Summary
NbNHX1 affects the cellular pH and oxidation state by regulating the vacuolar H+ flux, which primes the antioxidative system associated with Phytophthora parasitica var. nicotianae resistance in tobacco.
Key words: Cellular redox homeostasis, disease resistance, NAD(P) (H) pool, NHX1, tobacco, vacuolar H+ flux and pH.
Abstract
Despite the importance of NHX1 (Na+/H+ exchanger 1) in plant salt tolerance, little is known about its other functions. In this study, intriguingly, it was found that NHX1 participated in plant disease defence against Phytophthora parasitica var. nicotianae (Ppn) in Nicotiana benthamiana. NbNHX1 was originally isolated from N. benthamiana, and characterized. The subcellular localization of NbNHX1 with its C-terminus fused with green fluorescent protein indicated that NbNHX1 localized primarily to the tonoplast. Tobacco rattle virus-induced NbNHX1 silencing led to reduced H+ efflux from the vacuole to cytoplasts, and decreased Ppn resistance in N. benthamiana. After attack by Ppn, NbNHX1-silenced plants exhibited impaired ability to scavenge reactive oxidative species (ROS) induced by the pathogen. Pea early browning virus-mediated ectopic expression of SeNHX1 (from Salicornia europaea) or AtNHX1 (from Arabidopsis thaliana) both conferred enhanced Ppn resistance to N. benthamiana, with a lower H2O2 concentration after Ppn inoculation. Further investigation of the role of NHX1 demonstrated that transient overexpression of NbNHX1 improved the vacuolar pH and cellular ROS level in N. benthamiana, which was coupled with an enlarged NAD(P) (H) pool and higher expression of ROS-responsive genes. In contrast, NbNHX1 silencing led to a lower pH in the vacuole and a lower cellular ROS level in N. benthamiana, which was coupled with a decreased NAD(P) (H) pool and decreased expression of ROS-responsive genes. These results suggest that NHX1 is involved in plant disease defence; and regulation of vacuolar pH by NHX1, affecting the cellular oxidation state, primes the antioxidative system which is associated with Ppn resistance in tobacco.
Introduction
Due to their sessile nature, plants have developed various biochemical and physiological processes to respond to environmental stresses. They efficiently regulate redox homeostasis in response to abiotic and biotic stresses, and the redox state is regarded as one of the most important indicators for evaluating the situation of the cell (Foyer et al., 2009). The components in the plant cell that affect the redox potential and intracellular redox state include O2/H2O, OH·/H2O, oxidized/reduced glutathione (GSSG/GSH), oxidized/reduced NADs [NAD(P)/NAD(P)H], and oxidized/reduced ferredoxin (Fdox/Fdred) (Foyer and Noctor, 2003). Various compartments in plant cells retain redox homeostasis, and a new homeostasis is rapidly established when the original redox state is disrupted by stresses (Sharma and Dietz, 2009). Increasing the redox pool can boost resistance to abiotic or biotic stresses (Chen et al., 2003; Hayashi et al., 2005). The genetic engineering of redox components has been shown to improve disease resistance in plants. For example, overexpression of a glutathione reductase gene in wheat improves the resistance to powdery mildew and induces transcript accumulation of other pathogenesis-related genes (Y.P. Chen et al., 2007). Similarly, overexpression of a gene related to the NAD(P)H pool in rice improves the resistance to hydrogen peroxide (H2O2) and disease (Hayashi et al., 2005).
The NADPH oxidase (NOX) in the plasma membrane accepts electrons from NADPH at the cytosolic side of the membrane and donates them to molecular oxygen at the other side of the membrane, thus producing superoxide either outside the plasma membrane or in the endosomes (Sato et al., 2001). A major endocytotic route in plants is vesicle trafficking from the plasma membrane to the vacuole that plays an important role in many stresses (Leshem et al., 2006). The membrane trafficking provides the opportunity of endosomes to generate reactive oxygen species (ROS) by a mechanism similar to that in the plasmalemma–apoplast system, based on the activity of NOX (Andreev, 2012). Recently, many pieces of evidence point to the possibility that the tonoplast can generate ROS. Cytochemical visualization displays O2 − generation in the tonoplast (Romero-Puertas et al., 2004). The proteomics of the tonoplast demonstrate that it contains NOX-like proteins (Carter et al., 2004). Several enzymes associated with ROS generation in the vacuole have also been identified by biochemical analysis (Pradedova et al., 2011).
The vacuole, as the largest endosome, has been confirmed to participate actively in cellular oxidative events. Loss of vacuole function causes sensitivity to oxidative stress in the fission yeast Schizosaccharomyces pombe (Mikawa et al., 2010). ROS can diffuse out of the chloroplast at considerable rates and be transported to the vacuole by intrinsic proteins in the tonoplast (Brunetti et al., 2011). Maturation of endomembrane organelles involves luminal acidification driven by vacuolar H-ATPase and cation/H+ exchangers, which are also involved in charge balance during the NOX respiratory bust (El Chemaly et al., 2010). Transmembrane proton transfer in the vacuole is associated with superoxide production inside the endosome, which is dependent on the pH of the compartment (Lamb et al., 2009). Protons transported into the vacuole lumen are consumed in the dismutation of superoxide, which occurs rapidly under acidic conditions, but slows down remarkably under alkaline conditions (Plonka et al., 1986).
Na+/H+ exchanger 1 (NHX1) localized in the tonoplast affects transmembrane H+ flux in the vacuole by sequestering cytoplasmic Na+ in the vacuole and pumping vacuolar H+ into the cytoplasm (Blumwald, 2000). The function of NHX1 in the plant response to salt stress has been extensively studied. Overexpression of NHX1 improves the salt tolerance of many plant species, including Arabidopsis (Apse et al., 1999), tomato (Zhang and Blumwald, 2001), tall fescue (Tian et al., 2006), maize (M. Chen et al., 2007), and Nicotiana tabacum (Zhou et al., 2008). In contrast, the T-DNA insertional mutant of AtNHX1 leads to stronger sensitivity to NaCl in Arabidopsis seedlings (Apse et al., 2003). In addition, NHX1 also has important functions in cellular K+/Na+ homeostasis (Shabala and Cuin, 2007), vacuolar pH regulation (Yamaguchi et al., 2001), cold tolerance (Li et al., 2007), and regulation of plant growth, flower development, and reproduction (Bassil et al., 2011).
However, little is known about the role of NHX1 in biotic stresses such as pathogen attack. Biotrophic pathogens obtain nutrients from the plant and suppress host defence during the infection (O’Connell and Panstruga, 2006). Thus, the defence of plants against biotrophic pathogens relies on oxidative burst and induces cell death (Glazebrook, 2005). In contrast, necrotrophic pathogens are not restricted by cell death, but rather feed on the remains of dead organisms or their by-products (Glazebrook, 2005). Phytophthora parasitica is considered a hemibiotroph, which initially establishes itself in host tissues as a biotroph. It then switches to a necrotrophic type of growth, and rapidly invades and kills the host cells after disease burst which induces production of intracellular ROS in the host cells (Woltz, 1978; Galiana et al., 2005). Phytophthora parasitica var. nicotianae (Ppn) is regarded as one of the most destructive and widespread pathogens that causes black shank disease in tobacco. Thus, investigation of whether NHX1 is associated with plant defence against Ppn and responds to oxidative damage caused by Ppn in tobacco has gained considerable interest.
In the present work, NbNHX1 was originally isolated from Nicotiana benthamiana. NbNHX1 silencing led to stronger Ppn sensitivity in N. benthamiana. The general function of NHX1 on plant defence was confirmed by transformation of NHX1 from Salicornia europaea or Arabidopsis improving the Ppn resistance in tobacco. Further investigation demonstrated that NHX1 had functions in regulating the pH in the vacuole and cellular ROS level, which could prime the antioxidative system.
Materials and methods
Plant material
The seeds of N. benthamiana were spread on MS medium (Murashige and Skoog, 1962). After 2 weeks, tobacco seedlings were transferred into plastic pots containing a mixture of vermiculite, turf, and humus (1:1:1; v/v/v), and grown in a greenhouse under the following conditions: 16h light/8h dark photoperiod, 25±2 °C, and 50±10% relative humidity. The plants were watered weekly with half-strength Hoagland nutrient solution (Zhou et al., 2008).
Isolation of NbNHX1
First-strand cDNA was synthesized using total RNA isolated from N. benthamiana seedlings by the reverse transcription system (Promega, Madison, WI, USA) according to the manufacturer’s protocol. Full-length cDNA was obtained by using 3' RACE (rapid amplification of cDNA ends) and 5' RACE kits according to the manufacturer’s instructions (Invitrogen, Karlsuhe, Germany). The amplicon was cloned into the cloning vector pEASY-T-Simple (TransGen, China) and sequenced.
The nucleotide sequence, deduced amino acid sequence, and open reading frame encoded by NbNHX1, AtNHX1, and SeNHX1 were analysed by DNAman software. Multiple sequence alignment and the rooted phylogenetic tree were performed with ClustalW (http://www.genome.jp/tools/clustalw/). The transmembrane topology prediction was performed using TopPred2 (bioweb.pasteur.fr/seqanal/interfaces/toppred.html).
Green fluorescent protein (GFP) plasmid construction and microscopy analysis
The coding sequence of NbNHX1 was amplified and inserted into KpnI/BamHI sites of the pCAMBIA1300-35S::GFP vector to produce pCAMBIA1300-35S::NbNHX1-GFP. Three days after agroinfiltration into leaves of tobaccos, the protoplasts isolated from inoculated leaves were observed on a Zeiss LSM 510 META confocal microscope.
