Abstract
Significance: Oxidative stress is involved in the development of newborn lung diseases, such as bronchopulmonary dysplasia and persistent pulmonary hypertension of the newborn. The activity of antioxidant enzymes (AOEs), which is impaired as a result of prematurity and oxidative injury, may be further affected by specific genetic polymorphisms or an unfavorable combination of more of them. Recent Advances: Genetic polymorphisms of superoxide dismutase and catalase were recently demonstrated to be protective or risk factors for the main complications of prematurity. A lot of research focused on the potential of different antioxidant strategies in the prevention and treatment of lung diseases of the newborn, providing promising results in experimental models. Critical Issues: The effect of different genetic polymorphisms on protein synthesis and activity has been poorly detailed in the newborn, hindering to derive conclusive results from the observed associations with adverse outcomes. Therapeutic strategies that aimed at enhancing the activity of AOEs were poorly studied in clinical settings and partially failed to produce clinical benefits. Future Directions: The clarification of the effects of genetic polymorphisms on the proteomics of the newborn is mandatory, as well as the assessment of a larger number of polymorphisms with a possible correlation with adverse outcome. Moreover, antioxidant treatments should be carefully translated to clinical settings, after further details on optimal doses, administration techniques, and adverse effects are provided. Finally, the study of genetic polymorphisms could help select a specific high-risk population, who may particularly benefit from targeted antioxidant strategies. Antioxid. Redox Signal. 21, 1863–1880.
Oxidative Stress and Antioxidant Enzymes in the Newborn
Newborns, especially if preterm, encounter an elevated oxidative burden, as a consequence of hyperoxic events, leading to increased reactive oxygen species (ROS) production, mechanical ventilation, and inflammatory and infective complications (28, 29, 32, 92, 115, 147–149). Oxidative stress (OS), resulting from an unbalance between oxidant factors and antioxidant defenses, significantly contributes to different newborn pathologies, which are grouped as “oxygen radical diseases of neonatology” (149). According to this hypothesis, the main complications of prematurity, such as bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), intraventricular hemorrhage (IVH), and periventricular leukomalacia, can be considered organ-specific manifestations within a single spectrum of OS-related injury (21, 29, 32, 55, 58, 78, 147, 149). Significant OS also occurs in persistent pulmonary hypertension of the newborn (PPHN), resulting in cardiopulmonary transition failure from fetal to extrauterine life (164).
Preterm newborns and depressed term newborns may experience sustained hyperoxia during resuscitation and respiratory support, especially if the fractional inspired oxygen (FiO2) is inappropriately targeted. In fact, resuscitation of term infants with ambient air relative to FiO2 100% is associated with reduced mortality and shorter need for resuscitation (122, 125, 126, 145, 146, 149, 169, 177–179), and resuscitation of preterm newborns with a low oxygen strategy significantly reduces the need for respiratory support, the incidence of BPD and OS markers (180). Indeed, a significant correlation was established between cord levels of total hydroperoxides, advanced oxidation protein products, non-protein bound iron, and the risk of free-radical-related diseases of prematurity, including BPD (116), suggesting that a very early activation of oxidative pathways significantly impacts long-term outcomes. In addition, it is well known that mechanical ventilation for respiratory distress syndrome (RDS) behaves similar to a double-edged sword in preterm newborns, as it is a major activator of inflammation and remodeling processes in the developing lung (28, 29, 78, 147).
In addition, neonates with PPHN encounter significant OS, as treatment with high oxygen concentration increases lung ROS burden, which can directly damage pulmonary parenchyma and vessels (84, 85). As inhaled nitric oxide (iNO) is administered, ROS can propagate lung damage through the formation of reactive nitrogen species (RNS), leading to further oxidative damages and NO depletion, with worsening of arterial pulmonary vessels constriction (130, 153).
In these settings, antioxidant enzymes (AOEs) activity is expected to play a crucial role in the resultant outcome. Several observations demonstrated that superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and reductase (GRd) increase during gestation, paralleling the maturation of the surfactant system (11, 12, 49, 50, 110, 112, 121, 152, 183). After preterm birth, newborns lack proper levels of antioxidants, due to placental-fetal transfer interruption and insufficient endogenous production (20, 32, 55, 115). Indeed, preterm newborns exhibit significantly lower erythrocyte levels of SOD until 20 days of life and of CAT until 100 days if compared with term controls (107). Moreover, while term newborns own the capacity to induce AOEs in case of OS, as fetal distress (87) or resuscitation with FiO2 100% (179), preterm animal models fail to induce SOD and GPx in response to oxidative challenges (48, 104), even if this aspect shows significant differences between animal species (11–13, 50).
SOD2 is strictly necessary for surviving in room air, as demonstrated in knockout models, and it is induced under hyperoxic conditions in order to cope with mitochondrial ROS overproduction (89). The study of SOD2 expression profile in human lung postmortem materials demonstrated a differential location according to gestational age, probably reflecting the different role of SOD2 during fetal lung development (11), although the total amount of SOD2 did not consistently increase during gestation. Such an observation is in agreement with other data obtained from human specimens (12, 165), but it is discordant from the results obtained in preterm newborns and baboon models (41, 104). Lung specimens of RDS or BPD patients did not show different SOD2 expression relative to age-matched controls, suggesting the inability of preterm newborns to induce lung SOD2 in response to OS (11).
AOEs are also crucial for the establishment of proper feto-placental-uterine interactions, as their expression profile shows spatiotemporal developmental peculiarities at the feto-maternal interface (33). SOD2 is particularly abundant in the fetal villous endothelium, reflecting a preferential role in the trophism of chorionic villi (65); while SOD3 is mainly intra-cytoplasmic and located in the cyto- and syncytiotrophoblast in the first trimester, but it is later relocated to the extracellular matrix of peri-villous and peri-vascular spaces, probably taking part in placental vasculogenesis (65).
According to a recent pathogenetic model of the complications of prematurity, genetic factors play a central role in determining the entity of the inflammatory response to specific stimuli occurring in the fetus and in the preterm newborn (68). It has been proposed that newborns with hyper-responsive phenotype inducing exaggerated inflammatory cytokines and ROS production are at a high risk of free-radical-related complications, while newborns with hyporesponsive phenotype show a high risk of extensive infective invasion, tissue destruction, and mortality. Instead, the induction of proper levels of inflammatory response is associated with higher probability of survival without major complications. Among other factors, genetic predisposition may include not only specific polymorphisms of mediators that are involved in inflammatory pathways, which have been extensively studied, but also polymorphisms which are involved in antioxidant defense, as preliminarily demonstrated (59, 118).
Genetic Polymorphisms and Lung Diseases
A single nucleotide polymorphism (SNP) is a modification in the DNA that consists of a substitution or a deletion of a single nucleotide from the original sequence, occurring every 100–300 base pairs and present, by definition, in at least 1% of the population (42). The study of SNPs represents a useful tool in the investigation of the genetic susceptibility to develop a certain disease, as it consists of the sequencing of particular regions of interest within a candidate gene, which is certainly or putatively involved in the pathogenesis of the disease (42).
SNPs produce different effects on the resultant protein according to their location within a gene. When located in the promoter region, an SNP can alter the amount of the resultant protein, while it can alter the protein function if located in the coding regions. On the contrary, if an SNP is located within a non-coding region, it will usually not affect the product. However, some SNPs occurring in non-coding regions actually interfere with protein expression, particularly affecting post-translational control and mRNA stability (40, 42). Microarray chips are provided and may contain approximately 500,000 SNPs, in order to screen contemporarily for multiple SNPs associated with a specific disease (42).
SNPs of AOEs
In the adult population, SNPs of AOEs have been extensively investigated and have been linked to the risk of several different diseases in cardiovascular, respiratory, oncologic, ophthalmologic, and neurodegenerative fields. With regard to lung pathologies and respiratory outcomes in adults, specific SNPs in SOD2 were found to influence the risk of asthma (77, 91); while SNPs in SOD3 were observed to affect the risk of obstructive lung disease (95, 192), and were associated with reduced pulmonary function (30) without influencing the risk of asthma (77). Moreover, SNPs of CAT (69, 91) and inducible heme-oxygenase (69) were proved to influence the risk of asthma.
On the contrary, in the population of preterm newborns, SNPs of AOEs have been poorly studied. However, based on the pathogenesis of the major complications of prematurity which definitely involves oxidative injury, it is reasonable to speculate that SNPs of AOEs likely affect the outcomes of prematurity. The SNP Val105Ile in the subclass pi of Glutathione-S-transferase (GST), which encodes for a low-activity variant of the enzyme, was positively linked to the risk of BPD in a small population of preterm newborns (93). Such observations can be explained while considering the dual role of GST in the lung, which consists not only of the enhancement of glutathione (GSH) recycle, thus incrementing antioxidant potentials, but also of the modulation of the synthesis of eicosanoids and other inflammatory mediators (51).
Despite the small amount of data about SNPs of AOEs in preterm newborns, they may play a key role in the pathogenesis of the complications of prematurity, similar to several adult diseases. This may be a more crucial point than the demonstration of the involvement of SNPs in pro-inflammatory and oxidant mediators in the occurrence of FRDs of prematurity. In fact, the observation of SNPs with deleterious effects on AOEs activity may pose the basis for specifically targeted therapeutic interventions. Therefore, we studied a group of 10 SNPs in SOD and CAT genes in a cohort of 152 preterm newborns ≤28 weeks of gestational age, in order to assess their possible correlation with RDS requiring mechanical ventilation, BPD, ROP, and IVH. According to multivariate logistic regression analysis, the SNP rs2536512 in SOD3 showed a trend toward a protective association with BPD, and the SNP rs8192287 in SOD3 was proved to be an independent protective factor for IVH (59, 118). The SOD3 rs2536512 SNP is located in the region expressing the enzyme N-terminal domain (23) and results in reduced SOD3 activity (168). This SNP was previously associated with the risk of hypertension and non-insulin-dependent diabetes mellitus in adults (142, 168); while in pregnant women with pre-eclampsia, it was linked to the risk of intrauterine growth restriction (136). Instead, the rs8192287 SNP is located in the 5′ untranslated region but probably intervenes in the control of mRNA stability; however, the effects of this SNP on enzymatic activity are not fully elucidated (30). Such an SNP was previously related to adverse respiratory outcomes in adults (30) but has never been linked to intracranial bleeding events. However, SOD3 over-expression in the animal model was proved to confer protection against focal stroke and global cerebral ischemia; while SOD3 knockout animals exhibited worse cerebrovascular outcomes (154–156). This observation is concordant with the protective role of SOD3 in the endothelial cells, where the scavenging of superoxide both prevents oxidative damage to the endothelium and the nearby cells of arterial walls and hinders the superoxide-induced inactivation of NO-mediated signaling pathways, thus preventing endothelial dysfunction (43). Haplotype reconstruction analysis was performed in order to assess the effect on adverse outcomes of combinations of different SNPs of one enzyme. The risk of RDS requiring mechanical ventilation was reduced by specific haplotypes of SOD1, SOD2, and SOD3; while the risk of BPD was increased by a specific haplotype of SOD2, and it was increased by a haplotype in SOD3 (59, 118). Moreover, specific haplotypes of SOD1 and SOD2 were also proved to reduce the risk of IVH and ROP, while a haplotype of SOD3 was shown to reduce the risk of IVH alone (Table 1) (59, 118). It is reasonable to speculate that protective haplotypes are associated to increased antioxidant activity in preterm infants, resulting in lower incidence of free-radical-related diseases, while unfavorable haplotypes may be associated with impaired antioxidant defense. However, data on SNP effects on proteomics are highly incomplete (Table 2).
Table 1.
Haplotypes of Superoxide Dismutase and Catalase Significantly Associated with Adverse Outcomes
| Gene symbol | Outcome | Haplotypes | Frequency in subjects without adverse outcomes | Frequency in subjects with adverse outcomes | Coef. | SE | p |
|---|---|---|---|---|---|---|---|
| CAT | |||||||
| RDS | CTC | 0.122 | 0.085 | −0.18 | 0.09 | 0.0430 | |
| SNP1=rs1001179; SNP2=rs1049982; SNP3=rs769217 | |||||||
| SOD1 | |||||||
| IVH | GG | 0.005 | <0.0001 | −0.05 | 0.01 | <0.0001 | |
| ROP | GG | 0.004 | <0.0001 | −0.08 | 0.005 | <0.0001 | |
| RDS | GG | 0.007 | <0.0001 | −0.29 | 0.01 | <0.0001 | |
| SNP1=rs17880135; SNP2=rs204732 | |||||||
| SOD2 | |||||||
| BPD | GT | NA | 0.014 | 0.57 | 0.001 | <0.0001 | |
| IVH | GT | 0.005 | <0.0001 | −0.23 | 0.001 | <0.0001 | |
| ROP | GT | 0.004 | 0.00003 | −0.08 | 0.001 | <0.0001 | |
| RDS | GT | 0.007 | <0.0001 | −0.46 | 0.001 | <0.0001 | |
| SNP1=rs4880; SNP2=rs5746136 | |||||||
| SOD3 | |||||||
| BPD | TGC | 0.116 | 0.058 | −0.21 | 0.084 | 0.0153 | |
| IVH | TGC | 0.119 | 0.033 | −0.18 | 0.083 | 0.0263 | |
SNP1=rs8192287; SNP2=rs2536512; SNP3=rs1799895.
BPD, bronchopulmonary dysplasia; CAT, catalase; IVH, intraventricular hemorrhage; RDS, respiratory distress syndrome; ROP, retinopathy of prematurity; SOD, superoxide dismutase; SNP, single nucleotide polymorphism.
