Abstract
A significant feature of the cortical neuropathology of schizophrenia is a disturbance in the biogenesis of short non-coding microRNA (miRNA) that regulate translation and stability of mRNA. While the biological origin of this phenomenon has not been defined, it is plausible that it relates to major environmental risk factors associated with the disorder such as exposure to maternal immune activation (MIA) and adolescent cannabis use. To explore this hypothesis, we administered the viral mimic poly I:C to pregnant rats and further exposed some of their maturing offsprings to daily injections of the synthetic cannabinoid HU210 for 14 days starting on postnatal day 35. Whole-genome miRNA expression analysis was then performed on the left and right hemispheres of the entorhinal cortex (EC), a region strongly associated with schizophrenia. Animals exposed to either treatment alone or in combination exhibited significant differences in the expression of miRNA in the left hemisphere, whereas the right hemisphere was less responsive. Hemisphere-associated differences in miRNA expression were greatest in the combined treatment and highly over-represented in a single imprinted locus on chromosome 6q32. This observation was significant as the syntenic 14q32 locus in humans encodes a large proportion of miRNAs differentially expressed in peripheral blood lymphocytes from patients with schizophrenia, suggesting that interaction of early and late environmental insults may affect miRNA expression, in a manner that is relevant to schizophrenia.
Introduction
The post-mortem cortical neuropathology of schizophrenia is characterised by significant disturbances in the translation of large numbers of genes. The principal mechanisms involved in producing these diffuse yet wide-scale disturbances have been difficult to identify. Recent evidence, however, has suggested a role for small non-coding microRNA (miRNA).1,2 These short non-coding sequences are recognised to have a critical role in modifying gene expression by repressing translation or inducing mRNA degradation.3 A key feature of miRNA is the ability of one sequence to modulate the expression of a relatively large number of genes. It is this promiscuity that places miRNAs in an ideal position to drive the diffuse translational disturbances observed in schizophrenia. There have been a number of studies that have now shown that miRNAs have a central role in brain development4, 5, 6 and cellular response to stress.7, 8, 9 Despite this, there have been relatively few studies that have examined the relationship between major environmental risk factors for schizophrenia and cortical miRNA expression. Development of the mammalian brain involves a complex sequence10 requiring precise temporal and spatial regulation of gene expression.11 Environment–genome interactions, therefore, are vital to understand how environmental risk factors can influence neurodevelopmental processes.
One brain region strongly implicated in the pathophysiology of schizophrenia is the entorhinal cortex (EC), a region important for higher-level cognitive functioning, behaviours compromised in patients with schizophrenia.12,13 Post-mortem analyses of EC brain tissue in schizophrenia have been shown to display cytoarchitectural abnormalities,14,15 with evidence of abnormal development during early neonatal stages.14,16 In accordance with these findings, several neuroimaging studies have shown reduced EC volume in schizophrenia patients,17, 18, 19 while both EC volume19 and left EC cortical surface area16 have been found to positively correlate with the severity of delusions in patients with schizophrenia. In the majority of studies, these alterations occur predominantly in the left hemisphere of the EC; however, the mechanisms behind these alterations are yet to be elucidated.
Maternal immune activation (MIA) is arguably one of the most significant and intensively investigated environmental phenomena associated with schizophrenia. In animal studies following prenatal treatment with the dsRNA viral mimic polyriboinosinic-polyribocytidilic acid (poly I:C), in vivo structural imaging demonstrates administration of poly I:C on gestational day (GD) 15 interferes with fetal brain development, leading to aberrant postnatal brain development with structural abnormalities associated with schizophrenia. In poly I:C males, these abnormalities are evident by postnatal day (PND) 56 and are accompanied by an excessive response to acute amphetamine treatment, mimicking the exacerbation of psychotic symptoms in response to amphetamine in schizophrenia patients.20 Following maternal poly I:C administration, Piontkewitz et al.21 found that offspring had smaller volumes of the hippocampus, striatum and prefrontal cortex, whereas Zuckerman et al.22 demonstrated histopathological abnormalities in the EC of adult rat offspring. Interestingly, in both cases, changes in the brain structure were accompanied by behavioral abnormalities that include disrupted latent inhibition. Several other reports indicate that prenatal administration of poly I:C induces behavioural abnormalities in offspring that include excessive behavioral switching23 and impaired working memory,24 deficits also observed in subjects with schizophrenia.25 Recent studies suggest that MIA has a key role in determining the physiological and behavioral outcome of offspring,26, 27, 28 inducing long-term effects on limbic volumes as well as cortical cytoarchitecture and structure during postnatal life.21,29 Interestingly, Ellman et al.30 recently reported that fetal exposure to increased levels of the maternal cytokine interleukin-8 was associated with significant decreases in left EC volumes in subjects with schizophrenia.
While early environmental insults such as MIA have been strongly associated with increased risk for schizophrenia, current data indicate that human neurodevelopment is a protracted process continuing well into adolescence and early adulthood.31 This ongoing development extends the opportunity for environmental perturbation, with numerous neuropathologies, including schizophrenia, manifesting at the end of adolescence and in early adulthood, a time of high synaptic density. During adolescence, changes in the maturing brain can have an impact on behavioral attributes, increasing the propensity to use drugs such as cannabis,32 with various lines of evidence suggesting an association between adolescent drug use and long-term neurological consequences.33 The main psychoactive component of cannabis, Δ9-tetrahydrocannabinol (THC), produces its effects in the brain by interacting with the cannabinoid receptor (CB1). These receptors are also activated by endogenous cannabinoids (endocannabinoids). In the brain, endocannabinoid signalling regulates the function of both the immune and nervous systems via the CB1 receptor, having a functional role in sustaining a protective and healthy central nervous system microenvironment.34 During postnatal life, this signalling system has an important role in brain organisation, critically modulating both memory processing and emotional responses. Accordingly, current animal research suggests that exposure to cannabinoid compounds during development can induce long-lasting changes in both cognitive and emotional behaviors.35 The potent CB1 agonist HU210 [3-(1,1-dimethylheptyl)-(-)-11-hydroxy-Δ8-tetrahydrocannabinol]36 is known to mimic the biphasic pharmacological profile observed following the administration of THC or cannabis preparations37 and has demonstrated a high degree of correlation between its ability to bind to CB1 receptors and its efficacy in producing in vivo effects.38 Although much more potent than THC, the dose of HU210 used in many animal studies produces near maximal behavioral effects similar to 10 mg kg−1 THC.39 Human cannabis users on average smoke 0.2–40 cannabis cigarettes per day,40 with up to 50% of the THC content being absorbed into the bloodstream.41 THC content can vary immensely between different sources and preparations of cannabis, with potency increasing dramatically in the past 50 years;41 however, smoking an average joint is believed to result in the ingestion of up to 35 mg of THC. For a 70-kg human, this equates to 0.5 mg kg−1 THC. In this study, the dose of 100 μg kg−1 HU210, which is 30- to 100-fold more potent than THC, equates to 3–10 mg kg−1 THC.
However, although adolescent cannabis use has been determined to confer a twofold increase in the relative risk for psychotic disorder,42,43 only a minority of cannabis users will develop psychosis. It is therefore likely that interaction of cannabis with other genetic and/or environmental insults is needed to trigger the onset of psychotic or neurocognitive symptoms. This hypothesis is supported by recent evidence, which suggests that multiple factors are involved in the aetiology of schizophrenia, where early developmental disturbances enhance susceptibility to neurological disorders, and the activation or amplification of symptoms occur only after a pathological response to a secondary factor.44,45
In the present study, we examine the role of miRNA in the EC of late adolescent rats (PND 55) in response to an early developmental disturbance (MIA; poly I:C) alone and in combination with a later second insult (adolescent cannabinoid exposure; HU210). We report that prenatal poly I:C in combination with adolescent cannabinoid treatment induces an miRNA expression signature that is opposite to each of the single treatments alone, with a large proportion of this expression signature derived from a cluster of miRNA residing within the DLK1-DIO3 genomic region. This discovery has implications for our understanding of epigenetic regulation in response to environmental stressors and may also provide insight on the development of neurological disorders.
Materials and methods
Neurodevelopmental model
Rat brain tissue was prepared as previously described.46 Briefly, pregnant Wistar rats received an intravenous injection of 4 mg kg−1 poly I:C or vehicle on GD 15. Poly I:C at this dose has been shown to provoke a systemic immune response in the pregnant dams, significantly increasing levels of tumour necrosis factor-α, interleukin-6 and corticosterone compared with vehicle-treated dams.46 Beginning on PND 35, male offspring were treated daily with 100 μg kg−1 of the synthetic cannabinoid, HU210, or vehicle for 14 days. This dosage has been shown to be most effective for producing the physiological and behavioral symptoms associated with THC in humans.39,46, 47, 48 Animals were euthanized on PND 55 and their brains were removed, frozen in liquid nitrogen and stored at −80 °C. In rats, PND 55 corresponds to the period of human late adolescence, and in this animal model has been shown to be consistent with the appearance of structural alterations in the brain.20 All handling of animals and procedures were approved by the Animal Care and Ethics Committee at the Australian Nuclear Science and Technology Organisation.