Generating NbNHX1-silenced tobacco
The available sequences of NHX genes in N. benthamiana were obtained from the website of the ‘Sol genomics network’ (http://solgenomics.net/). It was found that unigene information of NHX2 (ID: SGN-U515339) and NHX3 (IDs: SGN-U518331 and SGN-U521516) have been annotated in N. benthamiana. However, the unigenes SGN-U518331 and SGN-U521516 are different fragments of the NHX3 gene, which was confirmed by the expressed sequence tag (EST) sequence of NHX3 cloned in the present study (Supplementary Fig. S1A available at JXB online). Therefore, a 263bp sequence from NbNHX1 (nucleotides 1321–1583) was selected as a distinctive sequence after alignment with NHX2 and NHX3, respectively (Supplementary Fig. S1B, C).
Tobacco rattle virus (TRV)-induced gene silencinge was used in N. benthamiana (Liu et al., 2004). The distinctive sequence from NbNHX1 described above was constructed into the pTRV2 vector as pTRV2-NbNHX1 (Supplementary Fig. S2A at JXB online). Agroinfiltration of 4-week-old N. benthamiana plants with pTRV1 was in combination with pTRV2-NbNHX1, pTRV2-PDS, and pTRV2 empty vector. To test whether the TRV clones could induce gene silencing in tobacco plants, the ability of the TRV-VIGS (virus-induced gene silencing) vector to suppress the expression of the endogenous phytoene desaturase gene (PDS), which was used as the reporter in the system (Liu et al., 2002), was examined. Four weeks after agroinfiltration, when leaves infected with pTRV2-PDS exhibited bleaching (Supplementary Fig. S2B), the expression of NbNHX1 was tested in agroinfiltrated tobacco plants by real-time PCR (Supplementary Fig. S4C). The NbNHX1 primers were: forward primer 5' GTTCAAGAGTTACTACAAGGCACG 3' and reverse primer 5' CAATGGTAATGGTGCTGGTAATC 3'. MxPro software was used to quantify gene expression. TRV-Nb plants as NbNHX1-silenced tobacco were created by transformation of pTRV2-NbNHX1; TRV plants as control were created by transformation of pTRV2 empty vector into N. benthamiana (Supplementary Fig. S2A, C). Relative expression of NbNHX1 in TRV-Nb plants was normalized against that in TRV plants. TRV-Nb and TRV plants did not display any difference in growth phenotype after 4 weeks of agroinfiltration.
Preparation of protoplasts and isolation of vacuoles
The isolation of protoplasts from N. benthamiana was based on a previous report (Chen et al., 2011b ). To release the vacuoles, the solution containing the protoplasts was diluted to a final concentration of 0.2M mannitol with 25mM TRIS-HCl (pH 7.5) and gently pipetted three or four times at 2min intervals for 10min. The suspension was then loaded onto the top of 8% (w/v) Ficoll 400 in 25mM TRIS-HCl (pH 7.5) and 0.5M mannitol. The gradient was centrifuged at 2000 g for 30min at 4 °C in a swinging bucket rotor. Vacuoles collected in the top layer were removed and examined using an A/O Spencer Bright Line Hemacytometer with a Nikon inverted phase contrast microscope.
Measurement of net H+ flux with non-invasive micro-test electrophysiological technology (NMT)
Non-invasive measurement of net H+ flux in vacuoles using NMT (NMT system, BIO-001A, Younger USA Sci. & Tech. Corp., Amherst, MA, USA) was performed based on a previous report (Chen et al., 2011b ).
The vacuolar net H+ flux was detected in the measuring solution that simulates the intracellular ionic environment (Chen et al., 2011b ). Prior to the measurement, the solution containing vacuoles was placed at the centre of the coverslip treated with 0.008% (w/v) poly-l-lysine. After vacuoles settled onto the coverslip (~15min), the residual solution was removed with a pipette, and then 3ml of measuring solution [0.05mM MES, 0.5M mannitol, 0.1mM NaCl, 0.1mM CaCl2, and 100mM K+ (potassium gluconate, C6H11O7K) pH 6.8] was added slowly to the container. The H+ flux of the sample vacuole was recorded from 0.5min to 5min under normal conditions. Then, NaCl (1M), KCl (1M), ATP (150mM), or PPi (150mM) stock solution was added slowly to reach the final concentration in the buffer. After the ions stabilized in solution for 1–2min, the H+ flux measurement was restarted and continued for a further minute. The mean net H+ flux was calculated based on all the transient H+ flux data recorded during the period of treatment. The value obtained from NMT indicates net ion flux, and the positive values of ion flux in the figures represent cation efflux from the vacuole into the cytoplast, and vice versa.
Pathogen challenge
The Ppn (race 0, pathogen of black shank disease) was cultured on PDA medium (potato, 200g l–1; sucrose, 20g l–1; agar, 15g l–1; pH 6.5). Ppn inoculation was based on the method of Guo et al. (2004). When the fungal mycelia had spread throughout the PDA plate, a plug of medium containing the fungal mycelia was removed using a plastic borer. Detached leaves (the third from top of the plant) were used for pathogen challenge. The leaf was wounded with a toothpick; wounds were located on the each side of the main vein. The mycelia were inoculated onto the right side of the main vein at the wound site; the left side of the leaf was used as a wound-only control site (Guo et al., 2004). The wounded leaves were kept in Petri dishes on water-soaked filter paper at 28±2 °C, 16h light/8h dark photoperiod until measurement.
The area of the wilt spot was measured using Matlab software. The range of grey values in the control part of each leaf was first scanned, and then the area with a continuous grey value higher than the maximum value of the control was calculated as the wilt spot area.
The symptoms caused by Ppn infection were classified into three ranks based on the areas of the wilt spots (Guo et al., 2004): rank 1, no symptom or area of wilt spots <8cm2; rank 2, the area of wilt spots is >8cm2 and <12cm2; and rank 3, the area of wilt spots is >12cm2.
Oxidative resistance analysis
The leaf discs from NbNHX1-silenced or Se/AtNHX1-YFP ectopically expressed N. benthamiana were detached using a plastic borer. The leaf discs were immersed in half-strength Hoagland nutrient solution with 0, 1, or 10mM methyl viologen (MV) for 2 d. The contents of H2O2 or total chlorophyll were measured by the method of Chen et al. (2011a ).
Determination of H2O2
Leaf tissues from around the infected spots were used for determination of H2O2 content. The measurement of H2O2 was based on the peroxide-mediated oxidation of Fe2+, followed by the reaction of Fe3+ with xylenol orange, according to the method of Bellincampi et al. (2000).
PEBV-mediated ectopic gene expression
Based on a previous study, the Pea early browning virus (PEBV) system was used to mediate ectopic expression of SeNHX1 (identified from S. europaea, GenBank accession no. AY131235.1) and AtNHX1 (identified from Arabidopsis, TAIR accession no. AT5G27150.1) in N. benthamiana (Chen et al., 2011a ). Two pCAPE2 derivative clones (pCAPE2-SeNHX1 or AtNHX1) were prepared using pCAPE2-YFP as the cloning vector (Supplementary Fig. S2E at JXB online). Agroinfiltration of 4-week-old N. benthamiana plants with pCAPE1 was in combination with pCAPE2-SeNHX1-YFP, pCAPE2-AtNHX1-YFP, pCAPE2-YFP, and pCAPE2-PDS, while pCAPE2-PDS was used as the positive control (Constantin et al., 2004). Four weeks after agroinfiltration, when leaves of the plants infected with pCAPE2-PDS appeared bleached (Supplementary Fig. S4F), pCAPE2-SeNHX1-YFP (Se-YFP)- and pCAPE2-AtNHX1-YFP (At-YFP)-transformed tobacco plants were ready for further study. PEBV-mediated SeNHX1–YFP, AtNHX1–YFP, and YFP expression in N. benthamiana was first observed using a fluorescence microscope, and the expression of NHX1 genes in N. benthamiana was confirmed by real-time PCR (Supplementary Fig. S2G). The primers for NbNHX1 and SeNHX1 were: forward primer 5' CAGGTAAAAAAGAAGCAATTCTTCC 3' and reverse primer 5' GAATCGCATTGAAAAGCACCACCGA 3'. The primers for NbNHX1 and AtNHX1 were: forward primer 5' CATCTTCTCGTCTTTAGTGAAG 3' and reverse primer 5'CAATGTCCAACTTCTTAAAGAA 3'. Relative expression of NHX1 in Se/At-YFP plants was normalized against that in YFP plants.
Measurement of vacuolar pH
Vacuolar pH was measured using the pH-sensitive dye 2',7'-difluorofluorescein (Oregon Green 488) (Wilson et al., 1998). Oregon Green 488 has spectral properties which allow dual excitation at 490nm (the pH-dependent wavelength near its absorption maximum) and at 440nm (the second wavelength, relatively pH independent near to its isobestic point); single emissions between 525nm and 550nm were collected for each excitation wavelength. The fluorescence intensity at an excitation of 488nm against that at 458nm indicates the pH in situ (Wilson et al., 1998).
For the calibration curve, before pH staining and detection, the epidermis of leaves was detached and incubated in equilibration buffer (half-strength Hoagland nutrient solution, containing 50mM HEPES or MES, 50mM ammonium acetate, pH 5.0–7.0) for 1h. Dye fluorescence images were collected using a confocal microscope (Zeiss, LSM 510 META) after excitation at 458nm and 488nm, and analysed by Image J (National Institutes of Health). The calibration curve indicating the 488/458 ratio was correlated with the pH in the cell (Supplementary Fig. S3A at JXB online).
For measurement of the vacuolar pH in N. benthamiana, the epidermis of leaves was detached and incubated in half-strength Hoagland nutrient solution, containing 50mM MES (pH 6.0), 20 μM Oregon Green 488 (Molecular Probes), for 1h in darkness at 23 °C. The pH value was calculated based on the488/458 ratio, using the calibration curve. For the ratio image, colour applied to the drawing was generated by Matlab, which converted the ratio of the grey value in each picture pixel at an excitation wavelength of 488nm against that at 458nm to a specific colour.