Table 2.
Main Characteristics of Single Nucleotide Polymorphisms of Superoxide Dismutase and Catalase
| Gene | dbSNP | Region | Nucleotide substitution | Aminoacid variation | Effects on AOE activity |
|---|---|---|---|---|---|
| SOD1 | rs17880135 | 3′ UTR | T>G | Not applicable | Unknown |
| rs204732 | 3′ near gene | G>A | Not applicable | Unknown | |
| SOD2 | rs4880 | Exon | G>A | Ala16Val (missense) | Unknown |
| rs5746136 | 3′ UTR | C>T | Not applicable | Less efficient transport into mitocondria; possible reduction of activity but not fully elucidated (157) | |
| SOD3 | rs8192287 | 5′ near gene | G>T | Not applicable | Probably relevant for translational control and mRNA stability; unknown effects on activity (30) |
| rs2536512 | Exon | A>G | Thr40Ala | Reduced activity (168) | |
| rs1799895 | Exon | C>G | Arg213Gly (missense) | Increased circulating levels (144) | |
| CAT | rs1001179 | 5′ near gene | −262C>T | Not applicable | Unknown |
| rs1049982 | 5′ UTR | −20T>C | Not applicable | Unknown | |
| rs769217 | Exon | C>T | Asp389Asp (synonymous) | Unknown |
Data regarding SNP localization and effects on nucleotide and amino-acid sequences were extracted from www.ncbi.nlm.nih.gov/SNP/accessed on 2013/07/15.
AOE, antioxidant enzyme; UTR, untranslated region.
Interestingly, the analysis of genotype distribution showed that homozygous patients for the SNP rs4880 in SOD2 had significantly lower gestational age and birth weight than heterozygous and homozygous wild-type infants; while homozygous patients for the SNP rs5746136 in SOD2 exhibited significantly lower gestational age. The SNP rs4880 is located in a coding region, while SNP rs5746136 is located in the mitochondrial targeting sequence (157); however it is unclear whether the resultant enzymatic activities are increased or reduced. These observations may be explained speculatively with a possible interference of SNPs of SOD2 with the activity of the enzyme at the feto-maternal interface, finally affecting the pregnancy outcome. In fact, AOEs are believed to participate in the formation of proper vascular connections and in determining adequate local tissues trophism and functionality, thus playing a pivotal role in the maintenance of pregnancy (33). At the interface, SNPs that decrease the activity of AOEs would likely result in oxidative unbalance, favoring local oxidative damage and preterm birth. Our observations are concordant with previous reports of several SNPs in mediators of inflammation or remodeling involved in the pathogenesis of preterm birth if present in the fetus and/or in the mother (68), such as tumor necrosis factor-alpha (TNF-α) (7, 103, 132), interleukin (IL)-6 (184), IL-4 (74), IL-1 receptor antagonist (53), matrix metalloproteinase (MMP)-9 (46), MMP-1 (52), and Fas (73). These data suggest that SNPs which enhance inflammation and OS or reduce antioxidant defenses may putatively participate not only in the development of the complications of prematurity, but also in the pathogenesis of prematurity itself.
SNPs of inflammatory mediators and remodeling molecules
Since the pathogenesis of BPD involves a complex mixture of alveolar and vascular developmental arrest, inflammation, and oxidative damage to the developing lung (78, 147, 149), several studies investigated the role of SNPs of genes promoting inflammation. Particularly, SNPs of TNF have been extensively investigated with conflicting results. TNF 308A polymorphism, which is linked to TNF-α overproduction, was observed to increase the risk of BPD (76), but the following studies did not confirm this observation (1, 18) and a recent meta-analysis excluded this association (166). IFNG +874T SNP, which induces interferon-γ overproduction, was associated with a shorter need for supplemental oxygen and mechanical ventilation and resulted a protective factor against BPD in preterm infants (17). Mixed results were reported for SNPs of ILs (17, 176, 191), and only SNP−634 of IL6 resulted in association to prolonged oxygen therapy in a large cohort of very low-birth-weight infants (176). The toll-like receptors (TLRs) family, which is believed to contribute to lung development by enhancing pathogen removal and regulating tissue repair, has also been investigated. The TLR5 (1174C>T) variant resulted in association with the risk of severe BPD, and SNP (2054C>T) of the toll-IL-1 domain containing adaptor protein, which is involved in the TLR4 signaling pathway, also resulted in association with the risk of BPD (143); moreover, TLR6 rs5743827 SNP was recently proved to reduce the risk of BPD (188). Among the factors regulating inflammatory cells' recruitment, the SNP −173C of macrophage migration inhibitory factor, which was associated with increased expression, resulted in an independent protective factor for BPD (120); while L-selectine Ser213 allele was associated with an increased risk of BPD (39). MMP are key regulators of the extracellular matrix remodeling process, which is of primary importance for a proper alveolarization of the preterm lung (78). Two SNPs of MMP-16 (rs2664352 and rs2664349), which are associated with reduced levels of MMP16 and MMP-2 in the tracheal aspirates, were found to be protective against BPD (62). Finally, since NO may be a source of RNS, especially in a hyperoxic environment, SNPs of nitric oxide synthase (NOS) were also studied. Different SNPs of inducible and endothelial nitric oxide synthase were associated to the risk of ROP (139), likely affecting retinal vascularization at different stages (19, 22, 24) and to the risk of cerebral palsy (108); however, in contrast to the adult population (66, 67, 80, 167), SNPs of NOS have not been assessed with regard to respiratory outcomes in the preterm newborn.
Antioxidant Strategies
Based on the evidence that OS plays a pivotal role in the pathogenesis of BPD and PPHN, it appears reasonable to include antioxidant strategies in the prevention and treatment of such conditions. New potential treatments for newborn lung diseases are a matter of great importance, as current therapies are far from optimal (78). Several lines of evidence suggest the beneficial effects of antioxidant therapies in the prevention of hyperoxia-induced lung injury and in the treatment of PPHN in vitro and in animal models (Tables 3 and 4). Despite this, antioxidant treatments cannot be regarded at present as a part of the routine equipment for such indications, as clinical data are insufficient or inconsistent. Therefore, future research in this field should focus on transferring experimental studies to clinical settings, in order to provide possible evidence that supports the clinical use of antioxidant treatment. The study of SNPs of AOEs could be of great importance from this perspective. In fact, the assessment of SNPs in AOEs or a combination of more SNPs with unfavorable effects on antioxidant activities could be considered a helpful tool to identify a specific subpopulation of newborns with a particularly high risk of adverse outcomes, who could maximally benefit from antioxidant strategies.
Table 3.
Studies of Exogenous Administration of SOD/CAT or SOD Overexpression in Respiratory Distress Syndrome Patients or Hyperoxic Lung Injury Models
| Patients or models | Administration details | Principal outcomes | Ref. |
|---|---|---|---|
| Rats, hyperoxic | IV liposome-encapsulated bovine SOD and CAT | Increased survival rate | (174) |
| Reduced pleural effusion | |||
| Rats, hyperoxic | ET liposome-encapsulated bovine SOD and CAT | Increased survival rate | (113) |
| Reduced lung histological abnormalities | |||
| Piglets, hyperventilated and hyperoxic | ET rhSOD | Reduced neutrophils chemotactic activity, total cell count, elastase activity, and albumin in TA | (37) |
| 5 mg/kg | |||
| Rabbits, lungs challenged with xanthine and xanthine oxidase | ET liposome-encapsulated bovine SOD and CAT | Preserved lung filtration coefficient | (15) |
| Preterm infants (n=26) | ET rhSOD | Reduced neutrophil chemotactic activity and albumin in TA (high dose) | (135) |
| BW 750–1250 g | 0.5 or 5 mg/kg within 30 min of surfactant, single dose | ||
| <24 h of life | |||
| Preterm infants (n=33) | ET rhSOD | Reduced neutrophil chemotactic activity and albumin in TA | (34, 36) |
| BW 700–1300 g | 2.5 or 5 mg/kg within 2 h of surfactant, up to 7 doses | Reduced wheezing requiring bronchodilators or steroids at 1 year follow up | |
| <24 h of life | |||
| Transgenic mice, hyperoxic | Lung-targeted SOD3 overexpression | Reduced lung neutrophils and oxidized GSH | (4) |
| Increased alveolar surface and lung volume density | |||
| Transgenic mice, hyperoxic | Lung-targeted SOD3 overexpression | Preserved alveolar and bronchiolar epithelium proliferation | (14) |
| Reduced DNA damage | |||
| Preserved apical protein expression in type I cells | |||
| Rabbit, hyperoxic | Transfection with hSOD3 containing plasmid, AER | Increased lung cGMP | (3) |
| Decreased lung NF-κB expression | |||
| Transgenic mice, hyperoxic | Lung-targeted SOD3 overexpression+IP Antileukinate for 7 days | Reduced lung neutrophils, 8-isoprostane and oxidized GSH | (102) |
| Reduced myeloperoxidase activity in TA |
TA, tracheal aspirate; IV, intravenous; ET, endotracheal; IP, intraperitoneal; AER, aerosolized; GSH, glutathione; rhSOD, recombinant human SOD; NF-κB, nuclear factor kappa B.
Table 4.
Studies of Exogenous Administration of SOD/CAT or SOD Overexpression in PPHN Models
| Models | Administration details | Principal outcomes | Refs. |
|---|---|---|---|
| Piglets, hyperoxic and iNO | ET rhSOD | Reduced neutrophil chemotactic activity, total cell count, and protein concentration in TA | (131) |
| 0.5 mg/kg single dose or | |||
| 5 mg/kg, 2 doses or | |||
| AER rhSOD | Reduced lung malondialdehyde | ||
| 10 mg/kg, single dose | |||
| PPHN lambs | ET rhSOD 5 mg/kg+iNO 5 or 80 ppm | Reduced PPA and PVR vs. iNO or rhSOD alone | (162) |
| PPHN lambs | ET rhSOD 5 mg/kg+iNO 20 ppm | More rapid increase in oxygenation and reduced mean airway pressure vs. rhSOD or iNO alone | (86) |
| Reduced nitrotyrosine in PA vs. iNO alone | |||
| Premature RDS lambs | ET rhSOD | No increase in oxygenation vs. iNO alone | (79) |
| 2.5 or 5 or 10 mg/kg, single dose+iNO 5 ppm | |||
| PPHN lambs | ET rhSOD 5 mg/kg or iNO 10 ppm | rhSOD or iNO reduced lung ROS content vs. untreated | (45) |
| rhSOD, but not iNO, restored eNOS function and increased BH4 in PA vs. untreated | |||
| PPHN lambs | ET rhSOD 5 mg/kg or iNO 20 ppm | rhSOD or iNO reduced PDE5 activity and increased cGMP in PA vs. untreated | (44) |
| PPHN lambs | ET CAT 20,000 U/kg | Increased oxygenation vs. untreated | (187) |
| PPHN transgenic mice | Lung-targeted hSOD3 overexpression | Reduced RV pressure, RV mass, and pulmonary vasculature wall thickness vs. PPHN wild type | (5) |
| PPHN lambs | Isolated PA cells transfected with SOD2 containing vector | Reduced SOD2 Tyrosine nitration, increased eNOS activity, and relaxation of PA vs. non-transfected | (2) |
PA, pulmonary artery; PPA, pulmonary arterial pressure; PVR, pulmonary vasculature resistance; RV, right ventricle; eNOS, endothelial nitric oxide synthase; PDE5, phosphodiesterase 5; PPHN, persistent pulmonary hypertension of the newborn; ROS, reactive oxygen species; iNO, inhaled nitric oxide.
AOEs replacement
BPD and hyperoxic lung injury
Several animal studies proved that SOD administration is effective in reducing lung injury induced by hyperoxia and mechanical ventilation (15, 37, 113, 174), in accordance with the observation that absent SOD3 activity is associated with increased hyperoxia-induced lung injury; while SOD2 over-expression in type II alveolar cells is associated with prolonged survival in hyperoxic conditions (27, 189).
Endotracheal administration of recombinant human SOD (rhSOD) has been proved to reduce lung injury in preterm newborns receiving mechanical ventilation for RDS (36, 135). After one administration of rhSOD soon after birth, SOD levels in serum, tracheal aspirates, and urine significantly increased with regard to placebo-treated controls. Neutrophil chemotactic activity in tracheal aspirates resulted in significantly lower SOD-treated newborns for the first week of life, suggesting that rhSOD administration reduces the activation of lung inflammation. The duration of such an increase was dose dependent, lasting for 3 days for the lowest administered dose and 4 days for the highest dose (135). However, the duration of increased SOD levels in body fluids was likely too limited to affect the development of BPD (135). Repeated endotracheal administration of SOD, started within 2 h of surfactant treatment for a total of seven doses, induced a four- to seven-fold increase of SOD activity, in serum, urine, and tracheal aspirates, which lasted for 14 days. Despite prolonged action and significant reduction in lung inflammatory markers observed with such regimen, no differences in BPD occurrence were detected (36). A one-year follow up of this cohort demonstrated that rhSOD-treated newborns had a lower frequency of repeated wheezing episodes requiring bronchodilators or corticosteroids administration (34), suggesting that early reduction of oxidative lung injury may prevent subsequent long-term pulmonary damages. Such beneficial effects were particularly evident for the subgroup of rhSOD-treated newborns <27 weeks of gestational age, who experienced a 55% reduction in wheezing episodes, a 55% decrease in emergency department accesses, and a 44% reduction in hospitalization (34). RhSOD was well tolerated, and treated newborns did not differ from controls with regard to the incidence of short-term and long-term complications (35, 36).
