Abstract
Objective: The purpose of this study was to evaluate the efficacy of once-daily guanfacine extended release (GXR) monotherapy administered either in the morning or evening, using a modified Conners' Parent Rating Scale–Revised: Short Form (CPRS–R:S) assessed three times/day in children with attention-deficit/hyperactivity disorder (ADHD).
Methods: This multicenter, double-blind, placebo-controlled study randomized children 6–12 years of age with ADHD into three groups: GXR a.m. (GXR in the morning and placebo in the evening), GXR p.m. (placebo in the morning and GXR in the evening), or twice-daily placebo. The CPRS–R:S, administered in the morning, afternoon, and evening prior to each study visit, was a secondary measure of efficacy.
Results: A total of 333 subjects were included in the analysis population (GXR a.m., n=107; GXR p.m., n=114; placebo, n=112). At visit 10, last observation carried forward (LOCF), subjects receiving GXR demonstrated significantly greater improvement from baseline in the daily mean CPRS–R:S total score, as well as in each of the morning, afternoon, and evening CPRS–R:S assessments, compared with placebo, regardless of the time of GXR administration (p<0.001 vs. placebo for GXR a.m. and GXR p.m.). In addition, subjects receiving GXR showed significantly greater improvements from baseline in each subscale score (oppositional, cognitive problems/inattention, hyperactivity, and ADHD index) compared with those receiving placebo, regardless of time of administration (p<0.003 vs. placebo across all subscales for GXR a.m. and GXR p.m.).
Conclusions: These results provide further support for the demonstrated efficacy of once-daily GXR in reducing ADHD symptoms, and demonstrate that response is consistent throughout the day regardless of the time of administration, with improvement seen in ratings of oppositional as well as of ADHD symptoms.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a disorder of hyperactivity, impulsivity, and inattention estimated to affect ∼9.5% of children and adolescents 4–17 years of age in the United States (Biederman and Faraone 2005; Centers for Disease Control and Prevention 2010). In ADHD, school/work functioning, psychological functioning, and/or social functioning are impaired, and ADHD often persists into adulthood (Biederman and Faraone 2005; Faraone et al. 2009). Stimulant medications are the most widely used pharmacological treatments for ADHD; however, in some patients, these medications have been associated with suboptimal efficacy and poor tolerability (Banaschewski et al. 2004; Faraone et al. 2009).
Nonstimulants, such as α2A-adrenoceptor agonists (e.g., guanfacine extended release [GXR] and clonidine XR), have also demonstrated efficacy in the treatment of ADHD (Biederman et al. 2008; Sallee et al. 2009; Jain et al. 2011; Wolraich et al. 2011). GXR is indicated in the United States for the treatment of ADHD in children and adolescents 6–17 years of age, both as monotherapy and used adjunctively to stimulants (INTUNIV 2011). The efficacy and safety of once-daily GXR monotherapy administered in the morning have been evaluated in a number of studies, including two large, randomized, double-blind, placebo-controlled trials in children and adolescents with ADHD (Biederman et al. 2008; Sallee et al. 2009). Recently, the efficacy and tolerability of once-daily GXR administered in the morning or evening were also evaluated in a double-blind, randomized, placebo-controlled trial in children 6–12 years of age with ADHD (Newcorn et al. 2013). GXR monotherapy administered either in the morning or evening resulted in significant and clinically meaningful reductions in ADHD symptoms compared with placebo, as measured by the ADHD Rating Scale IV (ADHD-RS-IV), the study's primary efficacy outcome. In addition, the tolerability profile of GXR administered either in the morning or evening was similar to that of previous clinical trials of GXR (Biederman et al. 2008; Sallee et al. 2009).
The objective of the current exploratory analyses was to examine additional potential efficacy differences between once-daily GXR (1–4 mg/day) monotherapy, administered either in the morning or evening, compared with placebo in children 6–12 years of age—specifically, consistency of response throughout the day and effect on oppositional as well as ADHD symptoms. The Conners' Parent Rating Scale–Revised: Short Form (CPRS–R:S), a parent-report measure designed to assess children's problem behaviors over the previous month, has become a useful tool for assessing treatment outcomes in children with ADHD; the CPRS–R:S can provide ADHD-related, behavior-specific outcome measures (e.g., hyperactivity/impulsivity) of treatment over time (Conners et al. 1998). In this study, the CPRS–R:S administration schedule was modified to assess ADHD symptoms at three time points (morning, afternoon, and evening) throughout the day, and analyzed as a secondary measure of efficacy. A modified CPRS has been utilized as an efficacy measure at similar time points throughout the day in previous studies of stimulants for the treatment of ADHD (Lopez et al. 2008; Coghill et al. 2013; VYVANSE 2013). The CPRS–R:S has widespread clinical utility and provides information on oppositional symptoms (Conners et al. 1998), a parameter not examined by the ADHD-RS-IV. Because α agonists have been associated with sedative adverse events (AEs), including somnolence, sedation, and hypersomnia (Jain et al. 2011; Kollins et al. 2011), the Pediatric Daytime Sleepiness Scale (PDSS) was also administered as an exploratory secondary measure of safety and tolerability, to determine the extent of daytime sleepiness reported by children treated with GXR monotherapy (and whether this varied as a function of time of administration).
