Summary
Extended darkness induces a transient increase in sugars and trehalose pathway gene expression.
Key words: Maize; shade stress,; trehalose-6-phosphate; trehalose gene family; diurnal cycle,; quantitative RT-PCR.
Abstract
Energy resources in plants are managed in continuously changing environments, such as changes occurring during the day/night cycle. Shading is an environmental disruption that decreases photosynthesis, compromises energy status, and impacts on crop productivity. The trehalose pathway plays a central but not well-defined role in maintaining energy balance. Here, we characterized the maize trehalose pathway genes and deciphered the impacts of the diurnal cycle and disruption of the day/night cycle on trehalose pathway gene expression and sugar metabolism. The maize genome encodes 14 trehalose-6-phosphate synthase (TPS) genes, 11 trehalose-6-phosphate phosphatase (TPP) genes, and one trehalase gene. Transcript abundance of most of these genes was impacted by the day/night cycle and extended dark stress, as were sucrose, hexose sugars, starch, and trehalose-6-phosphate (T6P) levels. After extended darkness, T6P levels inversely followed class II TPS and sucrose non-fermenting-related protein kinase 1 (SnRK1) target gene expression. Most significantly, T6P no longer tracked sucrose levels after extended darkness. These results showed: (i) conservation of the trehalose pathway in maize; (ii) that sucrose, hexose, starch, T6P, and TPS/TPP transcripts respond to the diurnal cycle; and(iii) that extended darkness disrupts the correlation between T6P and sucrose/hexose pools and affects SnRK1 target gene expression. A model for the role of the trehalose pathway in sensing of sucrose and energy status in maize seedlings is proposed.
Introduction
A central feature of plant metabolism is the photosynthetic conversion of light energy into stored chemical energy. Every 24h, plants cycle from net energy production to net energy consumption. During the day, plants produce sucrose and reducing sugars used in the synthesis of amino acids, lipids, nucleic acids, and complex carbohydrates. As light energy wanes at dusk and throughout the night, the plant transitions from a net producer of sugars to a net consumer. During the night, the plant utilizes stored carbohydrates as a source of carbon skeletons and chemical energy (Baena-González et al., 2007; Stitt and Zeeman, 2012). In some plants, like Arabidopsis, the vast majority of the stored carbohydrate is in the form of starch (Gibon et al., 2009; Sulpice et al., 2010, 2014), but in other plants, sugars including hexoses and sucrose can play a role in maintaining energy balance throughout the transition between light and dark. Trehalose [α-d-glucopyranosyl-(1→1)-α-d-glucopyranoside] is an important osmotic protectant in bacteria, fungi, and insects where it accumulates to high concentrations (Avonce et al., 2006). Most plants accumulate only trace amounts of trehalose and its intermediates, where it is unlikely to function as an osmoprotectant (Paul et al., 2008). Rather, the role of the trehalose metabolic pathway and its intermediates is to sense and communicate energy status (Lunn, 2007; Lunn et al., 2014). As examples, exogenously applied trehalose altered physiology and gene expression, such as induction of the AGPase gene in Arabidopsis (Wingler, 2002), and resulted in increased biomass yield and water-deficit stress tolerance (Rodríguez-Salazar et al., 2009; Sciences and Zeid, 2009; Ali and Ashraf, 2011). The inflorescences of the ramosa3 mutant of Zea mays have significantly reduced trehalose (Carillo et al., 2013) and excessive branching (Satoh-Nagasawa et al., 2006). An induced increase in trehalose-6-phosphate (T6P) inhibits starch degradation in Arabidopsis, and changes in T6P modulate the photoperiod and flowering patterns (Wahl et al., 2013).
Plants have a conserved three-step metabolic pathway for the synthesis and degradation of trehalose. In the first step, trehalose-6-phosphate synthase (TPS) catalyses the condensation of glucose-6-phosphate (G6P) and uridine diphosphate glucose (UDPG) to form T6P. Trehalose-6-phosphate phosphatase (TPP) subsequently removes phosphate to form trehalose. Trehalase (TRE) then hydrolyses trehalose into two glucose residues. Plant TPS and TPP are encoded by multigenic families, while the trehalase (TRE) gene is present in a single copy (Lunn, 2007). Arabidopsis and rice genomes each encode 11 TPS genes and, respectively, 13 and 10 TPP genes (Yang et al., 2012). TPS genes are divided into two classes. Class I TPS genes are generally present in a single copy, and they usually encode catalytically active TPS enzymes that have both TPS and TPP domains, with inactive phosphatase boxes. Class II TPS genes have both TPS and TPP domains but lack residues in the TPS domain needed for interaction with the substrate. Most class II TPS genes have conserved phosphatase domains; however, they do not possess TPS or TPP activity (Vandesteene et al., 2010). In rice, some class II TPS proteins interact to form high-molecular-weight complexes, and a regulatory role is suspected (Zang et al., 2011). All plant TPP genes are composed of a unique TPP domain with conserved phosphatase domains, and all encode functional TPP enzymes in Arabidopsis. Since they have similar activity but differential expression patterns, TPP genes probably have a tissue-, stage-, and/or process-specific function (Vandesteene et al., 2012).
The diurnal switch from energy production to energy consumption requires a global change in gene expression and metabolic networks. In concert with the internal clock, sugar levels are a key regulator of this switch. Sugar levels fluctuate during the diurnal cycle, and sugars and circadian rhythm have an approximately equal and interactive effect on gene expression (Bläsing et al., 2005). In maize, at least 10% of transcripts display circadian expression patterns, with peak expression at dawn and/or dusk in preparation for the periodic change in environment (Khan et al., 2010). Not surprisingly, many diurnally regulated transcripts encode proteins involved in photosynthesis, respiration, carbohydrate metabolism, and cell elongation (Harmer et al., 2000). Understanding how this switch takes place is of fundamental importance to improve crop productivity.
Plants have complex sugar signalling networks to maintain energy status regardless of photosynthetic output or growth rate (Sheen, 2010). Hexoses are sensed through hexokinase (HXK)-dependent and HXK-independent pathways (Sheen, 2010). Sucrose sensing is less well understood; however, a correlation between sucrose and T6P levels strongly suggests that one route may involve a T6P inhibitory effect on sucrose non-fermenting-related protein kinase 1 (SnRK1), a global integrator of energy balance (Polge and Thomas, 2007; Zhang et al., 2009; Nunes et al., 2013). When energy levels decrease due to starvation or stress, SnRK1 is activated and triggers induction or repression of ~1000 genes to switch from anabolism to catabolism, promoting survival in lieu of growth (Baena-González et al., 2007). This effect on gene expression probably involves the basic region leucine zipper transcription factor 11 (bZIP11) (Delatte et al., 2011; Ma et al., 2011).
As a consequence of altered carbohydrate metabolism (Lunn, 2007), dramatic phenotypes of plants with altered expression of trehalose pathway genes include effects on flowering, embryogenesis, branching, plant stature, biomass, grain yield, and abiotic/biotic stress tolerance (Wingler, 2002; Lunn et al., 2014). The role of the trehalose pathway is significant; however, the details remain unclear. Recent evidence points to T6P having a central role in sugar sensing (Paul, 2007; Zhang et al., 2009; Ponnu et al., 2011; Wingler et al., 2012; Wahl et al., 2013). Sucrose and T6P levels were correlated in Arabidopsis meristems (Wahl et al., 2013) and seedlings recovering from starvation (Lunn et al., 2006), and developing wheat grain showed a close correlation between sucrose, T6P, and SnRK1 levels (Martínez-Barajas et al., 2011), suggesting that T6P can act as a signal to indicate sucrose levels (Lunn et al., 2014). Recent work in Arabidopsis showed the ratio between T6P and sucrose to be tightly regulated and critical to maintaining homeostasis throughout the diurnal cycle and during periods of stress (Yadav et al., 2014).
Most work describing T6P and trehalose in energy-sensing networks has used Arabidopsis, which is a reference species for dicots and for C3 photosynthesis. Little is known about the trehalose pathway gene structure, regulation, or role in central metabolism in the C4 monocot maize, although maize is a major world crop that impacts on human and animal nutrition, and is an alternative energy source. The biodiversity in maize and availability of ‘omics’ data will be synergistic tools to investigate the impact of this pathway on plant growth and development.
This study aimed to identify and classify maize TPS/TPP/TRE gene families, and to determine their response to fluctuations in sugar and energy levels throughout the day/night cycle, after extended darkness (48h) to impose an energy deficit, and during recovery from this dark treatment. Additionally, we compared starch/sucrose/hexose/T6P levels with TPS/TPP gene expression during recovery from extended darkness. A model is presented to integrate these new data into a more general view of the role of this pathway in plant growth and its response to the environment.
Materials and methods
Plant growth, treatment and harvest
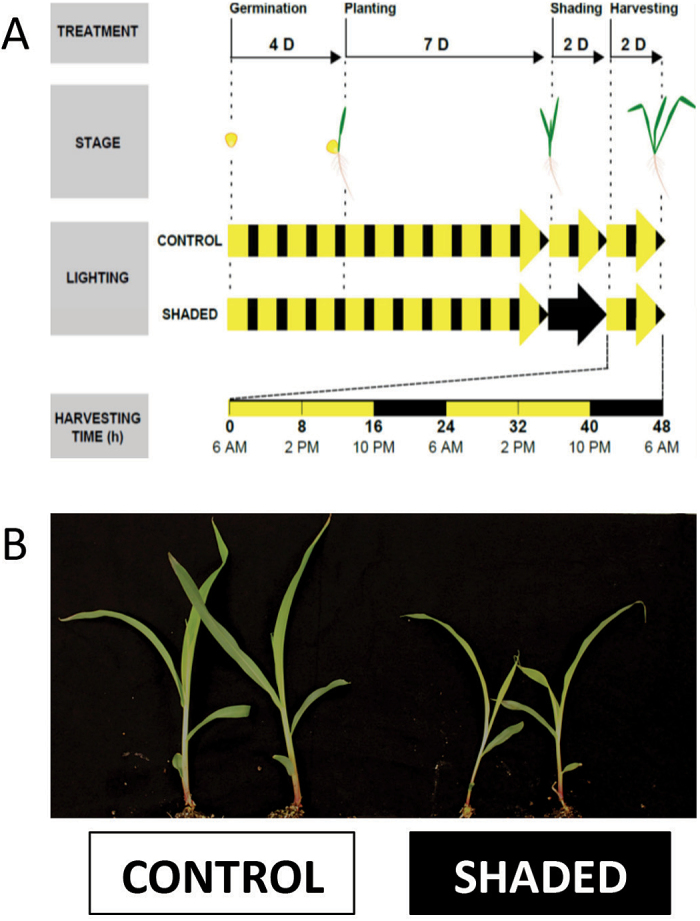
Inbred B73 maize (Z. mays L.) plants were used. Seeds were sterilized for 15min with 15% bleach (v/v), rinsed thoroughly with sterile water, stirred for 1min in 70 % ethanol, rinsed again, and soaked for 5min in sterile water. Seeds were then rolled in germinating paper (Anchor Paper) and germinated for 4 d in the presence of 1mM CaSO4 solution in a growth chamber (16h day/8h night, 220±30 µmol m–2 s–1, 24 °C, 50% relative humidity). Seedlings were planted in germinating trays containing potting mix (34% peat, 31% perlite, 31 % vermiculite, 4% soil), grown under the same conditions as described previously and watered daily with nutrient solution (20-20-20; J. R. Peters). Thirteen-day-old juvenile plants were then placed under a control photoperiod (same as above) or shaded for 48h (frame covered with a thick black fabric shading cloth, 75×75×45cm; 0 µmol m–2 s–1), and the frame was then removed to permit recovery for an additional 48h (Brouquisse et al., 1998; Mutisya et al., 2009). Leaf 3 (fully-expanded) was harvested every 8h for 48h starting at the end of the dark period (first time point: 6 a.m., end of the night) from plants randomly picked in the tray (Fig. 1). The centre one-third of the leaf (100–200mg) was collected into pre-chilled microcentrifuge tubes, instantly frozen in liquid nitrogen, and stored at −80 °C until use.