Following these encouraging results, the enhancement of AOEs activity in the prevention of hyperoxia-induced lung injury has been further investigated. Based on the observation that SOD3 overexpression reduced lung adhesion molecules and inflammatory cytokines in the adult models exposed to hyperoxia (47), lung-targeted human SOD3 overexpression was studied in the newborn mice model of hyperoxia-induced lung injury (4). Transgenic mice overexpressing SOD3 showed significantly lower lung content of neutrophils and oxidized GSH after 7 days of hyperoxia and preserved higher alveolar surface area and lung volume density after 21 days in comparison with wild-type animals, suggesting that the beneficial effects on alveolarization may be due to the early reduction of OS and inflammation. Since SOD3 is a secreted extracellular protein, it has been hypothesized to protect from superoxide-induced damages in compartments that cannot be reached by intracellular AOEs, such as the surface of alveolar epithelium and alveolar hypophase (25). However, in the newborn, SOD3 is also represented in the intracellular space, suggesting that SOD3 overexpression possibly affects the redox state of both cells and the extracellular environment. The authors also suggested that SOD3 would exert beneficial effects not only because of scavenging activity but also through the formation of hydrogen peroxide, which is involved in processes that are fundamental to tissue development and remodeling, such as apoptosis and cell proliferation (4). Lung-targeted human SOD3 overexpression was further demonstrated to exert beneficial effects on lung development in preterm animal models under hyperoxic conditions during the transition from saccular to alveolar stages (14). Transgenic mice showed proper proliferation of alveolar and bronchiolar epithelial cells at 3 days, which was significantly impaired in wild-type hyperoxic animals, and they also presented less DNA damage and preservation of specific apical protein expression in type I cells (14).
It is well known that hyperoxic damage to the lung is the result of a double mechanism, involving both the direct toxic effects of ROS and the accumulation of inflammatory cells and their mediators (70, 90). Alveolar macrophages are believed to control the initial inflammatory response through the secretion of pro-inflammatory mediators, such as IL-1β and TNF-α, which are responsible for the recruitment of further inflammatory cells, perpetuating the activation of inflammatory and oxidative cascades (90). Antileukinate, a potent inhibitor of CXC chemokines receptors 1 and 2, which specifically suppresses neutrophils chemotaxis, was actually demonstrated to reduce lung inflammations in several models (64, 88, 99). Based on the beneficial effects of SOD3 per se on neutrophil recruitment in the lung, it has been recently studied in combination with Antileukinate in a mice neonatal model of hyperoxia-induced lung injury (102). Targeted lung overexpression of human SOD3 and antileukinate treatment showed synergistic activity, as transgenic mice receiving the treatment showed a lower content of neutrophils in the lung and of myeloperoxidase in the bronchoalveolar lavage as well as lower levels of OS markers, such as 8-isoprostane and oxidized GSH, in comparison with wild-type treated animals; alveolar density was also improved in the former group (102). Moreover, the administration of a plasmid containing human SOD3 via aerosol to animal models under hyperoxic conditions was associated with increased levels of lung cGMP relative to untreated controls at 3 and 7 days of hyperoxia, indicating a significant preservation of NO bioavailability in treated animals (3), which is of fundamental importance in RDS patients, in order to prevent further impairment of the ventilation/perfusion ratio. Interestingly, such effects were associated with reduced nuclear factor kappa B (NF-κB) concentration in treated animals, thus indicating a protective role of SOD3 in the downstream inflammatory pathways (3).
Persistent pulmonary hypertension of the newborn
The study of AOEs in the treatment of PPHN is based on the observation that elevated ROS and RNS burden in this condition, as a result of the administration of high concentrations of oxygen along with iNO, may be involved in the extensive lung damage observed in this population (130, 153). The assessment of genetic variants of AOEs and NOS could be a crucial point in properly directing antioxidant treatments in PPHN.
Based on the hypothesis that SOD could reduce the formation of peroxynitrite thanks to the scavenging of superoxide in newborns with PPHN, rhSOD treatment was preliminary assessed in newborn piglets receiving high FiO2 and iNO, and was demonstrated effective in reducing inflammatory cell count and neutrophil chemotactic activity in bronchoalveolar fluid, and malondialdehyde in lung tissue, both in case of endotracheal administration or nebulization (131). It was later demonstrated that endotracheal rhSOD administration not only mitigates oxidative injury, but also potentiates the beneficial effects of iNO on lung vascular tone, resulting in additive benefits on lung hemodynamic measures (162). The combination of iNO and endotracheal rhSOD induced a significantly greater decrease in pulmonary arterial pressure and pulmonary vascular resistance than iNO or rhSOD alone, and the ratio of pulmonary arterial pressure/aortic pressure remained significantly lower in the former group throughout the study period. The addictive effects of the treatments could be probably attributed both to the scavenging of superoxide with RNS reduction and to the resultant increased iNO bioavailability with consequent enhancement of iNO effects. This hypothesis is concordant with the observation that in vitro SOD enhances pulmonary arterial vessel relaxation induced by an NO-donor compound and that this effect is not abated by CAT (2, 16), excluding the fact that it is mediated by increased H2O2, which is known to induce vasorelaxation (22). Further animal experiments demonstrated that the combined treatment of iNO+rhSOD in a single dose produced a more rapid improvement in oxygenation than the treatment with rhSOD or iNO alone and was associated with a need for lower medium airway pressure (86). Such promising results were partly mitigated by the observation that the alveolar/arterial oxygen ratio did not significantly improve in the group treated with rhSOD+iNO in comparison with either intervention alone. However, endotracheal rhSOD abated lung levels of 3-nitrotyrosine, a marker for peroxynitrite formation, suggesting a key role in preventing iNO-associated lung injury, probably through a multiple doses strategy, in order to achieve a more stable enzymatic action. Endotracheal rhSOD administration was also studied in a lamb model of RDS of prematurity (79), with analogous results on pulmonary hemodynamics, as iNO+rhSOD treatment did not improve oxygenation with regard to iNO alone. However, early treatment with high-dose rhSOD was associated with improved oxygenation in comparison to the control group not receiving iNO; while no beneficial effects were observed on lung edema and compliance. Delayed rhSOD administration (2 h after birth) did not produce any beneficial effect in this study, but it was reported to be effective in another study (4 h after birth) performed with term animal models, suggesting a probable differential response depending on gestational age, with more premature lungs resulting in being more vulnerable to OS and ventilation-induced injury (86).
More recent insights into the activity pathways of SOD (Figs. 1 and 2) provided evidence for the possible mechanisms responsible for the observed beneficial effects of SOD on lung vasculature in PPHN (44, 45, 151). The activity of endothelial NOS (eNOS) in pulmonary vasculature increases after birth as a consequence of relative hyperoxia and mechanical forces on lung vasculature in both healthy and ventilated animals (16, 44, 45, 164), taking part in the feto-neonatal transition, but such an increase is suppressed in PPHN models (45). Endotracheal rhSOD or iNO, in addition to ventilation with FiO2 100%, induces a significant eNOS mRNA increase in PPHN models with regard to PPHN animals receiving only ventilation with FiO2 100%, but rhSOD was proved more effective in increasing protein expression, and either rhSOD or iNO reduced lung ROS accumulation in PPHN (45). As demonstrated by the contractile response to noradrenaline in the presence of an eNOS inhibitor, rhSOD, but not iNO, restored eNOS functionality, as rhSOD restored the contraction response to the inhibitor; while iNO did not influence the test. Interestingly, rhSOD, but not iNO, significantly increased lung levels of tetra-hydro-byopterin (BH4), which is an essential cofactor for eNOS activity, and also increased the rate-limiting enzyme for the synthesis of BH4 (45). These observations demonstrate that SOD and iNO may exert additive but also partially different effects on lung vasculature in PPHN, thus possibly serving as adjunctive or alternative treatments for such a condition. Furthermore, while guanilate cyclase (GC) is overexpressed after birth in healthy models and phosphodiesterase 5 (PDE5) is down-regulated, resulting in increased cGMP relative to the fetus and relaxation of pulmonary vasculature, in the PPHN animals both GC and PDE5 are overexpressed, with lung cGMP content comparable to fetal levels (43a). Either iNO or endotracheal rhSOD decreased PDE5 activity and restored normal postnatal levels of cGMP (44). These data are concordant with the observation that ROS may compromise GC activity (161) and activate PDE5 expression (45, 106), while rhSOD would prevent such detrimental effects and are also in agreement with clinical studies indicating the usefulness of PDE5-inhibitors, as Sildenafil, in the treatment of PPHN (105, 163). A recent study also reported that cyclic stretch is associated with increased OS in pulmonary artery smooth cells along with increased GC expression (151). In this setting, rhSOD administration abated OS markers, but preserved induced GC levels (151).
FIG. 1.
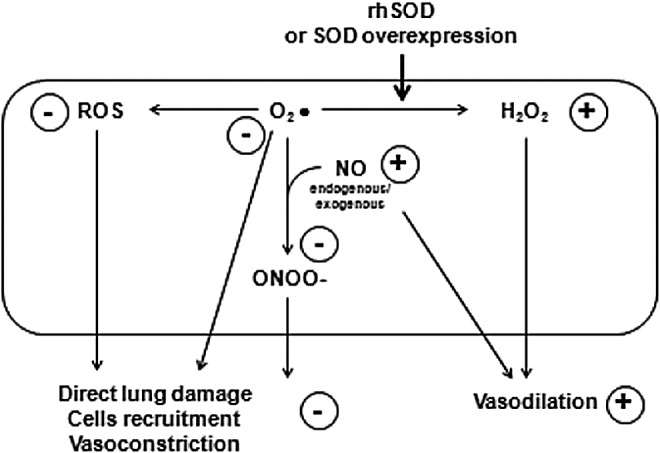
Effects of rhSOD or SOD overexpression on ROS, RNS, and NO availability in pulmonary arterial smooth muscle cells. Resultant effects are highlighted in circles. NO, nitric oxide; ROS, reactive oxygen species; RNS, reactive nitrogen species; rhSOD, recombinant human superoxide dismutase.
FIG. 2.
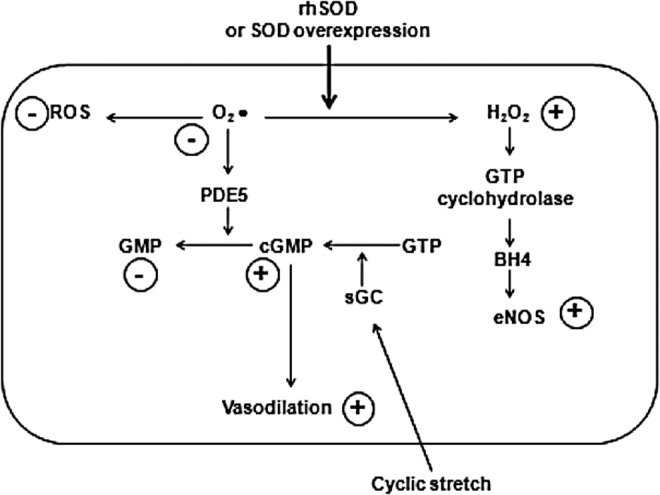
Effects of rhSOD on PDE5 and eNOS. Resultant effects are highlighted in circles (44, 45, 86, 151). eNOS, endothelial nitric oxide synthase; PDE5, phosphodiesterase 5.
Moreover, the development of antioxidant treatments that are capable of influencing SOD3 activity definitely deserves further investigation, as SOD3 appears abundantly expressed within lung tissue and smooth muscle (112), serving as a key regulator of vasodilation (71) and of antioxidant status in PPHN (187). In pulmonary arterial smooth muscle cells of PPHN lambs, SOD3 was suppressed relative to controls and hyperoxia or H2O2 addition similarly suppressed SOD3 in control cells. In agreement with these data, SOD3 activity was lower in PPHN animals than in controls. Interestingly, the addition of CAT to cultured control cells incubated with H2O2 restored proper SOD3 activity, while endotracheal administration of CAT to PPHN lambs increased SOD3 lung activity and oxygenation and reduced lung superoxide levels (187). Therefore, H2O2 formation during PPHN has been hypothesized to impair SOD3 activity, resulting in excessive superoxide load and vasoconstriction, which would be abated by specific treatments that are aimed at increasing SOD3 lung activity. A recent experiment of SOD3 transfection in mice pulmonary microvascular endothelial cells was recently associated with reduced intracellular ROS and xanthine oxidase accumulation after hypoxia exposure, and transgenic mice with lung targeted human SOD3 overexpression showed significantly lower signs of PPHN after prolonged hypoxia relative to PPHN wild-type animals, as demonstrated by lower right ventricle systolic pressure and mass and lower pulmonary vascular wall thickness, indicating less vascular remodeling (5). Transfection of PPHN wild-type animals with plasmid containing DNA encoding human SOD3 significantly reversed the indexes of PPHN, thus suggesting that SOD3 activity enhancement not only prevents PPHN development but could also be effective in treating already established cases, with possible crucial clinical implications (5).
Since reduced SOD2 expression in pulmonary arterial cells of PPHN lambs was proved to contribute to OS and endothelial dysfunction (2), it could be regarded as a further useful therapeutic target for antioxidant treatments. SOD2 activity was increased in PPHN cells relative to controls, while SOD2 tyrosine nitration was increased. SOD2 viral transduction in PPHN cells resulted in reduced superoxide mithocondrial content with increased H2O2, increased eNOS expression, and enhanced relaxation of pulmonary arteries, suggesting multiple roles of SOD2 in modulating vascular reactivity and OS in PPHN.
