later, PET images of the brain were obtained.
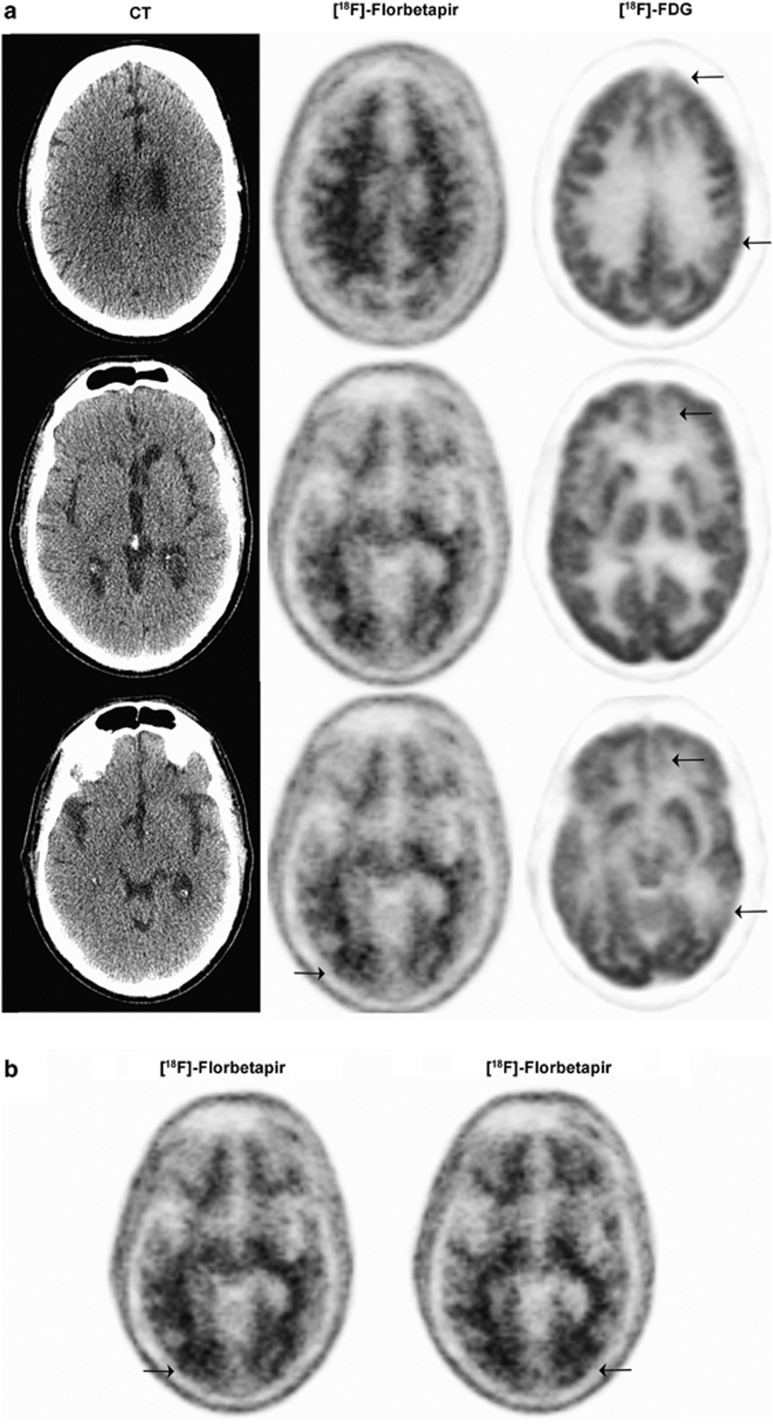
FDG PET showed decreased uptake in the medial portions of both frontal lobes, mildly on the right, moderately on the left (left > right), decreased uptake in the posterior portion of the left temporal lobe and mildly decreased activity in the right anterior temporal lobe. Otherwise, the distribution of radiotracer was normal in cortical and subcortical structures (Figure 4). The impression was of abnormal FDG PET scan that demonstrated decreased metabolism in the medial portions of the frontal lobes, more pronounced in the left side than the right, and the posterior portion of the left temporal lobe. The patchy nature of hypometabolic regions raised the possibility of a vascular etiology.
Figure 4.
Imaging from a 59-year-old, physician with a sports-related injury (case 2). [18F]-Florbetapir PET imaging findings were negative for amyloid accumulation except for focal [18F]-Florbetapir retention at the site of impact in the occipital region (arrows). (a) CT (left panel) [18F]-Florbetapir PET (middle panel) and FDG PET (right panel) at various depths of the brain. (b) [18F]-Florbetapir PET indicating amyloid accumulation. CT, computed tomography; FDG, [18F]-fluorodeoxyglucose; PET, positron emission tomography.