Flow cytometric analysis
The GFP and CellROX Deep Red (DR) fluorescence in the protoplasts was detected or screened by a MoFlo XDP high-speed flow cytometer (Beckman-Coulter, USA) with a 70 μm ceramic nozzle at 60 psi sheath pressure. The biparametrically analysed outputs were shown as dot plots in which the viable cell populations were gated based on forward and side scatter (FSC) values. GFP fluorescence was excited with 488nm, and detected with a 530nm band-pass filter; and DR fluorescence was excited with 640nm, and detected at 670nm (http://www.lifetechnologies.com/order/catalog/product/C10422#). The positive protoplasts with green or DR fluorescence were screened by selecting box R6 or R7, in which a near zero percentage (<1% in the present study) of control protoplasts (which completed the transfection procedure without plasmids or without staining) showed green or DR fluorescence. The average fluorescence intensity was obtained automatically from flow cytometry.
Measurement of nicotinamide coenzymes
Leaves (the third leaf from the top of the plant) of tobacco seedlings were detached for measurements. The contents of nicotinamide coenzymes were determined as described by Hayashi et al. (2005). Tobacco leaves homogenized with 0.1M HCl (for NAD and NADP) or 0.1M NaOH (for NADH and NADPH) at 95 °C were cooled, and then the pH was adjusted to 6.5 for NAD and NADP, or to 7.5 for NADH and NADPH. For NAD and NADH measurements, samples were added to the reaction mixture containing 50mM glycylglycine (pH 7.4), 20mM nicotinamide, 1mM phenazine methosulphate, 1mM thiazolyl blue, and 40 μg ml–1 alcohol dehydrogenase. For NADP and NADPH measurements, the samples were added to a reaction mixture containing 50mM glycylglycine (pH 7.4), 20mM nicotinamide, 1mM phenazine methosulphate, 1mM thiazolyl blue, and 2mM glucose-6-phosphate. The reaction mixture was measured in a UV-visible spectrophotometer at 570nm.
The expression of ROS-responsive genes
ROS-responsive genes were selected from the unigene database in the Sol genomics network (http://solgenomics.net/). The expression patterns of the selected genes in each tobacco plant were analysed by real-time PCR based on the primers described in Supplementary Table S1 at JXB online.
Statistical analysis
One-way analysis of variance (ANOVA) in the SPSS 13.0 statistical package was used for statistical analysis. SE indicatess the standard error, and the repetitions are indicated in every experiment. The significance was tested using the least significant difference (LSD) at the 5% level. Asterisks indicate significant differences from the control in the same treatment at P≤0.05.
Results
NbNHX1 as an Na+/H+ exchanger localized in the tonoplast regulated vacuolar H+ flux in tobacco
NbNHX1 (GenBank accession no. JX987081) was originally cloned from N. benthamiana, and encodes a polypeptide of 536 amino acids. Hydropathy plot analysis showed that NbNHX1 is similar to SeNHX1 (from S. europaea) as well as AtNHX1 (from Arabidopsis thaliana) containing 12 transmembrane domains typical for a vacuolar-type Na+/H+ antiporter. All three amino acid sequences contain a conserved putative binding site for amiloride (‘LFFIYLLPPI’) (Fig. 1A), which is an inhibitor of NHX1 activity (Qiu et al., 2004). The phylogenetic analysis showed that NbNHX1 and SeNHX1 are more closely related to AtNHX1 compared with other NHX members in Arabidopsis (Fig. 1B).
Fig. 1.
Characterizations of NbNHX1. (A) The alignment of the deduced amino acid sequences of NbNHX1, SeNHX1, and AtNHX1. The box indicates a putative amiloride binding site. NHX1 proteins contain 12 transmembrane domains which are indicated as TM1–TM12. (B) Phylogenetic analysis of NbNHX1, SeNHX1, and the NHX family in Arabidopsis. The NHX family (with their TAIR accession numbers) is as follows: AtNHX2-1 (AT3G05030.1); AtNHX2-2 (AT3G05030.2); AtNHX3 (AT5G55470); AtNHX4 (AT3G06370); AtNHX5 (AT1G54370); AtNHX6 (AT1G79610). (C) Vacuolar H+ net flux in NbNHX1-silenced tobacco plants. Mean H+ net fluxes for a period of 12min. (D, E) Mean H+ net fluxes in vacuoles supplied with 0, 25, and 50mM NaCl (D) or KCl (E). The value obtained from NMT indicates net ion flux, and the positive values of ion flux in the figures represent cation efflux from the vacuole into the cytoplast, and vice versa. Data are means ±SE (n = 36 vacuoles from six independent NbNHX1-silenced lines). The asterisks on the bars indicate significant differences from the TRV plants (C) or untreated plants (D and E) in the same treatment at P≤0.05.
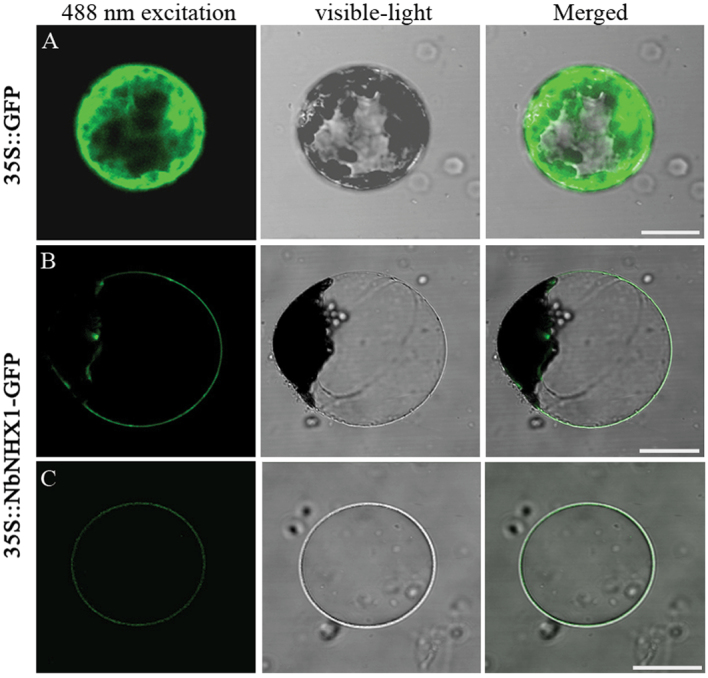
To detect the subcellular localization of NbNHX1, the fusion protein of NbNHX1 with GFP at the C-terminus was expressed in protoplasts of N. benthamiana. NbNHX1–GFP exhibited fluorescence in the endomembrane of the vacuolar membranous invagination, with discontinuous fluorescence in the cellular contour (Fig. 2B). Isolated vacuoles from 35S::NbNHX1-GFP-transformed tobacco showed obvious fluorescence in the tonoplast (Fig. 2C). These results indicated that NbNHX1 localized primarily in the tonoplast.
Fig. 2.
Subcellular localization of NbNHX1 (scale bar=20 μm). (A) Expression of 35S::GFP in protoplasts isolated from leaves of N. benthamiana. (B, C) Expression of 35S::NbNHX1-GFP in protoplasts (B) and vacuole (C) from leaves of N. benthamiana. (This figure is available in colour at JXB online.)
NbNHX1-silenced N. benthamiana (TRV-Nb plants) were created by a TRV-VIGS approach; TRV plants as control were created by transformation of the pTRV2 vector into N. benthamiana (Supplementary Fig. S2A at JXB online). Six NbNHX1-silenced N. benthamiana plants (TN1–TN6) were used to detect vacuolar H+ net flux; the results showed a dose-dependent effect of NHX1 silencing on the decrease in net vacuolal H+ efflux (Supplementary Fig. S4B). The average vacuolar net H+ efflux decreased to 1.65 pmol m–2 s–1 in TRV-Nb plants compared with 1.74 pmol m–2 s–1 in TRV plants (Fig. 1C). To confirm the association between NbNHX1 as an Na+/H+ exchanger 1 and the increased H+ efflux, NaCl and KCl were added into the measuring buffer with a concentration gradient. The vacuolar H+ efflux in TRV plants increased under NaCl treatment, whereas that in TRV-Nb plants remained unchanged with the application of NaCl and KCl (Fig. 1D, E).
It is reported that V-ATPase, PPase, and NHX1 act synergistically in the plant vacuole (Blumwald, 2000). Therefore, it was investigated whether the activity of V-ATPase changed in NbNHX1-silenced plants. Based on a previous study, ΔH+ flux in the vacuole (the change of vacuolar H+ flux) after 1.5mM ATP or PPi supply was used to indicate the activities of V-ATPase and PPase in the tonoplast, respectively (Chen et al., 2011b ). As shown in Supplementary Fig. S5 at JXB online, ΔH+ flux in the vacuole was comparable between TRV and TRV-Nb plants after 1.5mM ATP or PPi supply, indicating that NbNHX1 silencing did not impact the activities of V-ATPase and PPase.
Silencing of endogenous NbNHX1 decreased Ppn resistance in tobacco
Thirty TRV-Nb plants with different expression levels of NbNHX1 were selected for Ppn inoculation (Supplementary Fig. S2C at JXB online). No wilt spots were observed in TRV-Nb and TRV plants at 0 hours post-inoculation (hpi), whereas all the leaves exhibited water-soaked wilt after Ppn infection (Fig. 3A). At 60 hpi, TRV-Nb plants exhibited more serious disease symptoms and accumulated more H2O2 than the TRV plants (Fig. 3A–D). Moreover, among the 30 TRV-Nb plants, seedlings with lower NHX1 expression were more sensitive to Ppn (Fig. 3A; Supplementary Fig. S2C, D). The correlation analysis showed that the Ppn resistance was positively correlated with NHX1 expression in TRV-Nb plants, and the correlation coefficient was –0.764 (P<0.01) (Fig. 3E).
Fig. 3.