Fig. 1.
Experimental design (A) and plant phenotype after 48h of treatment (B). (A) B73 maize seeds were germinated for 4 d, planted, and cultivated under control diurnal cycles (16h day (D)/8h night (N), 220±30 µmol m–2 s–1, 24 °C, 50% relative humidity) for 7 d. The plants were then kept under the same conditions (control) or under total darkness (shaded) for 48h. They were then returned to regular diurnal cycles for recovery. Sample harvesting was done every 8h for 48h, starting at the end of the dark period when plants were still under darkness. (B) Control and shaded plants grew at different rates as a result of the treatment.
Gene identification and bioinformatic analysis
Genes were identified using a name search and BLAST with Arabidopsis sequences in the http://www.maizesequence.org and http://bioinformatics.psb.ugent.be/plaza/databases. Predicted protein sequences were then compared with those of rice, Arabidopsis, and poplar (Supplementary Tables S1 and S2 at JXB online) using the following website to generate classification and phylogenic relationships: http://www.phylogeny.fr/ (alignment with MUSCLE, phylogeny with PhyML, and tree rendering with TreeDyn). Protein sequences were also analysed using http://myhits.isb-sib.ch/ software with the following parameters (hamap, pat, prf, pre, pfam_fs, and pfam_Is) to identify conserved TPS and TPP protein domains.
Gene expression analysis
Frozen tissues were grounded to a fine powder using a Tissue Lyser II (Qiagen). RNA was extracted using a Trizol protocol as described by the provider with the addition of 1 µl of glycogen (Invitrogen) at the beginning. RNA samples were DNase treated using RQ1 RNase-free DNase (Promega) as recommended by the supplier and stored at –80 °C until use. RNA quantity and quality were checked using a Nanodrop 8000 spectrophotometer (ThermoScientific) and electrophoresis on a 1.2% agarose gel, respectively.
Reverse transcription (RT) was performed on 1 µg of total RNA using a SuperScript III First-Strand Synthesis Supermix kit (Life Technology) with random hexamer primers. RT quality and absence of genomic DNA contamination was then checked by semi-quantitative PCR using 5 µl of cDNA at a 1:100 dilution in a final volume of 25 µl using GoTaq® DNA Polymerase (Promega). ZmEF1-1α primers (forward: 5′-AGACTCACATCAACATTGTGGTCAT-3′, reverse: 5′-GT TGT CAC CT TCAAAACCAGAGATT-3′) were designed around an intron. For real-time RT-PCR, 5 µl of cDNA at a 1:50 dilution was used for reactions with SsoAdvancedTM SYBR® Green Supermix (Bio-Rad) and 167nM primers (Supplementary Table S2) in a final volume of 15 µl. Real-time amplification was performed in the LightCycler® 480 II (Roche) using the following program: 30 s at 95 °C; and 45 cycles of 5 s at 95 °C, 30 s at 60 °C, and 10 s at 72 °C. A melting-curve analysis was performed for 5 s at 95 °C, followed by 5 °C increments from 65 to 95 °C. For each time point and three biological replicates, quantitative PCR was performed three times. Six reference genes (Supplementary Table S2) were tested and three were selected using the Genorm software (Vandesompele et al., 2002). Relative gene expression was then calculated using the formula of Hellemans et al. (2007). Primer efficiency was determined using the method described by (Pfaffl (2001).
Carbohydrate metabolite analysis
Frozen tissues (20–100mg) were weighed and ground for 30–60 s while frozen using a Tissue Lyser II (Qiagen). Sugars (sucrose, fructose, and glucose) were then extracted following the method of Lunn et al. (2006) using lactose as an internal standard. Starch was extracted from the pellet generated during the extraction of soluble sugars,and quantified by analysis of glucose resulting from hydrolysis (Supplementary Methods S1 at JXB online). Samples were analysed with a high-pressure capillary ion chromatograph system (ICS-5000, PA-20 column; Thermo Scientific Dionex) using a 1 µl injection volume and 45mM KOH eluent. Sugar peaks were identified in comparison with known sugars, and data were analysed using the formulae described in Supplementary Methods S2 at JXB online. The method of Lunn et al. (2006) using anion-exchange liquid chromatography, linked to tandem mass spectrometry, was used to quantify T6P.
Statistical methods
Pearson correlation coefficient matrices between transcripts, sugars, and transcript versus sugars were determined were computed using the stats package from R software version 3.0.1 (R Core Team, 2013, http://www.r-project.org/). The average of three biological replicates was used to perform tests. Heat maps were then generated in MS Excel using conditional formatting functions.
Student’s t-test was used to compare differences between control and shaded condition for each time point. Analysis was performed using the following website: http://www.physics.csbsju.edu/stats/t-test.html. Significant differences with a value of P<0.05 are indicated by an asterisk.
Results
Classification of maize TPS/TPP genes
We identified 14, 11, and one genes predicted to encode for TPS, TPP, and TRE maize enzymes, respectively (gene accession numbers in Table S1) from maize genome databases (http://www.maizesequence.org and http://bioinformatics.psb.ugent.be/plaza/). The maize genome also encodes several genes with truncated TPS/TPP domains, named TPS-like or TPP-like. These are unlikely to be functional trehalose pathway enzymes based on domain analysis using the MyHits tool; therefore, we did not investigate them further.
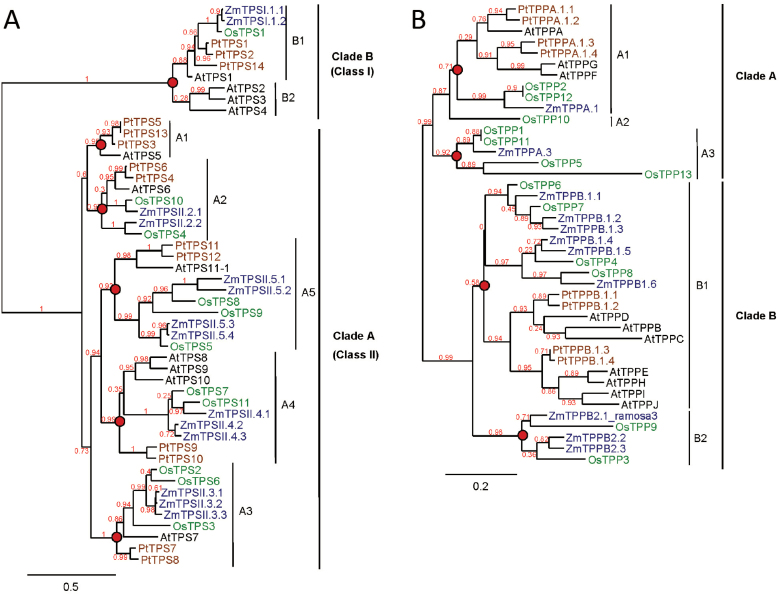
As described by Yang et al. (2012), TPS genes were divided into two clades: clade B included all class I TPS genes while clade A included all class II TPS genes. Clades B and A subdivided into two and five subclades, respectively, corresponding to groups with common ancestors before the split between monocots and dicots. Class A was found in all dicots and subclade B2 was specific to Arabidopsis. Maize encoded two class I TPS genes (clade B) and 12 class II TPS genes (clade A), named according to their position in the phylogenetic tree (Fig. 2A). All maize TPS proteins included both a TPS and TPP domain. Among the class I TPS genes, clade B1 contained the functional TPS from rice and Arabidopsis and both maize TPS class I genes. ZmTPSI.1.1 (previously named ZmTPS1) encoded a functional TPS enzyme and had all conserved TPS motifs (Table 1, Supplementary Fig. S2 at JXB online) (Jiang et al., 2010). Structurally, ZmTPSI.1.2 was a truncated version of ZmTPSI.1.1 and was missing amino acids required for substrate binding. This gene is therefore unlikely to encode a functional TPS enzyme. Interestingly, all class I TPS proteins lacked the first phosphatase motif required for the catalytic activity, although they possessed a full TPP domain. Maize class II TPS genes were composed of subclades A2–A5 with ZmTPSII.3.1, -3.2, and -3.3; ZmTPSII.4.2 and -4.3; ZmTPSII.5.1 and -5.2; and ZmTPSII.5.3 and -5.4, respectively. Maize class II TPS enzymes had a substitution of arginine with aspartic acid in the UDPG phosphate-binding pocket (Table 1). Most maize class II TPS displayed substitution of three to four amino acids in the UDPG- and G6P-binding sites, while class II TPS genes belonging to clade A5 showed a higher number of substitutions in the UDPG-binding site but had a highly conserved G6P-binding site.
Fig. 2.
Phylogenic trees of TPS and TPP genes from Z. mays, Arabidopsis thaliana, Oriza sativa, and Populus trichocarpa. A phylogenic tree was developed using predicted protein TPS (A) and TPP (B) sequences from maize (blue), Arabidopsis (black), rice (green), and poplar (brown) identified from genomic databases (http://www.maizesequence.org and http://bioinformatics.psb.ugent.be/plaza/). Common ancestors before the split between monocots and dicots are indicated by red circles. Clades and subclades are indicated and bootstrap values are shown in red. Bars, amino acid substitutions per site.
Table 1.
Conserved amino acids required for TPS and TPP activity in TPS predicted proteins from E. coli, Arabidopsis and maizeResidues involved in substrate interaction of the TPS domain are designed by a letter associated with a number indicating their position (Gibson et al., 2002; Vandesteene et al., 2010). Deletions are represented by a X. Presence (+) or absence (–) of the three phosphatase motifs required for the activity in the TPP domain is also indicated (Avonce et al., 2006; Lunn, 2007). Conservation of residues or motifs required for TPS and TPP activity is highlighted by shading.
