Abstract
Human decision making in situations of inequity has long been regarded as a competition between the sense of fairness and self-interest, primarily based on behavioral and neuroimaging studies of inequity that disfavor the actor while favoring others. Here, we use functional magnetic resonance imaging experiments to study refusals and protests using both favoring and disfavoring inequity in three economic exchange games with undercompensating, nearly equal, and overcompensating offers. Refusals of undercompensating offers recruited a heightened activity in the right dorsolateral prefrontal cortex (dlPFC). Accepting of overcompensating offers recruited significantly higher node activity in, and network activity among, the caudate, the cingulate cortex, and the thalamus. Protesting of undercompensating fixed offers activated the network consisting of the right dlPFC and the left ventrolateral prefrontal cortex and midbrain in the substantia nigra. These findings suggest that perceived fairness and social decisions are the results of coordination between evaluated fairness norms, self-interest, and reward.
Key words: : economic decision making, functional magnetic resonance imaging (fMRI), overcompensation, protest
Introduction
Our sense of fairness helps us to regulate our lives in society. Our perception of inequity leads to a range of emotions (Adams, 1965; Lind and Tyler, 1988; Sprecher, 1986; Walster et al., 1978) and often motivates us to react negatively (Fehr and Fischbacher, 2004; Fehr and Gachter, 2002; Guth et al., 1982), even when we know that such a reaction may lead to a personal cost (Fehr and Gachter, 2002). Such negative reactions have been observed to varying degrees across diverse cultures (Henrich et al., 2001), and nonhuman animals share a similar trait (Price and Brosnan, 2012), indicating a strong biological predisposition. Human social decision making in situations of inequity is often viewed as a competition between the norms of fairness and self-interest (Fehr and Fischbacher, 2003; Knoch et al., 2006). Thus, deviations from self-interested behavior leading to reciprocal fairness are hypothesized to reflect our values for social norms and inequity aversion (Fehr and Gintis, 2007). This notion of social decision making as a result of competition between sense of fairness and self-interest is primarily based on the studies of human responses in the two-person (proposer and responder) economic exchange using only one aspect of inequity, how individuals respond when inequity does not favor them (Glimcher et al., 2009; Houser and McCabe, 2008; Houser and Xiao, 2010). Inequity has, however, two sides: the responder may be over-benefitted (or over-compensated, which is advantageous inequity) or under-benefitted (or undercompensated, which is disadvantageous inequity). Is there an asymmetry in our responses and, hence, in our sense of fairness between these two inequity conditions? If yes, what are the brain mechanisms underlying this difference? To what degree do reactions depend on whether the responder's actions can influence the players' outcomes, and what are the differences in brain mechanisms for protests as compared to refusals? This study addresses these questions using functional neuroimaging techniques in 3 two-person economic exchange games: the ultimatum game (UG), the impunity game (IG), and a new fixed decision game (FDG).
The UG is the game most commonly used to determine how people make decisions in situations of inequity (Guth et al., 1982). In the UG, the first player, the proposer, splits a sum of money with the second player, the responder. If the responder accepts the offer, both participants are rewarded accordingly, while if the responder rejects the offer, neither player receives any money. Thus, a refusal by the responder leads to him or her receiving absolutely less, but relatively the same as, the partner. The responder's rejection in the UG has been interpreted as a way by which he or she punishes the unfair proposer and/or signals to the proposer (or others) that the unfair treatment has occurred. A variant of the UG is the IG (Bolton et al., 1998). The IG is procedurally identical to the UG, except that the responder's rejection response affects only his or her own payoff, and not the proposer's. Unlike in the UG, in the IG, the responder cannot punish an unfair proposer, nor does a refusal result in equality. In fact, a refusal results in both an absolutely and relatively less good outcome for the responder. Nonetheless, responders still refuse, possibly due to frustration or to reinforce their commitment to fairness (Yamagishi et al., 2009). Finally, we were interested in how responders would use a “protest” option that did not change either the proposer's or the responder's outcome. Thus, we developed a new game, the FDG, a variation on the UG and IG. In the FDG, a distribution of offers was shown to the responder, who could choose to either protest or not protest these decided outcome offers, but in neither case were the outcomes to either player altered. Additionally, in contrast to the UG, in the FDG there is no competition between economic self-interest and fairness norms, particularly in the case of overcompensation, because the responder still gets the designated monetary amount even if he or she protests a decision.
Fairness-related social decisions are known to be motivated by self-interest, self-versus-other comparisons and fairness norms (Fehr and Fischbacher, 2003; Guth et al., 1982). Neuroimaging studies have shown that such decisions activate several brain regions: the insula, dorsolateral prefrontal cortex (dlPFC), and the anterior cingulate cortex for the perception of unfair offers (Sanfey et al., 2003), the ventral striatum for fair offers (Tabibnia et al., 2008), and the ventromedial prefrontal cortex and caudate for social rewards (Smith et al., 2014). The competition between self-interest and fairness norms (Guth et al., 1982; Knoch et al., 2006) is neutrally instantiated in the right dlPFC, where activity is found to be associated with undercompensating (low unfair) offers (Knoch et al., 2006; Sanfey et al., 2003). The dlPFC is believed to play a role in limiting the selfish motive and implementing fairness (Sanfey et al., 2003). Disruption of the right, but not the left dlPFC [using repetitive transcranial magnetic simulation; (Knoch et al., 2006)] substantially reduces participants' willingness to reject their partners' unfair offers. However, the right dlPFC is also found to be recruited when responders decide whether or not to punish a partner in a two party economic exchange by rejecting an unfair economic deal proposed by that partner. Thus, the precise role of the dlPFC in these refusals is still debated; evidence supports both inhibition of self-interest (Knoch et al., 2006) and punishment of norm violators (Buckholtz et al., 2008; Sanfey et al., 2003). We hypothesized that, if the role of the dlPFC is to inhibit the self-interest, dlPFC activity would be elevated in the UG and IG rejection where the self-interested motivation is suppressed, but not necessarily the FDG game, in which a protest does not influence outcomes. If the dlPFC has a role in punishing the norm violators, dlPFC activity should be higher in the UG than in the IG or FDG when responders are rejecting undercompensating offers.
One limitation of the previous fairness-related studies is that they primarily consider only one aspect of inequity—disadvantageous inequity, or participants' reactions when they received less than a partner. Inequity aversion includes two components, both disadvantageous inequity aversion and advantageous inequity aversion, or an aversion to outcomes that overly benefit the individual as compared to a partner (Fehr and Schmidt, 1999). In this study, we included overcompensated offers. First, this allowed us to explore whether individuals would refuse rewards to bring about equity when they are overcompensated with respect to a partner. Second, we explored whether undercompensated and overcompensated offers equally triggered the disapproval response (Fehr and Gachter, 2002; Sanfey et al., 2003). One of our main foci was to determine the brain mechanism that differentiates between these two conditions. Third, we explored whether individuals would protest more frequently in the FDG condition, where the protest does not affect their earnings, which may allow them to appear to be “nicer” people without paying any cost to do so. This could potentially uncover situations in which people have a taste for fairness, but one that does not appear in traditional games due to the high cost of being fair (e.g., conflict between their taste for equity and their self-interest in their own material outcomes).
We hypothesized that there would be a low rejection rate for overcompensated offers, which is in line with previous behavioral studies (Dana et al., 2007; Lowewenstein et al., 1989). For the associated brain response, we hypothesized that the activity would be different between overcompensated and undercompensated offers, and between overcompensated and fair offers. The manifested behavioral outcome in overcompensated offers might be triggered by feelings similar to those experienced for being rewarded, which would invoke the brains' reward circuitry (Delgado et al., 2003, 2004; Hikosaka et al., 2006; Kim et al., 2009; Knutson et al., 2001; Martin-Soelch et al., 2003; Samejima et al., 2005). We additionally predicted that there would be a higher protest rate in the FDG than the refusal rate in the UG. We hypothesized that the brain activity would differ between the protested decisions and not protested decisions, allowing us to learn about the brain mechanisms of protesting decisions (Greenberg and Cohen, 1982). Here, we predicted that protesting a decision would involve the brain network that coordinates between the inner speech of subjective feeling and protest (McGuire et al., 1996; Verstichel et al., 1997) and social norm compliance (Spitzer et al., 2007).