Comparison of disease development between NbNHX1-silenced and control tobacco plants. (A) Symptoms of TRV plants (control) and three NbNHX1-silenced seedlings at 0 and 60 hpi after Ppn inoculation. N6/14/26 represents tobacco seedlings with different expression of NbNHX1 based on Supplementary Fig. S2C at JXB online. Wounding-only treatment on the left side of the leaf served as a control (scale bar=2cm). (B–D) Area of wilt spots (B), H2O2 content (C), and development of disease course (D) after Ppn infection. (E) Correlation analysis between NHX1 expression and wilt spot area in NbNHX1-silenced tobaccos. Data are means ±SE (n=30 leaves from 30 independent NbNHX1-silenced lines). The asterisks on the bars indicate significant differences from the TRV plants in the same treatment at P≤0.05. (This figure is available in colour at JXB online.)
Transformation of SeNHX1 and AtNHX1 improved Ppn resistance in tobacco
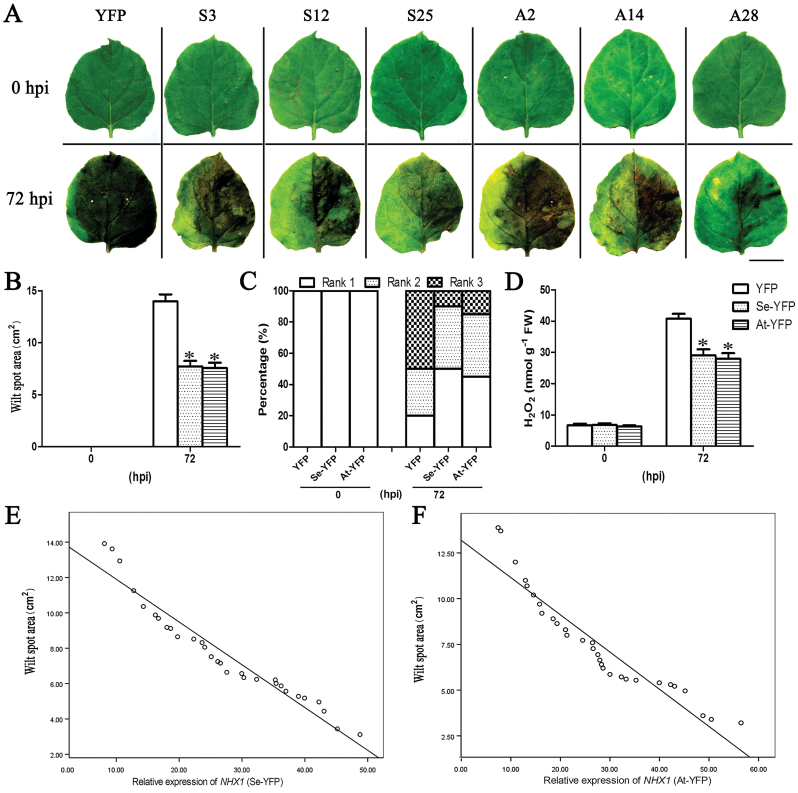
NHX1 genes from S. europaea and Arabidopsis (SeNHX1 and AtNHX1) were constructed into a viral vector (Supplementary Fig. S2E at JXB online), and then expressed in N. benthamiana to create ectopic expression of SeNHX1 or AtNHX1 tobacco plants (Se-YFP or At-YFP plants) by using the PEBV-mediated ectopic gene expression system. Their controls (YFP plants) were created by transformation of pCAPE2-YFP vector into N. benthamiana (Supplementary Fig. S2E). Thirty tobacco seedlings with different expression levels of the NHX1 gene were selected for further study (Supplementary Fig. S2G).
There were no wilt spots among YFP plants and Se/At-YFP plants before Ppn inoculation. At 72 hpi, all tobacco leaves exhibited disease symptoms, while Se/At-YFP plants showed smaller wilt spots and lower H2O2 contents compared with YFP plants (Fig. 4A–D). Among the 30 transgenic tobacco plants, those with higher NHX1 expression displayed stronger Ppn resistance (Fig. 4A; Supplementary Fig. S4G, H at JXB online). The correlation coefficient between the expression of NHX1 and the wilt spot area was –0.957 (P<0.01) in Se-YFP plants and –0.938 (P<0.01) in At-YFP plants (Fig. 4E, F).
Fig. 4.
Disease development in Se/AtNHX1 transgenic plants after Ppn inoculation. (A) Disease symptoms on tobacco leaves at 0 and 72 hpi. Wounding-only treatment on the left side of the leaf served as a control (scale bar=2cm). S3/12/25 and A2/14/28 represent tobacco seedlings with different expression of NHX1 based on Supplementary Fig. S2G at JXB online. (B–D) Area of wilt spots (B), development of disease course (C), and H2O2 content (D). (E, F) Correlation analysis between NHX1 expression and wilt spot area in SeNHX1 transgenic tobacco (E) and AtNHX1 transgenic tobacco (F). YFP indicates pCAPE2-YFP vector-transformed tobacco plants as control and Se/At-YFP indicates pCAPE2-Se/AtNHX1-YFP vector-transformed tobacco plants. Data are means ±SE (n=30 leaves from 30 independent SeNHX1 and AtNHX1 transgenic lines, respectively). The asterisks on the bars indicate significant differences from the YFP plants in the same treatment at P≤0.05. (This figure is available in colour at JXB online.)
NHX1 was associated with oxidative resistance in N. benthamiana
Six NbNHX1-silenced or Se/AtNHX1-YFP ectopically expressed N. benthamiana with different expression levels of NHX1 were selected for analysis of oxidative resistance (Supplementary Fig. S4C–E at JXB online). The detached leaf discs were treated with 0, 1, or 10mM MV for 2 d. Without MV treatment, there was no difference in H2O2 and total chlorophyll contents between TRV and TRV-Nb plants, or among YFP, Se-YFP, and At-YFP plants. However, the TRV-Nb plants exhibited greater H2O2 and lower total chlorophyll contents than TRV plants under 1mM or 10mM MV treatment (Fig. 5A, B). Although there were comparable H2O2 and total chlorophyll contents among YFP, Se-YFP, and At-YFP plants under 1mM MV treatment, Se-YFP and At-YFP plants exhibited lower H2O2 and higher total chlorophyll contents than YFP plants under 10mM MV treatment (Fig. 5A, C).
Fig. 5.
Response of NbNHX1-silenced or At/SeNHX1 ectopically expressed N. benthamiana to MV. (A) Leaf discs from N. benthamiana were treated with different concentrations of MV (0, 1, and 10mM) for 2 d (scale bars=1cm). The total chlorophyll and H2O2 contents were detected in NbNHX1-silenced plants (B) or At/SeNHX1 ectopically expressed plants (C), and are expressed as fold changes compared with the value in 0mM MV treatment. TRV represents pTRV2 empty vector-transformed tobacco (control plants), TRV-Nb represents pTRV2-NbNHX1 vector-transformed tobacco (NbNHX1-silenced plants), YFP indicates pCAPE2-YFP vector-transformed tobacco as control, and Se/At-YFP indicates pCAPE2-At/SeNHX1-YFP vector-transformed tobacco. Data are means ±SE (n=36 leaf discs of 12 leaves from six independent NbNHX1-silenced lines or SeNHX1 and AtNHX1 transgenic lines, respectively). The asterisks on the bars indicate significant differences from TRV or YFP plants in the same treatment at P≤0.05. (This figure is available in colour at JXB online.)
NbNHX1 regulated the pH in vacuole
NbNHX1-overexpressing plants (Nb-GFP) were created by transformation of the pCAMBIA1300-35S::NbNHX1-GFP vector into N. benthamiana as well as their controls (GFP plants) transformed with pCAMBIA1300-35S::GFP. The pH in vacuoles of GFP plants and Nb-GFP plants, or of TRV and TRV-Nb plants was detected by using the ratiometric fluorescein-based pH sensitive dye, 2',7'-difluorofluorescein (Oregon Green 488), in an image-based approach (Wilson et al., 1998). Six NbNHX1-overexpressing (NOE1–NOE6) or silenced (NS1–NS6) plants with different expression levels of NbNHX1 were selected for further analysis (Supplementary Fig. S4C, F at JXB online).
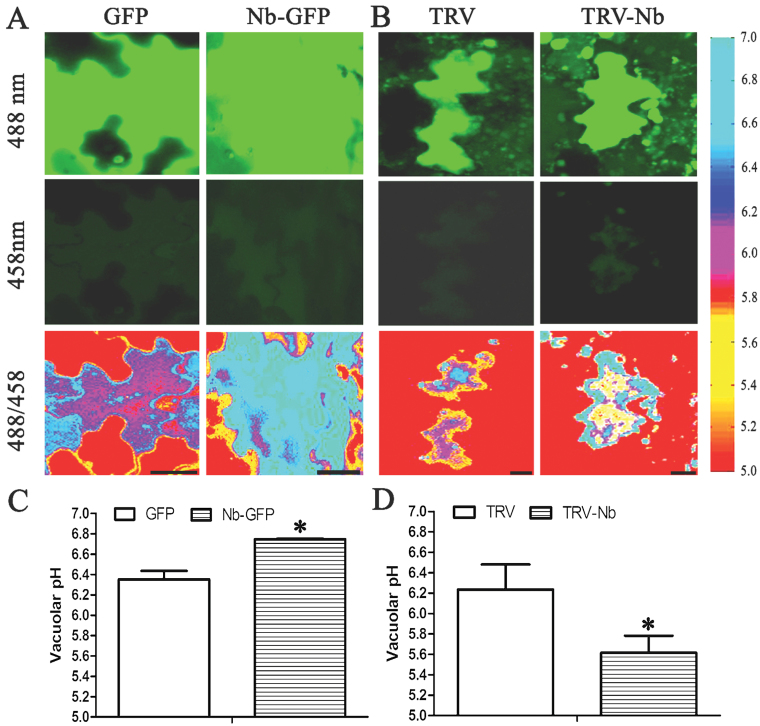
First, a calibration curve was created, which was used to quantify the vacuolar pH (Supplementary Fig. S3A at JXB online). Then positive control treatments were carried out to validate that the pH quantification method used here worked well. The epidermis of tobacco leaves was detached and incubated in acidic solution (half-strength Hoagland nutrient solution, containing 50mM MES and 50mM ammonium acetate) with pH 5.1 (TEST1) or 5.4 (TEST2) for 1h. Then, the vacuolar pH was calculated based on the calibration curve. As shown in Supplementary Fig. S3B, after staining by Oregon Green 488, the vacuolar pH was 5.12±0.057 in TEST1 and 5.38±0.035 in TEST2, indicating that the quantification method was appropriate for leaves of N. benthamiana.