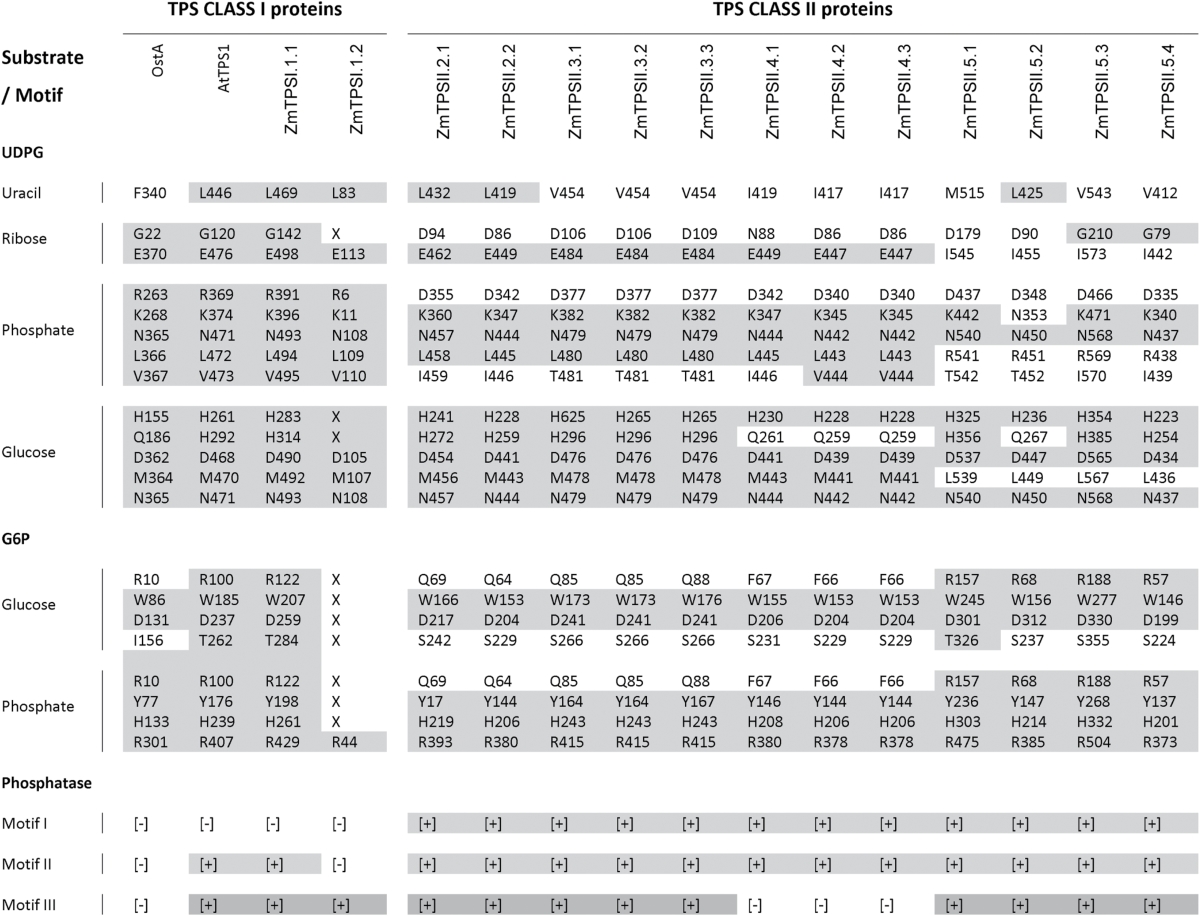
The TPP family also had two clades that were divided into three and two subclades (Fig. 2B). Subclades A1 and B1 included genes from both monocots and dicots, while other subclades were specific to monocot species. The position of the maize TPP genes within subclades was used for their nomenclature. TPP genes mainly evolved through duplication events after the monocot/dicot split or even after speciation, in contrast to TPS genes. All maize TPP genes displayed three conserved motifs required for TPP activity: (i) DXDX(T/V)(L/V/I); (ii) (S/T)(GX) in an hydrophobic context; and (iii) K(X)16–30(G/S)(D/S)XXX(D/N) (Table 1, Fig. S2) (Avonce et al., 2006; Lunn, 2007).
Maize TPS/TPP and SnRK1 targets gene expression
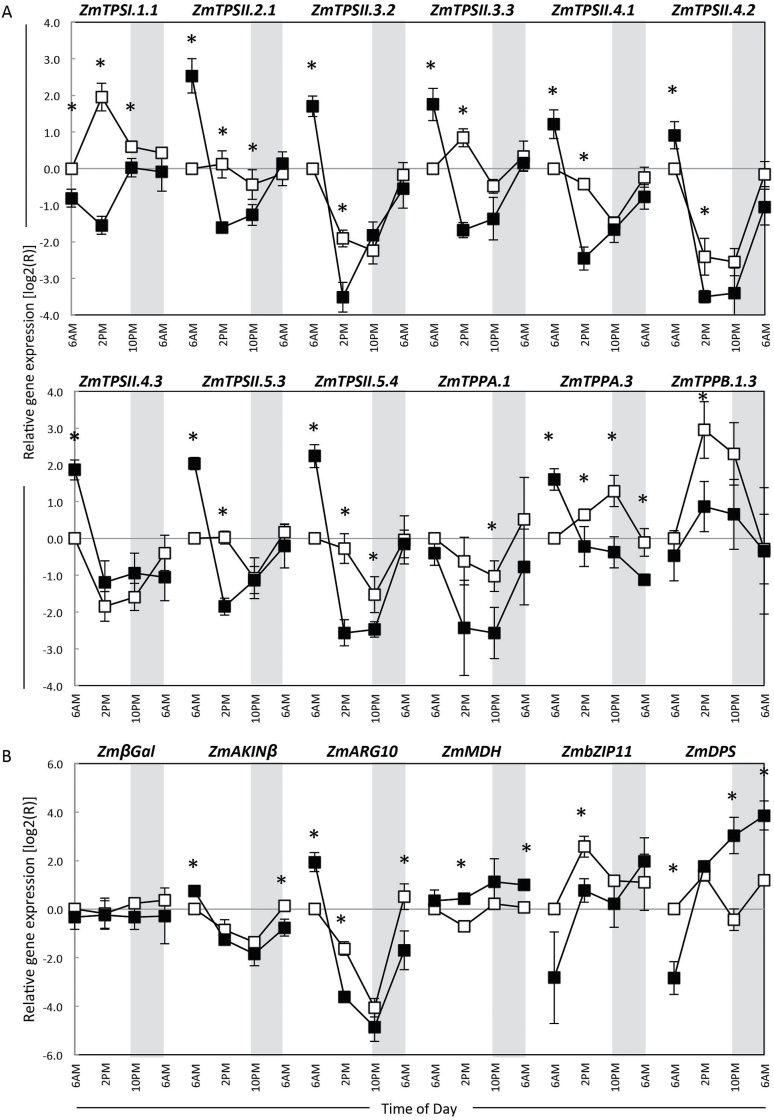
Maize TPS/TPP gene expression was characterized throughout the regular diurnal cycle and during the recovery from 48h of extended darkness. In plants with regular diurnal cycles, gene expression patterns were quite varied among TPS/TPP genes and putative SnRK1 targets (Fig. 3). Expression of the catalytically active ZmTPSI.1.1, ZmbZIP11, and ZmTPPB.1.3 increased throughout the morning, peaking at 2 p.m., and then decreased in the late afternoon and night. Most class II genes and ZmTPPA.1 had their highest transcript levels at the end of the night period and decreased throughout the day. Several SnRK1 target genes were selected as indicators of a possible SnRK1 activity. We looked at the expression of some targets shown to be upregulated (βGal, AKINβ, and ARG10) or downregulated (MDH, bZIP11, and DPS) by SnRK1 in Arabidopsis (Supplementary Fig. S3D and E at JXB online) (Baena-González et al., 2007; Usadel et al., 2008). As with the class II TPS genes, SnRK1 inducible transcripts were highest at the end of the night period and SnRK1-repressible transcripts were lowest. ZmTPSII.2.1, ZmβGal, and ZmMDH showed no significant change in transcript levels throughout the day/night period.
Fig. 3.
Relative gene expression for selected maize TPS, TPP, and SnRK1 putative target genes in control and shaded seedlings. Leaf tissue were collected from the V3 stage control (16h day/8h night, open squares) and shaded (48h, filled squares) plants. Sampling was done every 8h for 24h starting at the end of the night or extended shading period (recovery phase). TPS and TPP transcript levels were specifically measured using quantitative RT-PCR. Transcript levels are expressed relatively to the first time point of control treatment. Expression was normalized to the geometric mean of stably expressed genes: ZmPP2AA2-2 and ZmCACS, and transformed using a log2 function. Data are presented as means±standard error (SE) of independent biological samples (n=3). Genes are grouped in three classes (A, B, and C) based on their response to regular circadian cycles (open squares).
In plants recovering from extended darkness, all of the class II TPS genes had significantly higher transcript levels at the end of the dark period compared with control plants at the end of an 8h night. Transcript levels fell between 8 and 16h in the first light period after extended darkness (2 and 10 p.m.) and returned to normal levels by 24h after shading ended. Dark stress resulted in reduced expression of ZmTPSI.1.1 at a time it normally peaks during day/night cycle, and then returned to cycling similar to the control plants. A similar but attenuated pattern was observed for ZmTPPA.3 and ZmTPPB.1.3. Among the putatively upregulated SnRK1 targets, two responded accordingly: ZmAKINβ and ZmARG10 were both decreased during the day and increased at night in control plants (Fig. 3). These genes were induced by extended darkness and then repressed during the day during recovery, similarly to all class II TPS genes tested. Among the putatively downregulated targets, two, ZmbZIP11 and ZmDPS, responded as expected: under extended darkness they were strongly repressed and then induced during recovery.
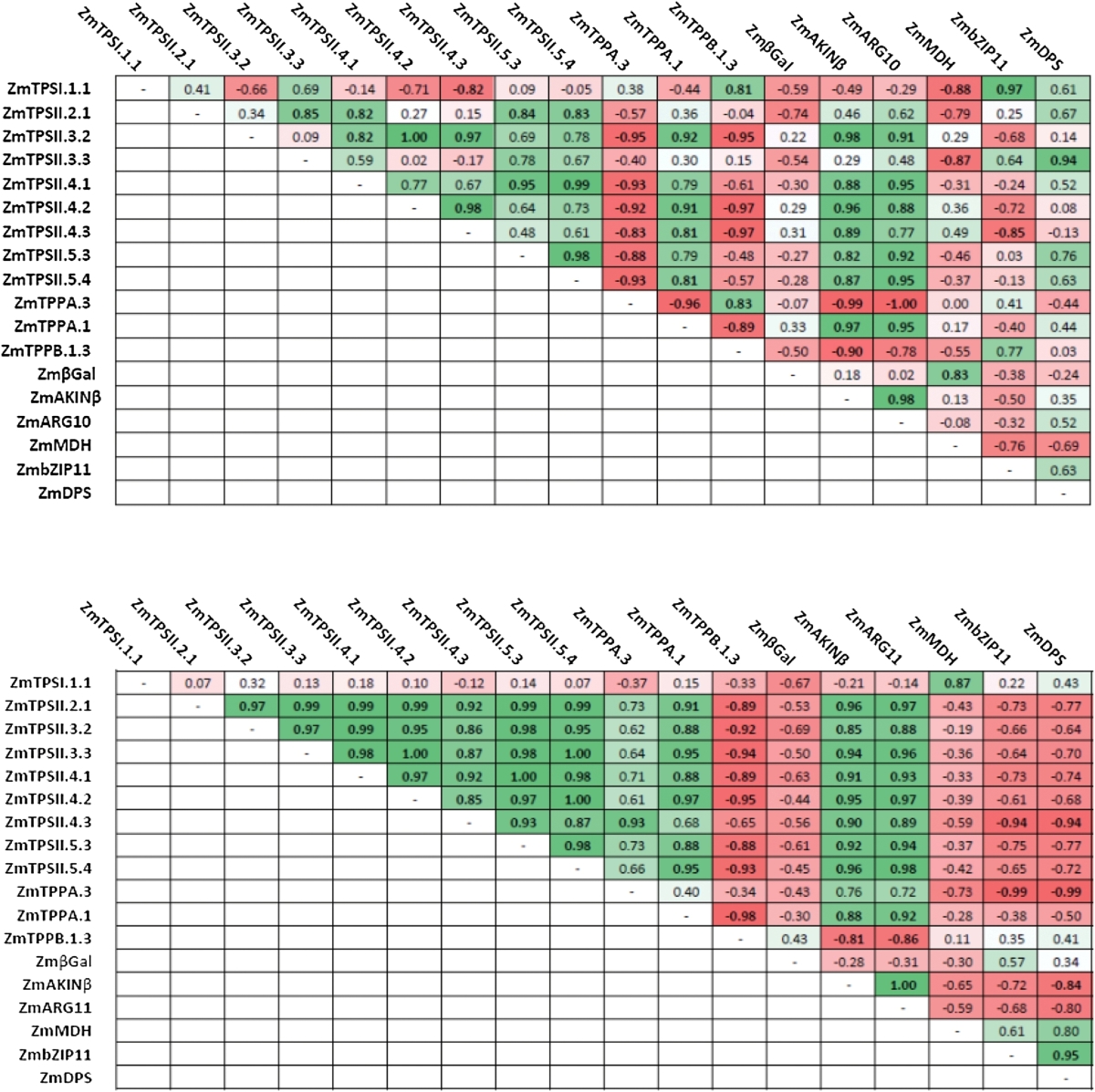
To determine if genes were regulated in a similar fashion, we determined their coefficient of correlation in control conditions and in dark-treated plants in the 24h following the treatment (Table 2). In control conditions, expression of some class II TPS genes positively correlated to each other, while most of them negatively correlated with ZmTPPA.3 expression. Most of class II TPS genes positively correlated with upregulated targets of SnRK1 (ZmAKINβ and ZmARG10). Other correlations were not as clear. In dark-treated plants, correlations between gene expression were much more obvious. All class II TPS transcripts were positively correlated to each other, to ZmTPPA.1, and to SnRK1 upregulated targets. In contrast, their expression was negatively correlated to ZmTPPB1.3 expression and negatively correlated with putative SnRK1 downregulated targets (ZmbZIP11 and ZmDPS). Expression of ZmTPSI.1.1 positively correlated with ZmMDH in dark-treated plants.