RNA extraction and analysis of integrity
Brains were removed from −80 °C and placed at −20 °C overnight before being placed on a stage chilled with dry ice and allowed to slightly thaw (maximum 5 min). A scalpel was used to make manual coronal sections of 1.5- to 2.0-mm thickness expected to correspond to 4.8- to 8.0-mm posterior to the Bregma in adult rat brain. According to the atlas of Paxinos and Watson,49 this area encompasses the lateral EC, located immediately lateral to the external branch of the external capsule and ~1 mm dorsal from the base of the brain, which was set as the tip of posteromedial cortical amygdala nucleus. From this coronal section, the entire lateral EC of both hemispheres was dissected using the rhinal fissure and the external branch of the external capsule as anatomical landmarks. As the left EC is predominantly altered in schizophrenia, we removed both cortices to compare miRNA expression between hemispheres. The dissected tissue was immediately homogenised, and total RNA extracted from biological replicates (n=5 per group) of poly I:C-treated, HU210-treated, combined poly I:C/HU210-treated and vehicle-only-treated animals using TRIzol reagent (Invitrogen, Life Technologies, Carlsbad, CA, USA) as described previously.50 RNA concentration and purity were assessed using the Agilent small RNA kit in the 2100 Bioanalyser according to the manufacturer's instructions (Agilent Technologies, Santa Clara, CA, USA). RNA integrity numbers were automatically calculated with the provided system software.51 All samples showed RNA integrity number values superior to 8.8.
Labelling and hybridisation for microarray analysis
Total RNA (700–1000 ng) was labelled using a FlashTag Biotin HSR RNA labelling kit according to the manufacturer's instructions (Genisphere, Hatfield, PA, USA). Labelled RNA was hybridised to the Affymetrix Genechip miRNA 2.0 microarrays for 16 h at 48 °C and 60 r.p.m. in a hybridisation oven (Affymetrix, Santa Clara, CA, USA), washed and stained with Fluidics Station 450 (Affymetrix). Hybridisation, wash and stain reagents were from the Hyb, Wash and Stain kit (Affymetrix). Arrays were scanned with Affymetrix GeneChip Scanner 3000 7G (Affymetrix) and the data were imported into GeneSpring GX 11.0 (Agilent Technologies) for statistical analysis. Differential miRNA expression was assessed as having a minimum twofold difference in either directions between treatment and controls with the threshold for significance at P<0.05.
Quantitative reverse transcription-PCR
Validation of differentially expressed miRNA was performed using quantitative reverse transcription-PCR on a subset of the differentially expressed miRNA. Briefly, 500 ng of sample RNA was treated with DNase-I (Invitrogen), and multiplex reverse transcription was performed with Superscript II reverse transcriptase (Invitrogen), a 3-nmol l−1 mix of miRNA sequence-specific primers and primers for small nucleolar RNAs (for primer sequences, see Supplementary Table 1). Triplicate reactions were set up in a 96-well format with the epMotion 5070-automated pipetting system (Eppendorf, Hamburg, Germany) and carried out with the Applied Biosystems (Foster City, CA, USA) 7500 real-time PCR machine. Relative quantification was assessed using the formula 2−ΔCt.52 The delta Ct was calculated by subtracting the Ct of the endogenous controls (geometric mean of small nucleolar RNA) from the Ct of the miRNA.
Validation of differentially expressed genes was performed using a pre-designed TaqMan Gene Expression Assay (Applied Biosystems, Life Technologies) as described previously.53 Briefly, 500 ng of total cellular RNA was treated with DNase I, and random-primed reverse transcription was performed with Superscript II reverse transcriptase (both Invitrogen, Life Technologies). Quadruplicate reactions were set up in a 96-well format using the epMotion 5070-automated pipetting system (Eppendorf) and carried out using the Applied Biosystems 7500 real-time PCR machine. All primer pairs were from Applied Biosystems: Mef2c (myocyte enhancer factor-2c): rn01494046_m1; Mef2d: Rn00578329_m1; Gusb: Rn00566655_m1; Hmbs: Rn00565886_m1. Gene expression was normalised to the geometric mean of control β-glucuronidase (Gusb) and hydroxymethylbilane synthase (Hmbs), and relative gene expression was calculated using the formula 2−ΔCt. Statistical significance of differential miRNA and mRNA expression between treatment and control samples were assessed using the Student's t-test with the threshold for significance at P<0.05.
Target gene prediction and pathway analyses
Putative target genes were identified using the Ingenuity Pathway Analysis software (IPA; Ingenuity Systems, www.ingenuity.com). The miRNA Target Filter in IPA uses experimentally validated interactions from TarBase and miRecords, a large number of miRNA-related findings from the peer-reviewed literature, as well as predicted miRNA–mRNA interactions from TargetScan to identify putative mRNA targets. Predicted targets of differentially expressed miRNAs were assessed for enrichment in gene ontology categories and involvement in biologic pathways using IPA and the Gene Annotation Tool to Help Explain Relationships (GATHER; available at http://gather.genome.duke.edu/) online database.54
Differential expression and statistical analysis
Expression data were analysed using two-way analysis of variance with Benjamini Hochberg multiple comparison testing with a Tukey's post hoc test (GeneSpring GX 11.5; Agilent Technologies). The threshold for significance was determined with respect to the fold change. A fold change of ⩽2 or ⩾2 (P<0.05) was considered as significantly altered. Statistical significance analysis was assessed using one-way or two-way analysis of variance with Benjamini Hochberg multiple comparison testing (Prism software version 5.0; GraphPad Software, San Diego, CA, USA). A value of P<0.05 was considered significant. The Tukey-HSD test with a level of significance set at P<0.05 was used for post hoc comparisons. Unsupervised hierarchical clustering was performed in Cluster (Stanford University, Palo Alto, CA, USA).55 Data were log-transformed and median centred by genes. Genes and arrays were clustered, correlation uncentred, by average linkage clustering and visualised through Java Treeview V.1.1.6r2 (http://jtreeview.sourceforge.net).56
Results
miRNA expression analysis
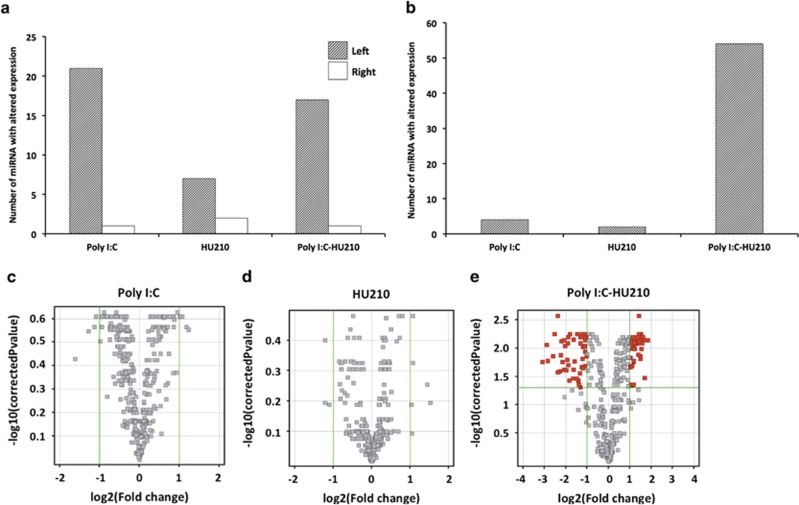
miRNA expression in the EC was analysed following treatment with poly I:C and the synthetic cannabinoid HU210, both alone and in combination, using Affymetrix Genechip miRNA 2.0 microarrays. Statistical analysis revealed a significant effect of treatment and a significant interaction between hemisphere and treatment on miRNA expression (Figures 1a–e). When comparing miRNA expression in the EC of treated groups compared with vehicle-treated controls, all treatments induced significant alterations in miRNA expression; however, surprisingly these alterations in expression occurred predominantly in the left hemisphere (Figure 1a; Supplementary Table 2). In total, 30 miRNA displayed altered expression following treatment with several miRNAs unique to each treatment group. Animals exposed to MIA alone were observed to have 21 differentially expressed miRNA, whereas the adolescent cannabinoid-exposure-only group had seven miRNAs with differential expression. The combination treatment was similar to the MIA-only group, with 18 differentially expressed miRNA compared with the untreated control group.
Figure 1.
Differential expression of miRNAs in the entorhinal cortex (EC). (a) The number of significantly differentially expressed miRNA (fold-change 0.5⩽ and ⩾2, P<0.05) in the left and right hemispheres of the EC following treatment as compared with non-treated controls. (b) The number of significantly differentially expressed miRNA (fold change 0.5⩽ and ⩾2, P<0.05) in the left hemisphere as compared with the right hemisphere of the EC, following treatment with poly I:C alone, HU210 alone and a combination of both. (c–e) Volcano plots comparing miRNA expression in specific treatment groups in the left hemisphere as compared with the right. miRNAs with a twofold difference (P<0.05) are shown in logarithmic scale (log2). miRNAs that were changed are represented as dark-red squares, in top-left (downregulated) and top-right (upregulated) parts of each figure. (c) Poly I:C-only-treated animals. (d) Rats exposed to HU210 alone in adolescence. (e) Poly I:C-treated animals that were also exposed to HU210 during adolescence.