[18F]-Florbetapir PET imaging: The patient received 403.3 MBq (10.9 mCi) of [18F]-Florbetapir through intravenous injection. Sixty minutes later, PET images of the brain were obtained. The [18F]-Florbetapir PET images showed normal distribution of radiotracer uptake throughout the cortical and subcortical structures, except for a small area of increased cortical uptake in the right occipital region. The scan was read as negative for significant amyloid deposition, thus the diagnosis of AD was not supported. However, there was amyloid accumulation focally and specifically in the region of the patient's TBI (right occipital) but also on the left side as well (Figure 4). The final diagnosis was frontotemporal lobar degeneration. What appeared to be a rapidly progressive dementing process looking like AD after a head injury (before [18F]-Florbetapir PET imaging) was ultimately diagnosed as frontotemporal lobar degeneration, albeit with focal posttraumatic amyloidosis. Although focal amyloidosis is the most parsimonious interpretation, we cannot exclude the possibility that the [18F]-Florbetapir is binding instead to chronic astrocytosis or hemosiderin in the resolving contusion in that region.
Discussion
We present two cases in which amyloid imaging clarified uncertain diagnoses. Case 1 may be the first assessment demonstrating, during life, lack of AD pathology in an NFL player with a remote history of multiple concussions and current cognitive decline that had many of the features of AD. A panel of expert clinicians were unable to reach unanimity on the inclusion of possible AD as a diagnosis. The patient's clinical presentation with memory loss was suggestive of AD. The absence of amyloid as revealed by [18F]-Florbetapir PET imaging excluded AD pathology and thereby prevented his inappropriate inclusion in an amyloid-lowering medication clinical trial.
Omalu et al.1 revived the term CTE (originated by Critchley in 1949, in a book chapter entitled, ‘Punch-drunk syndrome: The chronic traumatic encephalopathy of boxers') in their report of the case of a retired NFL player with progressive neurological dysfunction. Thereafter, evidence of CTE in American football players became increasingly evident.1,2,4,22,23 Currently, the challenge is no longer the acceptance of CTE as a diagnostic entity associated with repetitive head trauma, but rather a much needed accounting of the actual numbers of affected persons as well as the numbers of those who remain unaffected despite exposure to identical repetitive head traumas.24
With regard to the short-term memory problems in case 1, Guskiewicz et al.25 reported a strong relationship between TBI history and memory complaints in former NFL players. In that study, individuals with a history of at least three concussions were three times more likely to report significant memory problems and five times more likely than those with no history of concussions to have been diagnosed with mild CI.25 In their review of 48 cases of neuropathologically confirmed CTE, McKee et al.9 found that memory loss was reported in over half of the individuals. As in AD, loss of insight often precluded patients from recognizing their deficits; this valuable information was derived from friends or family, and the patients frequently demonstrated anosognosia during the course of the evaluation.
Impairment in executive function was common in cases of neuropathologically confirmed CTE.8 Executive functions are a collective set of higher-order abilities (judgment, self-inhibitory behaviors, decision-making, planning and organization) considered to be dependent primarily upon adequate functioning of frontal lobe networks. Damage to various regions of the frontal cortex can disrupt these higher-order abilities, leading to poor impulse control, and socially inappropriate, avolitional and/or apathetic behaviors. For example, damage to the orbitofrontal regions can result in significant changes in personality. Thus, changes in personality, apathy, impulsivity, aggression and ‘short fuse' behaviors typical of CTE14 are consistent with the atrophy, structural damage and other neuropathological changes of the frontal lobes that have been described in nearly all reported cases of CTE.8,9,14 Given these findings, the overlap in neurocognitive and behavioral symptoms may make the distinction among AD, CTE and FTD difficult, in the absence of molecular evidence of disease-specific proteins. In the cases reported here Florbetapir imaging served as an in vivo method of discrimination that resulted in diagnostic clarification that would ultimately guide treatment planning and intervention.
The [18F]-T807 imaging in case 1 yielded somewhat unexpected results. The preponderance of the ligand retention was subcortical and localized to the basal ganglia and substantia nigra. This distribution is not typical for CTE and is more similar to that of progressive supranuclear palsy, although our case did not manifest the typical clinical symptoms of progressive supranuclear palsy. Ling et al.26 recently reported a patient with concurrent CTE and progressive supranuclear palsy, in whom they proposed that this atypical phenotype arose because of the superimposition of the brain trauma and CTE on a genetic background already predisposed toward progressive supranuclear palsy. The prominent amnesia, the absence of a movement disorder and the involvement of the hippocampi in the [18F]-T807 retention all support the formulation of the diagnosis of CTE. Such a coincidence has also been proposed for the handful of concurrent cases of CTE and amyotrophic lateral sclerosis.11 This result emphasizes the need for novel ligands such as [18F]-T807 and for heightened suspicion for atypical phenotypes in the clinical setting of TBI. Other ligands that recognize microglia or TDP43 (ref. 11) might also be useful in providing a fuller appreciation of the spectrum of CTE. Indeed, the neuroimaging of CTE may lead to expansion and/or revision of the definition and staging10 of CTE beyond the current pathology-based system.