Finally, since NADPH oxidase was proved a major source of superoxide anions in pulmonary arteries in different models of PPHN (38, 60) and the treatment with NADPH oxidase inhibitor was proved effective in reducing ROS in pulmonary arteries of PPHN models (186), the associated therapy of rhSOD and NADPH oxidase may offer additive benefits and, therefore, deserves to be investigated for this purpose.
With regard to SOD2, the possibility to develop a recombinant protein may be likely challenged by the large size and high complexity of the enzyme (119). A possible tool to overcome this issue may be offered by SOD mimetics, which are low-molecular-mass compounds with specific catalytic activity developed to avoid the problems of dimensions, stability, immunogenicity, and delivery of natural enzymes (119, 141). SOD mimetics include Mn-based metalloporphyrins and Mn-salen complexes, which also possess minor CAT activity, and Mn-containing macroyclics, which selectively mimic SOD activity (141). SOD mimetics have been studied in animal cell models of different adult disease states that are characterized by OS and inflammation, such as neurological diseases, cancers, and ionizing radiation-induced injury (119, 141). These antioxidants have not yet been studied in models of neonatal lung diseases; however, they represent a possible future research topic, as they could also offer the theoretical advantage of enhanced penetration into the lung tissues in comparison with native or recombinant SOD.
Surfactant administration in RDS: potential antioxidant contribution
Endotracheal surfactant administration is one of the milestones of RDS treatment, as it was proved to reduce mortality, mechanical ventilation need, and air leaks, although it did not affect the occurrence of BPD (159). Besides the well-known beneficial effects on alveolar surface tension and mechanics (123, 124), the surfactant was also proved to possess anti-inflammatory and antioxidant properties (31, 96, 97), possibly contributing to the benefits of surfactant administration in RDS. The exogenous surfactant not only replaces the pre-existing surfactant partially inactivated by ROS (97), but also naturally contains specific proteins and minor lipids with antioxidant activities (Table 5). Both enzymatic and non-enzymatic antioxidants would contribute to reduce alveolar ROS accumulation, preventing local damage and ROS diffusion (97, 137). Indeed, lower levels of OS markers were found in tracheal aspirates of surfactant-treated preterm neonates relative to untreated preterm controls (101).
Table 5.
Antioxidant Compounds in Natural Surfactants
| Curosurf poractant | Survanta beractant | Alveofact bovactant | Infasurf calfactant | |
|---|---|---|---|---|
| Doses | ||||
| mg of PLs/kg | 200 | 100 | 100 | 100 |
| ml of surfactant/kg | 2.5 | 4 | 2.2 | 2.86 |
| SOD | ||||
| U/mg of PLs | 0.396a | 0.474 | 0.027b | 0.383c |
| U/ml of surfactant | 31.7 | 11.9 | 1.21 | 13.4 |
| U/dose per kg | 73.3 | 47.6 | 2.6 | 38.3 |
| CAT | ||||
| nmol/min/ml of PLs | 0.81d | 2.60 | 1.58 | 3.23 |
| nmol/min/ml of surfactant | 64.80 | 65.00 | 71.10 | 113.10 |
| U/dose per kg | 149.80 | 260.00 | 157.80 | 323.50 |
| Plasmalogens | ||||
| mol% of total PLs | 3.8±0.1 | 1.5±0.2 | 0.9±0.3 | n.a. |
| PUPLs | ||||
| mol% of total PLs | 26±1 | 6±1 | 11±1 | n.a. |
p=0.019 versus Survanta.
p<0.0001 versus Survanta.
p=0.003 versus Survanta.
p<0.0001 versus Infasurf.
PLs, phospholipids; PUPLs; polyunsaturated phospholipids; n.a., not available.
AOEs and other antioxidant molecules are commonly found in the epithelial lining fluid of the normal human lower airways (25, 26). Natural calf lung surfactant was proved to contain measurable amounts of SOD and CAT and to exert scavenging activity against H2O2 (97). Endotracheal administration of the surfactant also induced a significant increase of SOD content in alveolar type II cells, demonstrating the occurrence of enzyme uptake via liposome during the surfactant recycle process (97). On the contrary, a surfactant extract containing 1% proteins was shown to contain no significant amount of AOEs and did not exert any scavenging activity (97), strengthening the concept that protein compounds are of crucial importance to surfactant activities, which should be strongly considered in the setting of synthetic surfactant development. A recent study confirmed that four natural surfactants contain definite amounts of SOD and CAT, which have been detailed for Poractant, Beractant, Calfactant, and Bovactant (31). Poractant provided the highest content of SOD per ml, while Calfactant was proved to contain the highest amount of CAT. Moreover, Poractant provided the major amount of SOD per recommended dose for clinical use, while Calfactant was proved to contain the major amount of CAT per dose. Poractant also showed the highest scavenger activity when incubated with H2O2 (31). Despite relevant results obtained in vitro, the role of surfactant AOEs in vivo remains to be established. Moreover, the assessment of the role of surfactant AOEs in the prevention of ROS-mediated lung injury in the newborn would require specific measurements of alveolar lining fluid, AOE content, and antioxidant capacity after natural and synthetic surfactant administration in model systems.
Besides AOEs, natural surfactants also contain non-enzymatic antioxidants, such as plasmalogens and polyunsaturated phospholipids (PUPLs) (137). Plasmalogens were proved to work synergistically with cholesterol, SP-B, and SP-C in the formation of the surfactant reservoir and in the spreading of dipalmitoylphosphatidylcholine; in the experimental models, plasmalogens addiction to the surfactant achieved a further reduction of surface tension and viscosity in comparison to the surfactant alone (124, 137). Plasmalogens were also recently demonstrated to confer consistent antioxidant protection against ultraviolet light-induced lipid peroxidation within cell membranes (193) and also to exert antioxidant functions in low-density lipoproteins (72). The content of plasmalogens resulted in being consistently higher for Poractant than Beractant and Bovactant (124, 137). Poractant presents the highest concentration of PUPLs among natural surfactants, which are considered one of the main substrates for lipid peroxidation in the lung surfactant (137). Plasmalogens and polyunsatured fatty acids in tracheal aspirates at birth in preterm neonates resulted in being significantly lower in patients who developed BPD in comparison to non-BPD controls (138).
AOE addition to surfactants is based on the rationale to vehiculate them into the alveolar epithelial cells. The incubation of lung epithelial cells with an emulsion of Beractant plus SOD and CAT resulted in significantly higher SOD and CAT activity relative to cells incubated with enzymes or Beractant alone (109). These results were confirmed in lung homogenates obtained from animal models after endotracheal administration of Beractant plus SOD and CAT, suggesting that surfactant supplementation with AOEs is an efficacious way to increase antioxidant defenses in the alveolar environment (109). Liposome encapsulation of SOD and CAT was also studied to enhance intracellular delivery of the enzymes, and such a method was proved effective in increasing AOEs activity in the alveolar cells of preterm animal models of hyperoxia-induced lung injury (182). In vitro, the addition of SOD to natural surfactants resulted in increased scavenger activities for Poractant, Beractant, and Bovactant; while CAT addition resulted in increased scavenger activity for Poractant, Bovactant, and Calfactant (31). The addition of both SOD and CAT induced a further increase in scavenger activity in comparison with SOD or CAT addition alone, suggesting a possible synergic activity (31). Our recent data, which are still unpublished, demonstrate that SOD and CAT addition to natural surfactants reduces lung OS markers in preterm lambs with RDS.
In addition to direct antioxidant properties, the surfactant also exerts a complex web of anti-inflammatory and immune-modulating activities, which indirectly contribute to lower OS. Surfactant proteins A and D (SP-A and SP-D), which belong to the family of collectins, are involved at multiple levels in the immune response to several pathogens (61). SP-A and SP-D have the property to bind to alveolar pathogens, favoring their clearance (61, 98). SP-A was recently demonstrated to down-regulate inflammation in the presence of LPS, suppressing the pro-inflammatory pathways mediated by NF-κB (190), and both SP-A and SP-D down-regulate specific immune responses through the inhibition of pro-inflammatory cytokine secretion and the modulation of lymphocyte proliferation (61). The role SP-D may be of particular importance, as lower levels of SP-D were detected in tracheal aspirates of preterm newborns who subsequently developed BPD relative to age-matched non-BPD controls (82). Furthermore, SP-D modified by RNS was proved to lose aggregating activity (98), and a genetic variant of SP-D was recently found to be over-represented in RDS patients, relative to non-RDS controls (140).
Melatonin and other antioxidants
Several antioxidant therapies different from direct AOEs replacement were studied in preterm newborns for the prevention of respiratory morbidities. Vitamins A, E and N-acetyl-cysteine failed to reduce the occurrence of BPD in preterm newborns (175, 185), and intravenous GSH did not improve acute respiratory outcomes in preterm baboons (160). In contrast to previous observations (6), a recent study in the animal model of hyperoxic resuscitation after perinatal asphyxia suggested a potential role for N-acetylcysteine in lung protection, as it was proved to reduce lung and systemic levels of OS and inflammatory markers (111). Moreover, pentoxifillyne, a phosphodiesterase inhibitor with immunomodulatory and antifibrotic features, has been investigated in the animal model of hyperoxia, and it was proved to reduce lung edema and neutrophils influx, while it increased lung activity of SOD, CAT, and GPx (9), in agreement with previous reports of reduced inflammatory cytokines in tracheal aspirates (173) and lung protection against mechanical ventilation-induced injury (81, 158). Besides antioxidant activity, pentoxyfilline also induces VEGF expression and enhances pulmonary vasculature development (9), which could be a major issue for the prevention of BPD-associated vascular abnormalities.
Melatonin (MT), which is secreted by the pineal gland as a circadian rhythm transducer and presents multiple antioxidant properties, was proved effective in the prevention of different OS-related processes in adults, such as aging and neurodegenerative diseases (8, 75, 127, 128, 134, 150, 181). In neonatal medicine, MT is at present one of the most promising treatments for hypoxic-ischemic encephalopathy in addition to hypothermia (133). With regard to respiratory outcomes, MT administration was studied in a small cohort of preterm newborns with RDS, and it was shown to reduce the nitrite/nitrate ratio and the circulating levels of IL-6, IL-8, and TNF-α, which are typically over-represented in the lungs of BPD patients (57). The same group of authors reported 110 preterm newborns ≤32 weeks of gestational age ventilated with different strategies, who were randomly assigned to receive an MT treatment course or placebo (56). The MT-treated group showed significantly lower ventilator parameters and lower circulating levels of IL-6, IL-8, and TNF-α (56). These data, which preliminarily assess the efficacy and safety of MT administration in preterm newborns, suggest a possible role for MT in the prevention of BPD, as one of the crucial points in the pathogenesis is the secretion of inflammatory cytokines and inflammatory cell recruitment in the lung (78). Unfortunately, data on long-term respiratory outcomes of the studied cohorts were not provided. These clinical observations are in agreement with the demonstration in the mouse model of BPD that MT treatment reduces lung myeloperoxidase and nitrite/nitrate ratio and increases GPx, SOD and CAT activity (114). MT-treated animals showed significantly higher number of alveoli and less fibrotic aspects, suggesting that antioxidant effects of MT may positively influence alveolization and lung development under stressful conditions after preterm birth. MT was also proved to reduce circulating markers of lipid peroxidation in a small cohort of septic newborns as soon as 1 h after the first dose, with regard to the untreated septic newborn (54). Cytokines levels were not assessed in this group; however, since infectious events in both prenatal and postnatal life are clearly related with the risk of BPD (78), MT appears as a possible prevention tool, as it lowers both OS markers and inflammatory cytokines.
The antioxidant action of MT influences multiple pathways (134). MT possesses direct antioxidant properties (117, 171), as it works as a powerful scavenger of ROS, particularly hydroxyl radical, with the formation of metabolites, which, in turn, shows scavenging activity (63, 94, 170, 172). Beyond this, MT also up-regulates AOEs, particularly GPx, GRx, SOD, and CAT (10, 83, 100, 129, 134). MT, after binding to specific receptors MT1 and MT2, would activate different signaling pathways starting off from G-proteins and involving protein kinase A, phospholipase C, extracellular regulated kinase, and Jun N-terminal kinase, which would then result in the modulation of gene transcription. Moreover, MT could directly interact with the calcium-calmodulin complex, further affecting transcription processes, and, because of ROS reduction, it would also affect the activity of NFκB and would prevent ROS-mediated enzyme inactivation (134). Putatively, MT could offer clinical benefits in newborns with SNPs related to the impaired activity of AOEs. The study of MT treatment may be of particular interest in those newborns who present SNPs related to the impaired activity of AOEs, as it could putatively compensate for the antioxidant deficit. We suggest that the beneficial effects of MT treatment in newborns may occur as a consequence of MT anti-inflammatory and scavenging activities, particularly in case of favorable effects on OS markers observed very early after MT administration (54). On the contrary, MT-induced AOE overexpression has never been measured in newborns; neither as enzymatic activity nor as mRNA production. However, we can speculate that this aspect could be of major importance in determining the long-term effects of MT administration, particularly in case of a prolonged treatment course. The reliable route of administration and the reassuring safety profile (56, 57) makes MT a valuable candidate for further studies on BPD prevention.
Conclusions
OS stress plays a pivotal role in the pathogenesis of BPD and other complications of prematurity, such as IVH, PVL, and ROP, because of reduced antioxidant capacity of preterm newborns and high oxidative burden. OS also significantly occurs in PPHN, as a result hyperoxic ventilation and iNO administration. Genetic predisposition to induce excessive levels of inflammatory and oxidative mediators may be associated with increased risk of OS-related complications. In preterm newborns, specific SNPs of AOEs and inflammatory mediators were found to affect the risk of respiratory morbidities, as RDS requiring mechanical ventilation and BPD.