Nine miRNAs with differential expression were unique to animals exposed to MIA alone, including miR-148b-3p, miR-379*, miR-384-5p, miR-352, let-7f, miR-598-3p, miR-542-5p, miR-325-3p and miR-770. By contrast, in the adolescent cannabinoid-exposure group, only miR-23a was unique displaying a 2.85-fold upregulation with respect to the control group (P=2.02E−02). The combination treatment was similar to the MIA-only group, with eight differentially expressed miRNAs unique to that treatment group including pre-miR-214, miR-431, miR-370, miR-330*, miR-125a-3p, miR-708*, miR-665 and miR-301b. Four miRNAs with significantly altered expression were common to all three treatment groups, miR-340, miR-374, miR-300 and miR-499. Interestingly, miR-340 and miR-499 are differentially expressed in the superior temporal gyrus in schizophrenia.57,58
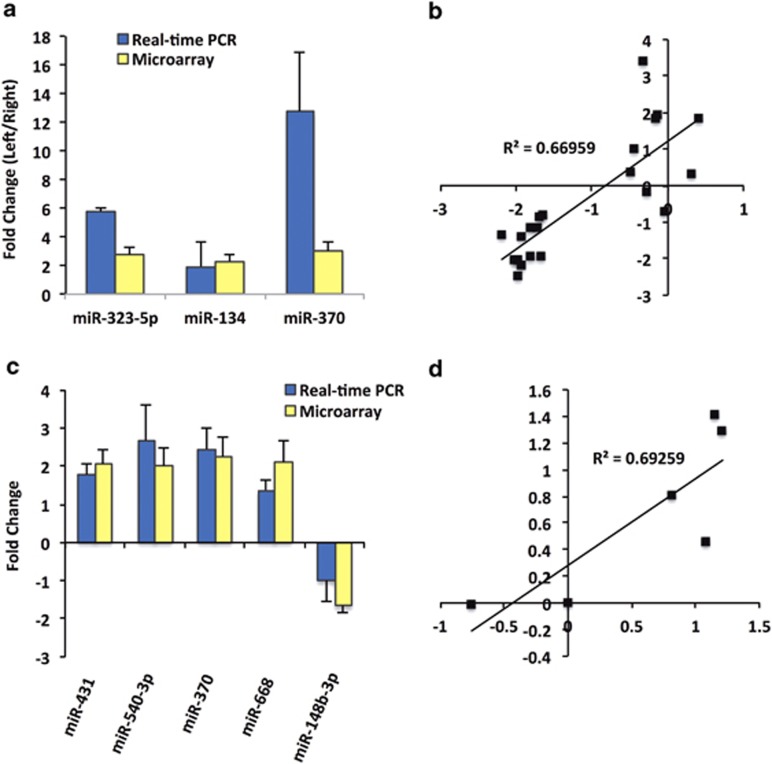
To further investigate the apparent hemispheric bias on miRNA expression, we compared expression between the left and right hemispheres following treatment. This revealed that poly I:C-treated animals that were also exposed to HU210 during adolescence had the greatest number of differentially expressed miRNAs, with 54 displaying a difference between hemispheres, compared with animals exposed to HU210 only and poly I:C only, with two and three altered miRNAs, respectively (Figures 1b–e; Supplementary Table 3). This included a number of brain-enriched miRNAs including miR-134, miR-148b and miR-7. Seven of the differentially expressed miRNAs were further analysed using quantitative reverse transcription-PCR on the basis of strong differential expression on the microarray and/or biological significance. The results were highly concordant with the microarray analysis, with expression levels of all seven miRNAs confirmed to be significantly altered following treatment (Figures 2a–d). Three miRNAs including miR-134, miR-323-5p and miR-370-3p were confirmed to have significantly altered expression between brain hemispheres in the two-hit group (Figures 2a and b), and five miRNAs, miR-431, miR-540-3p, miR-370, miR-668 and miR-148b-3p, were shown to have significantly altered expression following MIA and subsequent adolescent cannabinoid exposure as compared with non-treated controls (Figures 2c and d).
Figure 2.
Real-time PCR validation of miRNA microarrays. (a, c) miRNA expression as a result of maternal immune activation (MIA) with subsequent adolescent exposure to cannabinoid treatment. Data are expressed as a ratio of (a) left to right hemispheres and (c) treatment as compared with controls. miRNA expression as determined using microarray appear as yellow bars and reverse transcriptase (RT)-PCR results appear as blue bars. For RT-PCR, relative miRNA expression was determined by the difference between their individual cycle threshold (Ct) value and the geometric mean for the U6 and SNORD95 snRNA (ΔCt). These constitutively expressed small RNAs are not structurally associated with the large small nucleolar RNA (snoRNA)/miRNA cluster at 6q32. Microarray and RT-PCR data arose from five and three biological replicates, respectively. Bars are mean±s.e.m. (b) Microarray data (a) were validated using RT-PCR with a correlation coefficient of 0.67 (P<0.0001). (d) Microarray data (c) were validated using RT-PCR with a correlation coefficient of 0.69 (P<0.05).
Effects between treatments on miRNA expression
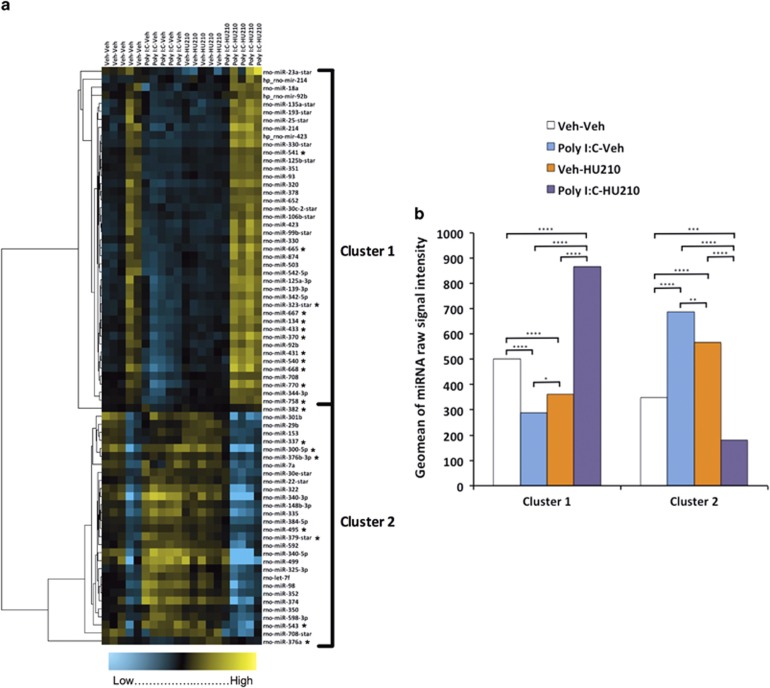
When clustered by expression and visualised by heat map (Figure 3a), unsupervised clustering of differentially expressed miRNA in the left hemisphere was characterized by the segregation of the participants into two main groups, in which poly I:C-only-treated animals and rats exposed to HU210 alone in adolescence showed similar expression, in contrast to the two-hit animals that clustered with the non-treated controls. Data were sorted into two visible clusters where miRNA expression in the two-hit group displayed a significantly opposite trend (P<0.0001), up or down, compared with poly I:C-only-treated animals and rats exposed to HU210 alone in adolescence (Figure 3b). To further investigate the effects of the combined treatments on miRNA expression, miRNA expression was compared between the single-hit groups and combined treatment group. In the left hemisphere, comparison of miRNA expression between the poly I:C-only and the combined treatment group revealed an even greater difference with 67 miRNAs with differential expression. Similarly, when we compared the combined treatment group to those exposed to HU210 only, 60 miRNAs were revealed (Supplementary Tables 4 and 5). There were 57 miRNAs in common to both these analyses, suggesting that the combined treatment is altering a common subset of miRNA. By contrast, in the right hemisphere, comparison of miRNA expression between the poly I:C-only and the combined treatment groups revealed only one miRNA with differential expression, miR-330-3p. Similarly, comparison of miRNA expression between the HU210-only and the combined treatment groups revealed two miRNAs with differential expression, miR-153 and miR-344-3p.
Figure 3.
Hierarchical clustering of differentially expressed miRNA. (a) Expression data of miRNA differentially expressed in the left hemisphere of the entorhinal cortex (EC) following treatment (n=5 per group) were subjected to hierarchical clustering (correlation uncentred, average linkage; Cluster 3.0). Blue indicates low expression and yellow indicates high expression. * denotes differentially expressed miRNA located on 6q32. Produced with Java Treeview 1.1.6r2 (http://jtreeview.sourceforge.net).56 (b) Expression trends of miRNA within Clusters 1 and 2. For display purposes, expression was derived by calculating the geomean of signal intensity values for all miRNAs within each treatment group. All data were analysed for significance by two-way analysis of variance (ANOVA), followed by Tukey–Kramer's post hoc t-testing (*P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001).