Materials and Methods
Participants
Eighteen people (10 male and 8 female; age: 25.2±6.2 years [mean±standard deviation]) participated in the experiment. A prescanning written interview was conducted. Participants were asked to fill an interview form with a number of questions related to magnetic resonance imaging (MRI) safety and medical history. All of our participants reported that they were right-handed and had normal or corrected-to-normal vision, no history of medical, psychiatric, or neurological diagnoses, and they were not taking any medication. A written informed consent was obtained from each participant before the experiment according to the procedures approved by the Institutional Review Board of Georgia State University.
Experimental task
We used two established economics games, the UG and IG, and included a new game, which we called the FDG. In all of these games, there were three offer distributions: fair, unfair-low (e.g., disadvantageous to the participant), and unfair-overcompensated (e.g., advantageous to the participant). The distributions were from the random splits of $100 in the above-mentioned three contexts as: fair offers ($40<offer<$60), unfair low (undercompensated) offers ($0<offer<$20), and unfair high (overcompensated) offers ($80<offer<$100) in all three games. There were thus nine offer conditions (three games type×three offer conditions), but during testing only one offer condition at a time was presented in the computer screen (Fig. 1). Offer conditions were randomized across the session. Presentation (Neurobehavioral systems, www.neurobs.com) was used to display the offers to the participants and to record participants' behavioral responses. The responder (the participant in our study) could either reject or accept the proposer's offers in the UG and IG, and could choose either to protest or not to protest the fixed offers in the FDG. The outcome of the responder's action varied according to the rules of these games (Table 1). The responder's rejection led to no pay-offs to both players in the UG, but only the responder lost their payoff in the IG. In the FDG, the responder could change neither their own nor the partners' outcomes, but could choose to protest the decided offers.
FIG. 1.
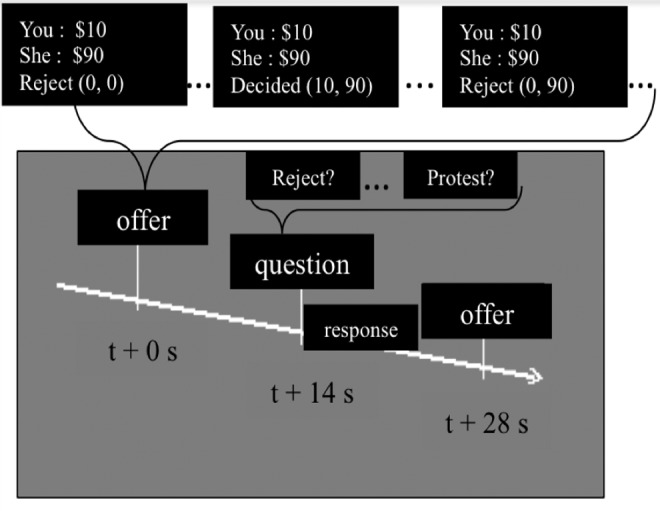
Task paradigm. A single round of the economic games consisted of the proposer's offer, the question, and the responder's yes or no choice. The time interval between an offer and a question was 14 sec. Offers, along with the game outcomes should the offer be rejected, were displayed for 4 sec on a computer-projected screen. Here, in three sample offers, participants saw both the offer and the payoffs to both parties should they reject the offer. Regarding the latter, “Reject (0, 0)” means that if the offer is rejected, $0 will go to the responder and the proposer (this is ultimatum game [UG]); “Reject (0, 90)” means $0 to the responder and $90 to the proposer (impunity game [IG]); “Decided (10, 90)” means a fixed offer of $10 to the responder and $90 to the proposer (fixed decision game [FDG]). Responders could indicate their yes or no choices by pressing one of the two buttons on a response box after the question, “Reject?” for the UG or IG and “Protest?” for the FDG, appeared on the screen.
Table 1.
Economic Games and Outcomes
| Outcomes for reject/protest | |||
|---|---|---|---|
| Game | Option | Proposer | Responder |
| Ultimatum | Reject/Accept | $0 | $0 |
| Impunity | Reject/Accept | Offer amount | $0 |
| Fixed decision | Protest/Not protest | Offer amount | Offer amount |
Before scanning, each participant saw the pictures and names of possible players (proposers), who would be referred as “he” or “she” in each trial (Fig. 1). They were also told that they would be playing with real money, and that they would be compensated for a percentage (∼2%) of their earnings, up to $50 in total. The participants were told how the games were played and what the possible outcomes of each game were. The participants were asked to practice a few rounds of the task in a computer setup before going into the scanner. In the scanner, each participant played 30 rounds of each of the three games from each of the three distributions discussed above, with games and payoff conditions randomized across the session. This was done in three 860 sec-long functional runs. Each run had two 10 sec rest (no task) durations, one in the beginning and the other at the end. Each round (trial) consisted of an offer and the question. The offer and the associated question (“Reject?” or “Protest?”) were each displayed for 4 sec. The time between the onset of an offer and a question was 14 sec. Participants decided whether to reject or not to reject the offers in the between offer-to-question block. The participants were asked to make a response after the question mark (?) appeared on the screen.
Data acquisition and analysis
Participants were scanned on 3-T Siemens functional MRI (fMRI) scanner in the Biomedical Imaging Technology Center at Emory University while they played three economic games and decided whether (i) to reject or not to reject unfair (under- or overcompensated) and fair monetary offers (UG and IG), and (ii) to protest or not protest a fixed monetary offers (FDG). The functional scans were acquired with T2*-weighted gradient echo-planar imaging sequence (repetition time [TR], 2000 msec; echo time [TE], 32 msec; flip angle, 90°; field of view, 256×256 mm2; dimensions, 64×64×33; voxel dimensions, 3×3×4 mm). For each of the three functional runs, 430 volume images were taken. Behavioral responses were analyzed using Matlab. The analysis of fMRI images was carried out by using Statistical Parametric Mapping (SPM8, www.fil.ion.ucl.ac.uk/spm/software/spm8). Slice timing and motion-corrected images were spatially normalized to the Montreal Neurological Institute (MNI) template. The voxels were resized to 3×3×3 mm3 per voxel resolution. Finally, images were spatially smoothed using an 8-mm full width at half maximum (FWHM) Gaussian Kernel. A random-effect, model-based, univariate statistical analysis was performed in a two-level procedure. At the first level, a separate general linear model (GLM) was specified for 18 task conditions (3 offer types×3 games×2 response conditions [that is accepted/not protested and rejected/protested conditions]) for each decision and response block plus time courses of six motion parameters (as nuisance covariates) were entered in GLM. The individual contrast images of all participants from the first level GLM were then taken into a second level analysis for a separate one-sample t-test. Resulting summary statistical maps were then corrected for multiple comparisons by using AlphaSim command in AFNI (Ward, 2000). These maps were overlaid on a high-resolution structural image in MNI orientation.
Behavior data analysis
In all of the games, we used three offer distributions mentioned above: undercompensating, nearly equal and overcompensating. We planned to test whether there was an asymmetry in behavioral responses and, hence, in the sense of fairness between these three conditions. Among games, we were interested in the degree to which the responder's actions could influence the players' outcomes in both undercompensated and overcompensated offer conditions. The rejection or protest rates for three distinct distributions for each game were calculated for individual participants and averaged for the group in each condition. Wilcoxon sum rank tests were performed to compute the significance levels of behavioral differences across conditions.