The pH of the leaf epidermal cells was shown in situ via ratio colour images by using the Matlab software, which showed that the pH of vacuolar zones in Nb-GFP plants was higher than that of GFP plants (Fig. 6A). The calculated vacuolar pH was 6.75 in Nb-GFP plants, which was significantly higher than the pH of 6.36 in GFP plants (Fig. 6C). In contrast, the ratio image showed that the pH of vacuolar zones in TRV-Nb plants was lower than that of TRV plants (Fig. 6B), and the vacuolar pH of 5.61 in TRV-Nb plants was significantly lower than the pH of 6.24 in TRV plants (Fig. 6D).
Fig. 6.
Vacuolar pH in epidermal cells of N. benthamiana leaves. (A, B) Ratio images indicating the vacuolar pH in epidermal cells of GFP and Nb-GFP tobacco leaves (A), or TRV and TRV-Nb tobacco leaves (B). Scale bars=20 μm. (C, D) Vacuolar pH quantification in GFP and Nb-GFP tobacco plants (C), or TRV and TRV-Nb tobacco plants (D). GFP indicates pCAMBIA1300-35S::GFP vector-transformed tobacco (control plants), Nb-GFP indicates pCAMBIA1300-35S::NbNHX1-GFP vector-transformed tobacco (NbNHX1-overexpressing plants), TRV represents pTRV2 empty vector-transformed tobacco (control plants), and TRV-Nb indicates pTRV2-NbNHX1 vector-transformed tobacco (NbNHX1 silenced plants). Data are means ±SE (n = 360 cells from 36 leaves of six independent NbNHX1-silenced or overexpressing lines, respectively). The asterisks on the bars indicate significant differences from the control plants in the same treatment at P≤0.05. (This figure is available in colour at JXB online.)
NbNHX1 affected the cellular oxidation level
It has been reported that the vacuolar pH is associated with ROS generation (Lamb et al., 2009). Therefore, DR dye was used to monitor the cellular oxidation levels in protoplasts of GFP and Nb-GFP plants, as well as TRV and TRV-Nb plants via flow cytometry, which can precisely detect subtle fluctuation of the cellular oxidation level in high-throughput mode. Six NbNHX1-overexpressing plants (Nb-GFP-1, Nb-GFP-3, Nb-GFP-5, Nb-GFP-6, Nb-GFP-8, and Nb-GFP-9, Supplementary Fig. S4G at JXB online) and six NbNHX1-silenced plants (TRV-Nb-4, TRV-Nb-10, TRV-Nb-12, TRV-Nb-16, TRV-Nb-22, and TRV-Nb-25, Supplementary Fig. S2C) were selected for further study.
For GFP and Nb-GFP plants, the protoplasts expressing green fluorescence should first be screened. According to the MoFlo XDP manual, the select box R6 was set so that <1% of control protoplasts showed fluorescence (Fig. 7A), and then the protoplasts from GFP or Nb-GFP plants in R6 were regarded as positive cells transformed successfully, and were used for further analysis (Fig. 7B, C). Similarly, the select box R7 was set so that <1% of protoplasts showed fluorescence without DR staining (Fig. 7D); the protoplasts after DR staining in R7 were regarded as cells stained successfully (Fig. 7E). Then, the protoplasts from GFP and Nb-GFP plants screened by R6 (Fig. 7B, C) were investigated for DR staining using the R7 select box (Fig. 7F, G). Upon counting the protoplasts in R7, Nb-GFP plants showed increased average fluorescence intensity compared with the GFP plants (Fig. 7H).
Fig. 7.
Flow cytometric analysis of the cellular oxidation level in GFP and Nb-GFP tobacco plants. (A–C) Flow cytometric analysis on protoplasts with fluorescence in control plants (A), GFP plants (B), and Nb-GFP plants (C). Biparametric outputs are displayed by the intensity of fluorescence and FSC (forward and side scatter values). The select boxes R6 or R7 are set so that near zero levels (<1%) of control protoplasts show fluorescence. (D, E) Protoplast without DR treatment (D), or with DR treatment (E). (F, G) Protoplasts screened by R6 are analysed on the DR fluorescence level by R7. (H) Average fluorescence intensity of the protoplasts in (F) and (G) screened by R7. Data are means ±SE (n=6 batches of protoplasts from six independent NbNHX1-overexpressing lines). The asterisks on the bars indicate significant differences from the GFP plants in the same treatment at P≤0.05. (This figure is available in colour at JXB online.)
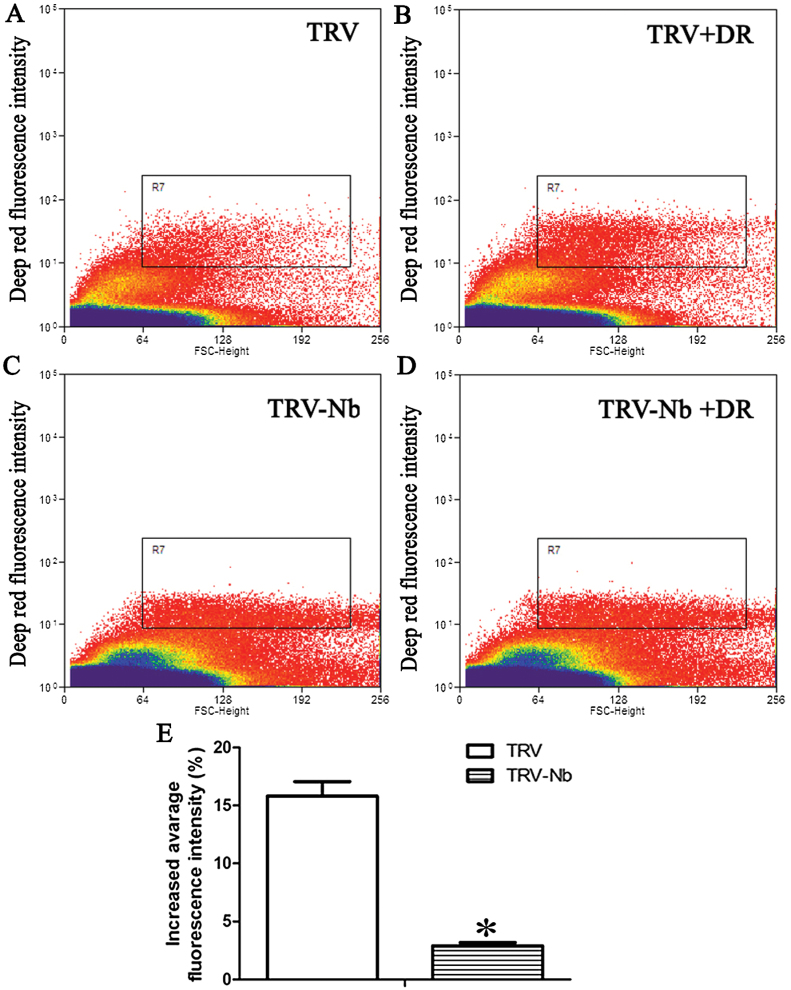
Similarly, the cellular oxidation level was also analysed in protoplasts from TRV and TRV-Nb tobaccos by flow cytometry. The average fluorescence intensity increased by 17% in TRV tobacco after DR staining (Fig. 8A, B, E), whereas an increase of only 3% was observed in TRV-Nb plants (Fig. 8C, D, E), which suggested that NbNHX1 silencing led to a decreased cellular oxidation level in N. benthamiana.
Fig. 8.
Flow cytometric analysis of the cellular oxidation level in TRV and TRV-Nb tobacco plants. (A–D) Flow cytometric analysis of protoplasts without DR treatment in TRV (A) and TRV-Nb (C); and protoplasts with DR treatment in TRV (B) and TRV-Nb (D). Protoplasts are displayed in biparametric outputs with intensity of fluorescence and FSC. The R7 box is set so that near zero levels (<1%) of control protoplasts show fluorescence. (E) Increased average fluorescence intensity of protoplasts in TRV and TRV-Nb plants. The increased average fluorescence intensity (%) was calculated as follows: [(fluorescence intensity of R7 in protoplasts with DR treatment/fluorescence intensity of R7 in protoplasts without DR treatment)–1]×100%. Data are means ±SE (n=6 batches of protoplasts from six independent NbNHX1-silenced lines). The asterisks on the bars indicate significant differences from the TRV plants in the same treatment at P≤0.05. (This figure is available in colour at JXB online.)