Differential miRNA expression on chromosome 6q32
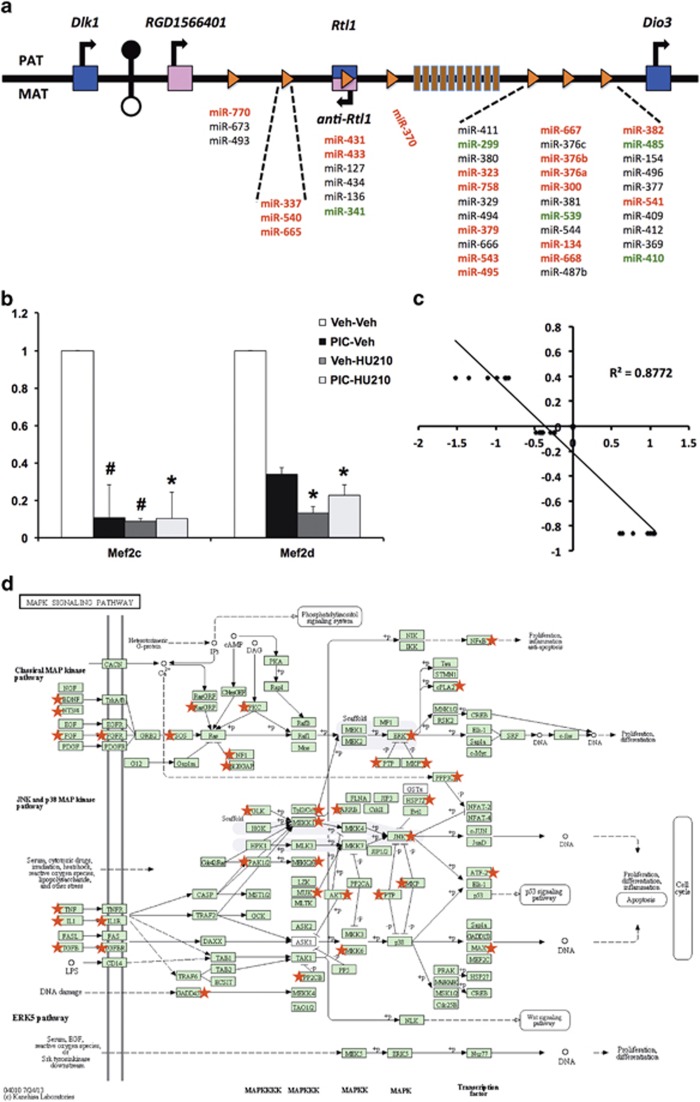
The most surprising observation from the microarray analysis was the high proportion of differentially expressed miRNAs identified, which were structurally associated by their genomic position to the long arm of chromosome 6 (6q32). All of these miRNAs reside within two closely neighbouring segments and encode at least 45 miRNA genes, of which 20 were differentially expressed in the left hemisphere (Figure 4a). This indicated that ~44% of the miRNAs expressed at this locus were significantly altered following treatment with poly I:C alone, HU210 alone or a combination of both (Supplementary Table 2), whereas 27% demonstrated significant differences in expression between the left and right hemispheres in the offspring of poly I:C-treated animals that were also exposed to HU210 during adolescence. Furthermore, when we examined miRNAs expressed in this cluster that were not significantly altered after correction for multiple testing, a further five miRNAs, or 11% (miR-299, miR-341, miR-410, miR-485 and miR-539), also displayed differential expression following treatment with either poly I:C alone, HU210 alone or a combination of both.
Figure 4.
The 6q32 miRNA cluster. (a) Schematic representation of the imprinted rat distal 6 domain (human 14q32). The positions of maternally expressed non-coding RNA genes are indicated by pink squares and paternally expressed genes are indicated by blue squares. The positions of small nucleolar RNA (snoRNA) and miRNA genes are indicated by vertical bars and triangles, respectively. The intergenic germline-derived differentially methylated region (IG-DMR) located between Dlk1 and RGD1566401 (better known as MEG3 or GTL2) genes is methylated on the paternal chromosome (represented by circles; filled indicates hypermethylated; open indicates hypomethylated). PAT indicates paternal chromosome; MAT indicates maternal chromosome. miRNAs in dark-red font were significantly differentially expressed in the left hemisphere following treatment and miRNAs in green were also differentially expressed, although did not reach significance after correction for multiple testing. The figure is not drawn to scale. The figure is adapted from Seitz et al.59 (b) Expression of the Ca2+-activated transcription factors, myocyte enhancer factor 2C and myocyte enhancer factor 2D, as validated using quantitative reverse transcription-PCR. Mef2c was significantly downregulated 9.9-fold (P=0.026) following maternal immune activation (MIA) combined with adolescent HU210 exposure, whereas Mef2d was significantly downregulated 7.6-fold (P=0.037) following HU210 exposure alone and 4.4-fold (P=0.045) following MIA combined with adolescent HU210 exposure. Bars indicate the mean fold change (treatment to control) mean±s.e.m.; (n=3–4); #P<0.06,*P<0.05 unpaired Student's t-test. (c) Mef2c expression plotted against the expression of 6q32 miRNA (miRs −134, −431, −433, −540, −668, −758 and −770) attained a correlation coefficient of 0.88 (P<0.0001). (d) Schematic of the KEGG mitogen-activated protein (MAP) kinase signalling pathway generated using DAVID. Putative miRNA target genes of the miRNA differentially expressed in the left hemisphere of poly I:C-treated animals, which were also exposed to HU210 during adolescence, were identified. The predicted gene lists were subjected to KEGG pathway analysis, which identified these genes as being highly enriched in the MAP kinase signalling pathway. Putative target genes are indicated with a red star.
We next examined expression of Mef2 transcription factors as they have been shown to regulate expression of miRNA in the 6q32 cluster.60 We found that expression of Mef2c was significantly downregulated 9.9-fold (P=0.026) following MIA combined with adolescent HU210 exposure, whereas Mef2d was significantly downregulated 7.6-fold (P=0.037) following HU210 exposure alone and downregulated 4.4-fold (P=0.045) following MIA combined with adolescent HU210 exposure (Figure 4b). As Mef2 expression is necessary for activity-dependent regulation of this miRNA cluster, we examined the correlation between Mef2 expression and the differentially expressed 6q32 miRNA. This identified seven significantly correlated miRNAs with respect to Mef2c expression including: miR-134 (R2=0.91, P=0.046); miR-431 (R2=0.92, P=0.039); miR-433 (R2=0.91, P=0.048); miR-540 (R2=0.94, P=0.031); miR-668 (R2=0.93, P=0.037); miR-758 (R2=0.96, P=0.021) and miR-770 (R2=0.94, P=0.033), with an overall correlation coefficient of 0.88 (P<0.0001; Figure 4c).
Target prediction and pathway analysis for miRNA altered in the left hemisphere
Events that affect miRNA expression have the potential to alter many regulatory networks, as each miRNA has the ability to modify the expression of hundreds of genes. In order to understand potential functional relevance of changes in miRNA expression in the left hemisphere following treatment, putative miRNA target genes of the differentially expressed miRNA were identified and subjected to pathway analysis using the IPA software (Ingenuity Systems) and GATHER.54 The offspring of rats administered poly I:C only had enrichment (P<0.0001) of predicted target genes in several pathways including mitogen-activated protein (MAP) kinase signalling, oxidative phosphorylation and focal adhesion (Supplementary Table 6). Animals administered HU210 only during adolescence also displayed enrichment (P<0.0001) of predicted target genes in the MAP kinase signalling pathway (Supplementary Table 6). The combination of MIA and adolescent cannabinoid exposure showed enrichment (P<0.0001) of predicted target genes in the MAP kinase signalling pathway, transforming growth factor-beta signalling pathway (P<0.0001), Wingless/int (Wnt) signalling pathway and oxidative phosphorylation (Supplementary Table 6).
Target prediction and pathway analysis for miRNA altered between hemispheres
When comparing the left hemisphere with the right hemisphere in poly I:C-treated animals that were also exposed to HU210 during adolescence, the gene ontology annotation provided highlighted processes such as development, neurogenesis and regulation of transcription as likely to be targeted by differentially expressed miRNA (Supplementary Table 7). There was also enrichment of target genes in the MAP kinase signalling pathway (Figure 4d) in KEGG pathway annotation. When comparing the two-hit group to their respective single treatments, predicted target genes were found to be highly enriched in the oxidative phosphorylation pathway, Wnt signalling pathway and the MAP kinase signalling pathway. These pathways are involved in neurogenesis, neural activity and synaptic plasticity and have been repeatedly implicated in the pathophysiology of schizophrenia.
Target prediction and pathway analysis for 6q32-associated miRNA
In view of the high representation of the 6q32-associated miRNA, potential miRNA target genes and associated pathways relating to this region were also identified as described above. These analyses suggested that 6q32 miRNAs from the combination treatment group were also highly enriched in the MAP kinase signalling pathway (P=0.0008). Gene ontology term analysis further highlighted MAP kinase activity, neuron recognition, neuron differentiation, neuron projection, synapse and regulation of gene expression (epigenetic) as likely targets of differentially expressed miRNA in the 6q32 cluster (Supplementary Table 8).
Discussion
Post-mortem analyses of cortical miRNA expression in schizophrenia have identified significant changes, which most likely reflect both the underlying genetic background and epigenetic influences. Environmental factors that can cause epigenetic modifications are important in the pathogenesis of schizophrenia, yet despite this, there have been relatively few studies that have examined the relationship between environmental risk factors for schizophrenia and cortical miRNA expression. In the current study, we sought to investigate specifically how miRNA expression in the EC of the developing mammalian brain is affected by exposure to MIA, adolescent cannabinoid exposure or the dual insult of a combination of treatments.