Since the treatment options for lung diseases of the newborn are far from optimal, new preventive and treatment strategies should be urgently assessed. Recent evidence suggests potential benefits of AOE supplementation or hyperexpression in the prevention and treatment of BPD, RDS, and PPHN. Moreover, surfactant replacement for RDS may exert antioxidant effects, thanks to AOEs and non-enzymatic antioxidants that are naturally present in animal surfactants, and such antioxidant potentials could be usefully enhanced by the addition of AOE to surfactants. Since the very main part of available data regarding antioxidant options comes from animal models and in vitro experiments, clinical trials are mandatory in order to assess the therapeutic potentials of these strategies. Among non-enzymatic antioxidants, MT was investigated for the prevention of adverse respiratory outcomes in the preterm newborns, with preliminary positive results, which should be further addressed.
A more extensive assessment of SNPs involved in the pathogenesis of newborn lung diseases may be particularly helpful to identify specific populations with a particularly high risk of adverse outcome, who may maximally benefit from antioxidant therapies, and the clarification of the effects of SNPs on proteomics may pose the basis for an individually targeted AOE supplementation.
Finally, antioxidant supplementation or induction during pregnancy should be studied as a potential strategy to reduce fetal and neonatal adverse outcomes in models of OS-related complications of pregnancies, such as pre-eclampsia.
Abbreviations Used
- AER
aerosolized
- AOE
antioxidant enzyme
- BPD
bronchopulmonary dysplasia
- CAT
catalase
- eNOS
endothelial nitric oxide synthase
- ET
endotracheal
- FiO2
fractional inspired oxygen
- GC
guanilate cyclase
- GPx
glutathione peroxidase
- GRd
glutathione reductase
- GSH
glutathione
- GST
glutathione-S-transferase
- IFN-γ
interferon-gamma
- IL
interleukin
- iNO
inhaled nitric oxide
- IP
intraperitoneal
- IV
intravenous
- IVH
intraventricular hemorrhage
- MMP
matrix metalloproteinase
- MT
melatonin
- n.a.
not available
- NF-κB
nuclear factor kappa B
- NO
nitric oxide
- NOS
nitric oxide synthase
- OS
oxidative stress
- PA
pulmonary artery
- PDE5
phosphodiesterase 5
- PLs
phospholipids
- PPA
pulmonary arterial pressure
- PPHN
persistent pulmonary hypertension of the newborn
- PUPLs
polyunsaturated phospholipids
- PVR
pulmonary vasculature resistance
- RDS
respiratory distress syndrome
- rhSOD
recombinant human SOD
- ROP
retinopathy of prematurity
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- RV
right ventricle
- SNP
single nucleotide polymorphism
- SOD
superoxide dismutase
- TA
tracheal aspirate
- TNF-α
tumor necrosis factor-alpha
- TLR
toll-like receptor
- UTR
untranslated region
Acknowledgments
The authors are most grateful to Rosanna Abbate, Betti Giusti, and the whole staff at Atherothrombotic Diseases Laboratory of Careggi University Hospital, Florence, Italy, for encouraging their interest in the field of genetic polymorphisms in the preterm newborns and for supporting their research with incomparable technical expertise.
Author Disclosure Statement
Carlo Dani declares that he does not have any conflict of interest to disclose. Chiara Poggi declares that she does not have any conflict of interest to disclose.
References
- 1.Adcock K, Hedberg C, Loggins J, Kruger TE, and Baier RJ. The TNF-alpha-308, MCP-1-2518 and TGF-beta1þ915 polymorphisms are not associated with the development of chronic lung disease in very low birth weight infants. Genes Immun 4: 420–426, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Afolayan AJ, Eis A, Teng RJ, Bakhutashvili I, Kaul S, Davis JM, and Konduri GG. Decreases in manganese superoxide dismutase expression and activity contribute to oxidative stress in persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 303: L870–L879, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed MN, Codipilly C, Hogg N, and Auten RL. The protective effect of overexpression of extracellular superoxide dismutase on nitric oxide bioavailability in the lung after exposure to hyperoxia stress. Exp Lung Res 37: 10–17, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Ahmed MN, Suliman HB, Folz RJ, Nozik-Grayck E, Golson ML, Mason SN, and Auten RL. Extracellular superoxide dismutase protects lung development in hyperoxia-exposed newborn mice. Am J Respir Crit Care Med 167: 400–405, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Ahmed MN, Zhang Y, Codipilly C, Zaghloul N, Patel D, Wolin M, and Miller EJ. Extracellular superoxide dismutase overexpression can reverse the course of hypoxia-induced pulmonary hypertension. Mol Med 18: 38–46, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahola T, Lapatto R, Raivio KO, Selander B, Stigson L, Jonsson B, Jonsbo F, Esberg G, Stovring S, Kjartansson S, Stiris T, Lossius K, Virkola K, and Fellman V. N-Acetylcysteine (NAC) does not prevent bronchopulmonary dysplasia in immature infants: a randomized controlled trial. J Pediatr 143: 713–719, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Aidoo M, McElroy PD, Kolczak MS, McElroy PD, Kolczak MS, Terlouw DJ, ter Kuile FO, Nahlen B, Lal AA, and Udhayakumar V. Tumor necrosis factor-alpha promoter variant 2 (TNF2) is associated with pre-term delivery, infant mortality, and malaria morbidity in western Kenya: Asembo Bay Cohort Project IX. Genet Epidemiol 21: 201–211, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Allegra M, Reiter RJ, Tan D-X.Reiter RJ, Tan DX, Gentile C, Tesoriere L, and Livrea MA. The chemistry of melatonin's interaction with reactive species. J Pineal Res 34: 1–10, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Almario B, Wu S, Peng J, Alapati D, Chen S, and Sosenko IR. Pentoxifylline and prevention of hyperoxia-induced lung-injury in neonatal rats. Pediatr Res 71: 583–589, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Antolin I, Rodriguez C, Sainz RM, Mayo JC, Uría H, Kotler ML, Rodríguez-Colunga MJ, Tolivia D, and Menéndez-Peláez A. Neurohormone melatonin prevents cell damage: effect on gene expression for antioxidant enzymes. FASEB J 10: 882–890, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Asikainen T, Heikkilä P, Kaarteenaho-Wiik R, Kinnula VL, and Raivio KO. Cell-specific expression of manganese superoxide dismutase protein in the lungs of patients with respiratory distress syndrome, chronic lung disease or persistent pulmonary hypertension. Pediatr Pulmonol 32: 193–200, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Asikainen TM, Raivio KO, Saksela M, and Kinnula VL. Expression and developmental profile of antioxidant enzymes in human lung and liver. Am J Respir Cell Mol Biol 19: 942–949, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Asikainen TM. and White CW. Pulmonary antioxidant defenses in the preterm newborn with respiratory distress and bronchopulmonary dysplasia in evolution: implications for antioxidant therapy. Antioxid Redox Signal 6: 155–167, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Auten RL, O'Reilly MA, Oury TD, Nozik-Grayck E, and Whorton MH. Transgenic extracellular superoxide dismutase protects postnatal alveolar epithelial proliferation and development during hyperoxia. Am J Physiol Lung Cell Mol Physiol 290: L32–L40, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnard ML, Baker RR, and Matalon S. Mitigation of oxidant injury to lung microvasculature by intratracheal instillation of antioxidant enzymes. Am J Physiol 265: L340–L345, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Black SM, Johengen MJ, Ma ZD, Bristow J, and Soifer SJ. Ventilation and oxygenation induce endothelial nitric oxide synthase gene expression in the lungs of fetal lambs. J Clin Invest 100: 1448–1458, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bokodi G, Derzbach L, Banyasz I, Tulassay T, and Vasarhelyi B. The association of interferon-gamma Tþ874A and interleukin-12 p40 promoter CTCTAA/GC polymorphism with the need for respiratory support and perinatal complications in low birth weight neonates. Arch Dis Child Fetal Neonatal Ed 92: F25–F29, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bokodi G, Treszl A, Derzbach L, Balogh A, and Vasarhelyi B. The association of the carrier state of the tumor necrosis factor-alpha (TNFalpha)-308A allele with the duration of oxygen supplementation in preterm neonates. Eur Cytokine Netw 16: 78–80, 2005 [PubMed] [Google Scholar]
- 19.Brooks SE, Gu X, Samuel S, Marcus DM, Bartoli M, Huang PL, and Caldwell RB. Reduced severity of oxygen-induced retinopathy in eNOS-deficient mice. Invest Ophthalmol Vis Sci 42: 222–228, 2001 [PubMed] [Google Scholar]
- 20.Buhimschi IA, Buhimschi CS, Pupkin M, and Weiner CP. Beneficial impact of term labor: nonenzymatic antioxidant reserve in the human fetus. Am J Obstet Gynecol 189: 181–188, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Buonocore G, Perrone S, and Tataranno ML. Oxygen toxicity: chemistry and biology of reactive oxygen species. Semin Fetal Neonatal Med 15: 186–190, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Burke TM. and Wolin MS. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol 252: H721–H732, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Campo S, Sardo AM, and Campo GM. Extracellular superoxide dismutase (EC-SOD) gene mutations screening in a sample of Mediterranean population. Mutat Res 578: 143–148, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Campochiaro PA. Retinal and choroidal neovascularization. J Cell Physiol 184: 301–310, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Cantin AM, Fells GA, Hubbard RC, and Crystal RG. Antioxidant macromolecules in the epithelial lining fluid of the normal humal lower respiratory tract. J Clin Invest 86: 962–971, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantin AM, Hubbard RC, and Crustal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Resp Dis 139: 370–372, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Carlsson LM, Jonsson J, Edlund T, and Marklund SL. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc Natl Acad Sci U S A 92: 6264–6268, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakraborty M, McGreal EP, and Kotecha S. Acute lung injury in preterm newborn infants: mechanisms and management. Paediatr Respir Rev 11: 162–170, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Coalson J. Pathology of new bronchopulmonary dysplasia. Semin Neonatol 8: 73–81, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Dahl M, Bowler RP, Juul K, Crapo JD, Levy S, and Nordestgaard BG. Superoxide dismutase 3 polymorphism associated with reduced lung function in two large populations. Am J Respir Crit Care Med 178: 906–912, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dani C, Buonocore G, Longini M, Felici C, Rodriguez A, Corsini I, and Rubaltelli FF. Superoxide dismutase and catalase activity in naturally derived commercial surfactants. Pediatr Pulmonol 44: 1125–1131, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Dani C, Cecchi A, and Bertini G. Role of oxidative stress as physiopathologic factor in the preterm infant. Minerva Pediatr 56: 381–394, 2004 [PubMed] [Google Scholar]