NbNHX1 was involved in cellular redox homeostasis
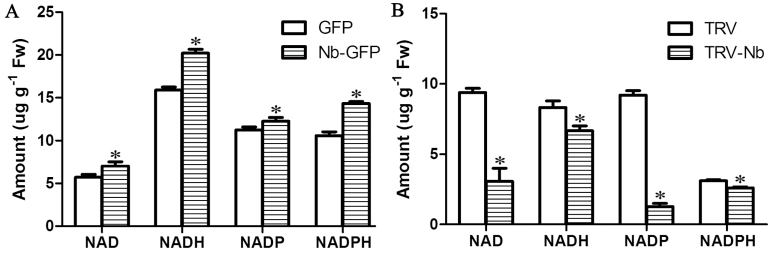
To investigate whether overexpression or silencing of NbNHX1 could affect the cellular NADPH homeostasis, the stability of the NAD(P) (H) pool indicating redox homeostasis was calculated in GFP and Nb-GFP (Nb-GFP-1, Nb-GFP-3, Nb-GFP-5, Nb-GFP-6, Nb-GFP-8, and Nb-GFP-9, Supplementary Fig. S4G at JXB online) plants, as well as TRV and TRV-Nb (TRV-Nb-4, TRV-Nb-10, TRV-Nb-12, TRV-Nb-16, TRV-Nb-22, and TRV-Nb-25, Supplementary Fig. S2C) plants. Compared with the GFP plants, the Nb-GFP plants exhibited higher contents of NAD(P) (H) components (Fig. 9A), resulting in a significant increase in the NAD(P) (H) pool [NAD(P)+NAD(P)H]. In contrast, silencing of NbNHX1 decreased the contents of NAD(P) (H) components and the NAD(P) (H) pool in tobacco plants (Fig. 9B).
Fig. 9.
Measurement of the NAD(P) (H) pool. (A) The contents of NAD(P) (H) components in GFP and Nb-GFP plants. (B) The contents of NAD(P) (H) components in TRV and TRV-Nb plants. Data are means ±SE (n=6 leaves from six independent NbNHX1 silenced or overexpressing lines, respectively). The asterisks on the bars indicate significant differences from the control plants in the same treatment at P≤0.05.
NbNHX1 regulated the expression of ROS-responsive genes
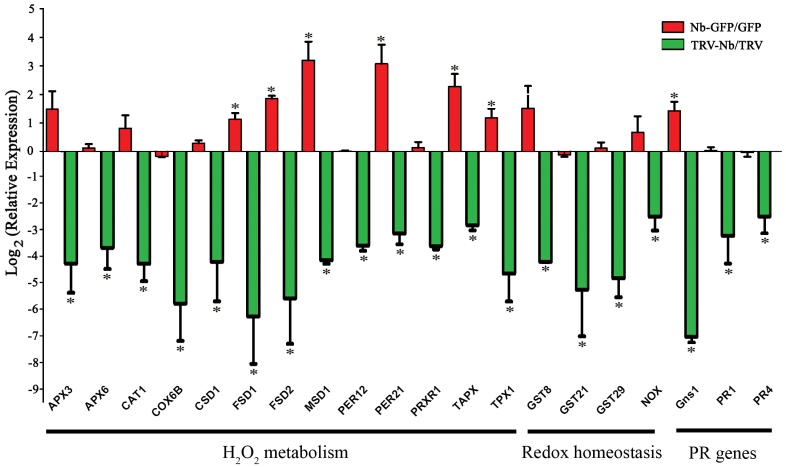
The expression of 20 ROS-responsive genes in NbNHX1-overexpressing (Nb-GFP-5, Nb-GFP-6, and Nb-GFP-9, Supplementary Fig. S4G at JXB online) or silenced (TRV-Nb-10, TRV-Nb-16, and TRV-Nb-25, Supplementary Fig. S2C) N. benthamiana was examined. These genes were selected from the unigene database in the Sol genomics network (http://solgenomics.net/), and were divided into three categories. The first category consisted of genes related to H2O2 metabolism, including ascorbate peroxidase genes, APX3, APX6, and TAPX; a catalase gene, CAT1; a cytochrome c oxidase gene, COX6B; superoxide dismutase genes, CSD1, FSD1, FSD2, and MSD1; and peroxidase genes, PER12, PER21, PRXR1, and TPX1. Category II were genes related to redox homeostasis, including GST8, GST21, GST29, and NOX. Category III were PR (pathogenesis-related) genes, including PR1, PR2 (Gns1), and PR4. The results showed that overexpression of NbNHX1 increased expression of FSD1, FSD2, MSD1, PER21, TAPX, TPX1, and Gns1 in Nb-GFP plants, while silencing of NbNHX1 decreased the expression of all genes (Fig. 10).
Discussion
Thus far, little is known about the function of NHX1 in biotic stresses. In the present study, it was found that endogenous NbNHX1 silencing led to more serious damage after pathogen inoculation in N. benthamiana (Fig. 3). Although the expression of NbNHX1 was found to be induced by Ppn infection (Supplementary Fig. S4A at JXB online), the enhanced expression could not compensate the reduction by gene silencing. Furthermore, the finding that ectopic expression of At/SeNHX1 improved resistance to Ppn in N. benthamiana confirmed the general characteristic of NHX1 in plant disease resistance (Fig. 4). The overexpression of NbNHX1 by PEBV was not used in the present study because endogenous genes can be silenced easily by this system (Constantin et al., 2004). It should be noted that although all the NHX1-transformed tobacco plants succumbed after Ppn attack, the alleviated oxidative damage caused by pathogen infection was important for agriculture production, in that potentially use of the system could buy the time for subsequent chemical prevention.
It has been reported that NHX1 transports both Na+ and K+ cations with similar affinities (Yamaguchi et al., 2003). Although the mechanism of the regulation of potassium transportation by NHX1 is still unclear (Martinoia et al., 2007), some evidence supports that NHX1 can mediate potassium compartmentation in vacuoles (Apse et al., 2003; Leidi et al., 2010). In the present study, the effect of exogenous Na+ or K+ application on the activity of NHX1 in transporting protons was investigated. In particular, the measurement simulated the intracellular ionic environment with 100mM potassium gluconate (Chen et al., 2011b ). As an increasing concentration of NaCl was added to the measuring buffer, TRV plants exhibited enhanced H+ efflux in the vacuole; whereas TRV-Nb plants exhibited unchanged H+ efflux in vacuoles, which was due to the silencing of NbNHX1 (Fig. 1D). When extra 25mM or 50mM KCl was added into the measuring buffer, the net H+ efflux in the vacuoles remained unchanged in TRV plants (Fig. 1E), which may be attributed to little change in the K+ concentration in the measuring buffer. These results suggest that because of a high concentration of K+ in the cytoplasm, a change in Na+ concentration may more easily affect vacuolar proton transport than K+.
Notably, it is reported that tonoplast Na+/H+ antiporters are involved in cytoplasmic acidification in response to microbial elicitors (Viehweger et al., 2002), which is known to induce oxidative burst. The present results support that the tonoplast-localized Na+/H+ exchangers regulating vacuolar pH are involved in cellular oxidative events. The concentration of superoxide in the endosomes depends on many factors, including lumen pH (Lamb et al., 2009). Due to the proton dependence of dismutation, decomposition of superoxide is prolonged ~10-fold for every 10-fold decrease in the proton concentration between pH 6 and 14 (Valentine and Curtis, 1975). It has also been confirmed that the contents of superoxide in vesicles improve along with increasing pH, from 4.4 μM superoxide in pH 5.0 to 30 μM in pH 8.0 (Mumbengegwi et al., 2008). In addition, superoxide produced in endosomes always affects the cellular oxidative state, since superoxide can diffuse easily via transmembranes of endosomes (Brunetti et al., 2011), and the tonoplast and other cellular membranes are quite permeable to Н2О2 (Andreev, 2012). Therefore, it is understandable that increased pH in the vacuole due to overexpression of NbNHX1 resulted in an improved cellular oxidation level in tobacco (Figs 6A, 7), and silencing of NbNHX1 reducing the pH in the vacuole led to a decreased cellular oxidation state (Figs 6B, 8). The conclusion that the pH in the vacuole affected the cellular oxidation state is consistent with the results of Mumbengegwi et al. (2008) and Pradedova et al. (2011), which implies that either accumulation of superoxide in the vacuole, or its being shielded from dismutation, is based on proton transportation across the tonoplast.
The change in cellular oxidation state triggers physicochemical responses, which results in a rapid re-establishment of redox homeostasis (Luthje et al., 2009). In the present study, an enhanced cellular oxidation level was also found to lead to a larger NAD(P) (H) pool and higher expression of redox homeostasis-related genes in NbNHX1-overexpressing plants (Figs 7, 9, and 10), and vice versa in NbNHX1-silenced plants (Figs 8, 9, 10). The NAD(P) (H) components are important molecules in plant response to oxidative stress (Foyer and Noctor, 2003), and the content of NADPH in particular can be significantly increased when cells suffer oxidative damage (Valderrama et al., 2006; Y.P. Wang et al., 2014). It has also been reported that increasing the content of one of the NAD(P) (H) components results in greater contents of both the oxidized and reduced forms of NADs as a larger NAD(P) (H) pool (Hayashi et al., 2005). The evidence also confirms that the NAD(P) (H) pool can be regulated by the cellular oxidation level (Cueno et al., 2014). The present study also found that transformation of NbNHX1 conferred a higher cellular oxidation level on tobacco, resulting in a larger NAD(P) (H) pool (Figs 7, 9). In addition, increased vacuolar H+ efflux might boost proton supplementation for NADPH oxidation mediated by the tonoplast-localized NOX, which would speed up NADP(H) recycling. Therefore, it is understandable that the NbNHX1-overexpressing plants with a higher cellular oxidation level exhibited a larger NAD(P) (H) pool. It should be noted that the NAD(P) (H) components in GFP plants were measured after 3 d agroinfiltration, and those in TRV plants were detected after 4 weeks agroinfiltration for gene silencing, and hence there were some differences in NAD(P) (H) levels between GFP and TRV plants (Fig. 9).
Fig. 10.