Functional connectivity analysis
The analysis of functional relationship among the brain regions, the functional connectivity, was done by defining the regions of interest (ROIs). ROIs were defined by generating a sphere of 6 mm radius around the coordinate of local maxima from the group level analysis of fMRI data by using MarsBar (Brett et al., 2002). The time courses from all the voxels within each ROI and all participants were extracted from the sets of the ROIs. To investigate the brain mechanism for accepting overcompensated offers and to explore how the brain internalized a overcompensated offer, we contrasted the brain activity of accepting overcompensated offers with that of fair offers (Delgado et al., 2003; Martin-Soelch et al., 2003) as “High unfair>fair (UG+IG).” We hypothesized that a network of brain regions involved in reward processing (Delgado et al., 2003, 2004; Hikosaka et al., 2006; Kim et al., 2009; Knutson et al., 2001; Martin-Soelch et al., 2003; Samejima et al., 2005) would be activated. This was the first set of ROIs. The second set of ROIs was chosen from the group level analysis of contrast “Low unfair protested>fair not protested (in FDG),” to investigate the neural correlated of protesting a decision. Time courses were then segmented into trials for accepting overcompensated versus fair offers from the first set of ROIs and for protesting versus not protesting the fixed decided undercompensated offers condition from the second set of ROIs. We calculated pairwise correlation coefficients from trial by trial time series between the ROIs of the respective sets. To estimate the average effect, we used Fisher's z-transformation (Bond and Richardson, 2004; Cox, 2008; Silver and Dunlap, 1987) on cross-correlation values. The correlation coefficients were converted into their equivalent Fisher's z-values (z=arctanh(r)) to compute average Fisher's z-value. The average Fisher's z-values for each participant and each pair of ROIs were then used to calculate the grand average z-value, the significance level p and the corresponding correlation coefficient. This analysis was done separately for accepting overcompensated offers and fair offers from the first set of ROIs and for protesting and not protesting the fixed decided undercompensated offers from the second set of ROIs.
Directed functional connectivity analysis
We performed Granger causality (GC) analysis to characterize the directional influences between ROIs, as the functional connectivity does not reveal the direction of information flow. We extracted the voxels time courses for each ROI from all participants. Since fMRI-blood-oxygen-level dependent (BOLD) signals are believed to originate from smoothing of neuronal activity by the hemodynamic response function (Aguirre et al., 1998; Handwerker et al., 2004), we constructed hidden neural signals by hemodynamic deconvolution for each ROI as suggested in previous studies (David et al., 2008; Handwerker et al., 2004; Roebroeck et al., 2011; Valdes-Sosa et al., 2011; Wu et al., 2013). We used these deconvolved fMRI-BOLD time series for functional connectivity calculation.The ensemble-mean removed segmented deconvolved time series from separate voxels and participants were treated as trials for reliable estimates of the network measures. We calculated the frequency dependent nonparametric GC spectra (Dhamala et al., 2008) for pairs of ROIs, separately for both set of RIOs. From the spectral GC, the time-domain values were obtained by integrating the causality spectra over the entire frequency range. The significant GC spectra were defined by setting a GC threshold above the random-noise baseline. To compute the threshold value of GC, we constructed a set of surrogates by randomly permuting trials data from each participant and used the random permutation technique (Blair and Karniski, 1993; Brovelli et al., 2004). The threshold was thus based on the null hypothesis that there was no statistical interdependence between nodes when trials were randomized. We computed GC spectra from all possible pairs of ROIs with 300 random permutations and picked maximum GC on each permutation. By fitting the distribution with gamma-distribution function (Dhamala et al., 2008), we obtained the threshold for GC spectra at significance p<10−3 separately from each set of ROIs. This threshold GC was used to identify significantly active directed network activity among three ROIs for both sets of ROIs. We computed the time-domain GC values for significantly active network directions.
Results
Behavioral results
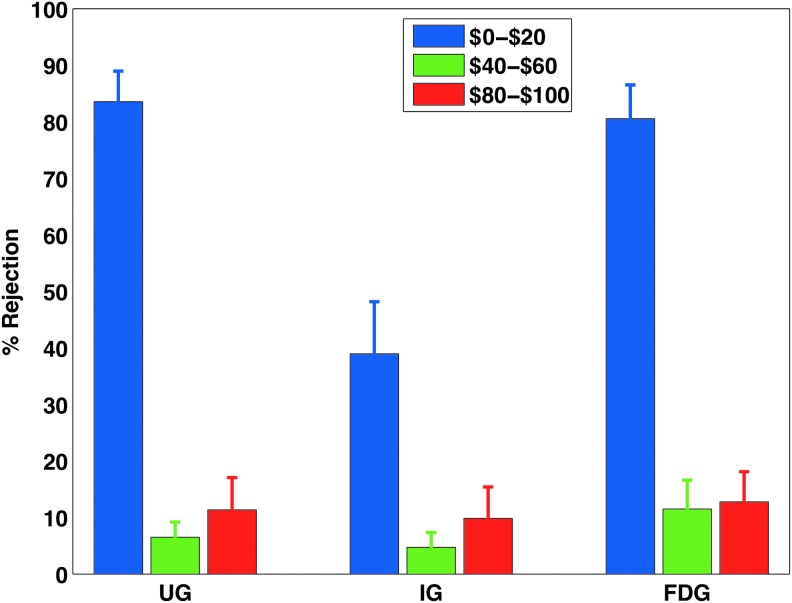
Most (88%) of the fair offers were accepted in all games, whereas most of the undercompensated offers were rejected and protested (>80%) in the UG and FDG. While we did not find any difference in the frequency with which undercompensated offers were rejected or protested between the UG and the FDG, the rejection rate of undercompensated offers in the IG was less (39.9%) than that of the UG (z-score=3.26, p<0.01) and than the protestation rate in the FDG (z-score=3.23, p<0.01). The overcompensated offers were not rejected to the same degree as undercompensated ones in any of the three games, including the FDG, where protest was not costly. Participants more frequently rejected or protested the undercompensated offers as compared to the fair offers and overcompensated offers in all games (z-score>3.3, p<0.001 in the UG and FDG; z-score=2.65, p<0.01 in the IG; Fig. 2 and Table 2). They did not respond differently to the overcompensated conditions as compared to the fair conditions in any of the games (Fig. 2).
FIG. 2.
Behavioral response. These are the group averages of rejection or protest rates for three distinct distributions of offers displayed to the responders, $0–$20 (unequal low offers), $40–$60 (nearly equal or equal offers), and $80–$100 (unequal high offers) out of $100. Each participant played 30 rounds of each game (UG, IG, and FDG) during three functional magnetic resonance imaging runs. Approximately 2% of the total earnings were given to the participants. Error bars here represent standard errors of the means. Color images available online at www.liebertpub.com/brain
Table 2.
Behavioral Rejection Rates in Economics Games
| Games | Choices | Undercompensated offers (%) | Fair offers (%) | Overcompensated offers (%) |
|---|---|---|---|---|
| UG | Reject | 84.5 | 6.5 | 11.3 |
| IG | Reject | 38.9 | 4.7 | 9.8 |
| FDG | Protest | 80.6 | 11.5 | 12.8 |
FDG, fixed decision game; IG, impunity game; UG, ultimatum game.