- 33.Davis JM. and Auten RL. Maturation of the antioxidant system and the effects on preterm birth. Semin Fetal Neonatal Med 15:191–195, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Davis JM, Parad RB, Michele T, Allred E, Price A, and Rosenfeld W. North American Recombinant Human CuZnSOD Study Group. Pulmonary outcome at 1 year corrected age in premature infants treated at birth with recombinant human CuZn superoxide dismutase. Pediatrics 111: 469–476, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Davis JM, Richter SE, Biswas S, Rosenfeld WN, Parton L, Gewolb IH, Parad R, Carlo W, Couser RJ, Baumgart S, Atluru V, Salerno L, and Kassem N. Long-term follow-up of premature infants treated with prophylactic, intratracheal recombinant human CuZn superoxide dismutase. J Perinatol 20: 213–216, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Davis JM, Rosenfeld WN, Richter SE, Parad MR, Gewolb IH, Spitzer AR, Carlo WA, Couser RJ, Price A, Flaster E, Kassem N, Edwards L, Tierney J, and Horowitz S. Safety and pharmacokinetics of multiple doses of recombinant human CuZn superoxide dismutase administered intratracheally to premature neonates with respiratory distress syndrome Pediatrics 100: 24–30, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Davis JM, Rosenfeld WN, Sanders RJ, and Gonenne A. Prophylactic effects of recombinant human superoxide dismutase in neonatal lung injury. J Appl Physiol 74: 2234–2241, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Dennis KE, Aschner JL, Milatovic D, Schmidt JW, Aschner M, Kaplowitz MR, Zhang Y, and Fike CD. NADPH oxidases and reactive oxygen species at different stages of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol 297: L596–L607, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derzbach L, Bokodi G, Treszl A, Vasarhelyi B, Nobilis A, and Rigo J, Jr. Selectin polymorphisms and perinatal morbidity in low birth weight infants. Acta Paediatr 95: 1213–1217, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Di X, Matsuzaki H, Webster TA, Hubbell E, Liu G, Dong S, Bartell D, Huang J, Chiles R, Yang G, Shen MM, Kulp D, Kennedy GC, Mei R, Jones KW, and Cawley S. Dynamic model based on algorithms for screening and genotyping more than 100 K SNPs on oligonucleotide microarrays. Bioinformatics 21: 1958–1963, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Dobashi K, Asayama K, Hayashibe H, Munim A, Kawaoi A, Morikawa M, and Nakazava S. Immunoistochemical study of copper-zinc and manganese superoxide dismutase in the lungs of human fetuses and newborn infants: developmental profile and alterations in hyaline membrane disease and bronchopulmonary dysplasia. Virchows Arch A Pathol Anat Histopathol 423: 177–184, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Esplin MS. Preterm birth: a review of genetic factors and future directions for genetic study. Obstet Gynecol Surv 61: 800–806, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Faraci FM. Protecting against vascular disease in brain. Am J Physiol Heart Circ Physiol 300: H1566–H1582, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43a.Farrow KN, Groh BS, Schumacker PT, Lakshminrushimha S, Czech L, Gugino SF, Russell JA, and Steinhorn RH. Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells. Circ Res 102: 226–233, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farrow KN, Lakshminrusimha S, Czech L, Gugino SF, Davis JM, Russell JA, and Steinhorn RH. SOD and inhaled nitric oxide normalize phosphodiesterase 5 expression and activity in neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 299: L109–L116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farrow KN, Lakshminrusimha S, Reda WJ, Wedgwood S, Czech L, Gugino SF, Davis JM, Russell JA, and Steinhorn RH. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L979–L987, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferrand PE, Parry S, Sammel M, Parry S, Sammel M, Macones GA, Kuivaniemi H, Romero R, and Strauss JF, 3rd. A polymorphism in the matrix metalloproteinase-9 promoter is associated with increased risk of preterm premature rupture of membranes in African Americans. Mol Hum Reprod 8: 494–501, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Folz RJ, Abushamaa AM, and Suliman HB. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J Clin Invest 103: 1055–1066, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frank L. and Sosenko IR. Failure of premature rabbits to increase antioxidant enzymes during hyperoxic exposure: increased susceptibility to pulmonary oxygen toxicity compared with term rabbits. Pediatr Res 29: 292–296, 1991 [DOI] [PubMed] [Google Scholar]
- 49.Frank L. and Groseclose EE. Preparation for birth into an O2-rich environment: the antioxidant enzymes in the developing rabbit lung. Pediatr Res 18: 240–244, 1984 [DOI] [PubMed] [Google Scholar]
- 50.Frank L. and Sosenko IR. Prenatal development of lung antioxidant enzymes in four species. J Pediatr 110: 106–110, 1987 [DOI] [PubMed] [Google Scholar]
- 51.Fryer AA, Bianco A, Hepple M, Jones PW, Strange RC, and Spiteri MA. Polymorphism at the glutathione S-transferase GSTP1 locus. A new marker for bronchial hyperresponsiveness and asthma. Am J Respir Crit Care Med 161: 1437–1442, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Fujimoto T, Parry S, Urbanek M, Parry S, Urbanek M, Sammel M, Macones G, Kuivaniemi H, Romero R, and Strauss JF, 3rd. A single nucleotide polymorphism in the matrix metalloproteinase-1 (MMP-1) promoter influences amnion cell MMP-1 expression and risk for preterm premature rupture of the fetal membranes. J Biol Chem 277: 6296–6302, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Genc MR, Gerber S, Nesin M, and Witkin SS. Polymorphism in the interleukin-1 gene complex and spontaneous preterm delivery. Am J Obstet Gynecol 187: 157–163, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Gitto E, Karbownik M, Reiter RJ, Tan DX, Cuzzocrea S, Chiurazzi P, Cordaro S, Corona G, Trimarchi G, and Barberi I. Effects of melatonin treatment in septic newborns. Pediatr Res 6: 756–760, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Gitto E, Pellegrino S, D'Arrigo S, Barberi I, and Reiter RJ. Oxidative stress in resuscitation and ventilation of newborns. Eur Resp J 34:1461–1469, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Gitto E, Reiter RJ, Amodio A, Romeo C, Cuzzocrea E, Sabatino G, Buonocore G, Cordaro V, Trimarchi G, and Barberi I. Early indicators of chronic lung disease in preterm infants with respiratory distress syndrome and their inhibition by melatonin. J Pineal Res 36: 250–255, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Gitto E, Reiter RJ, Cordaro SP, Reiter RJ, Cordaro SP, La Rosa M, Chiurazzi P, Trimarchi G, Gitto P, Calabrò MP, and Barberi I. Oxidative and inflammatory parameters in respiratory distress syndrome of preterm newborns: beneficial effects of melatonin. Am J Perinatol 21: 209–216, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Gitto E, Reiter RJ, Karbownik M, Tan DX, Gitto P, Barberi S, and Barberi I. Causes of oxidative stress in the pre- and perinatal period. Biol Neonate 81: 146–157, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Giusti B, Vestrini A, Poggi C, Magi A, Pasquini E, Abbate R, and Dani C. Genetic polymorphisms of antioxidant enzymes as risk factors for oxidative stress-associated complications in preterm infants. Free Radic Res 46: 1130–1139, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, and Black SM. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol 290: L1069–L1077, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Haagsman HP, Hogenkamp A, van Eijk M, and Veldhuizen EJ. Surfactant collectins and innate immunity. Neonatology 93: 288–294, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Hadchouel A, Decobert F, Franco-Montoya ML, Halphen I, Jarreau PH, Boucherat O, Martin E, Benachi A, Amselem S, Bourbon J, Danan C, and Delacourt C. Matrix metalloproteinase gene polymorphisms and bronchopulmonary dysplasia: identification of MMP16 as a new player in lung development. PLoS One 3: e3188, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hardeland R, Backhaus C, and Fadavi A. Reactions of the NO redox forms NO+, *NO and HNO (protonated NO-) with the melatonin metabolite N1-acetyl-5-methoxykynuramine. J Pineal Res 43: 382–388, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Hirayama S, Shiraishi T, Shirakusa T, Higuchi T, and Miller EJ. Prevention of neutrophil migration ameliorates rat lung allograft rejection. Mol Med 12: 208–213, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoang VM, Foulk R, Clauser K, Burlingame A, Gibson BW, and Fisher SJ. Functional proteomics: examining the effects of hypoxia on cytotrophoblast protein repertoire. Biochemistry 40:4077–4086, 2001 [DOI] [PubMed] [Google Scholar]
- 66.Holla LI, Jurajda M, Pohunek P, and Znojil V. Haplotype analysis of the endothelial nitric oxide synthase gene in asthma. Hum Immunol 69: 306–313, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Holla LI, Stejskalova A, Znojil V, and Vasku A. Analysis of the inducible nitric oxide synthase gene polymorphisms in Czech patients with atopic diseases. Clin Exp Allergy 36: 1592–1601, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Holst D. and Garnier Y. Preterm birth and inflammation-The role of genetic polymorphisms. Eur J Obstet Gynecol Reprod Bio 141: 3–9, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Islam T, McConnell R, Gauderman WJ, Avol E, Peters JM, and Gilliland FD. Ozone, oxidant defense genes, and risk of asthma during adolescence. Am J Respir Crit Care Med 177: 388–395, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Janssen YM, Van Houten B, Borm PJ, and Mossman BT. Cell and tissue responses to oxidative damage. Lab Invest 69: 261–274, 1993 [PubMed] [Google Scholar]
- 71.Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, and Brandes RP. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circ Res 93: 622–629, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Jurgens G, Fell A, Ledinski G, Chen Q, and Paltauf F. Delay of copper-catalyzed oxidation of low density lipoprotein by in vitro enrichment with choline or ethanolamine plasmalogens. Chem Phys Lipids 77: 25–31, 1995 [DOI] [PubMed] [Google Scholar]
- 73.Kalish RB, Nguyen DP, Vardhana S, Gupta M, Perni SC, and Witkin SS. A single nucleotide A>G polymorphism at position −670 in the Fas gene promoter: relationship to preterm premature rupture of fetal membranes in multifetal pregnancies. Am J Obstet Gynecol 192: 208–212, 2005 [DOI] [PubMed] [Google Scholar]
- 74.Kalish RB, Vardhana S, Gupta M, Perni SC, and Witkin SS. Interleukin 4 and 10 gene polymorphisms and preterm birth in multifetal gestations. Am J Obstet Gynecol 190:702–706, 2004 [DOI] [PubMed] [Google Scholar]
- 75.Karbownik M. and Reiter RJ. Antioxidative effects of melatonin in protection against cellular damage caused by ionizing radiation. Proc Soc Exp Biol Med 225: 9–22, 2000 [DOI] [PubMed] [Google Scholar]
- 76.Kazzi SN, Kim UO, Quasney MW, and Buhimschi I. Polymorphism of tumor necrosis factor-alpha and risk and severity of bronchopulmonary dysplasia among very low birth weight infants. Pediatrics 114: e243–e248, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Kinnula VL, Lehtonen S, Koistinen P, Kakko S, Savolainen M, Kere J, Ollikainen V, and Laitinen T. Two functional variants of the superoxide dismutase genes in Finnish families with asthma. Thorax 59:116–119, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kinsella JP, Greenough A, and Abman SH. Bronchopulmonary dysplasia. Lancet 367: 1421–1431, 2006 [DOI] [PubMed] [Google Scholar]
- 79.Kinsella JP, Parker TA, Davis JM, and Abman SH. Superoxide dismutase improves gas exchange and pulmonary hemodynamics in premature lambs. Am J Respir Crit Care Med 172: 745–758, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Konno S, Hizawa N, Yamaguchi E, Jinushi E, and Nishimura M. (CCTTT)n repeat polymorphism in the NOS2 gene promoter is associated with atopy. J Allergy Clin Immunol 108: 810–814, 2001 [DOI] [PubMed] [Google Scholar]
- 81.Korhonen K, Kiuru A, Svedstrom E, and Kaapa P. Pentoxifylline reduces regional inflammatory and ventilator disturbances in meconium-exposed piglets lungs. Pediatr Res 56: 901–906, 2004 [DOI] [PubMed] [Google Scholar]
- 82.Kotecha S, Davies PL, Clark HW, and McGreal EP. Increased prevalence of low oligomeric state surfactant protein D with restricted lectin activity in bronchoalveolar lavage fluid from preterm infants. Thorax 68: 460–467, 2013 [DOI] [PubMed] [Google Scholar]
- 83.Kotler ML, Rodriguez C, Sainz RM, Antolín I, and Menéndez-Peláez A. Melatonin increases gene expression for antioxidant enzymes in rat brain cortex. J Pineal Res 24: 83–89, 1998 [DOI] [PubMed] [Google Scholar]
- 84.Lakshminrusimha S, Russell JA, Steinhorn RH, Ryan RM, Gugino SF, Morin FC, 3rd, Swartz DD, and Kumar VH. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr Res 59: 137–141, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lakshminrusimha S, Russell JA, Steinhorn RH, Swartz DD, Ryan RM, Gugino SF, Wynn KA, Kumar VH, Mathew B, Kirmani K, and Morin FC, 3rd. Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatr Res 62: 313–318, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lakshminrusimha S, Russell JA, Wedgwood S, Gugino SF, Kazzaz AJ, Davis JM, and Steinhorn RH. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med 174: 1370–1377, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Laurie S, Matas Z, Boaz M, Fux A, Golan A, and Sadan O. Different degrees of fetal oxidative stress in elective and emergent caesarean section. Neonatology 92: 111–115, 2007 [DOI] [PubMed] [Google Scholar]
- 88.Lin X, Yang H, Sakuragi T, Hu M, Mantell LL, Hayashi S, Al-Abed Y, Tracey KJ, Ulloa L, and Miller EJ. A-chemokine receptor blockade reduces high mobility group box 1 (HMGB1) protein induced lung inflammation and injury and improves survival in sepsis. Am J Physiol Lung Cell Mol Physiol 289: L583–L590, 2005 [DOI] [PubMed] [Google Scholar]