Expression of ROS-responsive genes in NbNHX1-overexpressing and silenced plants. Relative expression of each gene was calculated by gene expression in Nb-GFP plants against that in GFP plants (above line), or gene expression in TRV-Nb plants against that in TRV plants (below line). Biparametric output was displayed with log2 (relative expression) and the name of each gene. APX3, ascorbate peroxidase 3 gene; APX6, ascorbate peroxidase 6 gene; CAT1, catalase 1 gene; COX6B, cytochrome c oxidase 6b gene; CSD1, copper/zinc superoxide dismutase 1 gene; FSD1, Fe superoxide dismutase 1 gene; FSD2, Fe superoxide dismutase 2 gene; MSD1, manganese superoxide dismutase 1 gene; PER12, peroxidase 12 gene; PER21, peroxidase 21 gene; PRXR1, secretory peroxidase gene; TAPX, l-ascorbate peroxidase gene; TPX1, thioredoxin-dependent peroxidase 1 gene; GST8, glutathione transferase 8 gene; GST21, glutathione transferase 21 gene; GST29, glutathione transferase 29 gene; NOX, NADPH oxidase gene; Gns1, beta-1,3-glucanase 1 gene; PR1, pathogenesis-related protein 1 gene; PR4, pathogenesis-related protein 4 gene. Data are means ±SE (n=3 independent NbNHX1-silenced or overexpressing lines, respectively). The asterisks on the bars indicate significant differences from the control plants in the same treatment at P≤0.05. (This figure is available in colour at JXB online.)
Ascorbate peroxidases (APXs), catalases (CATs), and peroxidases (PODs) are very important enzymes for H2O2 detoxification, cytochrome c oxidases (COXs) are important for reduction of O2 to H2O, and superoxide dismutases (SODs) catalyse the dismutation of O2- into H2O2, which are all associated with cellular ROS homeostasis (Garg and Manchanda, 2009). In this study, it was found that the genes involved in H2O2 metabolism: APX genes (APX3, APX6, and TAPX), CAT1, COX6B, POX genes (PER12, PER21, and TPX1), and SOD genes (CSD1, FSD1, FSD2, and MSD1) were regulated by the cellular oxidation level in Nb-GFP or TRV-Nb plants (Fig. 10). A changed cellular oxidation state served as a signal to trigger the expression of antioxidant-related genes (Liu et al., 2010). For example, it is reported that sulphur dioxide (SO2) can improve cellular ROS levels, leading to higher expression of genes encoding SODs (CSD1, CSD2) and PODs (POD) (Li and Yi, 2012). However, the gene expression of the ROS scavenging network in Arabidopsis indicates that there are more complicated molecular events in plant response to oxidative damage (Mittler et al., 2004). A mutant (knockout of SOD gene) affecting production of H2O2 exhibits down-regulated expression of nearly all ROS-responsive genes in Arabidopsis (Mittler et al., 2004), which to some extent supports that expression of ROS-responsive genes decreased in NbNHX1-silenced plants with a lower cellular oxidation level (Figs 8, 10). Another possible reason is that NbNHX1 silencing led to a significant decrease in cellular pH which was associated with classic apoptotis of acidified cells and DNA cleavage (Boyle et al., 1997), and hence TRV-Nb plants exhibited decreased expression of most genes. In addition, PR genes (PR1, Gns1, and PR4) were regulated by the cellular oxidation level in NbNHX1-silenced or overexpressing plants (Fig. 10). It has also been reported that in ZmSIMK1 transgenic tobacco regulating cellular ROS promotes transcription of PR genes such as PR1, PR2, and PR4 (L. Wang et al., 2014).
H2O2 plays different roles in plant defence against biotrophic and necrotrophic pathogens. Plants rely on oxidative burst against biotrophic pathogens but are dependent on the alleviation of H2O2 against necrotrophic pathogens (Glazebrook, 2005). It was further investigated whether NHX1 transgenic tobacco plants with enhanced abilities to alleviate H2O2 were more sensitive to biotrophic pathogens. As shown in Supplementary Fig. S6 at JXB online, the leaves of Se-YFP and At-YFP plants, along with those of YFP plants, were infected by the biotrophic pathogen Pseudomonas syringae pv. maculicola ES4326 (Moeder et al., 2005). The bacterial colonies were measured at 0, 72, and 96 hpi, and the results showed that SeNHX1 and AtNHX1 transgenic plants and control tobacco plants exhibited comparable growth of bacteria (Supplementary Fig. S6). It is assumed that the enhanced antioxidative system in NHX1 transgenic tobacco impaired the diffusion of ROS produced by Ppn infection but could not inhibit the oxidative burst induced by biotrophic pathogens. Although tests in more plant species are still needed to investigate further NHX1 function in disease resistance, the present results imply that NHX1 has the potential to be used to increase both salt tolerance and disease resistance in plant.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Screening the distinctive sequence of NbNHX1 for gene silencing.
Figure S2. Virus-induced gene silencing and ectopic expression in N. benthamiana.
Figure S3. In situ calibration curve and pH quantification test.
Figure S4. The relative expression of NHX1 in different tobacco genotypes.
Figure S5. Vacuolar H+ net fluxes in NbNHX1-silenced N. benthamiana under 0 and 1.5mM ATP or PPi treatment.
Figure S6. Responses of NHX1 transgenic tobacco to ES4326.
Table S1. The primers of ROS-responsive genes used in real-time PCR.
Acknowledgements
This work was supported by the Research Programs from the Chinese Ministry of Agriculture (grant no. 2013ZX08009-003-002) and the National Natural Science Foundation of China (grant no. 31200201).
Glossary
Abbreviations:
- At/Se-YFP plant
pCAPE2-At/SeNHX1-YFP vector-transformed tobacco
- DR
CellROX deep red reagent
- Fdox/Fdred
oxidized/reduced ferredoxin
- FSC
forward and side scatter value
- GFP
green fluorescent protein
- GFP plant
35S::GFP cassette-transformed tobacco
- GSSG/GSH
oxidized/reduced glutathione
- hpi
hours post-inoculation
- Nb-GFP
35S::NbNHX1-GFP cassette-transformed tobacco
- NHX1
Na+/H+ exchanger 1
- NMT
non-invasive micro-test electrophysiological technology
- NOX
NADPH oxidase
- PEBV
Pea early browning virus
- Ppn
Phytophthora parasitica var. nicotianae
- PR
pathogenesis related
- RACE
rapid amplification of cDNA ends
- ROS
reactive oxygen species
- TRV
Tobacco rattle virus
- TRV plant
pTRV2 empty vector-transformed tobacco
- TRV-Nb plant
pTRV2-NbNHX1 vector-transformed tobacco
- YFP
yellow fluorescent protein
- YFP plant
pCAPE2 empty vector-transformed tobacco.
References
- Apse MP, Aharon GS, Snedden WA, Blumwald E. 1999. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis . Science 285, 1256–1258 [DOI] [PubMed] [Google Scholar]
- Apse MP, Sottosanto JB, Blumwald E. 2003. Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. The Plant Journal 36, 229–239 [DOI] [PubMed] [Google Scholar]
- Andreev IM. 2012. Role of the vacuole in the redox homeostasis of plant cells. Russian Journal of Plant Physiology 5, 611–617 [Google Scholar]
- Bassil E, Tajima H, Liang YC, Ohto M, Ushijima K, Nakano R, Esumi T, Coku A, Belmonte M, Blumwald E. 2011. The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. The Plant Cell 23, 3482–3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellincampi D, Dipierro N, Salvi G, Cervone F, De Lorenzo G. 2000. Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants. Plant Physiology 122, 1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E. 2000. Sodium transport and salt tolerance in plants. Current Opinion in Cell Biology 12, 431–434 [DOI] [PubMed] [Google Scholar]
- Boyle KM, Irwin JP, Humes BR, Runge SW. 1997. Apoptosis in C3H-10T1/2 cells: roles of intracellular pH, protein kinase C, and the Na+/H+ antiporter. Journal of Cellular Biochemistry 67, 231–240 [PubMed] [Google Scholar]
- Brunetti FA, Ferdinando DM, Ferrini F, Tattini M. 2011. Stress induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signaling and Behavior 6, 709–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C, Songqin P, Zouhar J, Avila EL, Girke T, Raikhel NV. 2004. The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. The Plant Cell 16, 3285–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KM, Gong HJ, Chen GC, Wang SM, Zhang CL. 2003. Up-regulation of glutathione metabolism and changes in redox status involved in adaptation of reed (Phragmites communis) ecotypes to drought-prone and saline habitats. Journal of Plant Physiology 160, 293–301 [DOI] [PubMed] [Google Scholar]
- Chen M, Cheni QJ, Niu XG, Zhang R, Lin HQ, Xu CY, Wang XC, Wang GY, Chen J. 2007. Expression of OsNHX1 gene in maize confers salt tolerance and promotes plant growth in the field. Plant, Soil and Environment 53, 490–498 [Google Scholar]
- Chen XY, Han HP, Jiang P, Nie LL, Bao HXG, Fan PX, Lv SL, Feng JJ, Li YX. 2011a. Transformation of beta-lycopene cyclase genes from Salicornia europaea and Arabidopsis conferred salt tolerance in Arabidopsis and tobacco. Plant and Cell Physiology 52, 909–921 [DOI] [PubMed] [Google Scholar]
- Chen XY, Nie LL, Bao HXG, Jiang P, Lv SL, Li YX. 2011b. Modified non-invasive micro-test electrophysiological technology for vacuolar H+ flux detection. Analytical Biochemistry 418, 295–297 [DOI] [PubMed] [Google Scholar]
- Chen YP, Xing LP, Wu GJ, Wang HZ, Wang XE, Cao AZ, Chen PD. 2007. Plastidial glutathione reductase from Haynaldia villosa is an enhancer of powdery mildew resistance in wheat (Triticum aestivum). Plant and Cell Physiology 48, 1702–1712 [DOI] [PubMed] [Google Scholar]