Table 2.
Correlation coefficient between TPS/TPP and SnRK1 target transcriptsCoefficients of correlation were determined over 24h after control (top) and dark (bottom) treatment using a Pearson comparison test (n=3). Positive and negative correlations are indicated in shades of green and red, respectively.
Soluble sugars and starch in cycling and dark-treated plants
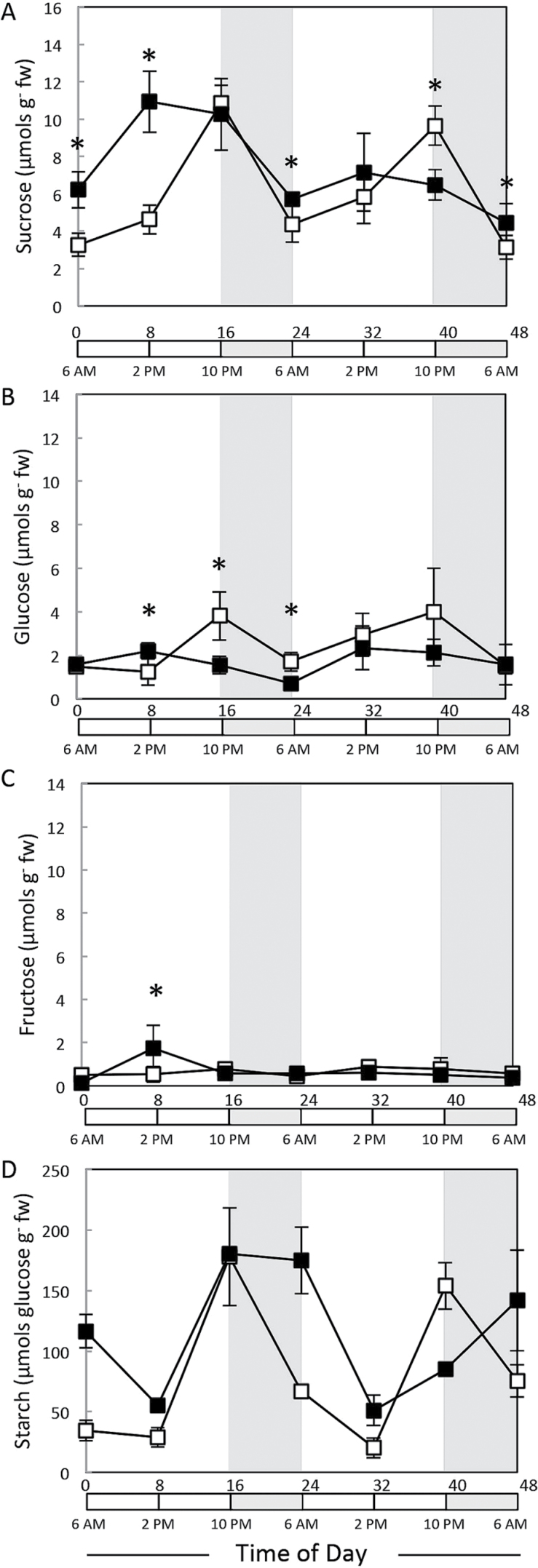
Under regular diurnal cycling, concentrations of sucrose (Fig. 4A) and starch (Fig. 4D) were lowest in the morning (6 a.m.), rose slightly in the first 8h of light, strongly between 8 and 16h in the light, and decreased overnight. Starch levels were higher than sucrose at dusk, indicating that it was the major transient carbon store in maize. The delay in the onset of starch accumulation resembled that seen in Arabidopsis in long photoperiods (Sulpice et al., 2014). Extended starvation stress affected starch and sucrose accumulation, but each in a different way (Fig. 4; filled squares). Unexpectedly, both were higher in leaf 3 at the end of a 48h period of shading than at the end of the 8h night, including a 3-fold higher level of starch. This was in contrast to whole Arabidopsis rosettes, where starch and sucrose were very low after 48h of darkness (Usadel et al., 2008). During recovery, sucrose accumulated during the light period, but this increase occurred earlier, by at least 8h (Fig. 4A). This response was attenuated on the second day, indicating a return to a regular diurnal pattern of regulation. During recovery from shading, starch showed a dramatically different response to that in an undisturbed light/dark cycling. Starch decreased during the first 8h in the light and rose to a high level at dusk similar to that in control plants but remained high at the end of the night instead of being degraded (Fig. 4D). The decrease in starch during the first part of the light period coincided with an increase in sucrose levels (compare Fig 4A and D). Glucose and fructose had low levels and a less distinct diurnal pattern, and shading had a minor effect on their levels (Fig. 4B and C).
Fig. 4.
Temporal levels of soluble sugars and starch in maize control or shaded seedlings. Starch and soluble sugars were extracted from the V3 stage control (16h day/8h night, open squares) and shaded (48h, filled squares) plants. Sampling was done every 8h for 48h starting at the end of the night or extended dark period (recovery phase). Sucrose (A, E), glucose (B, F), fructose (C, G), and glucose derived from hydrolysed starch (D, H) was determined by capillary high pressure ion chromatography. Values are presented as means±SE of independent biological replicates (n=5).
T6P response to diurnal cycling and recovery from extended darkness
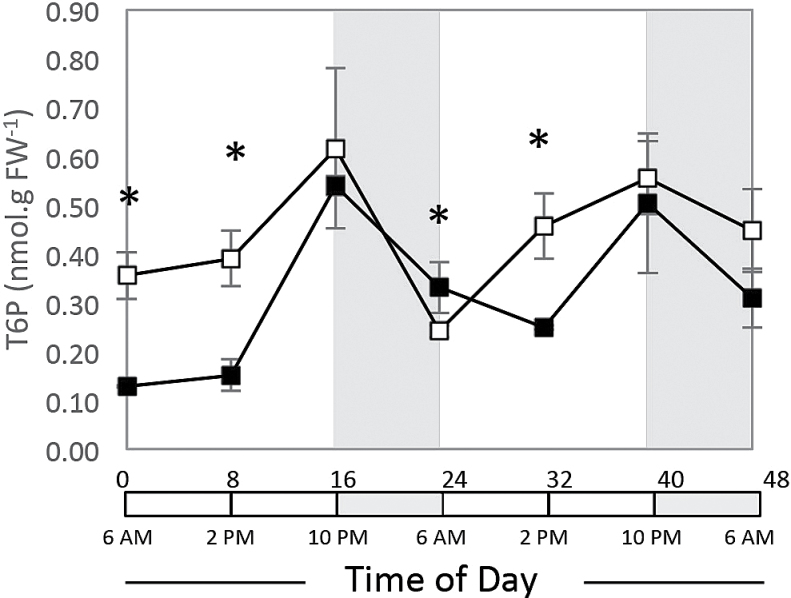
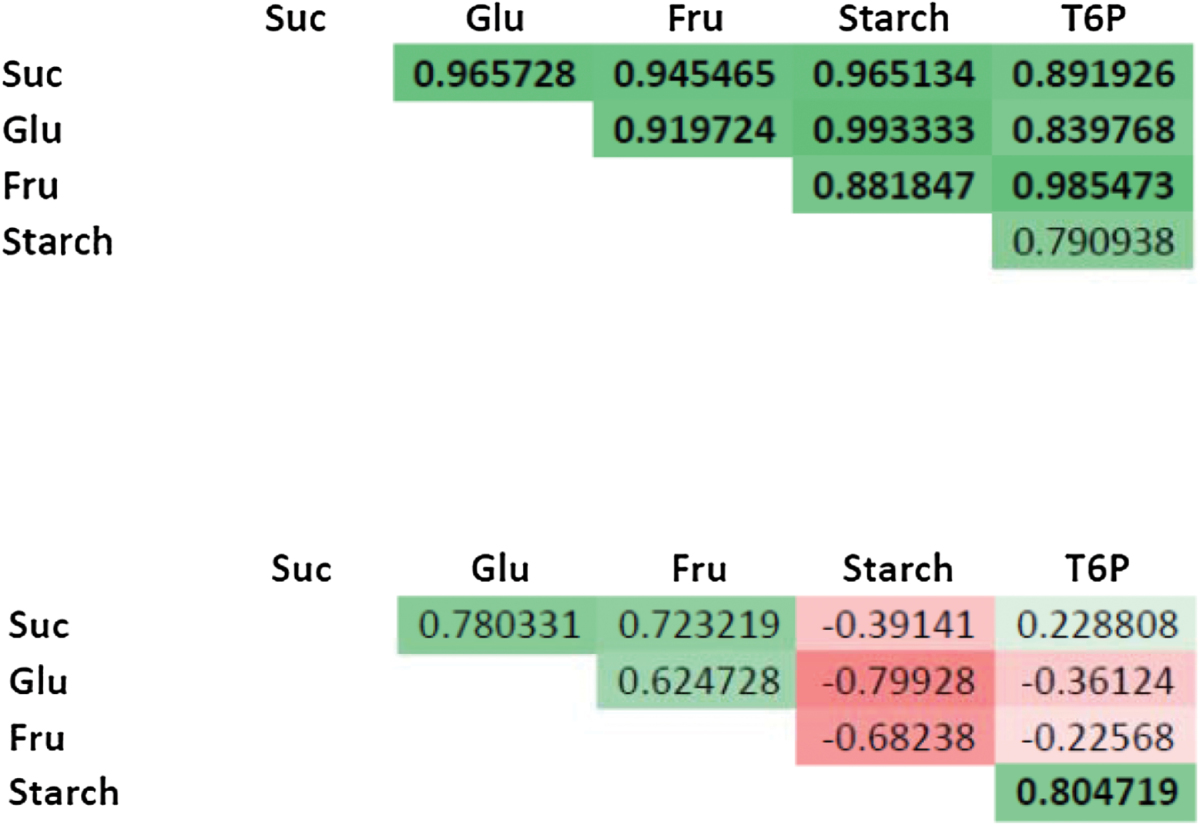
T6P levels were measured using liquid chromatography coupled with tandem mass spectrometry (Fig. 5, Table 3). Under regular diurnal cycles, T6P levels were low in the morning, went up during the day to reach their maximum in the evening, and then decreased overnight. After 48h of darkness, T6P levels were significantly lower than in control plants in the morning and at noon, and then rose to reach levels like those in the control in the evening. During the night, T6P levels decreased but less than in control plants, and again were lower than in controls at noon the on the second day of recovery, and then rose to levels like those in the control at dusk. Additionally, T6P levels were correlated with sugar and starch levels throughout the diurnal cycle but not after extended darkness (Table 3).