- 89.Lindau-Shepard B, Shaffer JB, and Del Vecchio PJ. Overexpression of manganous superoxide dismutase (MnSOD) in pulmonary endothelial cells confers resistance to hyperoxia. J Cell Physiol 161: 237–242, 1994 [DOI] [PubMed] [Google Scholar]
- 90.Lukacs NW. and Ward PA. Inflammatory mediators, cytokines, and adhesion molecules in pulmonary inflammation and injury. Adv Immunol 62: 257–304, 1996 [DOI] [PubMed] [Google Scholar]
- 91.Mak JC, Leung HC, Ho SP, Ko FW, Cheung AH, Ip MS, and Chan-Yeung MM. Polymorphisms in manganese superoxide dismutase and catalase genes: functional study in Hong Kong Chinese asthma patients. Clin Exp Allergy 36: 440–447, 2006 [DOI] [PubMed] [Google Scholar]
- 92.Maltepe E. and Saugstad OD. Oxygen in health and disease: regulation of oxygen homeostasis-clinical implications. Pediatr Res 65: 261–268, 2009 [DOI] [PubMed] [Google Scholar]
- 93.Manar MH, Brown MR, Gauthier TW, and Brown LAS. Association of glutathione-S-transferase-P1 (GST-P1) polymorphisms with bronchopulmonary dysplasia. J Perinatol 24: 30–35, 2004 [DOI] [PubMed] [Google Scholar]
- 94.Manda K, Ueno M, and Anzai K. AFMK, a melatonin metabolite, attenuates X-ray-induced oxidative damage to DNA, proteins and lipids in mice. J Pineal Res 424: 386–393, 2007 [DOI] [PubMed] [Google Scholar]
- 95.Marklund SL, Nilsson P, Israelsson K, Schampi I, Peltonen M, and Asplund K. Two variants of extracellular superoxide dismutase: relationship to cardiovascular risk factors in an unselected middle-aged population. J Intern Med 242: 5–14, 1997 [DOI] [PubMed] [Google Scholar]
- 96.Matalon S. and Wright JR. Surfactant proteins and inflammation: the Yin and the Yang. Am J Respir Cell Mol Biol 31: 585–586, 2004 [DOI] [PubMed] [Google Scholar]
- 97.Matalon S, Holm BA, Baker RR, Whitfield MK, and Freeman BA. Characterization of antioxidant activities of pulmonary surfactant mixtures. Biochim Biophys Acta 1035: 121–127, 1990 [DOI] [PubMed] [Google Scholar]
- 98.Matalon S, Shrestha K, Kirk M, Waldheuser S, McDonald B, Smith K, Gao Z, Belaaouaj A, and Crouch EC. Modification of surfactant protein D by reactive oxygen-nitrogen intermediates is accompanied by loss of aggregating activity, in vitro and in vivo. FASEB J 23: 1415–1430, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maus U, von Grote K, Kuziel WA, Mack M, Miller EJ, Cihak J, Stangassinger M, Maus R, Schlondorff D, and Seeger W. The role of CC chemokine receptor 2 in alveolar monocyte and neutrophil immigration in intact mice. Am J Respir Crit Care Med 166: 268–273, 2002 [DOI] [PubMed] [Google Scholar]
- 100.Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, and Rodriguez C. Melatonin regulation of antioxidant enzyme gene expression. Cell Mol Life Sci 59: 1706–1713, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Merritt TA, Hallman M, Holcomb K, Strayer D, Bloom B, Revak S, and Cochrane CG. Human surfactant treatment of severe respiratory distress syndrome: pulmonary effluent indicators of lung inflammation. J Pediatr 108: 741–748, 1986 [DOI] [PubMed] [Google Scholar]
- 102.Min JH, Codipilly CN, Nasim S, Miller EJ, and Ahmed MN. Synergistic protection against hyperoxia-induced lung injury by neutrophils blockade and EC-SOD overexpression. Resp Res 13: 58, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moore S, Ide M, Randhawa M, Walker JJ, Reid JG, and Simpson NA. An investigation into the association among preterm birth, cytokine gene polymorphisms and periodontal disease. BJOG 111: 125–132, 2004 [DOI] [PubMed] [Google Scholar]
- 104.Morton RL, Das KC, Guo XL, Ikle DN, and White CW. Effect of oxygen on lung superoxide dismutase activities in premature baboons with bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 276: L64–L74, 1999 [DOI] [PubMed] [Google Scholar]
- 105.Mukherjee A, Dombi T, Wittke B, and Lalonde R. Population pharmacokinetics of sildenafil in term neonates: evidence of rapid maturation of metabolic clearance in the early postnatal period. Clin Pharmacol Ther 85: 56–63, 2009 [DOI] [PubMed] [Google Scholar]
- 106.Muzaffar S, Shukla N, Bond M, Sala-Newby GB, Newby AC, Angelini GD, and Jeremy JY. Superoxide from NADPH oxidase upregulates type 5 phosphodiesterase in human vascular smooth muscle cells: inhibition with iloprost and NONOate. Br J Pharmacol 155: 847–856, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nassi N, Ponziani V, Becatti M, Galvan P, and Donzelli G. Anti-oxidant enzymes and related elements in term and preterm newborns. Pediatr Int 51: 183–187, 2009 [DOI] [PubMed] [Google Scholar]
- 108.Nelson KB, Dambrosia JM, Iovannisci DM, Cheng S, Grether JK, and Lammer E. Genetic polymorphisms and cerebral palsy in very preterm infants. Pediatr Res 57: 494–499, 2005 [DOI] [PubMed] [Google Scholar]
- 109.Nieves-Cruz B.Rivera A, Cifuentes J, Pataki G, Matalon S, Carlo WA, Tanswell AK, and Freeman B. Clinical surfactant preparations mediate SOD and catalase uptake by type II cells and lung tissue. Am J Physiol 270: L659–L667, 1996 [DOI] [PubMed] [Google Scholar]
- 110.Nozik-Grayck E, Dieterle CS, Piantadosi CA, Enghild JJ, and Oury TD. Secretion of extracellular superoxide dismutase in neonatal lungs. Am J Physiol Lung Cell Mol Physiol 279: L977–L984, 2000 [DOI] [PubMed] [Google Scholar]
- 111.Østerholt HC, Dannevig I, Wyckoff MH, Liao J, Akgul Y, Ramgopal M, Mija DS, Cheong N, Longoria C, Mahendroo M, Nakstad B, Saugstad OD, and Savani RC. Antioxidant protects against increases in low molecular weight hyaluronan and inflammation in asphyxiated newborn pigs resuscitated with 100% oxygen. PLoS One 7: e38839, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Oury TD, Day BJ, and Crapo JD. Extracellular superoxide dismutase in vessels and airways of humans and baboons. Free Radic Biol Med 20: 957–965, 1996 [DOI] [PubMed] [Google Scholar]
- 113.Padmanabhan RV, Gudapaty R, Liener IE, Schwartz BA, and Hoidal JR. Protection against pulmonary oxygen toxicity in rats by the intratracheal administration of liposome-encapsulated superoxide dismutase or catalase. Am Rev Respir Dis 132: 164–167, 1985 [DOI] [PubMed] [Google Scholar]
- 114.Pan L, Fu JH, Xue XD, Xu W, Zhou P, and Wei B. Melatonin protects against oxidative damage in a neonatal rat model of bronchopulmonary dysplasia. World J Pediatr 5: 216–221, 2009 [DOI] [PubMed] [Google Scholar]
- 115.Perrone S, Negro S, Tataranno ML, and Buonocore G. Oxidative stress and antioxidant strategies in newborns. J Matern Fetal Neonatal Med 23: 63–65, 2010 [DOI] [PubMed] [Google Scholar]
- 116.Perrone S, Tataranno ML, Negro S, Longini M, Marzocchi B, Proietti F, Iacoponi F, Capitani S, and Buonocore G. Early identification of the risk for free radical-related diseases in preterm newborns. Early Hum Dev 86: 241–244, 2010 [DOI] [PubMed] [Google Scholar]
- 117.Poeggeler B, Reiter RJ, Tan DX, Chen LD, and Manchester LC. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: a hypothesis. J Pineal Res 14: 151–168, 1993 [DOI] [PubMed] [Google Scholar]
- 118.Poggi C, Giusti B, Vestri A, Pasquini E, Abbate R, and Dani C. Genetic polymorphisms of antioxidant enzymes in preterm infants. J Matern Fetal Neonatal Med 25 (Suppl 4): 131–134, 2012 [DOI] [PubMed] [Google Scholar]
- 119.Pong K. Oxidative stress in neurodegenerative diseases: therapeutic implications for superoxide dismutase mimetics. Expert Opin Biol Ther 3: 127–139, 2003 [DOI] [PubMed] [Google Scholar]
- 120.Prencipe G, Auriti C, Inglese R, Devito R, Ronchetti MP, Seganti G, Ravà L, Orzalesi M, and De Benedetti F. A polymorphism in the macrophage migration inhibitory factor promoter is associated with bronchopulmonary dysplasia. Pediatr Res 69: 142–147, 2011 [DOI] [PubMed] [Google Scholar]
- 121.Qanungo S. and Mukherjea M. Ontogenic profile of some antioxidants and lipid peroxidation in human placental and fetal tissues. Mol Cell Biochem 215: 11–19, 2000 [DOI] [PubMed] [Google Scholar]
- 122.Raby Y, Rabi D, and Yee W. Room air resuscitation of the depressed newborn: a systematic review and meta-analysis. Resuscitation 72: 353–363, 2007 [DOI] [PubMed] [Google Scholar]
- 123.Ramathan. Animal-derived surfactants: where are we? The evidence from randomized, controlled clinical trials. J Perinatol 29: S38–S43, 2009 [DOI] [PubMed] [Google Scholar]
- 124.Ramathan. Choosing a right surfactant for respiratory distress syndrome treatment. Neonatology 95: 1–5, 2009 [DOI] [PubMed] [Google Scholar]
- 125.Ramji A, Ahuja S, Thirupuram S, Rootwelt T, Rooth G, and Saugstad OD. Resuscitation of asphyxic newborn infants with room air or 100% oxygen. Pediatr Res 34: 809–812, 1993 [DOI] [PubMed] [Google Scholar]
- 126.Ramji S, Rasaily R, Mishra PK, Narang A, Jayam S, Kapoor AN, Kambo I, Mathur A, and Saxena BN. Resuscitation of asphyxiated newborns with room air or 100% oxygen. Ind Pediatr 40: 510–507, 2003 [PubMed] [Google Scholar]
- 127.Reiter RJ. and Maestroni G. Melatonin in relation to the antioxidative defense and immune systems: possible implications for cell and organ transplantation. J Mol Med 77: 36–39, 1999 [DOI] [PubMed] [Google Scholar]
- 128.Reiter RJ. and Tan DX. Melatonin: a novel protective agent against oxidative injury of the ischemic/reperfused heart. Cardiovasc Res 58:10–19, 2003 [DOI] [PubMed] [Google Scholar]
- 129.Reiter RJ, Tan DX, Osuna C, and Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci 7: 444–458, 2000 [DOI] [PubMed] [Google Scholar]
- 130.Robbins CG, Davis JM, Merritt TA, Amirkhanian J, Sahgal N, Morin FC III, and Horowitz S. Combined effects of nitric oxide and hyperoxia on surfactant function and pulmonary inflammation. Am J Physiol 269: L545–L550, 1995 [DOI] [PubMed] [Google Scholar]
- 131.Robbins CG, Horowitz S, Merritt TA, Kheiter A, Tierney J, Narula P, and Davis JM. Recombinant human superoxide dismutase reduces lung injury caused by inhaled nitric oxide and hyperoxia. Am J Physiol 272: L903–L907, 1997 [DOI] [PubMed] [Google Scholar]
- 132.Roberts AK, Monzon-Bordonaba F, Van Deerlin PG, Holder J, Macones GA, Morgan MA, Strauss JF, 3rd, and Parry S. Association of polymorphism within the promoter of the tumor necrosis factor alpha gene with increased risk of preterm premature rupture of the fetal membranes. Am J Obstet Gynecol 180: 1297–1302, 1999 [DOI] [PubMed] [Google Scholar]
- 133.Robertson NJ, Faulkner S, Fleiss B, Bainbridge A, Andorka C, Price D, Powell E, Lecky-Thompson L, Thei L, Chandrasekaran M, Hristova M, Cady EB, Gressens P, Golay X, and Raivich G. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain 136: 90–105, 2013 [DOI] [PubMed] [Google Scholar]
- 134.Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, and Reiter RJ. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 36: 1–9, 2004 [DOI] [PubMed] [Google Scholar]
- 135.Rosenfeld WN, Davis JM, Parton L, Richter SE, Price A, Flaster E, and Kassem N. Safety and pharmacokinetics of recombinant human superoxide dismutase administered intratracheally to premature neonates with respiratory distress syndrome. Pediatrics 97: 811–817, 1996 [PubMed] [Google Scholar]
- 136.Rosta K, Molvarec A, Enzsöly A, Nagy B, Rónai Z, Fekete A, Sasvári-Székely M, Rigó J., Jr., and Vér A. Association of extracellular superoxide dismutase (SOD3) Ala40Thr gene polymorphism with pre-eclampsia complicated by severe fetal growth restriction. Eur J Obstet Gynecol Reprod Biol 142: 134–138, 2009 [DOI] [PubMed] [Google Scholar]
- 137.Rüdiger M, Tölle A, Meier W, and Rüstow B. Naturally derived commercial surfactants differ in composition of surfactant lipids and in surface viscosity. Am J Physiol Lung Cell Mol Physiol 288: L379–L383, 2005 [DOI] [PubMed] [Google Scholar]
- 138.Rüdiger M, von Baehr A, Haupt R, Wauer RR, and Rüstow B. Preterm infants with high polyunsaturated fatty acid and plasmalogens content in tracheal aspirates develop bronchopulmonary dysplasia less often. Ped Crit Care 28: 1572–1577, 2000 [DOI] [PubMed] [Google Scholar]
- 139.Rusai K, Vannay A, Szebeni B, Borgulya G, Fekete A, Vásárhelyi B, Tulassay T, and Szabó AJ. Endothelial nitric oxide synthase gene T-786C and 27-bp repeat gene polymorphisms in retinopathy of prematurity. Mol Vis 14: 286–290, 2008 [PMC free article] [PubMed] [Google Scholar]
- 140.Ryckman KK, Dagle JM, Kelsey K, Momany AM, and Murray JC. Genetic associations of surfactant protein D and angiotensin-converting enzyme with lung disease in preterm neonates. J Perinatol 32: 349–355, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Salvemini D, Riley DP, and Cuzzocrea S. SOD mimetics are coming of age. Nat Rev Drug Discov 1: 367–374, 2002 [DOI] [PubMed] [Google Scholar]