- Cueno ME, Imai K, Tamura M, Ochiai K. 2014. Butyric acid-induced rat jugular blood cytosolic oxidative stress is associated with SIRT1 decrease. Cell Stress and Chaperones 19, 295–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin GD, Krath BN, MacFarlane SA, Nicolaisen M, Johansen IE, Lund OS. 2004. Virus-induced gene silencing as a tool for functional genomics in a legume species. The Plant Journal 40, 622–631 [DOI] [PubMed] [Google Scholar]
- El Chemaly A, Okochi Y, Sasaki M, Arnaudeau S, Okamura Y, Demaurex N. 2010. VSOP/Hv1 proton channels sustain calcium entry, neutrophil migration, and superoxide production by limiting cell depolarization and acidification. Journal of Experimental Medicine 207, 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Bloom AJ, Queval G, Noctor G. 2009. Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annual Review of Plant Biology 60, 455–484 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. 2003. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiologia Plantarum 119, 355–364 [Google Scholar]
- Galiana E, Rivière MP, Pagnotta S, Baudouin E, Panabières F, Gounon P, Boudier L. 2005. Plant-induced cell death in the oomycete pathogen Phytophthora parasitica . Cellular Microbiology 7, 1365–1378 [DOI] [PubMed] [Google Scholar]
- Garg N, Manchanda G. 2009. ROS generation in plants: boon or bane? Plant Biosystems 143, 81–96 [Google Scholar]
- Glazebrook J. 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annual Review of Phytopathology 43, 205–227 [DOI] [PubMed] [Google Scholar]
- Guo ZJ, Chen XJ, Wu XL, Ling JQ, Xu P. 2004. Overexpression of the AP2/EREBP transcription factor OPBP1 enhances disease resistance and salt tolerance in tobacco. Plant Molecular Biology 55, 607–618 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Takahashi H, Tamura K, Huang J, Yu LH, Kawai-Yamada M, Tezuka T, Uchimiya H. 2005. Enhanced dihydroflavonol-4-reductase activity and NAD homeostasis leading to cell death tolerance in transgenic rice. Proceedings of the National Academy of Sciences, USA 102, 7020–7025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb FS, Moreland JG, Miller FJ. 2009. Electrophysiology of reactive oxygen production in signaling endosomes. Antioxidants and Redox Signaling 11, 1335–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidi EO, Barragan V, Rubio L, et al. 2010. The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. The Plant Journal 61, 495–506 [DOI] [PubMed] [Google Scholar]
- Leshem Y, Melamed-Book N, Cagnac O, Ronen G, Nishri Y, Solomon M, Cohen GA. 2006. Suppression of Arabidopsis vesicle-SNARE expression inhibited fusion of H2O2-containing vesicles with tonoplast and increased salt tolerance. Proceedings of the National Academy of Sciences, USA 103, 18008–18013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Jiang GQ, Huang P, Ma J, Zhang FC. 2007. Overexpression of the Na+/H+ antiporter gene from Suaeda salsa confers cold and salt tolerance to transgenic Arabidopsis thaliana . Plant Cell, Tissue and Organ Culture 90, 41–48 [Google Scholar]
- Li LH, Yi HL. 2012. Effect of sulfur dioxide on ROS production, gene expression and antioxidant enzyme activity in Arabidopsis plants. Plant Physiology and Biochemistry 58, 46–53 [DOI] [PubMed] [Google Scholar]
- Liu FX, Xu WY, Wei Q, et al. 2010. Gene expression profiles deciphering rice phenotypic variation between Nipponbare (Japonica) and 93-11 (Indica) during oxidative stress. PLoS One 5, e8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YL, Nakayama N, Schiff M, Litt A, Irish VF, Dinesh-Kumar SP. 2004. Virus induced gene silencing of a DEFICIENS ortholog in Nicotiana benthamiana . Plant Molecular Biology 54, 701–711 [DOI] [PubMed] [Google Scholar]
- Liu YL, Schiff M, Dinesh-Kumar SP. 2002. Virus-induced gene silencing in tomato. The Plant Journal 31, 777–786 [DOI] [PubMed] [Google Scholar]
- Luthje S, Hopff D, Schmitt A, Meisrimler CN, Menckhoff L. 2009. Hunting for low abundant redox proteins in plant plasma membranes. Journal of Proteomics 72, 475–483 [DOI] [PubMed] [Google Scholar]
- Martinoia E, Maeshima M, Neuhaus HE. 2007. Vacuolar transporters and their essential role in plant metabolism. Journal of Experimental Botany 58, 83–102 [DOI] [PubMed] [Google Scholar]
- Mikawa T, Kanoh J, Ishikawa F. 2010. Fission yeast Vps1 and Atg8 contribute to oxidative stress resistance. Genes to Cells 15, 229–242 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. 2004. Reactive oxygen gene network of plants. Trends in Plant Science 9, 490–498 [DOI] [PubMed] [Google Scholar]
- Moeder W, Yoshioka K, Klessig DF. 2005. Involvement of the small GTPase Rac in the defense responses of tobacco to pathogens. Molecular Plant-Microbe Interactions 18, 116–124 [DOI] [PubMed] [Google Scholar]
- Mumbengegwi DR, Li Q, Li C, Bear CE, Engelhardt JF. 2008. Evidence for a superoxide permeability pathway in endosomal membranes. Molecular and Cellular Biology 28, 3700–3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiologia Plantarum 15, 473–497 [Google Scholar]
- O’Connell RJ, Panstruga R. 2006. Tete a tete inside a plant cell: establishing compatibility between plants and biotrophic fungi and oomycetes. New Phytologist 171, 699–718 [DOI] [PubMed] [Google Scholar]
- Plonka A, Mayer J, Metodiewa D, Gebicki JL, Zgirski A, Grabska M. 1986. Superoxide radical dismutation by copper proteins. Journal of Radioanalytical and Nuclear Chemistry 101, 221–225 [Google Scholar]
- Pradedova EV, Isheeva OD, Salyaev RK. 2011. Antioxidant defense enzymes in cell vacuoles of red beet roots. Russian Journal of Plant Physiology 58, 36–44 [Google Scholar]
- Qiu QS, Guo Y, Quintero FJ, Pardo JM, Schumaker KS, Zhu JK. 2004. Regulation of vacuolar Na+/H+ exchange in Arabidopsis thaliana by the salt-overly-sensitive (SOS) pathway. Journal of Biological Chemistry 279, 207–215 [DOI] [PubMed] [Google Scholar]
- Romero-Puertas MC, Rodriguez-Serrano M, Corpas FJ, Gomez M, Del-Rio LA, Sandalio LM. 2004. Cadmium-induced subcellular accumulation of O2·– and H2O2 in pea leaves. Plant, Cell and Environment 27, 1122–1134 [Google Scholar]
- Sato TK, Overduin M, Emr SD. 2001. Location, location, location: membrane targeting directed by PX domains. Science 294, 1881–1885 [DOI] [PubMed] [Google Scholar]
- Shabala S, Cuin TA. 2007. Potassium transport and plant salt tolerance. Physiologia Plantarum 133, 651–669 [DOI] [PubMed] [Google Scholar]
- Sharma SS, Dietz KJ. 2009. The relationship between metal toxicity and cellular redox imbalance. Trends in Plant Science 14, 43–50 [DOI] [PubMed] [Google Scholar]
- Tian LM, Huang CL, Yu R, Liang RF, Li ZL, Zhang LS, Wang YQ, Zhang XH, Wu ZY. 2006. Overexpression AtNHX1 confers salt-tolerance of transgenic tall fescue. African Journal of Biotechnology 5, 1041–1044 [Google Scholar]
- Valderrama R, Corpas FJ, Carreras A, Gómez-Rodríguez MV, Chaki M, Pedrajas JR, Fernández-Ocaña A, Del Río LA, Barroso JB. 2006. The dehydrogenase-mediated recycling of NADPH is a key antioxidant system against salt-induced oxidative stress in olive plants. Plant, Cell and Environment 29, 1449–1459 [DOI] [PubMed] [Google Scholar]
- Valentine JS, Curtis AB. 1975. A convenient preparation of solutions of superoxide anion and the reaction of superoxide anion with a copper (II) complex. Journal of the American Chemical Society 97, 224–226 [DOI] [PubMed] [Google Scholar]
- Viehweger K, Dordschbal B, Roos W. 2002. Elicitor-activated phospholipase A2 generates lysophosphatidylcholines that mobilize the vacuolar H+ pool for pH signaling via the activation of Na+-dependent proton fluxes. The Plant Cell 14, 1509–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu Y, Cai GH, Jiang SS, Pan JW, Li DQ. 2014. Ectopic expression of ZmSIMK1 leads to improved drought tolerance and activation of systematic acquired resistance in transgenic tobacco. Journal of Biotechnology 172, 18–29 [DOI] [PubMed] [Google Scholar]
- Wang YP, Zhou LS, Zhao YZ, et al. 2014. Regulation of G6PD acetylation by SIRT2 and KAT9 modulates NADPH homeostasis and cell survival during oxidative stress. EMBO Journal 33, 1304–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GH, Grolig F, Kosegarten H. 1998. Differential pH restoration after ammonia-elicited vacuolar alkalisation in rice and maize root hairs as measured by fluorescence ratio. Planta 206, 154–161 [Google Scholar]
- Woltz SS. 1978. Nonparasitic plant pathogens. Annual Review of Phytopathology 16, 403–430 [Google Scholar]
- Yamaguchi T, Apse MP, Shi HZ, Blumwald E. 2003. Topological analysis of a plant vacuolar Na+/H+ antiporter reveals a luminal C terminus that regulates antiporter cation selectivity. Proceedings of the National Academy of Sciences, USA 100, 12510–12515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Fukada-Tanaka S, Inagaki Y, Saito N, Yonekura-Sakakibara K, Tanaka Y, Kusumi T, Iida S. 2001. Genes encoding the vacuolar Na+/H+ exchanger and flower coloration. Plant and Cell Physiology 42, 451–461 [DOI] [PubMed] [Google Scholar]
- Zhang HX, Blumwald E. 2001. Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nature Biotechnology 19, 765–768 [DOI] [PubMed] [Google Scholar]
- Zhou SF, Chen XY, Zhang XG, Li YX. 2008. Improved salt tolerance in tobacco plants by co-transformation of a betaine synthesis gene BADH and a vacuolar Na+/H+ antiporter gene SeNHX1 . Biotechnology Letters 30, 369–376 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.