Differential miRNA expression occurred predominantly in the left hemisphere
When comparing the effect of treatment to that of vehicle-treated controls, all treatments induced alterations in miRNA expression, with the effects occurring predominantly in the left hemisphere (Figure 1a). In addition, when comparing the effect of treatment on miRNA expression between the left and right hemispheres, the majority of changes occurred in the left hemisphere following combined treatments (Figure 1b). This is interesting as asymmetric anatomical abnormalities have been observed in the EC of subjects with schizophrenia,14,18 with smaller volumes occurring specifically in the left hemisphere.19,30 There have also been many reports of a loss of lateralization in schizophrenia.61 While rodents display less lateralization than humans, recent studies observe significant hemispheric patterns, particularly in reference to behaviour and memory. In vivo imaging in rats demonstrates strong left hemispheric dominance for the processing of vestibular information involving the EC62 with hemispheric lateralization central for processing taste saliency information.63 There is also a left predominance for the activity of proteins related to the modulation of behaviour and memory in the hippocampus of the rats.64 It is therefore plausible that some of these structural and functional lateralizations are supported by hemisphere-specific differences in gene–miRNA interactions. miRNAs have been shown to be crucial for establishing left–right asymmetries in the Caenorhabditis elegans nervous system65 and may therefore have a role in anatomical and functional brain lateralizations. There is also some support for the role of miRNA expression asymmetry in brain laterality in the rat.66 During neurodevelopment, miRNAs may influence the asymmetric and lateral projection of afferent connections from the EC to the hippocampus, which must enter in a layer-specific manner.67
Effect of combined treatments on miRNA expression
Comparison of miRNA expression between the combined group and MIA/cannabinoid alone revealed a large increase in the number of miRNA with differential expression. Interestingly, combination of MIA and adolescent cannabinoid exposure induced a response that was in the opposite direction to that observed in the MIA and adolescent cannabinoid-only groups (Figure 3; Supplementary Tables 4 and 5). This suggests that there is a regulatory relationship between the two environmental insults in the combined treatment, which leads to a change in the direction of the miRNA expression in the left EC. While the most significant overall change in miRNA expression was observed between each of the single insults and the combined treatments, the expression pattern in the combined treatment had more in common with the controls (Figure 3a), suggesting that the response to a second insult had the effect of resetting the pattern. Although the mechanism behind this response in the double-treated animals is not known, it is plausible that the second insult in adolescence opens a regulatory opportunity for homeostatic repositioning of the post-transcriptional regulatory matrix. If this is the case, then the large difference between the single and double exposures suggests that there is also significant overcorrection that may also have neurobehavioral consequences.
While providing new insights into our understanding of environmental interactions, these findings only begin to scratch the surface of the complex interplay. There are still numerous unanswered questions. For example, are these results exclusive to this model? What do these findings mean for other two-hit studies in animal models? What are the human implications for cannabis abuse in individuals who were exposed to in utero infection? The challenge going forward will be to answer these questions while beginning to understand the interactions between in utero infection and adolescent cannabis use using more targeted strategies. Focus should be directed towards understanding specific regulatory influences modifying these environment-associated miRNAs and their interactions with target genes. It is also critical to investigate the behavioral correlates in mature animals to observe their relationship with the molecular changes associated with these prenatal and early-life exposures.
In addition, while HU210 produces a number of similarities between animal and human behavior, including behavioral abnormalities,48 an anxiogenic-like effect similar to that seen in humans elicited by marijuana or THC38 and the persistence of enhanced emotional response to novel environments when the drug is discontinued,38 it is possible that this variation may also be partially attributed to the more than 400 compounds found in cannabis, some of which may interact in complex ways with human brain processes.37 Although marijuana contains many cannabinoids, the general consensus is that the major psychoactive effects are mediated by THC acting on CB1 receptors, with cannabidiol possibly having a modulatory or ameliorative effect by an as-yet-uncertain mechanism.
Differentially expressed miRNAs are contained within an imprinted locus
We identified several brain-enriched miRNAs including miR-134, miR-323 and miR-370 that were differentially expressed following treatment. The reduction of these miRNAs was particularly significant not only because of their roles in brain development and function6,68,69 but also as a consequence of their position within the Dlk1-Dio3 domain in a cluster of differentially expressed miRNAs on chromosome 6q32. In humans, the syntenic locus 14q32 is imprinted such that the associated miRNAs are expressed only from the maternal chromosome.70 This domain collectively comprises over 5% of known human miRNA genes and ~40–50% of all known eutherian-specific miRNA.70 All miRNA genes within this domain are processed from long non-coding RNAs only expressed from the maternal chromosome.59,71 The miRNAs are arranged within two clusters located in close proximity to each other, and their expression is confined mostly to the brain. The smaller cluster is transcribed and processed from a gene antisense to the paternally expressed retrotransposon-like gene Rtl1 (Figure 4a).71 Rtl1, also called Peg11 in sheep, displays homology with the Ty3/gypsy retrotransposon family and contains six miRNAs that have been shown to cause RNA-induced silencing complex (RISC)-mediated cleavage of the Rtl1 sense transcript.72 This suggests that the functions of miRNA contained within the Dlk1-Dio3 domain may not only be related to the post-transcriptional regulation of mRNA but may possibly be engaged in imprinting control. In sheep, miRNAs within this domain are thought to contribute to the polar overdominance phenomenon displayed by the Callipyge phenotype.72,73 Imprinting at the 14q32 region has been implicated in bipolar disorder74,75 and anxiety76 and recently has been found to encode a large proportion of miRNAs differentially expressed in white blood cells from patients with schizophrenia.58 Although the nature of their organisation is not entirely clear in humans, some of the miRNAs in this region could be processed from a single large polycistron such as Mirg (miRNA-containing gene, also known as Meg9), which appears to be the case in the mouse. However, it seems more likely that the majority of miRNAs in this cluster are encoded by tandem arrays of related intronic sequences.59,71 Following treatment, the miRNAs in this cluster demonstrate bidirectional expression changes, suggesting that the genes encoding these molecules are responding more to differences in their local transcriptional environment, which over-ride the prevailing cluster-wide influence. They may also display isolated differences in their splicing or primary transcript processing, or pre-miRNA cleavage and maturation.
It is possible that because of their involvement in brain development and dendritic re-modelling, these miRNAs may be involved in an adaptive response, altering neural circuitry associated with environmental stressors. Interestingly, a recent study by Meerson et al.9 identified stress-induced changes in miRNA expression in the hippocampus and central amygdala of adult rats. The study investigated miRNA involvement in governing region-specific stress-induced changes in alternative splicing; however, closer examination of the altered miRNA reveals changes in nine miRNAs mapped to the 6q32 cluster. In a recent study by Laufer et al.77 the expression of several miRNAs from this region were altered in the adult mouse brain following fetal alcohol exposure. Interestingly, the expression of these miRNAs showed the same trend, up or down, as those altered following combined prenatal poly I:C treatment and subsequent adolescent cannabinoid exposure in the current study. The study also demonstrated that a large proportion of the miRNAs from this region had differential DNA methylation. Disruptions in adult DNA methylation could potentially be responsible for the changes in miRNA expression observed in this region and certainly warrant further investigation.
Another possibility is that a cluster-associated transcription factor may be responsible for the observed expression changes in miRNA expression. This miRNA cluster has been shown previously to be regulated by the Ca2+/neural activity-depended Mef2 transcription factor.60 In support of this hypothesis, we also observed significant changes in Mef2c and Mef2d, which were highly correlated with several miRNAs encoded at 6q32 (Figure 4c). This transcription factor was recently shown to be directly modulated by MIA via major histocompatibility class I molecules, resulting in reduced cortical synapse density in offsprings.78 Mef2 isoforms have also been shown to regulate multiple aspects of synaptic development.79 Mef2c is normally highly expressed in the EC80 and is the major isoform involved in hippocampal synaptic function.81 This gene is known to have a crucial role in the development of specific cortical layers80,82 with the decreased expression of Mef2c during neurodevelopment resulting in abnormal distribution of neurons in the cortical plate and subsequent developmental abnormalities, such as abnormal anxiety-like behaviors and decreased cognitive function.82 As afferent connections from the EC must enter the hippocampus in a layer-specific manner during neurodevelopment,67 the reduced expression of Mef2c following treatment as seen in our study, along with its correlation with alterations in expression of seven of the 6q32 miRNA including miR-134, could potentially contribute to the altered transcription of this cluster and ultimately to impaired neurodevelopment of the EC.
Function of differentially expressed miRNA in the left EC
When comparing the effect of treatment to that of vehicle-treated controls, all treatments induced alterations in miRNA expression, with the effects occurring predominantly in the left hemisphere. To further understand how these changes might have an impact on the pathophysiology of schizophrenia, predicted targets of differentially expressed miRNA were subjected to pathway analysis. As neurogenesis in the rat EC begins on GD 15,83 the findings that prenatal administration of poly I:C induces alterations in the expression of miRNA predicted to regulate important neurodevelopmental processes suggest that MIA at this stage of development could lead to aberrant development of the EC. Similarly during adolescence, a time of continuing neurodevelopment, exposure to the cannabinoid HU210 was also found to induce alterations in the expression of miRNA predicted to regulate pathways important to neurodevelopmental processes. It is plausible that these alterations could result in malformation in this region as seen in schizophrenia, which leads to functional disturbances.