Imaging results
Brain activity associated with undercompensated offers
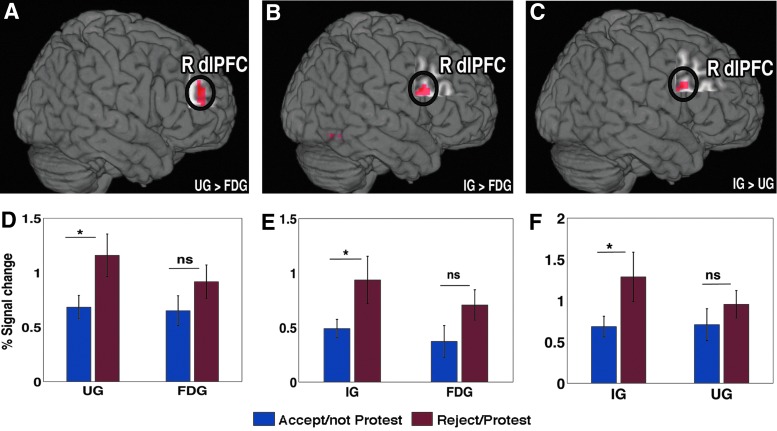
To explore the precise role of the dlPFC in two-person economic exchanges, we contrasted the conditions for undercompensating offers from the UG and IG with FDG as (i) UG>FDG, (ii) IG>FDG, and (iii) IG>UG. In all of these cases (i–iii), the dlPFC was activated (Fig. 3A–C). The details of the brain activations with the multiple comparisons correction is shown in Table 3. We also compared the level of activity (% signal change) in the dlPFC for each game during accepting (or not protesting) conditions with rejecting (or protesting) conditions (Fig. 3D–F). We found that the dlPFC activity was significantly higher (p<0.05) when rejecting offers as compared with accepting offers in the UG, IG, and in protesting offers compared to not protesting offers in the FDG.
FIG. 3.
Right dorsolateral prefrontal cortex (dlPFC) activity. (A, D) The right dlPFC (R dlPFC) showed heightened activity during the rejection of unfair undercompensated offer in the UG as compared to the FDG and corresponding % signal change for rejected/protested and accepted/not protested offers. (B, E) dlPFC activity while rejecting unfair undercompensated offers in the IG as compared to unfair undercompensated offers in the FDG and % signal change for rejected/protested and accepted or not protested offers. (C, F) dlPFC activity during the rejection of unfair undercompensated offers in the IG as compared to unfair undercompensated offers in the UG and % change for rejected and accepted offers. Here, *p<0.05, ns, not significant. Color images available online at www.liebertpub.com/brain
Table 3.
Brain Activations
| Contrast (economics game) | Brain region | Cluster size | Voxel t (z-equivalent) | MNI coordinates |
|---|---|---|---|---|
| Low UG>low FDG | R middle frontal gyrus (dlPFC) | 22** | 3.18 (2.79) | 30, 47, 28 |
| R thalamus | 14** | 3.24 (2.82) | 18, −10, 13 | |
| Low IG>low FDG | R inferior frontal gyrus and BA 46 (dlPFC) | 35** | 4.75 (3.74) | 42, 14, 22 |
| R inferior temporal cortex (BA 37) | 47** | 5.26 (4.00) | 48, −56, −17 | |
| L cuneus | 17 | 4.19 (3.43) | −12, −70, 4 | |
| Low unfair (IG)>Low unfair (UG) | L lingual gyrus | 820** | 4.21 (3.44) | −15, −67, −2 |
| L middle temporal gyrus | 78** | 4.10 (3.37) | −60, −58, −2 | |
| L superior temporal gyrus | 19 | 3.88 (3.24) | −45, 14, −23 | |
| R middle frontal gyrus (dlPFC) | 30* | 3.19 (2.79) | 45, 14, 31 | |
| L culmen | 23** | 3.28 (2.48) | −30, −64, −26 | |
| L fusiform gyrus | 78** | 3.42 (2.94) | −36, −52, −11 | |
| L thalamus | 22** | 4.77 (3.75) | −24, −25, −2 | |
| High unfair>fair (UG+IG) | R thalamus | 26** | 4.92 (3.83) | 9, −22, 7 |
| L caudate | 12* | 4.61 (3.66) | −12, −1, 22 | |
| R cingulate | 12* | 4.24 (3.46) | 18, 2, 31 | |
| Low unfair protested>fair not protested (FDG) | R middle frontal gyrus (dlPFC) | 10** | 3.80 (3.19) | 54, 17, 37 |
| L midbrain | 11** | 3.57 (3.04) | −6, −22, −20 | |
| L ventrolateral prefrontal cortex (BA 45/47) | 11** | 3.44 (2.95) | −48, 17, 4 |
All the activations survived a significance threshold at p<0.005 and cluster threshold of k>10. The activation clusters that survived multiple comparisons corrections of the AlphaSim in AFNI are marked with a star (*) for p<0.05 and double stars (**) for p<0.001.
dlPFC, dorsolateral prefrontal cortex; L, left; MNI, Montreal Neurological Institute; R, right.
Brain activity associated with overcompensated offers
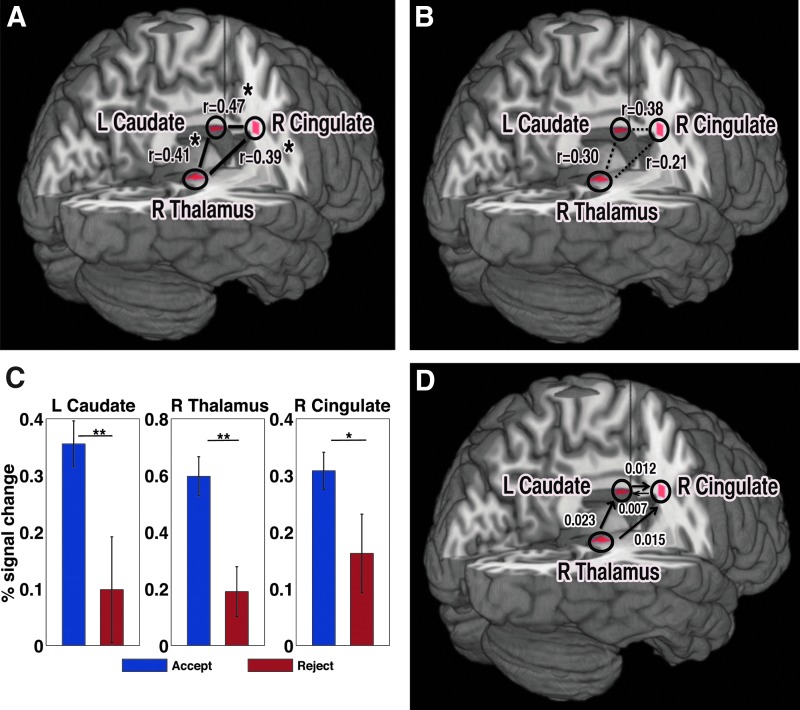
To understand the brain mechanism of accepting overcompensated offers, we contrasted the brain response from overcompensated trials in the UG and IG with that from fair trials (Martin-Soelch et al., 2003). We found significant activation in the left caudate in the dorsal striatum, the right middle cingulate gyrus, and the right thalamus when participants decided to accept overcompensated (OC) offers as compared with fair offers (Fig. 4). Further, the average percent signal changes in these regions significantly differed (p<0.05) between the trials when the participants accepted OC offers and the trials when they rejected these offers (Fig. 4C). Also these regions were found to be functionally connected while accepting the OC offers (Fig. 4A) but not in accepting the fair offers (Fig. 4B). We obtained the directed functional connectivity within this network based on the hidden neuronal responses derived from the deconvolution of hemodynamic responses (Fig 4D). The GC results showed that thalamus exerted causal influences to both the caudate and the cingulate. There were bidirectional influences between the caudate and the cingulate, but the influence from the caudate to the cingulate was stronger.
FIG. 4.