Fig. 5.
Temporal levels of T6P in maize control or shaded seedlings. Sugars were extracted from the V3 stage control (16h day/8h night, open squares) and shaded (48h, filled squares) plants. Sampling was done every 8h for 48h starting at the end of the night or extended dark period (recovery phase). T6P levels were determined using reversed-phase liquid chromatography, linked to tandem mass spectrometry. Values are presented as means±SE of independent biological replicates (n=3).
Table 3.
Correlation coefficients between sugars, starch, and T6PCoefficients of correlation were determined over 24h after control (top) and shading (bottom) treatment using a Pearson comparison test (n=3–6). Positive and negative correlations are indicated in shades of green and red, respectively.
TPS/TPP gene expression versus carbohydrate and T6P levels
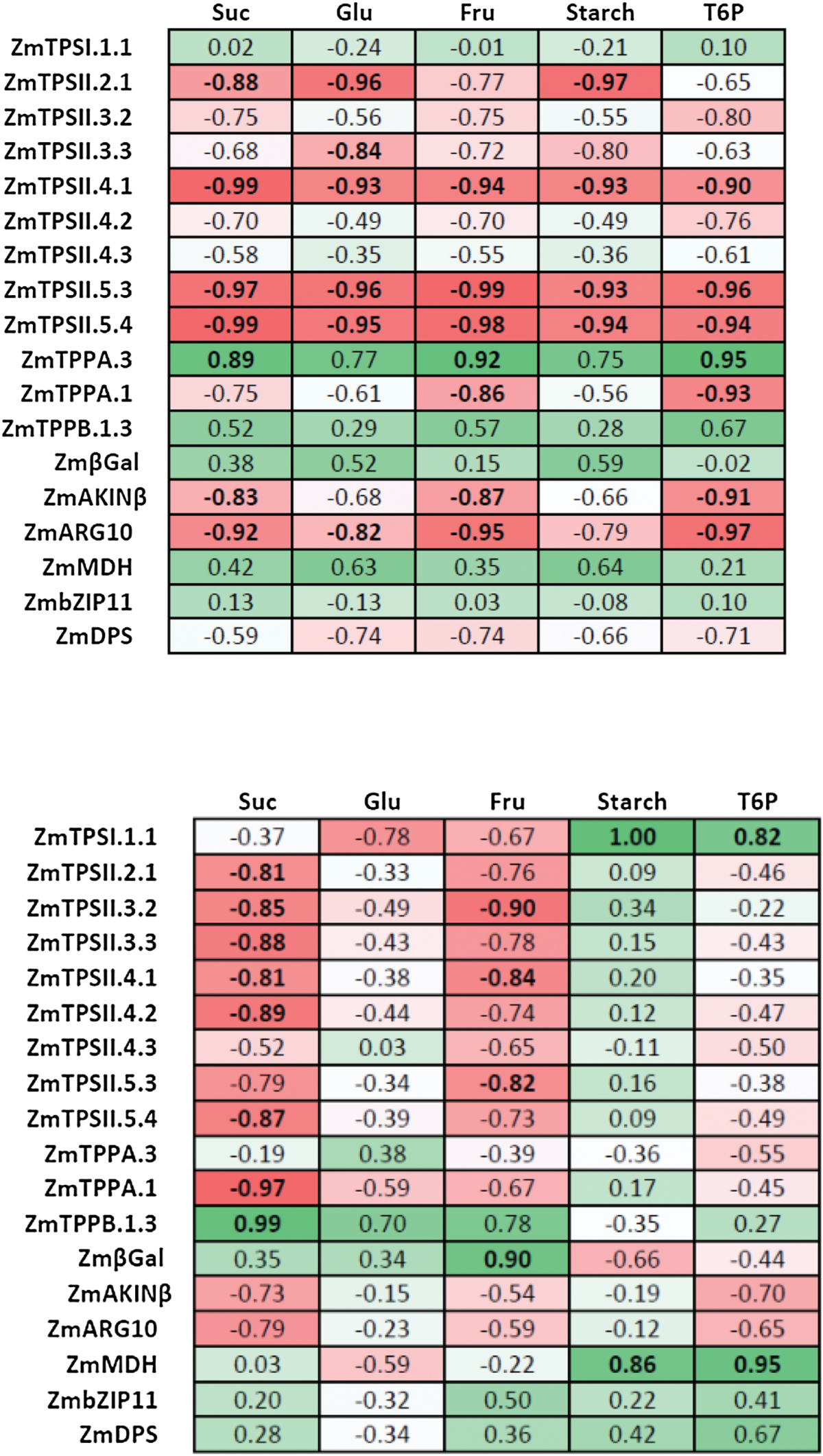
To help visualize the relationship between TPS/TPP gene expression and sugar levels, we determined the correlation between sugar and transcript levels over the first 24h of treatment using a Pearson test (Table 4). Under control conditions, transcript levels of some class II TPS (ZmTPSII.4.1, -5.3, and -5.4) and SnRK1 upregulated (ZmAKINβ and ZmARG10) targets negatively correlated with T6P, sucrose, and fructose, and sometimes with glucose and starch. ZmTPPA.1 behaved similarly and correlated negatively with T6P and fructose. ZmTPSII.2.1 negatively correlated with sucrose, glucose, and starch, while ZmTPSII.3.3 correlated negatively with glucose and starch only. Conversely, ZmTPPA.3 expression correlated positively with T6P, sucrose, and fructose levels. In plants recovering from extended darkness, most class II TPS transcript levels tended to correlate negatively with sucrose and sometimes fructose levels. Conversely, ZmTPPB.1.3 correlated with sucrose levels. Interestingly both ZmTPSI.1.1 and ZmMDH (malate dehydrogenase) correlated strongly with T6P and starch levels.
Table 4.
Correlation coefficients between transcripts and sugarsCoefficients of correlation were determined over 24h after control (top) and dark (bottom) treatment using a Pearson comparison test (n=3–6). Positive and negative correlations are indicated in shades of green and red, respectively.
Discussion
Crop productivity depends on the efficient conversion of solar energy into grain and biomass. Many of these efficiencies are realized through the carefully orchestrated metabolic switch between day and night. This large-scale metabolic switch is closely regulated by the circadian clock and through the sensing of intracellular sugar levels (Bläsing et al., 2005). Over the last decade, the trehalose pathway and its intermediate precursor, T6P, have surfaced as key regulators of carbohydrate metabolism, growth, and development in several plant species (reviewed by Paul et al., 2008; Schluepmann et al., 2011; Lunn et al., 2014). Much of the research on the trehalose pathway has used the model C3 plant Arabidopsis. However, to date little is known of the TPS/TPP gene family and the function of the trehalose pathway and T6P in the regulation of carbon flow and energy status in a C4 cereal crop such as maize.
TPS pathway is conserved in maize
The maize genome encodes families of 14 TPS and 11 TPP genes, while the TRE gene is present in a single copy, which is quite similar to what has been observed in rice, Arabidopsis, and poplar (reviewed by Avonce et al., 2006; Lunn, 2007; Li et al., 2008; Paul et al., 2008; Yang et al., 2012). According to evolutionary theories, the duplication process started earlier for TPS genes than for TPP genes. TPS genes have more common ancestors before the split between monocot and dicot species than do TPP genes, which were duplicated more after this event (Fig. 2). The maize class I full-length TPS gene has 16 introns, while most class II TPS genes have two introns (Fig. S1), similar to rice, Arabidopsis, and poplar (Yang et al., 2012). Maize TPP genes have a high number of introns (7–10), with the exception of ZmTPPB.1.2 and -1.3, which respectively display five and three introns (Supplementary Fig. S1 at JXB online). These results indicate a striking conservation of gene structure across species.
At least one class I TPS gene encodes a catalytic TPS. The maize genome has two class I TPS genes (Fig. 1A, Table 1), with one of them (ZmTPSI.1.1, also called ZmTPS1) encoding a functional enzyme (Jiang et al., 2010). The other (ZmTPSI.1.2) has a truncated TPS domain, which makes it unlikely to be functional. Since its sequence is very similar to ZmTPSI.1.1, it may have been duplicated recently, and its function remains unknown. Class I TPS genes also harbour a TPP domain; however, they lack some of the motifs forming the active site of phosphatase proteins belonging to the HAD superfamily (Lunn, 2007; Vandesteene et al., 2010). The maize genome encodes 12 class II TPS genes in four subclades (A2–A5) that include sequences from both monocot and dicots (Fig. 2A). Their structure is similar to Arabidopsis class II TPS genes, which have both TPS and TPP domains. As shown in Table 1, they all display both TPS and TPP domains with numerous substitutions in amino acids that are essential for substrate binding and conserved phosphatase motifs (Avonce et al., 2006; Lunn, 2007; Vandesteene et al., 2010). The role for the class II TPS enzymes has been mostly undefined (Chary et al., 2008; Singh et al., 2011); however, the class II TPS genes display remarkable differential spatial and temporal expression patterns in Arabidopsis (Ramon et al., 2009). To understand better the possible function for the maize class II TPS proteins, we reviewed the raw data from public genome-wide transcript analyses with attention to class II TPS genes. There were numerous instances where TPS/TPP transcripts showed remarkable spatial and temporal specificity; ZmTPSII.2.2 in the ovule, ZmTPSII.3.3, -4.2, -5.3, and -5.4 in the leaf, and ZmTPSII.5.3 in the endosperm (Davidson et al., 2011; Sekhon et al., 2011). Based on the pattern of expression observed during the diurnal cycle and recovery from darkness, we suggest that the maize class II TPS enzymes play a regulatory role in responding to and/or managing energy resources in seedling leaf tissue, perhaps through its interaction with sugar phosphates.
Close examination of the substrate binding and catalytic domains of the maize class II TPS proteins suggests that this group may not possess catalytic activity (Table 1). R391 has been shown to be required for binding UDPG in the catalytically active TPS1. Substitution of R391 with an aspartate residue in all maize class II TPS proteins may abolish enzymatic activity. This corresponds to the absence of TPS activity observed for most class II TPS in Arabidopsis (Vandesteene et al., 2010). Maize class II genes from subclade 5 display more variations in the binding site for UDPG than genes from subclades 2, 3, and 4. Few substitutions are observed, however, in the G6P-binding site (except a minor substitution of T284S). One possible explanation is that the class II TPS proteins have lost their catalytic activity but have retained the binding site for G6P. This may indicate a sensing as opposed to a catalytic function. Such is the case for the plant pathogenic fungi Magnaporthe grisea TPS1 gene, which has a regulatory function in the pentose pathway required for fungal virulence. This involves G6P binding without formation of T6P, and the association with a regulator protein, TPS3 (Wilson et al., 2007). Since plant and fungal trehalose pathways are somewhat similar (Avonce et al., 2010), a similar process could occur in maize. The existence of high-molecular-weight TPS complexes has already been demonstrated in rice (Zang et al., 2011).