In case 2, the patient had a history of personality change and a fall from a ski lift with head impact on concrete and some alteration in level of consciousness. When referred, the tentative diagnosis was AD. This initial formulation was ultimately rejected once it was clarified that the personality change preceded the TBI and the patient was given a diagnosis of FTD. The Florbetapir scan revealed only a small focal occipital retention of ligand at the site of impact. There was no amyloidosis elsewhere in the brain, and the MRI and FDG PET showed atrophy and hypometabolism in the frontal and temporal poles, consistent with a frontotemporal lobar degeneration etiology for his clinical FTD syndrome. The best explanation was that this patient had FTD due to frontotemporal lobar degeneration compounded by the diffuse and focal injuries induced and perhaps accelerated by the TBI; the focal occipital cortical amyloidosis itself had an uncertain contribution to his clinical symptoms.
Our findings in case 2 are somewhat consistent with those of Hong et al.27 who performed PET imaging in patients who had sustained moderate-to-severe TBI within 1 year of injury; they utilized [11C] Pittsburgh compound B (abbreviated PiB; the [18F] version of PiB is known as Flutemetamol or Vizamyl) PET imaging, the first amyloid labeling ligand to be developed. They found increased distribution of [11C]-PiB following TBI, the specificity of which was validated by neocortical binding of tritium-labeled PiB in regions of amyloid deposition in the postmortem tissue of another cohort of patients who had sustained a TBI and died at intervals of 3 h to 56 days after injury.28 Our patient had sustained TBI within 1 year of our evaluation and [18F]-Florbetapir PET imaging.
Our CTE/molecular imaging experience has included [18F]-Florbetapir PET imaging of young, active boxers post knockout (n=3, ages 35, 36 and 42 years), but these studies have been unrevealing (Jordan and Gandy, unpublished observations). A number of uncontrolled variables, including levels of amyloid upregulation insufficient to aggregate into diffuse plaque, rapid clearance of any aggregated amyloid post TBI, or insufficient sensitivity of florbetapir to detect low levels of diffuse amyloid could explain this.
In conclusion, amyloid imaging offers in vivo affirmative confirmation of the presence or absence of amyloid deposition and may increase the accuracy of the likely cause of CI or dementia in patients with a history of TBI. Future evaluation in repetitive head trauma where CTE is suspected may be best served by tau imaging because beta-amyloid is usually relatively sparse or absent in CTE brains.9 Therefore, selective tau binding ligands may be more useful for diagnosis or ruling out CTE. We are now testing this hypothesis in a new cohort of patients and research subjects.
Acknowledgments
We gratefully acknowledge Ash Rafique for technical support. EMM received grants from the Veterans Affairs Administration and the Icahn School of Medicine Clinical Research Center. SG thanks the Cure Alzheimer's Fund, the Department of Veteran Affairs, the Gideon and Sarah Gartner Foundation and the Louis B. Mayer Foundation. SG has received grants from NIA, NINDS, Baxter Pharmaceuticals, Polyphenolics and Amicus Pharmaceuticals. He has served as a member of the Data and Safety Monitoring Board for the Pfizer-Janssen Alzheimer's Immunotherapy Alliance, as a member of the Scientific Advisory Board of DiaGenic and as a consultant to Amicus Pharmaceuticals and to Cerora. This research was supported in part by the Icahn School of Medicine Alzheimer's Disease Research Center grant P50 AG005138.
The authors declare no conflict of interest.