Brain node and network activity related to accepting overcompensated offers in the UG and IG. (A) The left caudate, right thalamus and right cingulate cortex became relatively more active in unfair high offers (overcompensated offers) than in fair offers. This activation analysis included the trials from the UG and IG games. There was significant functional connectivity among these regions during unfair high offer trials. (B) There was not a significant connectivity pattern during fair offers. (C) The average BOLD signal changes in all of these regions were significantly different between the trials when the participants accepted overcompensated offers and the trials when they rejected these offers. (D) The directed functional connectivity pattern is significant at p<0.001 (with multiple comparisons corrections). The cingulate cortex receives a dominant information flow from the caudate and thalamus. Here, *p<0.05, **p<0.01. Color images available online at www.liebertpub.com/brain
Brain activity associated with protesting a decision
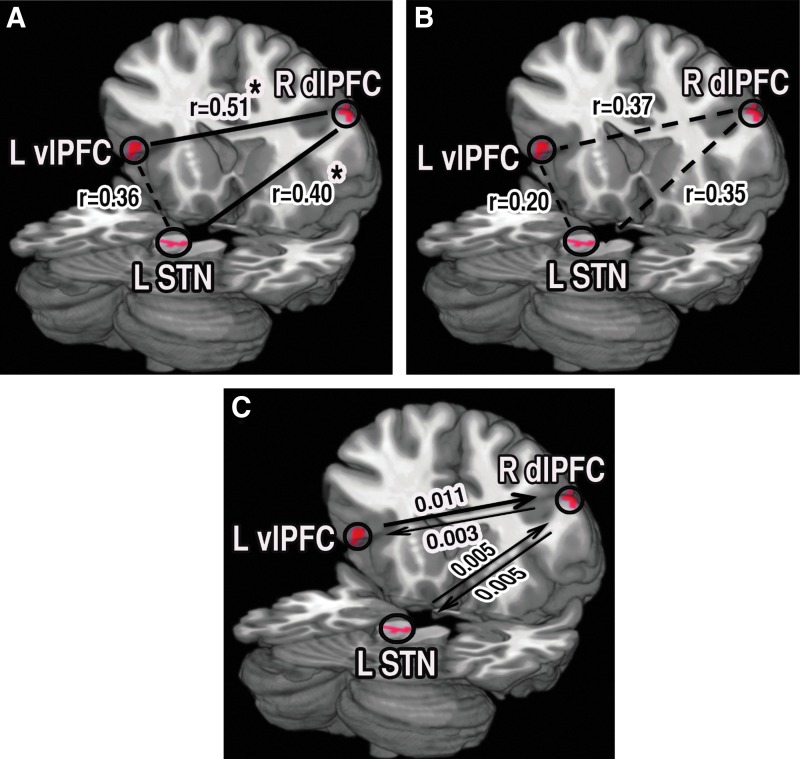
We isolated the brain areas that were activated when a responder was protesting a decision by contrasting the trials in which he or she protested versus did not protest in the FDG. This allowed us to further elucidate the neural circuitry associated with protesting a decision. Protesting a decision produced significant activation in two structurally (Blumenfeld et al., 2013) and functionally (Zysset et al., 2002) connected brain regions in the left ventrolateral prefrontal cortex (vlPFC) within the inferior frontal gyrus (BA 45/47), the right middle frontal gyrus (dlPFC [BA 9]), a subregion in the prefrontal cortex, and in the left midbrain in the substantia nigra (STN) (Fig. 5). These regions vlPFC and dlPFC; dlPFC and STN were found to be functionally connected during protest (Fig. 5A), but not when individuals chose not to protest (Fig. 5B). There was bidirectional directed functional connectivity between these pairs of regions; vlPFC and dlPFC; dlPFC and STN. The causal influence was stronger from vlPFC to dlPFC than for the opposite direction. The causal influences were nearly equal in strength from dlPFC to STN and to the opposite direction (Fig. 5C).
FIG. 5.
Brain node and network activity related to protesting fixed decided offers. (A) The left substantia nigra (L STN), R dlPFC, and left ventrolateral prefrontal cortex (L vlPFC) became more active during the protest of undercompensated offers than during not protesting fair offers. vlPFC and STN were functionally associated with dlPFC during protest. (B) This functional association was not significant in decided fair offers. (C) Although there were bidirectional network interactions of R dlPFC with L vlPFC and L STN, the R dlPFC received a dominant information flow from L vlPFC during the protest of undercompensated offers. Here, *p<0.05. Color images available online at www.liebertpub.com/brain
Discussion
Primarily based on the UG and the brain response to disadvantageous inequity, previous neuroimaging studies proposed that human decision making in the context of unfair offers is a result of competition between cognitive and emotional processes in the brain. In this study, we included both disadvantageous and advantageous inequity conditions to explore the asymmetry in the sense of fairness (e.g., whether reactions are the same in situations of over- versus undercompensation) and its neural substrates. Our result further showed that a different neural system comes into play in the situations when the responder protests against unfair outcomes without altering these outcomes. Here, we discussed the results of the behavioral responses, fMRI activations and brain networks obtained in three different economic games, the UG, IG, and FDG.
Behavioral response
In all three games, participants showed their sensitivity to being disadvantaged by refusing or protesting outcomes more often when they were offered less money than a social partner. On the other hand, participants did not show any sensitivity to overcompensation; we found no significant difference in rejection or the protest rate for overcompensated offers as compared to fair offers. These results add to the discussion about the degree to which humans are sensitive to the outcomes of others as compared to themselves. While it is very clear that humans care about others' outcomes (Fehr and Fischbacher, 2002, 2003), they are also more interested in their own outcomes than those of others (Lowewenstein et al., 1989) and when sensitivity to others' outcomes may result in a drop in one's own outcome, people prefer not to have that knowledge (Dana et al., 2007). Additionally, it is possible that even these prosocial outcomes may be overstated in the case of explicit experimental situations as compared to otherwise similar daily interactions (Winking and Mizer, 2013). However, despite disliking being disadvantaged, humans do not uniformly refuse disadvantageous offer (Zhou et al., 2014). Our results showed that participants were sensitive to how their refusal influenced both their own and their partner's outcomes; participants were less likely to refuse the unequal outcomes in the IG, where refusing resulted in $0 payoff for them but not the partner, than the UG, where refusing resulted in $0 payoff for both participants. This reflects previous work showing that people refuse unequal outcomes in the IG at about half the frequency for which they refuse the same distribution in the UG (Yamagishi et al., 2009). These results from the UG and IG games altogether suggest that the refusals in the UG are not about the punishment to the proposer. Similarly, the refusals in the IG are not an effort to engender equity.
In the UG, there is a tension between acquiring more resources and achieving equity, but in the FDG, individuals could protest such offers without losing resources, presumably dissolving this tension. Thus, we predicted higher protest rates in the FDG than the refusal rate in the UG. Nonetheless, in the FDG, when participants could protest without affecting their material outcomes, participants did not protest with any more frequency than they refused overcompensated outcomes in the IG and UG. That is, despite the essentially nonexistent cost of protesting, people frequently chose not to do so. This indicates that participants are not failing to refuse offers in the UG in order to gain resources. One possible explanation for this is that participants were not concerned about the inequity to their partners in the overcompensated conditions. This also extends to disadvantageous offers toward the responder him or herself.
Dorsolateral prefrontal cortex
The dlPFC is a part of cognitive system that represents goals and the means to achieve them (Miller and Cohen, 2001; Weissman et al., 2008), and it also plays a role in evaluating the fairness-related behaviors and outcomes (Wagner et al., 2001). In two-person economic exchange games, such as the UG, the dlPFC is usually activated while rejecting undercompensated offers. However, whether its role is to inhibit the self-interest (Knoch et al., 2006) or to punish the norm violators (Buckholtz et al., 2008; Sanfey et al., 2003) is not resolved yet. Our finding of significantly increased BOLD activity in the dlPFC while participants are rejecting undercompensated offers in the UG (where rejection needs to inhibit economic self interest) as compared to that of the FDG, (where monetary gain is fixed, meaning no concern about monetary loss and no self-control is required) supports the role of the dlPFC in self-control (Miller, 2000).