Similar to TPP from other plants, maize TPPs consist of a TPP domain with three conserved phosphatase domains required for activity. Only ZmTPPB.2.1, also called RA3, was demonstrated as a functional TPP enzyme in maize where it controls inflorescence branching (Satoh-Nagasawa et al., 2006; Carillo et al., 2013). Genes belonging to subclade A2, A3, or B2 are found only in monocot species, i.e. maize, rice, or sorghum (data not shown), which means that they could have arisen later in evolution or have been lost in dicots. As with TPS genes, several TPP genes show spatial and temporal expression patterns; ZmTPPA.3 and ZmTPPB.1.3 in the leaf, ZmTPPB.2.2 and -2.3 in anthers and pollen, ZmTPPA.3 in roots, and ZmTPPB2.1 in the endosperm, suggesting tissue-specific functions (Davidson et al., 2011; Sekhon et al., 2011).
TPS/TPP genes show a diurnal pattern of expression
Diurnally expressed genes participate in growth, development, reproduction, and metabolism (Smith et al., 2004; Bläsing et al., 2005; Osuna et al., 2007). So far, the relationship between the trehalose pathway genes and maintenance of energy balance throughout the day/night cycle is not well defined. TPS/TPP gene expression was shown in Arabidopsis to be sensitive to sucrose depletion (Thimm et al., 2004; Lunn et al., 2006). In maize, the highest mRNA levels for class II TPS genes were at the end of the regular 8h night period, corresponding to the lowest levels of sucrose, starch, and T6P (Fig. 6A). These results agree with those seen in Arabidopsis (Lunn et al., 2006; Wahl et al., 2013). In maize seedlings subjected to a typical diurnal cycle (16h day/8h night), we observed that all class II TPS genes demonstrated a diurnal pattern of gene expression with transcript levels decreasing throughout the day and increasing throughout the night (Fig. 3). Interestingly, T6P levels in the same samples showed a distinct diurnal pattern with levels increasing throughout the day and decreasing throughout the night (Fig. 5). Debast et al. (2011) used transgenic potato tubers to produce artificially elevated or reduced T6P levels. They observed that elevated T6P resulted in a reduction of transcripts for two class II TPS genes (TPS8 and TPS11), and repressed T6P levels induced the transcription of these genes. These results in potato corroborate our observation in maize that class II TPS transcripts are inverted with respect to T6P levels throughout the diurnal cycle.
Fig. 6.
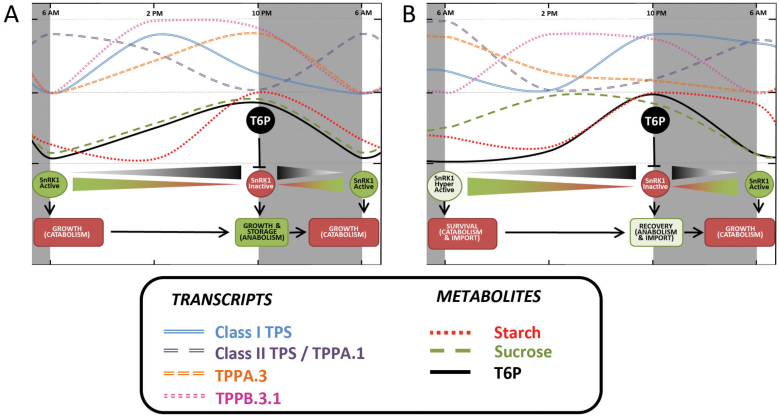
Model depicting a role for the maize trehalose pathway in regulating sugar metabolism and growth under regular day/night cycles and extended darkness in juvenile maize leaves. The model is based on data from the present and previously published work (Baena-González et al., 2007; Zeeman et al., 2007; Zhang et al., 2009; Ghillebert et al., 2011; Wahl et al., 2013) (A) Under regular day/night cycles, as day proceeds, photosynthesis induces the accumulation of sucrose and T6P. Rising levels of T6P gradually promote the inactivation of SnRK1 and the redox activation of AGPase in order to turn on anabolism, growth, and starch accumulation. During the night, starch and sucrose are used as a source of carbon and energy for growth to continue. Their levels, as well as those of T6P, decrease during the night and are low in the morning. Low T6P levels induce: (i) a minimal AGPase (starch synthesis enzyme) activation, preventing starch synthesis; and (ii) a maximal activation of SnRK1 (major energy sensor), enabling growth by partially activating catabolism through starch/sugar consumption. (B) During extended darkness, sucrose and starch immediately available are rapidly used. In order to keep cell stasis, leaf cells slow down metabolism and mobilize alternative carbon sources other leaves, roots, or the attached seed. This generates an accelerated accumulation of remobilized sucrose, negatively correlating with very low T6P levels and a strong activation of SnRK1 at the end of the stress period. SnRK1 activity correlates with the induction of ZmAKINβ and ZmARG10 (positive SnRK1 targets) and all the class II TPS genes, and the repression of ZmbZIP11 and ZmDPS (negative SnRK1 targets) by the end of extended dark period. During recovery, 8h after stress relief, the opposite phenomenon occurs. Photosynthesis is turned on when there remains an abundant supply of imported sucrose, while T6P levels continue to be low because of the perceived stress. Our results indicate that the cell detects intracellularly derived sucrose independently of that which is imported, possibly sensed through hexokinase. Eight hours after returning to the light, T6P levels remain low; however, they increase enough to inactivate SnRK1. Transcript levels change, with a strong repression of ZmAKINβ and ZmARG10 (positive SnRK1 targets) and all the Class II TPS genes, and there is induction of ZmbZIP11 and ZmDPS (negative SnRK1 targets). These SnRK1/T6P-mediated changes result in the switch from growth-from-catabolism to growth-from-anabolism. After 24–48h of recovery, the plant goes back to its regular cycle at the transcriptional level, while metabolites levels are still being affected.
Circadian clock-regulated genes participate in a large number of physiological processes, preparing the plant for the rhythmic change in its environment. In Arabidopsis, as much as 30% of the expressed transcripts cycle every 24h under constant light and temperature (Covington et al., 2008). The rhythmic control of circadian-regulated genes continues after cycling environmental cues, i.e. light and temperature, have been removed (Doherty and Kay, 2010; Khan et al., 2010). Kahn et al. (2010) set the circadian clock by exposing maize seedlings to 12h light/12h dark, and then switched to continuous light for 48h to identify those genes regulated by the circadian clock. They identified >1300 transcripts that maintained a circadian rhythm even after being switched to continuous light. Here, we identified four TPS genes that were among the data they collected; ZmTPSI.1.1 and ZmTPSII.5.3, -2.1, and -3.2. For these four TPS genes, transcript levels drift along with complete loss of circadian cycling. At least for these four TPS genes, it can be concluded that gene expression is not regulated by the circadian clock but rather by the energy status of the cell (Gibon et al., 2004).
All of the maize TPS and TPP genes examined in this work showed some degree of diurnal cycling and, based on the results of Khan et al. (2010), are regulated by energy status as opposed to an internal clock. This provides further evidence that regulation of the trehalose pathway is tightly linked to sugar levels and plays an important role in maintaining sensing and energy stasis. Usadel et al. (2008) observed transcriptome changes in vegetative Arabidopsis rosettes throughout the diurnal cycle and after 4h of extended night. Examination of their data revealed that, as with maize, under regular diurnal cycling all class II TPS genes showed diurnal cycling with highest expression at the end of the night period, with the exception of AtTPS5, which has no homologue in maize (Figs 2 and S3B).
In contrast to the class II genes, ZmTPSI.1.1 is expressed at its lowest levels during the night and rises during the day, which is out of phase with sucrose and T6P levels, again in agreement with that seen with all class I genes in Arabidopsis (Fig. S3A) (Usadel et al., 2008). Low transcript levels at the end of night and at end of the extended darkness indicate that ZmTPSI.1.1 is not likely to be under the control of SnRK1 as with the class II genes (Fig. 6). ZmTPSI.1.1 is perhaps regulated at the post-translational level through interaction with specific kinases, e.g. SnRK1, phosphatases, or class II TPS proteins (Glinski and Weckwerth, 2005; Harthill et al., 2006). We also observed that gene expression patterns for the three predominant maize TPP genes, ZmTPPA.3, ZmTPPB.1.3, and ZmTPPA.1, was varied and showed unique patterns of expression. ZmTPPA.3 and ZmTPPB.1.3 had the lowest expression at the end of the night period, in contrast to what was observed for class II TPS transcripts, and is consistent with their role in the dephosphorylation of T6P (Lunn et al., 2006). ZmTPPA.1 followed an expression pattern similar to the class II TPS genes, with the highest expression at the end of the dark period. This variable pattern for TPP genes is the same as seen in Arabidopsis (Fig. S3C) (Usadel et al., 2008).
Effect of extended darkness on energy status and TPS/TPP gene expression
It has been shown previously that reducing light by as little as 30% can have significant impact on grain production and total biomass yield in maize (Earley et al., 1966). Setter et al. (2001) imposed 5 d of shade stress on flowering maize plants. They observed a 66% reduction in kernel dry matter production, along with a 20–50% reduction in floret carbohydrates. Here, we also observed that extended darkness (48h) had a significant effect on starch and sucrose levels in maize seedlings (Fig. 4). Surprisingly, 48h of darkness resulted in higher levels of sucrose and starch, suggesting a slowing of carbon usage for growth including cell expansion (Fig. 2) and the mobilization of carbohydrate reserves stored in other leaves, roots, or the attached seed. During a regular light/dark cycle, the plant draws on cellular reserves of sugar and starch to fuel metabolism and growth. Our results indicate that, during prolonged darkness, the plant enters a metabolic stasis in order to survive.
Perhaps most surprising was the observation that when plants were returned to a light/dark cycle after extended darkness, T6P levels no longer followed the same time course as sucrose and hexose sugars. When Arabidopsis plants were subjected to extended nights (Usadel et al., 2009), during leaf senescence (Wingler et al., 2012), were starved for carbon (Yadav et al., 2014), or in the maize seedling leaf under a regular diurnal cycle (Fig. 6), T6P levels always followed the same time course as sucrose and hexose sugars. We suggest that, after a period of darkness, leaf 3 does not sense sugars imported from other parts of the plant in the same manner as cellular-derived sugar sources. Certainly the cell does not appear to sense imported sugars through T6P or SnRK1. The difference may lie in the mechanism of sucrose degradation i.e., invertase, sucrose synthase, and the products of these reactions, i.e. glucose, fructose, UDPG, or modifications in the sucrose sensing pathways.