- 142.Samoila OC, Carter AM, Futers ST, Otiman G, Anghel A, Tamas L, and Seclaman E. Polymorphic variants of extracellular superoxide dismutase gene in a romanian population with atheroma. Biochem Genet 46: 634–643, 2008 [DOI] [PubMed] [Google Scholar]
- 143.Sampath V, Garland JS, Le M, Patel AL, Konduri GG, Cohen JD, Simpson PM, and Hines RN. A TLR5 (g.1174C>T) variant that encodes a stop codon (R392×) is associated with bronchopulmonary dysplasia. Pediatr Pulmonol 47: 460–468, 2012 [DOI] [PubMed] [Google Scholar]
- 144.Sandstrom J, Nilsson P, Karlsson K, and Marklund SL. 10-fold increase in human plasma extracellular superoxide dismutase content caused by a mutation in heparin binding domain. J Biol Chem 269: 19163–19166, 1994 [PubMed] [Google Scholar]
- 145.Saugstad OD, Ramji S, and Vento M. Resuscitation of depressed newborn infants with ambient air or pure oxygen: a meta-analysis. Biol Neonate 87: 22–34, 2005 [DOI] [PubMed] [Google Scholar]
- 146.Saugstad OD, Rootwelt T, and Aalen O. Resuscitation of asphyxiated newborn infants with room air or oxygen: an international controlled trial, the Resair 2 study. Pediatr Res 102: e1, 1998 [DOI] [PubMed] [Google Scholar]
- 147.Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol 8: 39–49, 2003 [DOI] [PubMed] [Google Scholar]
- 148.Saugstad OD. Optimal oxygenation at birth and in the neonatal period. Neonatology 91: 319–322, 2007 [DOI] [PubMed] [Google Scholar]
- 149.Saugstad OD. Oxidative stress in the newborn—a 30-year perspective. Biol Neonate 88: 228–236, 2005 [DOI] [PubMed] [Google Scholar]
- 150.Sewerynek E, Abe M, Reiter RJ, Barlow-Walden LR, Chen L, McCabe TJ, Roman LJ, and Diaz-Lopez B. Melatonin administration prevents lipopolysaccharide-induced oxidative damage in phenobarbital-treated animals. J Cell Biochem 58: 436–444, 1995 [DOI] [PubMed] [Google Scholar]
- 151.Shah MR, Wedgwood S, Czech L, Kim GA, Lakshminrusimha S, Schumacker PT, Steinhorn RH, and Farrow KN. Cyclic stretch induces inducible nitric oxide synthase and soluble guanylate cyclase in pulmonary artery smooth muscle cells. Int J Mol Sci 14: 4334–4348, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sharma S, Grobe AC, Wiseman DA, Kumar S, Englaish M, Najwer I, Benavidez E, Oishi P, Azakie A, Fineman JR, and Black SM. Lung antioxidant enzymes are regulated by development and increased pulmonary blood flow. Am J Physiol Lung Cell Mol Physiol 293: L960–L971, 2007 [DOI] [PubMed] [Google Scholar]
- 153.Sheehy AM, Burson MA, and Black SM. Nitric oxide exposure inhibits endothelial NOS activity but not gene expression: a role for superoxide. Am J Physiol 274: L833–L841, 1998 [DOI] [PubMed] [Google Scholar]
- 154.Sheng H, Bart RD, Oury TD, Pearlstein RD, Crapo JD, and Warner DS. Mice overexpressing extracellular superoxide dismutase have increased resistance to focal cerebral ischemia. Neuroscience 88: 185–191, 1999 [DOI] [PubMed] [Google Scholar]
- 155.Sheng H, Brady TC, Pearlstein RD, Crapo JD, and Warner DS. Extracellular superoxide dismutase deficiency worsens outcome from focal cerebral ischemia in the mouse. Neurosci Lett 267: 13–16, 1999 [DOI] [PubMed] [Google Scholar]
- 156.Sheng H, Kudo M, Mackensen GB, Pearlstein RD, Crapo JD, and Warner DS. Mice overexpressing extracellular superoxide dismutase have increased resistance to global cerebral ischemia. Exp Neurol 163: 392–398, 2000 [DOI] [PubMed] [Google Scholar]
- 157.Shimoda-Matsubayashi S, Matsumine H, Kobayashi T, Nakagawa-Hattori Y, Shimizu Y, and Mizuno Y. Structural dimorphism in the mitochondrial targeting sequence in the human manganese superoxide dismutase gene. A predictive evidence for conformational change to influence mitochondrial transport and a study of allelic association in Parkinsons disease. Biochem Biophys Res Commun 226: 561–565, 1996 [DOI] [PubMed] [Google Scholar]
- 158.Smalling WE, Jr., Suguihara C, huang J, Rodriguez MM, and Bancalari E. Protective effect of pentoxyfilline on volume-induced lung injury in newborn piglets. Biol Neonate 86: 15–21, 2004 [DOI] [PubMed] [Google Scholar]
- 159.Soll RF. Prophylactic natural surfactant extract for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev CD000511, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Stabler SP, Morton RL, Winski SL, Allen RH, and White CW. Effects of parenteral cysteine and glutathione feeding in a baboon model of severe prematurity. Am J Clin Nutr 72: 1548–1557, 2000 [DOI] [PubMed] [Google Scholar]
- 161.Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY HS, Meurer S, Deile M, Taye A, Knorr A, Lapp H, Muller H, Turgay Y, Rothkegel C, Tersteegen A, Kemp-Harper B, Muller-Esterl W, and Schmidt HH. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest 116: 2552–2561, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Steinhorn RH, Albert G, Swartz DD, Russell JA, Levine CR, and Davis JM. Recombinant human superoxide dismutase enhances the effect of inhaled nitric oxide in persistent pulmonary hypertension. Am J Respir Crit Care Med 164: 834–839, 2001 [DOI] [PubMed] [Google Scholar]
- 163.Steinhorn RH, Kinsella JP, Pierce C, Butrous G, Dilleen M, Oakes M, and Wessel DL. Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension. J Pediatr 155: 841–847.e1, 2009 [DOI] [PubMed] [Google Scholar]
- 164.Steinhorn RH, Morin FC III, Gugino SF, Giese EC, and Russell JA. Developmental differences in endothelium-dependent responses in isolated ovine pulmonary arteries and veins. Am J Physiol Heart Circ Physiol 264: H2162–H2167, 1993 [DOI] [PubMed] [Google Scholar]
- 165.Strange RC, Cotton W, Fryer AA, Jones P, Bell J, and Hume R. Lipid peroxidation and expression of copper-zinc and manganese superoxide dismutase in lungs of premature infants with hyaline membrane disease and bronchopulmonary dysplasia. J Lab Clin Med 116: 666–673, 1990 [PubMed] [Google Scholar]
- 166.Strassberg SS, Cristea IA, Qian D, and Parton LA. Single nucleotide polymorphisms of tumor necrosis factor-alpha and the susceptibility to bronchopulmonary dysplasia. Pediatr Pulmonol 42: 29–36, 2007 [DOI] [PubMed] [Google Scholar]
- 167.Sun LR, Wang C, Wu AQ, Yan BM, Li DM, Zhang JL, Wang GJ, Zhang Y, Li XQ, and Zhang QZ. Gene polymorphism of the endothelial nitric oxide synthase enzyme and pulmonary hypertension in patient with chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi 31: 335–340, 2008 [PubMed] [Google Scholar]
- 168.Tamai M, Furuta H, Kawashima H, Doi A, Hamanishi T, Shimomura H, Sakagashira S, Nishi M, Sasaki H, Sanke T, and Nanjo K. Extracellular superoxide dismutase gene polymorphism is associated with insulin resistance and the susceptibility to type 2 diabetes. Diabetes Res Clin Pract 71: 140–145, 2006 [DOI] [PubMed] [Google Scholar]
- 169.Tan A, Schulze A, O'Donnell CP, and Davis PG. Air versus oxygen for resuscitation of infants at birth. Cochrane Database Syst Rev 18: CD002237, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tan DX, Manchester LC, Burkhardt S, Sainz RM, Mayo JC, Kohen R, Shohami E, Huo YS, Hardeland R, and Reiter RJ. N1-acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J 15: 2294–2296, 2001 [DOI] [PubMed] [Google Scholar]
- 171.Tan DX, Manchester LC, Reiter RJ, Qi WB, Karbownik M, and Calvo JR. Significance of melatonin in antioxidative defense system: reactions and products. Biol Signals Recept 9: 137–159, 2000 [DOI] [PubMed] [Google Scholar]
- 172.Tan DX, Manchester LC, Terron MP, Flores LJ, and Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res 42: 28–42, 2007 [DOI] [PubMed] [Google Scholar]
- 173.ter Horst SA, Wagenaar GT, de Boer E, van Gastelen MA, Meijers JC, Biemond BJ, Poorthuis BJ, and Walther FJ. Pentoxifylline reduces fibrin deposition and prolongs survival in neonatal hyperoxic lung injury. J Appl Physiol 97: 2014–2019, 2004 [DOI] [PubMed] [Google Scholar]
- 174.Turrens JF, Crapo JD, and Freeman BA. Protection against oxygen toxicity by intravenous injection of liposome-entrapped catalase and superoxide dismutase. J Clin Invest 73: 87–95, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, Stoll BJ, Lemons JA, Stevenson DK, Bauer CR, Korones SB, and Fanaroff AA. Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med 340: 1962–1968, 1999 [DOI] [PubMed] [Google Scholar]
- 176.Usuda T, Kobayashi T, Sakakibara S, Kobayashi A, Kaneko T, Wada M, Onozuka J, Numata O, Torigoe K, Yamazaki H, Sato T, Nagayama Y, and Uchiyama M. Interleukin-6 polymorphism and bronchopulmonary dysplasia risk in very low-birthweight infants. Pediatr Int 54: 471–475, 2012 [DOI] [PubMed] [Google Scholar]
- 177.Vento M, Asensi M, Sastre J, García-Sala F, and Viña J. Six years of experience with the use of room air for the resuscitation of the asphyxiated newly born term infants. Biol Neonate 79: 261–272, 2001 [DOI] [PubMed] [Google Scholar]
- 178.Vento M, Asensi M, Sastre J, García-Sala F, Pallardó FV, and Viña J. Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics 107: 642–647, 2001 [DOI] [PubMed] [Google Scholar]
- 179.Vento M, Asensi M, Sastre J, Lloret A, García-Sala F, and Viña J. Oxidative stress in asphyxiated infants resuscitated with 100% oxygen. J Pediatr 142: 240–246, 2003 [DOI] [PubMed] [Google Scholar]
- 180.Vento M, Moro M, Escrig R, Arruza L, Villar G, Izquierdo I, Roberts LJ, II, Arduini A, Escobar JJ, Sastre J, and Asensi MA. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation and chronic lung disease. Pediatrics 124: e439–e449, 2009 [DOI] [PubMed] [Google Scholar]
- 181.Wakatsuki A, Okatani Y, Shinohara K, Ikenoue N, Kaneda C, and Fukaya T. Melatonin protects fetal rat brain against oxidative mitochondrial damage. J Pineal Res 30: 22–28, 2001 [DOI] [PubMed] [Google Scholar]
- 182.Walther FJ, David-Cu R, and Lopez SL. Antioxidant-surfactant liposomes mitigate hyperoxic lung injury in premature rabbits. Am J Physiol 269: L613–L617, 1995 [DOI] [PubMed] [Google Scholar]
- 183.Walther FJ, Wade AB, Warburton D, and Forman HJ. Ontogeny of antioxidant enzymes in the fetal lamb lung. Exp Lung Res 17: 39–45, 1991 [DOI] [PubMed] [Google Scholar]
- 184.Wang H, Parry S, Macones G, Parry S, Macones G, Sammel MD, Kuivaniemi H, Tromp G, Argyropoulos G, Halder I, Shriver MD, Romero R, and Strauss JF, 3rd. A functional SNP in the promoter of the SERPINH1 gene increases risk of preterm premature rupture of membranes in African Americans. Proc Natl Acad Sci U S A 103: 13463–13467, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Watts JL, Milner R, Zipursky A, Paes B, Ling E, Gill G, Fletcher B, and Rand C. Failure of supplementation with vitamin E to prevent bronchopulmonary dysplasia in infants less than 1,500 g birth weight. Eur Respir J 4: 188–190, 1991 [PubMed] [Google Scholar]
- 186.Wedgwood S, Lakshminrusimha S, Farrow KN, Czech L, Gugino SF, Soares F, Russel JA, and Steinhorn RH. Apocynin improves oxygenation and increases eNOS in persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 302: L616–L626, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wedgwood S, Lakshminrusimha S, Fukai T, Russell JA, Schumacker PT, and Steinhorn RH. Hydrogen peroxide regulates extracellular superoxide dismutase activity and expression in neonatal pulmonary hypertension. Antioxid Redox Signal 15: 1497–1506, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Winters AH, Levan TD, Vogel SN, Chesko KL, Pollin TI, and Viscardi RM. Single nucleotide polymorphism in Toll-Like receptor 6 is associated with a decreased risk for ureaplasma respiratory tract colonization and bronchopulmonary dysplasia in preterm infants. Pediatr Infect Dis J 32: 898–904, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wispé JR, Warner BB, Clark JC, Dey CR, Neuman J, Glasser SW, Crapo JD, Chang LY, and Whitsett JA. Human Mn-superoxide dismutase in pulmonary epithelial cells of transgenic mice confers protection from oxygen injury. J Biol Chem 267: 23937–23941, 1992 [PubMed] [Google Scholar]
- 190.Wu Y, Adam S, Hamann L, Heine H, Ulmer AJ, Buwitt-Beckmann U, and Stamme C. Accumulation of inhibitory of kB-α as a mechanism contributing to the anti-inflammatory effects of surfactant protein A. Am J Respir Cell Mol Biol 31: 587–594, 2004 [DOI] [PubMed] [Google Scholar]
- 191.Yanamandra K, Boggs P, Loggins J, and Baier RJ. Interleukin-10-1082G/A polymorphism and risk of death or bronchopulmonary dysplasia in ventilated very low birth weight infants. Pediatr Pulmonol 39: 426–432, 2005 [DOI] [PubMed] [Google Scholar]
- 192.Zelko IN, Mariani TJ, and Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med 33: 337–349, 2002 [DOI] [PubMed] [Google Scholar]
- 193.Zoeller RA, Morand OH, Raetz CR, et al. A possible role for plasmalogens in protecting animal cells against photosensitized killing. J Biol Chem 263: 11590–11596, 1988 [PubMed] [Google Scholar]