To understand its possible role in punishment, we further contrasted the undercompensated offer condition in the IG (where there is no punishment goal) with that of the UG and FDG. We found significantly higher dlPFC activity while rejecting monetary offers in the IG compared with the UG and FDG. Because there is no direct punishment goal associated with rejection in the IG, our results do not support the role of dlPFC for maintaining punishment goals. The higher dlPFC activity while refusing undercompensated offers in the IG might be because it requires a higher level of self-control because refusing in the IG results in both an absolute and a relative loss, while refusing in the UG results only in an absolute loss. The role of the dlPFC in self-control is further supported by a recent study in which disrupting activity in the right dlPFC via transcranial magnetic stimulation, generated fewer rejections of unfair offers (Knoch et al., 2006). Moreover, the significant signal change (%) in the dlPFC while rejecting offers as compared to accepting offers in both the UG and IG game conditions suggests that the dlPFC might be involved in inhibiting the appetitive desire for money, which allows individuals to choose socially appropriate options in the implementation of fairness related goal rather than a punitive response to unfair treatment.
Neural correlates of accepting overcompensated offers
Reward plays a major motivational role in changing the behavior of humans and other animals. Our behavioral results clearly showed that people were motivated not to reject or protest overcompensated offers. Importantly, from the brain analysis, the approval response to the overcompensated offers was found to be triggered by the reward related brain circuit consisted of the left caudate, right cingulate gyrus, and right thalamus (Delgado et al., 2003, 2004; Hikosaka et al., 2006; Kim et al., 2009; Knutson et al., 2001; Martin-Soelch et al., 2003; Samejima et al., 2005). The role of the caudate in reward processing is further supported by a positive correlation of caudate activation with increased monetary reward (Delgado et al., 2003; Knutson et al., 2001; Martin-Soelch et al., 2003). Similarly, activity in the cingulate gyrus (Martin-Soelch et al., 2003) and the thalamus have been found to be associated with rewards (de Quervain et al., 2004; Delgado et al., 2000; Knutson et al., 2001; Martin-Soelch et al., 2003). Thus here, too, it is reasonable to argue that the brain might have internalized the overcompensated offers as rewards.
This argument is also supported by the low frequency of protestation of overcompensated offers in the FDG. The low rate of refusals may not be surprising in the IG and UG because responders lose materially by rejecting the offers. However, individuals who possess the taste for fairness should protest when there is no cost to doing so, as in the FDG. The failure to do this may indicate that they do not find these outcomes troubling. This argument is further supported by previous work (King-Casas et al., 2005) done by using a two-person economic exchange game (the trust game) in which hemodynamic responses in the caudate were found to be correlated with an increase in trust (i.e., an increase in payments to the partner). In the same direction, a study by Hikosaka et al. (2006) and single neuron recording in nonhuman (Apicella et al., 1991; Hikosaka et al., 1989; Schultz, 2000) have shown that the dorsal striatum is the main hub associated with reward and goal-directed behaviors. The cingulate is known to be involved in conflict monitoring (Botvinick et al., 2001, 2004), and it is active when choosing actions associated with reward (Rogers et al., 2004) and in reward related decision making (Bush et al., 2002; Martin-Soelch et al., 2003; Rogers et al., 2004; Williams et al., 2004).
Furthermore, the functional connectivity analysis revealed functional connection between them (Fig. 4A) while accepting overcompensated offers, but not in the fair offer condition (Fig. 4B). The caudate and cingulate were causally influenced from the thalamus and they also communicated when people accepted overcompensating offers (Fig. 4D). The caudate is well known for processing reward-related information (Delgado et al., 2000, 2004) and is thought to involve the outputs of the midbrain dopaminergic system (Delgado, 2007). The strong causal influence from the caudate to the cingulate might be the reward signals, or the incentive motivation, which helps the cingulate resolve conflict (Botvinick et al., 2001, 2004) between social fairness norms and accepting unfair overcompensation offers. This might lead toward the acceptance of unfair overcompensated offers by modifying or changing behavioral responses (Bush et al., 2002; Williams et al., 2004). The significantly higher BOLD signal during the acceptance of overcompensated compared to fair offers (Fig. 4C) showed the involvement of this brain network towards the acceptance of overcompensating offers. The involvement the caudate-cingulate–thalamus network might be either in the selection of action associated with higher value outcomes (Hikosaka et al., 2006; Kim et al., 2009; Rogers et al., 2004; Samejima et al., 2005) or in the processing of rewards (Bush et al., 2002; Delgado et al., 2003, 2004; Hikosaka et al., 2006; Knutson et al., 2001; Martin-Soelch et al., 2003; Williams et al., 2004), and does not support previous thinking that inequity aversion is symmetric in humans (Camerer et al., 2005; Fehr and Camerer, 2007; Fehr and Gachter, 2002; Guth et al., 1982).
Neural correlates of protesting a fixed decision
The monetary gain in the FDG is fixed and so, unlike in the UG, there is no conflict (or tension) between acquiring monetary gain and promoting (if not achieving) equity when protesting a decision in the FDG. As a result, the act of protesting a decision is based only on the responders' attitudes concerning fairness (e.g., the current reward is uninvolved) (Zysset et al., 2002), which are highly rooted on one's knowledge, awareness, personality traits (Gallagher, 2000; Morin, 2009), and perception of proposer's intention (Houser and Xiao, 2010), and it requires cognitive control (Miller, 2000; Miller and Cohen, 2001). The elevated right dlPFC activity while protesting the decision might indicate executive top-down control of such behavior (Mansouri et al., 2009; Miller and Cohen, 2001; Passingham and Sakai, 2004). This view is further supported by the previous findings (Damasio, 1995) that patients with right prefrontal lesions were characterized by the inability to behave in normatively appropriate ways, despite the fact that they were keenly aware of the prevailing norms, such as the fairness norm [in tasks similar to ours; (Fehr and Fischbacher, 2004)]. The higher dlPFC activity while protesting the undercompensated offers compared with not protested fair offers is also consistent with the previous finding (Knoch et al., 2006) that the dlPFC is involved in social norms enforcement.
However, the dlPFC is not the only region activated when protesting an undercompensated offer; the vlPFC, a region involved in verbal reasoning (Johnson et al., 2005) and inner speech (Verstichel et al., 1997), and the STN are coherently activated. The vlPFC has consistently been identified as the neuro-anatomical basis of inner speech and reliably gets activated when participants are asked to silently articulate sentences or single words (McGuire et al., 1996); dysfunction also disrupts inner speech (Verstichel et al., 1997). In our case, the vlPFC might be feeding (the observed dominant causal flow from the vlPFC to the dlPFC, Fig. 5C) its inner speech of frustration/protestation induced by unfair treatment to the dlPFC while protesting the decision. Further, the strong functional connectivity between the dlPFC and the vlPFC when protesting a decision (Fig. 5A), but not when participants choose not to protest (Fig. 5B), suggests that the interaction between these regions is important during the processing of participants' reaction to unfair offers. The similar connectivity pattern between the right dlPFC and the left ventral prefrontal cortex (Baumgartner et al., 2011) was observed in rejecting unfair offers. As these brain areas are not yet fully developed in children, adolescents, or even young adults (Gogtay et al., 2004), these groups are not fully able to evaluate appropriate social norms (Spitzer et al., 2007). Thus, the network formed by these regions might be a part of the neural circuitry involved in social norm compliance (Spitzer et al., 2007). Additionally, brain activity in the left STN (midbrain), a reward-related brain region (Chib et al., 2013; Delgado, 2007; Knutson et al., 2001), might reflect the pleasure feeling induced by decision of protesting (symbolically) unfair decision (de Quervain et al., 2004). Thus, the evidence suggests that the act of protestation or the expression of frustration (Greenberg et al., 1982) resulted from the coordination of neural activity among the dlPFC, vlPFC, and STN and was triggered by the frustration of a social norm violation.
Previous studies on human social decision making in the context of economic games have shown that there is competition between self-interest and fairness norms, and little is known about the neural basis of resolving such conflict. Here, by using three games, the UG, IG, and FDC, we find evidence supporting the role of the right dlPFC in the social decision-making process. By including an overcompensated offer condition, we found that, contrary to earlier reports (Fehr and Schmidt, 1999; Guth et al., 1982), people do not make decisions that benefit others at a cost to themselves. Further, accepting overcompensated offers was found to be associated with activity in the brain reward's system (Delgado et al., 2000, 2003, 2004; Knutson et al., 2001; Martin-Soelch et al., 2003). Finally, we found that the ability to protest without cost does not influence participants' tendency to do so, indicating that the cost involved in refusals is not sufficient to explain the lack of rejections.