Schussler and Westgate (1995) suggested that it is the flux of carbohydrates into the developing ovary as opposed to sugar concentration per se that determines kernel set. A possible mechanism for sugar sensing could be through the rapidly turning over pool of intermediates such as UDPG and G6P (Setter et al., 2001). These are also substrates for TPS, or are capable of binding to a catalytically inactive TPS protein. Usadel et al. (2009) observed the effect of extending the night by an additional 6h for maize seedlings. Using the maize 18K Affymetrix chip, they found that extending the dark (similar to short days for Arabidopsis) resulted in a 2- to 4-fold increase in transcripts for several class II TPS genes (ZmTPSII.4.1, -5.2, and -5.3), suggesting that class II TPS enzymes participate in maintaining the survival state through its sensing of sugars. We observed that all class II TPS transcripts that were typically at their highest level at 6 a.m. were several orders of magnitude higher after 48h of darkness, and dropped rapidly as sucrose levels rose in the light (Fig. 3). These results suggest an important role for the maize class II TPS enzymes in prolonging survival and in recovering from extended darkness. As before, Arabidopsis class II TPS genes are induced by extended night, with the exception of AtTPS5 (Usadel et al., 2008). Osuna et al. (2007) starved Arabidopsis seedlings grown in liquid culture under low light by withholding sucrose for 48h. They observed a rapid (30min) sucrose-dependent alteration in transcripts for more than 1000 genes, including a decrease in AtTPS8, AtTPS9, AtTPS10, and AtTPS11. One possible explanation for the pattern seen in Fig. 4A is that class II genes are expressed when sucrose levels are low and SnRK1 is active. This result is consistent with the transcriptional co-regulation of various TPS genes by energy related stresses (sucrose starvation, darkness, etc.), and the SnRK1 catalytic subunit KIN10 in Arabidopsis (Baena-González, 2007; Ghillebert et al., 2011).
A very different response was observed for the catalytically active ZmTPSI.1.1 in that extended darkness resulted in repression of the transcript at a time it normally peaks during the diurnal cycle (2 p.m.). Indeed we found that, in plants recovering from extended darkness, the ZmTPSI.1.1 transcript closely mimicked starch levels (R 2=99%). The relationship between the ZmTPSI.1.1 transcript and starch levels after extended darkness indicates a metabolic shift from short-term sugar depletion (8h) to long-term absence of photosynthate (48h) with a possible role for starch in the formation, hydrolysis, and/or sensing to regulate ZmTPSI.1.1 levels. This hypothesis could be supported by the results of Scialdone et al. (2013), indicating that Arabidopsis plants sense both starch and day length in order to regulate starch degradation rate. Such a phenomenon could be involved in regulation of target metabolic genes to enable the plant to adjust its environment according to its internal resources.
Role of TPS, TPP, and T6P in sugar sensing and maintenance of energy status
Based on literature from Arabidopsis and potato, as well as our results, we present a model for the regulation of energy balance throughout the diurnal cycle and the recovery from extended darkness that incorporates the trehalose pathway genes T6P and SnRK1 (Fig. 6). During a typical night period, starch is consumed to maintain growth and cellular metabolism. Starch breakdown provides less sucrose than carbon fixation in the light, and thus sucrose levels fall as reflected by a decrease in T6P levels, with a peak at dusk and a minimum at dawn (Lunn et al., 2006; Wahl et al., 2013). This occurs coincidentally with an observed increase in transcription of SnRK1 target (inducible) genes (Usadel et al., 2008) (Supplementary Fig. S3D, E). AGPase is then inactivated by changes in allosteric regulators and by light- and sucrose-dependent post-translational redox modification, while starch degradation is stimulated (Tiessen et al., 2003; Gibon et al., 2004; Kolbe et al., 2005; Lunn et al., 2006). SnRK1 activity is also induced during the night as the plant enters sink mode (Baena-González et al., 2007), correlated with the transcription of class II TPS genes in maize, such that their peak expression is at the end of the night period, and this may result in SnRK1-mediated phosphorylation of some TPS1 (Glinski and Weckwerth, 2005), resulting in feedback regulation of the trehalose pathway. Upon re-illumination, sucrose and starch accumulate and T6P levels rise (Wahl et al., 2013), inhibiting SnRK1 (Zhang et al., 2009). This is accompanied by the activation of AGPase, repression of starch degradation, upregulation of ZmTPSI.1.1 gene expression, and downregulation of class II TPS transcription. These transcriptional and metabolic changes are consistent with cell growth, with its optimum at the end of the day.
Interestingly, in maize seedling leaf tissue, each of the eight class II TPS genes showed the same pattern of transcript induction during the night, although these genes show quite varied expression throughout development and in response to environmental stimuli (Covington et al., 2008; Wahl et al., 2013). The only maize TPS gene known to have catalytic function, ZmTPSI.1.1, was not induced after extended darkness; however, was induced during the afternoon. Extended darkness resulted in even lower transcript levels for ZmTPSI.1.1, an indication that transcriptional regulation of this TPS gene is critical during normal growth and not while the plant is subjected to prolonged darkness. One can infer from this that: (i) ZmTPSI.1.1 expression does not require SnRK1 to be active; or (ii) its transcriptional regulation is not important in the production of stress-induced T6P. Our attention now turns to the class II TPS genes in maize in regard to their role in sugar metabolism and during the recovery from extended darkness.
Conclusions
The maize family of trehalose biosynthetic enzymes offers a fascinating system for the characterization of energy management with respect to sucrose and starch, and how it contributes to crop productivity and stress tolerance. Regarding the present results, recovery from extended darkness probably involved the participation of class II TPS proteins. It is of great interest to determine their role in this process, whether they can function catalytically alone or as regulatory elements of a high-molecular-weight complex, or if they act as signalling molecules or transcription factors. Further investigation into protein–protein interactions will validate this hypothesis. The use of mutants and transgenic plants will facilitate our understanding of how each TPS and TPP enzyme contributes to what is undoubtedly a complex regulatory apparatus. We also observed that extended darkness disrupted the connection between sucrose and T6P, suggesting multiple sucrose sensing pathways operating simultaneously.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Methods S1. Methods for sugars and starch analysis.
Supplementary Method S2. Formulae for sugar and starch (glucose) analysis.
Supplementary Table S1. Gene names and accession numbers for maize, rice, Arabidopsis, and poplar TPS and TPP.
Supplementary Table S2. Sequence of primers used for quantitative PCR.
Supplementary Fig. S1. Gene structures with introns for maize TPS I and II genes.
Supplementary Fig. S2. Predicted enzymatic domains for maize TPS and TPP genes.
Supplementary Fig. S3. Arabidopsis gene expression
Acknowledgements
Authors would like to thank Drs Harkamal Walia and James Specht/George Graef laboratories for sharing their equipment to grind tissue samples and perform quantitative PCR analysis with us. We also want to thank Drs Soulaiman Sakr (Agrocampus-ouest, INHP) and John Lunn (Max Planck Institute) for their time and valuable comments to significantly improve the manuscript.
Glossary
Abbreviations:
- G6P
glucose-6-phosphate
- HXK
hexokinase
- RT
reverse transcription
- SE
standard error
- T6P
trehalose-6-phosphate
- TPP
trehalose-6- phosphate phosphatase
- TPS
trehalose-6-phosphate synthase
- TRE
trehalose
- UDGP
uridine diphosphate glucose.
References
- Ali Q, Ashraf M. 2011. Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: growth, photosynthesis, water relations and oxidative defence mechanism. Journal of Agronomy and Crop Science 197, 258–271 [Google Scholar]
- Avonce N, Mendoza-Vargas A, Morett E, Iturriaga G. 2006. Insights on the evolution of trehalose biosynthesis. BMC Evolutionary Biology 6, 109–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avonce N, Wuyts J, Verschooten K, Vandesteene L, Van Dijck P. 2010. The Cytophaga hutchinsonii ChTPSP: first characterized bifunctional TPS-TPP protein as putative ancestor of all eukaryotic trehalose biosynthesis proteins. Molecular Biology and Evolution 27, 359–369 [DOI] [PubMed] [Google Scholar]
- Baena-González E, Rolland F, Thevelein JM, Sheen J. 2007. A central integrator of transcription networks in plant stress and energy signalling. Nature 448, 938–942 [DOI] [PubMed] [Google Scholar]
- Bläsing O, Gibon Y, Günther M, Hohne M, Morcuende R, Osuna D, Thimm O, Usadel B, Scheible W-R, Stitt M. 2005. Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell 17, 3257–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouquisse R, Gaudillere J, Raymond P. 1998. Induction of a carbon-starvation-related proteolysis in whole maize plants submitted to light/dark cycles and to extended darkness. Plant Physiology 117, 1281–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carillo P, Feil R, Gibon Y, Satoh-Nagasawa N, Jackson D, Bläsing OE, Stitt M, Lunn JE. 2013. A fluorometric assay for trehalose in the picomole range. Plant Methods 9, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chary SN, Hicks GR, Choi YG, Carter D, Raikhel N. V. 2008. Trehalose-6-phosphate synthase/phosphatase regulates cell shape and plant architecture in Arabidopsis. Plant Physiology 146, 97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington MF, Maloof JN, Straume M, Kay SA, Harmer SL. 2008. Global transcriptome analysis reveals circadian regulation of key pathways in plant growth and development. Genome Biology 9, R130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RM, Hansey CN, Gowda M, et al. 2011. Utility of RNA sequencing for analysis of maize reproductive transcriptomes. Plant Genome Journal 4, 191 [Google Scholar]
- Debast S, Nunes-nesi A, Hajirezaei MR, Hofmann J, Sonnewald U, Fernie AR, Bornke F. 2011. Altering trehalose-6-phosphate content in transgenic potato tubers affects tuber growth and alters responsiveness to hormones during sprouting 1. Plant Physiology 156, 1754–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatte TL, Sedijani P, Kondou Y, Matsui M, de Jong GJ, Somsen GW, Wiese-Klinkenberg A, Primavesi LF, Paul MJ, Schluepmann H. 2011. growth arrest by trehalose-6-phosphate: an astonishing case of primary metabolite control over growth by way of the SnRK1 signaling pathway. Plant Physiology 157, 160–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty CJ, Kay SA. 2010. Circadian control of global gene expression patterns. Annual Review of Genetics 44, 419–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley EB, Miller RJ, Reichert GL, Hageman RH. 1966. Effects of shade on maize production under field conditions. 6, 1–7 [Google Scholar]
- Ghillebert R, Swinnen E, Wen J, Vandesteene L, Ramon M, Norga K, Rolland F, Winderickx J. 2011. The AMPK/SNF1/SnRK1 fuel gauge and energy regulator: structure, function and regulation. FEBS Journal 278, 3978–3990 [DOI] [PubMed] [Google Scholar]