Following MIA in combination with adolescent cannabinoid treatment of offspring, pathway analysis of predicted target genes suggests that MAP kinase and Wnt signalling could be adversely affected. Our results are consistent with studies in animals showing that prenatal treatment with poly I:C in mice results in aberrant expression of proteins involved in MAP kinase signalling in the prefrontal cortex of adult offspring84 and with post-mortem human studies showing a disruption of the MAP kinase pathway in schizophrenia.85 Importantly, along with its role in neuronal maturation and plasticity, MAP kinase signalling is crucial for memory consolidation in the EC.86,87 Given that the EC is a relevant node in the network mediating learning and memory, alterations to this pathway may contribute to the cognitive deficits particularly memory impairment, that are frequently observed in schizophrenia. Not surprisingly, this pathway is also enriched when analysing predicted target genes of differentially expressed miRNA on 6q32. For example, two 6q32 miRNAs with significant hemispheric differences in the two-hit group, miR-134 and miR-323, are known to be crucial for learning and memory formation.88,89 In particular, overexpression of miR-134 has been demonstrated to significantly impair long-term memory formation89 and has been observed in post-mortem dorsolateral prefrontal cortex (Brodmann Area 46) of schizophrenia patients.53
We also observed that interaction between early and late environmental insults can induce significant differences in miRNA expression between the left and right hemispheres. Predicted targets of these miRNAs were subjected to pathway analysis that again revealed MAP kinase signalling as being highly enriched. Putative target genes in this pathway include Akt1, Bdnf, Pak2 and Plcb1, all associated with schizophrenia.90, 91, 92, 93
When comparing the combined treatment group with their single-exposure counterparts, we also observed Wnt signalling as being highly enriched. Putative target genes in this pathway include: Fzd3, Fzd5, Psen1, Prkca, Tp53, Wnt1, Wnt2b and Wnt7a. This finding is consistent with studies in animals showing that maternal infection in mice results in altered Wnt signalling, leading to decreased cellular proliferation in the cerebral cortex,29 and with post-mortem human studies showing a disruption in Wnt signalling in schizophrenia.94,95 During development, Wnt signalling is required for cell proliferation and differentiation, cell polarity generation and embryonic patterning. During adulthood, Wnt signalling mediates synapse density and numbers, and hippocampal network structure,96 and has a role in regulating the extension of dendrites to form functional synapses with projections from the EC.97 Along with MAP kinase signalling, Wnt signalling also has an important role in long-lasting spatial memory formation processing and long-term memory formation.98,99 It is therefore plausible that changes in these signalling pathways, because of perturbations in EC miRNA expression following the interaction of both an early and late environmental insult, may lead to aberrations in synaptic remodelling that ultimately contribute to the cognitive and memory deficits observed in schizophrenia. In accordance with this rationale, a series of human and animal studies suggest that antipsychotic drugs used in the treatment of schizophrenia may provide long-term benefits through their ability to modulate the MAP kinase signalling pathway and thereby have an impact on neuroplasticity (reviewed by Molteni et al.100). For example, the antipsychotic drug aripiprazole, used in the treatment of psychiatric disorders such as schizophrenia, has been shown to induce neurite outgrowth, an effect that can be blocked by MAP kinase inhibitors.101 Interestingly, Mef2c has also been shown to coordinate the biological response to different types of extracellular stimuli in vivo through the integration of MAP kinase signalling pathways.102 In support of the relevance of the EC in mediating learning and memory and the crucial role of MAP kinase signalling in memory consolidation, developmental deletion of Mef2c in the forebrain has been demonstrated to cause hippocampal-dependent learning and memory impairments.103 It is therefore plausible that MIA combined with adolescent cannabis use could alter expression of Mef2 transcription factors, and consequently the 14q32 miRNA cluster, resulting in alterations to MAP kinase signalling and ultimately cognitive deficits. As a caveat to the assertions made via functional annotation of miRNA target prediction approaches such as TargetScan, we note that these are prone to false-negative and false-positive attributions, which may have a significant influence on the composition of pathways.104 While the more inclusive method used in this study will have some false-positives, we are confident that it is more robust than using the stringent intersection of multiple algorithms, which incurs a significant cost in false-negatives. As a large number of predicted miRNA target genes have been shown to display positive correlation with the miRNA rather than the expected inverse correlation, with miRNA also being capable of protecting or even elevating the steady-state levels of their target mRNA,104 this method will accurately reflect the divergence of pathways enriched with target genes and possible alternative cellular functions.
Conclusions
This study represents the first comprehensive characterisation of miRNA expression using a novel two-hit model of environment-related psychopathology. Prenatal treatment with poly I:C, adolescent cannabinoid exposure and a combination of both were observed to induce significant changes in miRNA expression in the left hemisphere of the EC as compared with vehicle-treated controls. In particular, when comparing the effect of treatment alone between the left and right hemispheres, offspring of poly I:C-treated rats that were exposed to cannabinoid treatment during adolescence displayed sizeable differences (98%) in hemispheric miRNA expression compared with the single-hit groups. A large subset of these miRNAs are clustered within the Dlk1-Dio3-imprinted domain on 6q32, which is associated with the syntenic human locus in schizophrenia, and are predicted to regulate pathways involved in synaptic remodelling, learning and memory formation. Although alterations in Mef2c expression occur almost equally in response to both the individual and combined treatments, it may be that MIA triggers alterations in Mef2c-induced transcription of miRNA in the 6q32 cluster.14,16 As cognitive dysfunction is now recognised as a central characteristic of schizophreniaand has been consistently demonstrated to have a negative association with functional outcome,12 understanding the molecular basis of these deficits with respect to alterations in miRNA expression and the downstream effects on target genes is of great importance. Our results support the hypothesis that cortical miRNA expression is modified by environmental factors including early developmental immune activation and adolescent cannabinoid exposure, and these alterations may regulate changes in the molecular network dynamics underlying the synaptic reorganisation associated with mental illness.
Acknowledgments
This study was supported by the Schizophrenia Research Institute utilising funding from NSW Health and an MC Ainsworth Research Fellowship in Epigenetics (MC); an Australian Postgraduate Award (SH); a NARSAD Young Investigator Award; and an NHMRC project grant 631057.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Beveridge NJ, Tooney PA, Carroll AP, Gardiner E, Bowden N, Scott RJ, et al. Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum Mol Genet. 2008;17:1156–1168. doi: 10.1093/hmg/ddn005. [DOI] [PubMed] [Google Scholar]
- Beveridge NJ, Gardiner E, Carroll AP, Tooney PA, Cairns MJ. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol Psychiatry. 2010;15:1176–1189. doi: 10.1038/mp.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll AP, Tooney PA, Cairns MJ. Context-specific microRNA function in developmental complexity. J Mol Cell Biol. 2013;5:73–84. doi: 10.1093/jmcb/mjt004. [DOI] [PubMed] [Google Scholar]
- Maiorano NA, Mallamaci A. Promotion of embryonic cortico-cerebral neuronogenesis by miR-124. Neural Dev. 2009;4:1–16. doi: 10.1186/1749-8104-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5:R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, et al. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111–1124. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Uchida S, Nishida A, Hara K, Kamemoto T, Suetsugi M, Fujimoto M, et al. Characterization of the vulnerability to repeated stress in Fischer 344 rats: possible involvement of microRNA-mediated down-regulation of the glucocorticoid receptor. Eur J Neurosci. 2008;27:2250–2261. doi: 10.1111/j.1460-9568.2008.06218.x. [DOI] [PubMed] [Google Scholar]
- Meerson A, Cacheaux L, Goosens KA, Sapolsky RM, Soreq H, Kaufer D. Changes in brain microRNAs contribute to cholinergic stress reactions. J Mol Neurosci. 2010;40:47–55. doi: 10.1007/s12031-009-9252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108 (Suppl):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead JDH, Neal C, Meng F, Wang Y, Evans S, Vazquez DM, et al. Transcriptional profiling of the developing rat brain reveals that the most dramatic regional differentiation in gene expression occurs postpartum. J Neurosci. 2006;26:345–353. doi: 10.1523/JNEUROSCI.2755-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia. Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the ‘right stuff'. Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Jakob H, Beckmann H. Prenatal developmental disturbances in the limbic allocortex in schizophrenics. J Neural Transm. 1986;65:303–326. doi: 10.1007/BF01249090. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Ruscheinsky DD, Han LY. Further evidence of abnormal cytoarchitecture of the entorhinal cortex in schizophrenia using spatial point pattern analyses. Biol Psychiatry. 1997;42:639–647. doi: 10.1016/s0006-3223(97)00142-x. [DOI] [PubMed] [Google Scholar]