Behavioral results in our new FDG were very similar to those typically found in the UG in low unfair condition (Sanfey et al., 2003; Yamagishi et al., 2009), however, the difference in the rate of rejection of overcompensated offers was of the same degree as fair offers in all games. This is surprising, especially in the case of the FDG, because the responder could lodge a protest for a decision to unfair offers without affecting their material outcomes. Future neuroimaging studies on social decision-making processes can examine the interplay between over- and undercompensation, and the role of actual rejection versus protest. In particular, this sample space should be extended by investigating similarities and differences due to a variety of factors such as personality traits (Gallagher, 2000; Morin, 2009), perception of proposer's intention (Houser and Xiao, 2010), age (Bailey et al., 2013), and culture (Henrich et al., 2001). Levels of perceived fairness are known to change in certain psychiatric illnesses, such as bipolar disorder and personality disorders (Baez et al., 2013; King-Casas et al., 2008). This study with fairness related games and neuroimaging probes could provide some basis for future studies of cognitive functions and dysfunctions useful for the diagnosis and understanding of mental disorders.
Acknowledgments
A Georgia State University Brains and Behavior faculty seed grant to the authors M.D. and S.F.B financially supported this work. M.D. was also financially supported by a National Science Foundation (NSF) CAREER Award (BCS 0955037).
Author Disclosure Statement
No competing financial interests exist.
References
- Adams JS. 1965. Inequity in social exchange. In: Berkowitz L. (ed.) Advances in Experimental Social Psychology, Vol. 2. New York: Academic; pp. 267–299 [Google Scholar]
- Aguirre GK, Zarahn E, D'Esposito M. 1998. The variability of human, BOLD hemodynamic responses. Neuroimage 8:360–369 [DOI] [PubMed] [Google Scholar]
- Apicella P, Ljungberg T, Scarnati E, schultz W. 1991. Responses to reward in monkey dorsal and ventral striatum. Exp Brain Res 85:491–500 [DOI] [PubMed] [Google Scholar]
- Baez S, Herrera E, Villarin L, Theil D, Gonzalez-Gadea ML, Gomez P, Mosquera M, Huepe D, Strejilevich S, Vigliecca NS, Matthaus F, Decety J, Manes F, Ibanez AM. 2013. Contextual social cognition impairments in schizophrenia and bipolar disorder. PLoS One 8:e57664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey PE, Ruffman T, Rendell PG. 2013. Age-related differences in social economic decision making: the ultimatum game. J Gerontol B Psychol Sci Soc Sci 68:356–363 [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Knoch D, Hotz P, Eisenegger C, Fehr E. 2011. Dorsolateral and ventromedial prefrontal cortex orchestrate normative choice. Nat Neurosci 14:1468–1474 [DOI] [PubMed] [Google Scholar]
- Blair RC, Karniski W. 1993. An alternative method for significance testing of waveform difference potentials. Psychophysiology 30:518–524 [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Nomura EM, Gratton C, D'Esposito M. 2013. Lateral prefrontal cortex is organized into parallel dorsal and ventral streams along the rostro-caudal axis. Cereb Cortex 23:2457–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton GE, Katok E, Zwick R. 1998. Dictator game giving: rules of fairness versus acts of kindness. Int J Game Theory 27:269–299 [Google Scholar]
- Bond CF, Richardson K. 2004. Seeing the Fisher z-transformation. Psychometrika 69:291–303 [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. 2001. Conflict monitoring and cognitive control. Psychol Rev 108:624–652 [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. 2004. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 8:539–546 [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. 2002. Region of interest analysis using an SPM toolbox (abstract). In Presented at the 8th International Conference on Functional mapping of Humal Brain, Sendai, Japan, June2–6, 2002 [Google Scholar]
- Brovelli A, Ding M, Ledberg A, Chen Y, Nakamura R, Bressler SL. 2004. Beta oscillations in a large-scale sensorimotor cortical network: directional influences revealed by Granger causality. Proc Natl Acad Sci U S A 101:9849–9854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Asplund CL, Dux PE, Zald DH, Gore JC, Jones OD, Marois R. 2008. The neural correlates of third-party punishment. Neuron 60:930–940 [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. 2002. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A 99:523–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerer C, Loewenstein G, Prelec D. 2005. Neuroeconomics: how neoroscience can inform ecomonics. J Econo Lit XLIII:9–64 [Google Scholar]
- Chib VS, Yun K, Takahashi H, Shimojo S. 2013. Noninvasive remote activation of the ventral midbrain by transcranial direct current stimulation of prefrontal cortex. Transl Psychiatry 3:e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox NJ. 2008. Speaking stata: correlation with confodence, or Fisher's z revisited. Stata J 8:413–439 [Google Scholar]
- Damasio AR. 1995. Descartes' Error: Emotion, Reason, and the Human Brain. New York: Harper Collins [Google Scholar]
- Dana J, Weber R, kuang JX. 2007. Exploiting moral wiggle room: experiments demonstrating an illusory preference for fairness. Econ Theory 33:67–80 [Google Scholar]
- David O, Guillemain I, Saillet S, Reyt S, Deransart C, Segebarth C, Depaulis A. 2008. Identifying neural drivers with functional MRI: an electrophysiological validation. PLoS Biol 6:2683–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ, Fischbacher U, Treyer V, Schellhammer M, Schnyder U, Buck A, Fehr E. 2004. The neural basis of altruistic punishment. Science 305:1254–1258 [DOI] [PubMed] [Google Scholar]
- Delgado MR. 2007. Reward-related responses in the human striatum. Ann N Y Acad Sci 1104:70–88 [DOI] [PubMed] [Google Scholar]
- Delgado MR, Locke HM, Stenger VA, Fiez JA. 2003. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cogn Affect Behav Neurosci 3:27–38 [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. 2000. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol 84:3072–3077 [DOI] [PubMed] [Google Scholar]
- Delgado MR, Stenger VA, Fiez JA. 2004. Motivation-dependent responses in the human caudate nucleus. Cereb Cortex 14:1022–1030 [DOI] [PubMed] [Google Scholar]
- Dhamala M, Rangarajan G, Ding M. 2008. Analyzing information flow in brain networks with nonparametric Granger causality. Neuroimage 41:354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr E, Camerer CF. 2007. Social neuroeconomics: the neural circuitry of social preferences. Trends Cogn Sci 11:419–427 [DOI] [PubMed] [Google Scholar]
- Fehr E, Fischbacher U. 2002. Why social preferences matter—the impact of non-selfish motives on competition, cooperation and incentives. Econ J 112:C1–C33 [Google Scholar]
- Fehr E, Fischbacher U. 2003. The nature of human altruism. Nature 425:785–791 [DOI] [PubMed] [Google Scholar]
- Fehr E, Fischbacher U. 2004. Third-party punishment and social norms. Evol Hum Behav 25:63–87 [Google Scholar]
- Fehr E, Gachter S. 2002. Altrustic punishment in human. Nature 415:137–140 [DOI] [PubMed] [Google Scholar]
- Fehr E, Gintis H. 2007. Human motivation and social cooperation: experimental and analytical foundations. Annu Rev Sociol 33:43–64 [Google Scholar]
- Fehr E, Schmidt KM. 1999. A theory of fairness, competition and cooperation. Q J Econ 114:817–868 [Google Scholar]
- Gallagher S. 2000. Philosophical conception of the self: implications for cognitive science. Trends Cogn Sci 4:14–21 [DOI] [PubMed] [Google Scholar]
- Glimcher PW, Camerer CF, Fehr E, poldrack RA. 2009. Neuroeconomics—Decision Making and the Brain. London: Elsevier academic press [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A 101:8174–8179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JS, Cohen R. 1982. Equity and Justice in Social Behavior. New York: Acedemic Press [Google Scholar]