- Gibon Y, Bläsing OE, Palacios-Rojas N, Pankovic D, Hendriks JHM, Fisahn J, Höhne M, Günther M, Stitt M. 2004. Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the followin. The Plant Journal 39, 847–862 [DOI] [PubMed] [Google Scholar]
- Gibon Y, Pyl E-T, Sulpice R, Lunn JE, Höhne M, Günther M, Stitt M. 2009. Adjustment of growth, starch turnover, protein content and central metabolism to a decrease of the carbon supply when Arabidopsis is grown in very short photoperiods. Plant, Cell & Environment 32, 859–74 [DOI] [PubMed] [Google Scholar]
- Gibson RP, Turkenburg JP, Charnock SJ, Lloyd R, Davies GJ. 2002. Insights into trehalose synthesis provided by the structure of the retaining glucosyltransferase OtsA. Chemistry & Biology 9, 1337–46 [DOI] [PubMed] [Google Scholar]
- Glinski M, Weckwerth W. 2005. Differential multisite phosphorylation of the trehalose-6-phosphate synthase gene family in Arabidopsis thaliana: a mass spectrometry-based process for multiparallel peptide library phosphorylation analysis. Molecular & Cellular Proteomics 4, 1614–1625 [DOI] [PubMed] [Google Scholar]
- Harmer SL, Hogenesch JB, Straume M, Chang H-S, Han B, Zhu T, Wang X, Kreps JA, Kay SA. 2000. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290, 2110–2113 [DOI] [PubMed] [Google Scholar]
- Harthill JE, Meek SEM, Morrice N, Peggie MW, Borch J, Wong BHC, Mackintosh C. 2006. Phosphorylation and 14-3-3 binding of Arabidopsis trehalose-phosphate synthase 5 in response to 2-deoxyglucose. The Plant Journal 47, 211–223 [DOI] [PubMed] [Google Scholar]
- Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. 2007. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biology 8, R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Fu F-L, Zhang S-Z, Wu L, Li W-C. 2010. Cloning and characterization of functional trehalose-6-phosphate synthase gene in maize. Journal of Plant Biology 53, 134–141 [Google Scholar]
- Khan S, Rowe SC, Harmon FG. 2010. Coordination of the maize transcriptome by a conserved circadian clock. BMC Plant Biology 10, 126–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbe A, Tiessen A, Schluepmann H, Paul M, Ulrich S, Geigenberger P. 2005. Trehalose 6-phosphate regulates starch synthesis via posttranslational redox activation of ADP-glucose pyrophosphorylase. Proceedings of the National Academy of Sciences, USA 102, 11118–11123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Ma S, Bohnert HJ. 2008. Coexpression characteristics of trehalose-6-phosphate phosphatase subfamily genes reveal different functions in a network context. Physiologia Plantarum 133, 544–56 [DOI] [PubMed] [Google Scholar]
- Lunn JE. 2007. Gene families and evolution of trehalose metabolism in plants. Functional Plant Biology 34, 550. [DOI] [PubMed] [Google Scholar]
- Lunn JE, Delorge I, Figueroa CM, Van Dijck P, Stitt M. 2014. Trehalose metabolism in plants. The Plant Journal 49, n/a–n/a. [DOI] [PubMed] [Google Scholar]
- Lunn JE, Feil R, Hendriks JHM, Gibon Y, Morcuende R, Osuna D, Scheible W-R, Carillo P, Hajirezaei M-R, Stitt M. 2006. Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochemical Journal 397, 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Hanssen M, Lundgren K, et al. 2011. The sucrose-regulated Arabidopsis transcription factor bZIP11 reprograms metabolism and regulates trehalose metabolism. New Phytologist 191, 733–45 [DOI] [PubMed] [Google Scholar]
- Martínez-Barajas E, Delatte T, Schluepmann H, de Jong GJ, Somsen GW, Nunes C, Primavesi LF, Coello P, Mitchell RAC, Paul MJ. 2011. Wheat grain development is characterized by remarkable trehalose 6-phosphate accumulation pregrain filling: tissue distribution and relationship to SNF1-related protein kinase1 activity. Plant Physiology 156, 373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutisya J, Sun C, Jansson C. 2009. Circadian oscillation of starch branching enzyme gene expression in the sorghum endosperm. Plant Signaling & Behavior 4, 871–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes C, O’Hara LE, Primavesi LF, Delatte TL, Schluepmann H, Somsen GW, Silva AB, Fevereiro PS, Wingler A, Paul MJ. 2013. The trehalose 6-phosphate/SnRK1 signaling pathway primes growth recovery following relief of sink limitation. Plant Physiology 162, 1720–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuna D, Usadel B, Morcuende R, et al. 2007. Temporal responses of transcripts, enzyme activities and metabolites after adding sucrose to carbon-deprived Arabidopsis seedlings. The Plant Journal 49, 463–91 [DOI] [PubMed] [Google Scholar]
- Paul M. 2007. Trehalose 6-phosphate. Current Opinion in Plant Biology 10, 303–9 [DOI] [PubMed] [Google Scholar]
- Paul MJ, Primavesi LF, Jhurreea D, Zhang Y. 2008. Trehalose metabolism and signaling. Annual Review of Plant Biology 59, 417–441 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polge C, Thomas M. 2007. SNF1/AMPK/SnRK1 kinases, global regulators at the heart of energy control? Trends in Plant Science 12, 20–28 [DOI] [PubMed] [Google Scholar]
- Ponnu J, Wahl V, Schmid M. 2011. Trehalose-6-phosphate: connecting plant metabolism and development. Frontiers in Plant Science 2, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon M, De Smet I, Vandesteene L, Naudts M, Leyman B, Van Dijck P, Rolland F, Beeckman T, Thevelein JM. 2009. Extensive expression regulation and lack of heterologous enzymatic activity of the Class II trehalose metabolism proteins from Arabidopsis thaliana . Plant, Cell & Environment 32, 1015–1032 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Salazar J, Suárez R, Caballero-Mellado J, Iturriaga G. 2009. Trehalose accumulation in Azospirillum brasilense improves drought tolerance and biomass in maize plants. FEMS Microbiology Letters 296, 52–59 [DOI] [PubMed] [Google Scholar]
- Satoh-Nagasawa N, Nagasawa N, Malcomber S, Sakai H, Jackson D. 2006. A trehalose metabolic enzyme controls inflorescence architecture in maize. Nature 441, 227–230 [DOI] [PubMed] [Google Scholar]
- Schluepmann H, Berke L, Sanchez-Perez GF. 2011. Metabolism control over growth: a case for trehalose-6-phosphate in plants. Journal of Experimental Botany 63, 3379–3390 [DOI] [PubMed] [Google Scholar]
- Schussler JR, Westgate ME. 1995. Assimilate flux determines kernel set at low water potential in maize. Crop Science 35, 1074–1080 [Google Scholar]
- Sciences B, Zeid IM. 2009. Trehalose as osmoprotectant for maize under salinity-induced stress. Research Journal of Agriculture and Biological Sciences 5, 613–622 [Google Scholar]
- Sekhon RS, Lin H, Childs KL, Hansey CN, Buell CR, de Leon N, Kaeppler SM. 2011. Genome-wide atlas of transcription during maize development. The Plant Journal 66, 553–563 [DOI] [PubMed] [Google Scholar]
- Setter TL, Flannigan BA, Melkonian J. 2001. Loss of kernel set due to water deficit and shade in maize. Crop Science 41, 1530 [Google Scholar]
- Sheen J. 2010. Discover and connect cellular signaling. Plant Physiology 154, 562–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Louis J, Ayre BG, Reese JC, Shah J. 2011. TREHALOSE PHOSPHATE SYNTHASE11‐dependent trehalose metabolism promotes Arabidopsis thaliana defense against the phloem‐feeding insect Myzus persicae . The Plant Journal 67, 94–104 [DOI] [PubMed] [Google Scholar]
- Smith S, Fulton D, Chia T, Thorneycroft D, Chapple A, Dunstan H, Hylton C, Zeeman S, Smith A. 2004. Diurnal Changes in the transcriptome encoding enzymes of starch metabolism provide evidence for both transcriptional and posttranscriptional regulation of starch metabolism in Arabidopsis. Plant Physiology 136, 2687–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Zeeman SC. 2012. Starch turnover: pathways, regulation and role in growth. Current Opinion in Plant Biology 15, 282–92 [DOI] [PubMed] [Google Scholar]
- Sulpice R, Flis A, Ivakov AA, Apelt F, Krohn N, Encke B, Abel C, Feil R, Lunn JE, Stitt M. 2014. Arabidopsis coordinates the diurnal regulation of carbon allocation and growth across a wide range of photoperiods. Molecular Plant 7, 137–155 [DOI] [PubMed] [Google Scholar]
- Sulpice R, Trenkamp S, Steinfath M, et al. 2010. Network analysis of enzyme activities and metabolite levels and their relationship to biomass in a large panel of Arabidopsis accessions. Plant Cell 22, 2872–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimm O, Bläsing O, Gibon Y, Nagel A, Meyer S, Krüger P, Selbig J, Müller LA, Rhee SY, Stitt M. 2004. Mapman: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. The Plant Journal 37, 914–939 [DOI] [PubMed] [Google Scholar]
- Tiessen A, Prescha K, Branscheid A, Palacios N, McKibbin R, Halford NG, Geigenberger P. 2003. Evidence that SNF1-related kinase and hexokinase are involved in separate sugar-signalling pathways modulating post-translational redox activation of ADP-glucose pyrophosphorylase in potato tubers. The Plant Journal 35, 490–500 [DOI] [PubMed] [Google Scholar]
- Usadel B, Bläsing OE, Gibon Y, Retzlaff K, Höhne M, Günther M, Stitt M. 2008. Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiology 146, 1834–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usadel B, Poree F, Nagel A, Lohse M, Czedik-Eysenberg A, Stitt M. 2009. A guide to using MapMan to visualize and compare Omics data in plants: a case study in the crop species, maize. Plant, Cell & Environment 32, 1211–29 [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology 3, research0034.1–research0034.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesteene L, López-Galvis L, Vanneste K, et al. 2012. Expansive evolution of the trehalose-6-phosphate phosphatase gene family in arabidopsis. Plant Physiology 160, 884–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesteene L, Ramon M, Le Roy K, Van Dijck P, Rolland F. 2010. A single active trehalose-6-P synthase (TPS) and a family of putative regulatory TPS-like proteins in Arabidopsis. Molecular Plant 3, 406–19 [DOI] [PubMed] [Google Scholar]
- Wahl V, Ponnu J, Schlereth A, Arrivault S, Langenecker T, Franke A, Feil R, Lunn JE, Stitt M, Schmid M. 2013. Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana . Science 339, 704–7 [DOI] [PubMed] [Google Scholar]
- Wilson RA, Jenkinson JM, Gibson RP, Littlechild JA, Wang Z-Y, Talbot NJ. 2007. Tps1 regulates the pentose phosphate pathway, nitrogen metabolism and fungal virulence. EMBO Journal 26, 3673–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler A. 2002. The function of trehalose biosynthesis in plants. Planta 60, 437–440 [DOI] [PubMed] [Google Scholar]
- Wingler A, Delatte TL, O’Hara LE, Primavesi LF, Jhurreea D, Paul MJ, Schluepmann H. 2012. Trehalose 6-phosphate is required for the onset of leaf senescence associated with high carbon availability. Plant Physiology. 10.1104/pp.111.191908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav UP, Ivakov A, Feil R, et al. 2014. The sucrose-trehalose 6-phosphate (Tre6P) nexus: specificity and mechanisms of sucrose signalling by Tre6P. Journal of Experimental Botany 65, 1051–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H-L, Liu Y-J, Wang C-L, Zeng Q-Y. 2012. Molecular evolution of trehalose-6-phosphate synthase (TPS) gene family in Populus, Arabidopsis and rice. PLoS One 7, e42438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang B, Li H, Li W, Deng XW, Wang X. 2011. Analysis of trehalose-6-phosphate synthase (TPS) gene family suggests the formation of TPS complexes in rice. Plant Molecular Biology 76, 507–522 [DOI] [PubMed] [Google Scholar]
- Zeeman SC, Smith SM, Smith AM. 2007. The diurnal metabolism of leaf starch. Biochemical Journal 401, 13–28 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Primavesi LF, Jhurreea D, Andralojc PJ, Mitchell RAC, Powers SJ, Schluepmann H, Delatte T, Wingler A, Paul MJ. 2009. Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiology 149, 1860–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.