- Schultz CC, Koch K, Wagner G, Roebel M, Schachtzabel C, Nenadic I, et al. Psychopathological correlates of the entorhinal cortical shape in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2010;260:351–358. doi: 10.1007/s00406-009-0083-4. [DOI] [PubMed] [Google Scholar]
- Joyal CC, Laakso MP, Tiihonen J, Syva E, Vilkman H, Laakso A, et al. A volumetric MRI study of the entorhinal cortex in first episode neuroleptic-naive schizophrenia. Biol Psychiatry. 2002;51:1005–1007. doi: 10.1016/s0006-3223(01)01368-3. [DOI] [PubMed] [Google Scholar]
- Baiano M, Perlini C, Rambaldelli G, Cerini R, Dusi N, Bellani M, et al. Decreased entorhinal cortex volumes in schizophrenia. Schizophr Res. 2008;102:171–180. doi: 10.1016/j.schres.2007.11.035. [DOI] [PubMed] [Google Scholar]
- Prasad KMR, Patel AR, Muddasani S, Sweeney J, Keshavan MS. The entorhinal cortex in first-episode psychotic disorders: a structural magnetic resonance imaging study. Am J Psychiatry. 2004;161:1612–1619. doi: 10.1176/appi.ajp.161.9.1612. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. Tracing the development of psychosis and its prevention: what can be learned from animal models. Neuropharmacology. 2012;62:1273–1289. doi: 10.1016/j.neuropharm.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. Abnormal trajectories of neurodevelopment and behavior following in utero insult in the rat. Biol Psychiatry. 2011;70:842–851. doi: 10.1016/j.biopsych.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39:311–323. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Weiner I. The “two-headed” latent inhibition model of schizophrenia: modeling positive and negative symptoms and their treatment. Psychopharmacology (Berl) 2003;169:257–297. doi: 10.1007/s00213-002-1313-x. [DOI] [PubMed] [Google Scholar]
- Bronson SL, Ahlbrand R, Horn PS, Kern JR, Richtand NM. Individual differences in maternal response to immune challenge predict offspring behavior: contribution of environmental factors. Behav Brain Res. 2011;220:55–64. doi: 10.1016/j.bbr.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Rooney RJ, Mori S, Kornfield TE, Reutiman TJ, et al. The viral theory of schizophrenia revisited: Abnormal placental gene expression and structural changes with lack of evidence for H1N1 viral presence in placentae or brains of exposed offspring from infected mice. Neuropharmacology. 2011;62:1290–1298. doi: 10.1016/j.neuropharm.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SEP, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolp HB, Turnquist C, Dziegielewska KM, Saunders NR, Anthony DC, Molnár Z. Reduced ventricular proliferation in the foetal cortex following maternal inflammation in the mouse. Brain. 2011;134:3236–3248. doi: 10.1093/brain/awr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman LM, Deicken RF, Vinogradov S, Kremen WS, Poole JH, Kern DM, et al. Structural brain alterations in schizophrenia following fetal exposure to the inflammatory cytokine interleukin-8. Schizophr Res. 2010;121:46–54. doi: 10.1016/j.schres.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catts VS, Fung SJ, Long LE, Joshi D, Vercammen A, Allen KM, et al. Rethinking schizophrenia in the context of normal neurodevelopment. Front Cell Neurosci. 2013;7:60. doi: 10.3389/fncel.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Dudley KJ, Li X, Kobor MS, Kippin TE, Bredy TW. Epigenetic mechanisms mediating vulnerability and resilience to psychiatric disorders. Neurosci Biobehav Rev. 2011;35:1544–1551. doi: 10.1016/j.neubiorev.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, et al. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49:67–79. doi: 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Trezza V, Campolongo P, Manduca A, Morena M, Palmery M, Vanderschuren LJMJ, et al. Altering endocannabinoid neurotransmission at critical developmental ages: impact on rodent emotionality and cognitive performance. Front Behav Neurosci. 2012;6:2. doi: 10.3389/fnbeh.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little PJ, Compton DR, Mechoulam R, Martin BR. Stereochemical effects of in mice and dogs. Pharmacol Biochem Behav. 1989;32:661–666. doi: 10.1016/0091-3057(89)90014-2. [DOI] [PubMed] [Google Scholar]
- Dewey WL. Cannabinoid pharmacology. Pharmacol Rev. 1986;38:151–178. [PubMed] [Google Scholar]
- Giuliani D, Ferrari F, Ottani A. The cannabinoid agonist Hu 210 modifies rat behavioural responses to novelty and stress. Pharmacol Res. 2000;41:45–51. doi: 10.1006/phrs.1999.0560. [DOI] [PubMed] [Google Scholar]
- Ottani A, Giuliani D. HU 210: a potent tool for investigations of the cannabinoid system. CNS Drug Rev. 2001;7:131–145. doi: 10.1111/j.1527-3458.2001.tb00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly D, Didcott P, Swift W, Hall W. Long-term cannabis use: characteristics of users in an Australian rural area. Addiction. 1998;93:837–846. doi: 10.1046/j.1360-0443.1998.9368375.x. [DOI] [PubMed] [Google Scholar]
- Ashton CH. Pharmacology and effects of cannabis: a brief review. Br J Psychiatry. 2001;178:101–106. doi: 10.1192/bjp.178.2.101. [DOI] [PubMed] [Google Scholar]
- Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31:608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- Moore THM, Zammit S, Lingford-Hughes A, Barnes TRE, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Falkai P, Maier W. Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the “two hit hypothesis”. J Psychiatr Res. 1999;33:543–548. doi: 10.1016/s0022-3956(99)00039-4. [DOI] [PubMed] [Google Scholar]
- Maynard TM, Sikich L, Lieberman JA, LaMantia AS. Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophr Bull. 2001;27:457–476. doi: 10.1093/oxfordjournals.schbul.a006887. [DOI] [PubMed] [Google Scholar]
- Dalton VS, Verdurand M, Walker A, Hodgson DM, Zavitsanou K. Synergistic effect between maternal infection and adolescent cannabinoid exposure on serotonin 5HT1A receptor binding in the hippocampus: testing the “two hit” hypothesis for the development of schizophrenia. ISRN Psychiatry. 2012;2012:1–9. doi: 10.5402/2012/451865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton VS, Zavitsanou K. Cannabinoid effects on CB1 receptor density in the adolescent brain: an autoradiographic study using the synthetic cannabinoid HU210. Synapse. 2010;64:845–854. doi: 10.1002/syn.20801. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Rubio P, Menzaghi F, Merlo-Pich E, Rivier J, Koob GF, et al. Corticotropin-releasing factor (CRF) antagonist [D-Phel2,Nle21,38,CαMeLeu37]CRF attenuates the acute actions of receptor agonist HU-210 on defensive-withdrawal behavior in rats1. J Pharmacol Exp Ther. 1996;276:56–64. [PubMed] [Google Scholar]
- Paxino G, Watson C.In: Poncelet M, Chermat R, Soubrie P, Simon P.(eds). 6th edn, San Diego; Academic Press; 1998 [Google Scholar]
- Wu JQ, Wang X, Beveridge NJ, Tooney PA, Scott RJ, Carr VJ, et al. Transcriptome sequencing revealed significant alteration of cortical promoter usage and splicing in schizophrenia. PLoS ONE. 2012;7:e36351. doi: 10.1371/journal.pone.0036351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, et al. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Santarelli DM, Beveridge NJ, Tooney PA, Cairns MJ. Upregulation of dicer and microRNA expression in the dorsolateral prefrontal cortex Brodmann area 46 in schizophrenia. Biol Psychiatry. 2011;69:180–187. doi: 10.1016/j.biopsych.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Chang JT, Nevins JR. GATHER: a systems approach to interpreting genomic signatures. Bioinformatics. 2006;22:2926–2933. doi: 10.1093/bioinformatics/btl483. [DOI] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha AJ. Java Treeview-extensible visualization of microarray data. Bioinformatics. 2004;20:3246–3248. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- Beveridge NJ, Gardiner E, Carroll P, Tooney P, Cairns MJ. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol Psychiatry. 2010;15:1176–1189. doi: 10.1038/mp.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner E, Beveridge NJ, Wu JQ, Carr V, Scott RJ, Tooney PA, et al. Imprinted DLK1-DIO3 region of 14q32 defines a schizophrenia-associated miRNA signature in peripheral blood mononuclear cells. Mol Psychiatry. 2012;17:827–840. doi: 10.1038/mp.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz H, Royo H, Bortolin M-L, Lin S-P, Ferguson-Smith AC, Cavaillé J. A large imprinted microRNA gene cluster at the mouse Dlk1-Gtl2 domain. Genome Res. 2004;14:1741–1748. doi: 10.1101/gr.2743304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore R, Khudayberdiev S, Christensen M, Siegel G, Flavell SW, Kim T-K, et al. Mef2-mediated transcription of the miR379-410 cluster regulates activity-dependent dendritogenesis by fine-tuning Pumilio2 protein levels. EMBO J. 2009;28:697–710. doi: 10.1038/emboj.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel-Knöchel V, Linden DEJ. Cerebral asymmetry in schizophrenia. Neuroscientist. 2011;17:456–467. doi: 10.1177/1073858410386493. [DOI] [PubMed] [Google Scholar]
- Best C, Lange E, Buchholz H-G, Schreckenberger M, Reuss S, Dieterich M. Left hemispheric dominance of vestibular processing indicates lateralization of cortical functions inrats. Brain Struct Funct. advance online publication, 1 August 2013; doi:10.1007/s00429-013-0628. [DOI] [PubMed]