- Guth W, Schmittberger R, Schwarze B. 1982. An experimental analysis of ultimatum bargaining. J Econ Behav Organ 3:367–388 [Google Scholar]
- Handwerker DA, Ollinger JM, D'Esposito M. 2004. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage 21:1639–1651 [DOI] [PubMed] [Google Scholar]
- Henrich J, Boyd R, Bowles S, Camerer C, Fehr E, Gintis H, McElreath R. 2001. In search of homo economicus: Behavioral experiments in 15 small-scale societies. Am Econ Rev 91:73–78 [Google Scholar]
- Hikosaka O, Nakamura K, Nakahara H. 2006. Basal ganglia orient eyes to reward. J Neurophysiol 95:567–584 [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. 1989. Functional properties of monkey caudate neurons III. Activities related toexpectation of target and reward. J Neurophysiol 61:814–832 [DOI] [PubMed] [Google Scholar]
- Houser D, McCabe K. 2008. Neouroeconomics: Advance in Health Economics and Health Services Research. London: Emerald insight; [DOI] [PubMed] [Google Scholar]
- Houser D, Xiao E. 2010. Inequality-seeking punishment. Econ Lett 109:20–23 [Google Scholar]
- Johnson CS, Schmitz TW, Kawahara-Baccus TN, Rowley TA, Alexander AL, Lee J, Davidson RJ. 2005. The cerebral response during subjective choice with and without self-reference. J Cogn Neurosci 17:1897–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Sul JH, Huh N, Lee D, Jung MW. 2009. Role of striatum in updating values of chosen actions. J Neurosci 29:14701–14712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Casas B, Shaep C, Lomax-Bream L, Lohrenz T, Fonagy P, Montague PR. 2008. The rupture and repair of cooperation in borderline personality disorder. Science 321:806–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Casas B, Tomlin D, Anen C, Camerer CF, Quartz SR, Montague PR. 2005. Getting to know you: reputation and trust in a two-person economic exchange. Science 308:78–83 [DOI] [PubMed] [Google Scholar]
- Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. 2006. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science 314:829–832 [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. 2001. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21RC:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind EA, Tyler TR. 1988. The Social Psychology of Procedural Justic. New York: Plenum [Google Scholar]
- Lowewenstein GF, Thompson L, Bazerman MH. 1989. Interpersonal relations and group processes. J Pers Soc Phychol 57:426–441 [Google Scholar]
- Mansouri FA, Tanaka K, Buckley MJ. 2009. Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nat Rev Neursci 10:141–152 [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Missimer J, Leenders KL, Schultz W. 2003. Neural activity related to the processing of increasing monetary reward in smokers and nonsmokers. Eur J Neurosci 18:680–688 [DOI] [PubMed] [Google Scholar]
- McGuire P, Silbersweig D, Murray R, Frackowiak R, Frith D. 1996. The neural correlates of inner speech and auditory verbal imagery in schizophrenia: Relationship to auditory verbal hallucinations. Br J Psychiatry 167:148–159 [DOI] [PubMed] [Google Scholar]
- Miller EK. 2000. The prefrontal cortex and cognitive control. Nat Rev Neursci 1:59–65 [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. 2001. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202 [DOI] [PubMed] [Google Scholar]
- Morin A. 2009. Self-awareness deficits following loss of inner speech: Dr. Jill Bolte Taylor's case study. Conscious Cogn 18:524–529 [DOI] [PubMed] [Google Scholar]
- Passingham D, Sakai K. 2004. The prefrontal cortex and working memory: physiology and brain imaging. Curr Opin Neurobiol 14:163–168 [DOI] [PubMed] [Google Scholar]
- Price SA, Brosnan SF. 2012. To each according to his need? variability in the responses to inequity in non-human primates. Soc Just Res 25:140–169 [Google Scholar]
- Roebroeck A, Formisano E, Goebel R. 2011. The identification of interacting networks in the brain using fMRI: model selection, causality and deconvolution. Neuroimage 58:296–302 [DOI] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, Smith SM. 2004. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry 55:594–602 [DOI] [PubMed] [Google Scholar]
- Samejima K, Ueda Y, Doya K, Kimura M. 2005. Representation of action-specific reward values in the striatum. Science 310:1337–1340 [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. 2003. The neural basis of economic decision-making in the Ultimatum Game. Science 300:1755–1758 [DOI] [PubMed] [Google Scholar]
- Schultz W. 2000. Multiple reward signals in the brain. Nat Rev Neursci 1:199–207 [DOI] [PubMed] [Google Scholar]
- Silver NC, Dunlap WP. 1987. Averaging correlation coefficients: should Fisher's z transformation be used? J Appl Psychol 72:146–148 [Google Scholar]
- Smith DV, Clithero JA, Boltuck SE, Huettel SA. 2014. Functional connectivity with ventromedial prefrontal cortex reflects subjective value for social rewards. Soc Cogn Affect Neurosci [Epub ahead of print]; DOI: 10.1093/scan/nsu005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer M, Fischbacher U, Herrnberger B, Gron G, Fehr E. 2007. The neural signature of social norm compliance. Neuron 56:185–196 [DOI] [PubMed] [Google Scholar]
- Sprecher S. 1986. The relation between inequity and emotions in close relationships. Soc Psychol Quart 49:309–321 [Google Scholar]
- Tabibnia G, Satpute AB, Lieberman MD. 2008. The sunny side of fairness: preference for fairness activates reward circuitry (and disregarding unfairness activates self-control circuitry). Psychol Sci 19:339–347 [DOI] [PubMed] [Google Scholar]
- Valdes-Sosa PA, Roebroeck A, Daunizeau J, Friston K. 2011. Effective connectivity: influence, causality and biophysical modeling. Neuroimage 58:339–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstichel P, Bourak C, Font V, Crochet G. 1997. Langage intérieur après lésion cérébrale gauche: etude de la représentation phonologique des mots chez des patients aphasiques et non aphasiques [Inner speech and left brain damage: Study of the phonological analysis of words in aphasic and non-aphasic patients]. Rev Neuropsychol 7:281–311 [Google Scholar]
- Wagner AD, Maril A, Bjork RA, Schacter DL. 2001. Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex. Neuroimage 14:1337–1347 [DOI] [PubMed] [Google Scholar]
- Walster E, Walster GW, Berscheid E. 1978. Equity: Theory and Research. New York: Plenum [Google Scholar]
- Ward B. 2000. Simultaneous inference fo FMRI data. Available at http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf
- Weissman DH, Perkins AS, Woldorff MG. 2008. Cognitive control in social situations: a role for the dorsolateral prefrontal cortex. Neuroimage 40:955–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN. 2004. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci 7:1370–1375 [DOI] [PubMed] [Google Scholar]
- Winking J, Mizer N. 2013. Natural-field dictator game shows no altruistic giving. Evol Hum Behav 34:288–293 [Google Scholar]
- Wu GR, Liao W, Stramaglia S, Ding JR, Chen H, Marinazzo D. 2013. A blind deconvolution approach to recover effective connectivity brain networks from resting state fMRI data. Med Image Anal 17:365–374 [DOI] [PubMed] [Google Scholar]
- Yamagishi T, Horita Y, Takagishi H, Shinada M, Tanida S, Cook KS. 2009. The private rejection of unfair offers and emotional commitment. Proc Natl Acad Sci U S A 106:11520–11523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang Y, Rao LL, Yang LQ, Li S. 2014. Money talks: neural substrate of modulation of fairness by monetary incentives. Front Behav Neurosci 8:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zysset S, Huber O, Ferstl E, von Cramon DY. 2002. The anterior frontomedian cortex and evaluative judgment: an fMRI study. Neuroimage 15:983–991 [DOI] [PubMed] [Google Scholar]