References
- Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH, et al. Chronic traumatic encephalopathy in a National Football League player Neurosurgery 200557128–134.discussion 130–134. [DOI] [PubMed] [Google Scholar]
- Omalu BI, DeKosky ST, Hamilton RL, Minster RL, Kamboh MI, Shakir AM, et al. Chronic traumatic encephalopathy in a national football league player: part II Neurosurgery 2006591086–1092.discussion 1092–1083. [DOI] [PubMed] [Google Scholar]
- Shahim P, Tegner Y, Wilson DH, Randall J, Skillback T, Pazooki D, et al. Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol. 2014;71:684–692. doi: 10.1001/jamaneurol.2014.367. [DOI] [PubMed] [Google Scholar]
- Omalu BI, Fitzsimmons RP, Hammers J, Bailes J. Chronic traumatic encephalopathy in a professional American wrestler. J Forensic Nurs. 2010;6:130–136. doi: 10.1111/j.1939-3938.2010.01078.x. [DOI] [PubMed] [Google Scholar]
- Sivanandam TM, Thakur MK. Traumatic brain injury: a risk factor for Alzheimer's disease. Neurosci Biobehav Rev. 2012;36:1376–1381. doi: 10.1016/j.neubiorev.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia. Nat Rev Neurol. 2013;9:211–221. doi: 10.1038/nrneurol.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin L. Dementia pugilistica. J Insur Med. 2006;38:300–302. [PubMed] [Google Scholar]
- Omalu B, Bailes J, Hamilton RL, Kamboh MI, Hammers J, Case M, et al. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in American athletes. Neurosurgery. 2011;69:173–183. doi: 10.1227/NEU.0b013e318212bc7b. [DOI] [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanza A, Weber K, Gandy S, Bouras C, Hof PR, Giannakopoulos P, et al. Contact sport-related chronic traumatic encephalopathy in the elderly: clinical expression and structural substrates. Neuropathol Appl Neurobiol. 2011;37:570–584. doi: 10.1111/j.1365-2990.2011.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Bouras C, Buée L, Delacourte A, Perl DP, Morrison JH, et al. Differential distribution of neurofibrillary tangles in the cerebral cortex of dementia pugilistica and Alzheimer's disease cases. Acta Neuropathol. 1992;85:23–30. doi: 10.1007/BF00304630. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Blennow K, Ikonomovic MD, Gandy S. Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nat Rev Neurol. 2013;9:192–200. doi: 10.1038/nrneurol.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RA, Riley DO, Daneshvar DH, Nowinski CJ, Cantu RC, McKee AC, et al. Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PM R. 2011;3:S460–S467. doi: 10.1016/j.pmrj.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Stern RA, Daneshvar DH, Baugh CM, Seichepine DR, Montenigro PH, Riley DO, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81:1122–1129. doi: 10.1212/WNL.0b013e3182a55f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsis EM, Bender HA, Kostakoglu L, Machac J, Martin J, Woehr JL, et al. A consecutive case series experience with [18 F] florbetapir PET imaging in an urban dementia center: impact on quality of life, decision making, and disposition. Mol Neurodegen. 2014;9:10. doi: 10.1186/1750-1326-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lye TC, Shores EA. Traumatic brain injury as a risk factor for Alzheimer's disease: a review. Neuropsychol Rev. 2000;10:115–129. doi: 10.1023/a:1009068804787. [DOI] [PubMed] [Google Scholar]
- Xia CF, Arteaga J, Chen G, Gangadharmath U, Gomez LF, Kasi D, et al. [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer's disease. Alzheimers Dement. 2013;9:666–676. doi: 10.1016/j.jalz.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Carter SF, Rinne J, Drzezga A, Brooks DJ, Vandenberghe R, et al. A European multicentre PET study of fibrillar amyloid in Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2013;40:104–114. doi: 10.1007/s00259-012-2237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19:224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omalu BI, Hamilton RL, Kamboh MI, DeKosky ST, Bailes J. Chronic traumatic encephalopathy (CTE) in a National Football League Player: case report and emerging medicolegal practice questions. J Forensic Nurs. 2010;6:40–46. doi: 10.1111/j.1939-3938.2009.01064.x. [DOI] [PubMed] [Google Scholar]
- Omalu BI, Bailes J, Hammers JL, Fitzsimmons RP. Chronic traumatic encephalopathy, suicides and parasuicides in professional American athletes: the role of the forensic pathologist. Am J Forensic Med Pathol. 2010;31:130–132. doi: 10.1097/PAF.0b013e3181ca7f35. [DOI] [PubMed] [Google Scholar]
- Gandy S. Chronic traumatic encephalopathy: clinical-biomarker correlations and current concepts in pathogenesis. Mol Neurodegen (Under Revision) 2014. [DOI] [PMC free article] [PubMed]
- Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–726. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- Ling H, Kara E, Revesz T, Lees AJ, Plant GT, Martino D, et al. Concomitant progressive supranuclear palsy and chronic traumatic encephalopathy in a boxer. Acta Neuropathol Commun. 2014;2:24. doi: 10.1186/2051-5960-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YT, Veenith T, Dewar D, Outtrim JG, Mani V, Williams C, et al. Amyloid imaging with carbon 11-labeled Pittsburgh compound B for traumatic brain injury. JAMA Neurol. 2014;71:23–31. doi: 10.1001/jamaneurol.2013.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Abrahamson EE, Ciallella JR, Paljug WR, Wisniewski SR, Clark RS, et al. Association of increased cortical soluble abeta42 levels with diffuse plaques after severe brain injury in humans. Arch Neurol. 2007;64:541–544. doi: 10.1001/archneur.64.4.541. [DOI] [PubMed] [Google Scholar]