- Inberg S, Elkobi A, Edri E, Rosenblum K. Taste familiarity is inversely correlated with Arc/Arg3.1 hemispheric lateralization. J Neurosci. 2013;33:11734–11743. doi: 10.1523/JNEUROSCI.0801-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wang C, Wang X, Wang L, Chang C, Wang P, et al. Correlations between angiotensinase activity asymmetries in the brain and paw preference in rats. Neuropeptides. 2010;44:253–259. doi: 10.1016/j.npep.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Alqadah A, Hsieh Y-W, Chuang C-F. microRNA function in left-right neuronal asymmetry: perspectives from C. elegans. Front Cell Neurosci. 2013;7:158. doi: 10.3389/fncel.2013.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen L, Klausen M, Helboe L, Nielsen FC, Werge T. MicroRNAs show mutually exclusive expression patterns in the brain of adult male rats. PLoS ONE. 2009;4:e7225. doi: 10.1371/journal.pone.0007225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skutella T, Nitsch R. New molecules for hippocampal development. Trends Neurosci. 2001;24:107–113. doi: 10.1016/s0166-2236(00)01717-3. [DOI] [PubMed] [Google Scholar]
- Kim J, Krichevsky A, Grad Y, Hayes GD, Kosik KS, Church GM, et al. Identification of many microRNAs that copurify with polyribosomes in mammalian neurons. PNAS. 2004;101:360–365. doi: 10.1073/pnas.2333854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manakov SA, Grant SGN, Enright AJ. Reciprocal regulation of microRNA and mRNA profiles in neuronal development and synapse formation. BMC Genomics. 2009;10:419. doi: 10.1186/1471-2164-10-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royo H, Cavaillé J. Non-coding RNAs in imprinted gene clusters. Biol Cell. 2008;100:149–166. doi: 10.1042/BC20070126. [DOI] [PubMed] [Google Scholar]
- Seitz H, Youngson N, Lin S-P, Dalbert S, Paulsen M, Bachellerie J-P, et al. Imprinted microRNA genes transcribed antisense to a reciprocally imprinted retrotransposon-like gene. Nat Genet. 2003;34:261–262. doi: 10.1038/ng1171. [DOI] [PubMed] [Google Scholar]
- Davis E, Caiment F, Tordoir X, Cavaillé J, Ferguson-Smith A, Cockett N, et al. RNAi-mediated allelic trans-interaction at the imprinted Rtl1/Peg11 locus. Curr Biol. 2005;15:743–749. doi: 10.1016/j.cub.2005.02.060. [DOI] [PubMed] [Google Scholar]
- Byrne K, Colgrave ML, Vuocolo T, Pearson R, Bidwell CA, Cockett NE, et al. The imprinted retrotransposon-like gene PEG11 (RTL1) is expressed as a full-length protein in skeletal muscle from Callipyge sheep. PLoS ONE. 2010;5:e8638. doi: 10.1371/journal.pone.0008638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichon S, Schumacher J, Müller DJ, Hürter M, Windemuth C, Strauch K, et al. A genome screen for genes predisposing to bipolar affective disorder detects a new susceptibility locus on 8q. Hum Mol Genet. 2001;10:2933–2944. doi: 10.1093/hmg/10.25.2933. [DOI] [PubMed] [Google Scholar]
- Segurado R, Detera-Wadleigh SD, Levinson DF, Lewis CM, Gill M, Nurnberger JI, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part III: Bipolar disorder. Am J Hum Genet. 2003;73:49–62. doi: 10.1086/376547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp CM, Hottenga J-J, Slagboom PE, Sullivan PF, de Geus EJC, Posthuma D, et al. Linkage on chromosome 14 in a genome-wide linkage study of a broad anxiety phenotype. Mol Psychiatry. 2008;13:84–89. doi: 10.1038/sj.mp.4002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer BI, Mantha K, Kleiber ML, Diehl EJ, Addison SMF, Singh SM. Long-lasting alterations to DNA methylation and ncRNAs could underlie the effects of fetal alcohol exposure in mice. Dis Model Mech. 2013;6:977–992. doi: 10.1242/dmm.010975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer BM, Estes ML, Barrow SL, McAllister AK. MHCI requires MEF2 transcription factors to negatively regulate synapse density during development and in disease. J Neurosci. 2013;33:13791–13804. doi: 10.1523/JNEUROSCI.2366-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Kim T-K, Gray JM, Harmin DA, Hemberg M, Hong EJ, et al. Genome-wide analysis of MEF2 transcriptional program reveals synaptic target genes and neuronal activity-dependent polyadenylation site selection. Neuron. 2008;60:1022–1038. doi: 10.1016/j.neuron.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifer D, Golden J, Kowall NW. Myocyte-specific enhancer binding factor 2C expression in human brain development. Neuroscience. 1994;63:1067–1079. doi: 10.1016/0306-4522(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Akhtar MW, Kim M-S, Adachi M, Morris MJ, Qi X, Richardson JA, et al. In vivo analysis of MEF2 transcription factors in synapse regulation and neuronal survival. PLoS ONE. 2012;7:e34863. doi: 10.1371/journal.pone.0034863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Radford JC, Ragusa MJ, Shea KL, McKercher SR, Zaremba JD, et al. Transcription factor MEF2C influences neural stem/progenitor cell differentiation and maturation in vivo. Proc Natl Acad Sci USA. 2008;105:9397–9402. doi: 10.1073/pnas.0802876105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determermined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Deng MY, Lam S, Meyer U, Feldon J, Li Q, Wei R, et al. Frontal-subcortical protein expression following prenatal exposure to maternal inflammation. PLoS ONE. 2011;6:e16638. doi: 10.1371/journal.pone.0016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyosseva SV, Elbein AD, Griffin WS, Mrak RE, Lyon M, Karson CN. Mitogen-activated protein kinases in schizophrenia. Biol Psychiatry. 1999;46:689–696. doi: 10.1016/s0006-3223(99)00104-3. [DOI] [PubMed] [Google Scholar]
- Samuels IS, Karlo JC, Faruzzi AN, Pickering K, Herrup K, Sweatt JD, et al. Deletion of ERK2 mitogen-activated protein kinase identifies its key roles in cortical neurogenesis and cognitive function. J Neurosci. 2008;28:6983–6995. doi: 10.1523/JNEUROSCI.0679-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert AE, Dash PK. Extracellular signal-regulated kinase activity in the entorhinal cortex is necessary for long-term spatial memory. Learn Mem. 2002;9:156–166. doi: 10.1101/lm.48502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MJ, Grimm CH, Paya-Cano JL, Sugden K, Nietfeld W, Lehrach H, et al. Using hippocampal microRNA expression differences between mouse inbred strains to characterise miRNA function. Mamm Genome. 2008;19:552–560. doi: 10.1007/s00335-008-9116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Wang W-Y, Mao Y-W, Gräff J, Guan J-S, Pan L, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll LS, Williams HJ, Walters J, Kirov G, Donovan MCO, Owen MJ, et al. Mutation screening of the 3q29 microdeletion syndrome candidate genes DLG1 and PAK2 in schizophrenia. Am J Med Genet. 2011. pp. 844–849. [DOI] [PubMed]
- Van Beveren NJM, Buitendijk GHS, Swagemakers S, Krab LC, Röder C, de Haan L, et al. Marked reduction of AKT1 expression and deregulation of AKT1-associated pathways in peripheral blood mononuclear cells of schizophrenia patients. PLoS ONE. 2012;7:e32618. doi: 10.1371/journal.pone.0032618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry. 2011;16:960–972. doi: 10.1038/mp.2010.88. [DOI] [PubMed] [Google Scholar]
- Udawela M, Scarr E, Hannan AJ, Thomas EA, Dean B. Phospholipase C beta 1 expression in the dorsolateral prefrontal cortex from patients with schizophrenia at different stages of illness. Aust N Z J Psychiatry. 2011;45:140–147. doi: 10.3109/00048674.2010.533364. [DOI] [PubMed] [Google Scholar]
- Cotter D, Kerwin R, Al-Sarraji S, Brion JP, Chadwich A, Lovestone S, et al. Abnormalities of Wnt signalling in schizophrenia—evidence for neurodevelopmental abnormality. Neuroreport. 1998;9:1379–1383. doi: 10.1097/00001756-199805110-00024. [DOI] [PubMed] [Google Scholar]
- Miyaoka T, Seno H, Ishino H. Increased expression of Wnt-1 in schizophrenic brains. Schizophr Res. 1999;38:1–6. doi: 10.1016/s0920-9964(98)00179-0. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Galimberti I, Deguchi Y, Caroni P. Wnt signaling mediates experience-related regulation of synapse numbers and mossy fiber connectivities in the adult hippocampus. Neuron. 2009;62:510–525. doi: 10.1016/j.neuron.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Kumamoto N, Gu Y, Wang J, Janoschka S, Takemaru K-I, Levine J, et al. A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nat Neurosci. 2012;15:399–405. doi: 10.1038/nn.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguschak KA, Ressler KJ. Wnt signaling in amygdala-dependent learning and memory. J Neurosci. 2011;31:13057–13067. doi: 10.1523/JNEUROSCI.3248-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatadze N, Tomas C, McGonigal R, Lin B, Schook A, Routtenberg A. Wnt transmembrane signaling and long-term spatial memory. Hippocampus. 2012;22:1228–1241. doi: 10.1002/hipo.20991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Calabrese F, Racagni G, Fumagalli F, Riva MA. Antipsychotic drug actions on gene modulation and signaling mechanisms. Pharmacol Ther. 2009;124:74–85. doi: 10.1016/j.pharmthera.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Ishima T, Iyo M, Hashimoto K. Neurite outgrowth mediated by the heat shock protein Hsp90α: a novel target for the antipsychotic drug aripiprazole. Transl Psychiatry. 2012;2:e170. doi: 10.1038/tp.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Kravchenko VV, Tapping RI, Han J, Ulevitch RJ, Lee J. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 1997;16:7054–7066. doi: 10.1093/emboj/16.23.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa AC, Kim M-S, Ertunc M, Adachi M, Nelson ED, McAnally J, et al. MEF2C, a transcription factor that facilitates learning and memory by negative regulation of synapse numbers and function. Proc Natl Acad Sci USA. 2008;105:9391–9396. doi: 10.1073/pnas.0802679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll AP, Tran N, Tooney PA, Cairns MJ. Alternative mRNA fates identified in microRNA-associated transcriptome analysis. BMC Genomics. 2012;13:561. doi: 10.1186/1471-2164-13-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.