Methods
Subjects
Children 6–12 years of age with a primary diagnosis of ADHD with combined subtype or hyperactive/impulsive subtype, as defined by the Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM-IV-TR) (American Psychiatric Association 2000) based on psychiatric assessment using the Kiddie-Schedule for Affective Disorders and Schizophrenia–Present and Lifetime version (K-SADS-PL), were enrolled in this study. Subjects were required to have an ADHD-RS-IV total score ≥28 and a Clinical Global Impressions–Severity score ≥4 at baseline. Subjects were excluded if they had a current diagnosis of controlled or uncontrolled comorbid psychiatric disorders (except oppositional defiant disorder), including any severe comorbid Axis II or Axis I disorders that could potentially confound efficacy and safety measures or for which GXR is contraindicated, a previous or present risk for suicide, or a history or active presence of cardiac abnormalities or a primary sleep disorder.
Study design
This was an 8 week, multicenter, double-blind, randomized, placebo-controlled, dose optimization, phase 3 study. A screening visit (visit 1) to determine eligibility occurred in the month prior to randomization. Eligible subjects were randomized in a 1:1:1 ratio to the following treatment arms: GXR a.m. (GXR administered in the morning and placebo administered in the evening), GXR p.m. (placebo administered in the morning and GXR administered in the evening), or placebo (placebo administered in the morning and evening). The study consisted of a 5 week dose-optimization period, a 3 week dose-maintenance period, and a 9 day dose-taper period.
During dose optimization, a starting dose of 1 mg/day was titrated upward in 1 mg increments after a minimum of 1 week at the previous dose, based on clinical response and tolerability, up to a maximum of 4 mg/day (visits 2–7). The objective of dose optimization was to determine the dose required to achieve an adequate response (i.e., optimal dose), defined as a clinically significant reduction in ADHD symptoms (≥30% reduction in ADHD-RS-IV total score from baseline) with an acceptable level of side effects. If a ≥30% reduction in ADHD-RS-IV scores was achieved, the optimal dose was well tolerated, and the clinician felt that the subject could potentially achieve additional symptom reduction, the dose could be increased to the next dosage strength. The investigator could decrease the dose by 1 mg based on tolerability; however, only one dose reduction was allowed per subject during the study.
Subjects were maintained on their optimal dose for an additional 3 weeks, during which efficacy and safety were assessed weekly (visits 8–10). During the maintenance period, the subject's dose could be reduced by 1 mg based on tolerability, provided that the dose had not been decreased during the optimization period. Final on-treatment assessment was defined as the last non-missing postbaseline value while the subject was on treatment (before dose tapering) with study drug (analogous to visit 10, last observation carried forward [LOCF]).
Assessments
Results of the primary efficacy measure of change from baseline in the clinician-rated ADHD-RS-IV score have been reported previously (Newcorn et al. 2013). Secondary efficacy endpoints included the change from baseline in the parent/guardian-rated CPRS–R:S score; these results are reported herein. The CPRS–R:S consists of 27 questions grouped into four subscales: oppositional (6 items), cognitive problems/inattention (6 items), hyperactivity (6 items), and ADHD index (12 items). Each item is rated on a four point scale, from 0 (not true at all) to 3 (very much true); total score ranged from 0–81 (higher scores represent greater severity). In the current study, the administration of the CPRS−R:S administration was modified from the validated guidelines in order to evaluate the duration of efficacy response throughout the day (Lopez et al. 2008; VYVANSE 2013). The CPRS−R:S was administered upon awakening (6:00 a.m.), at midday (2:00 p.m.), and during the evening (8:00 p.m.) prior to each of the following visits: 2 (baseline), 7, 8, 9, and 10, and assessments were to be performed within 1 hour of the specified times, rating the child's behavior immediately before the assessment time.
Safety evaluations included assessments of AEs, vital signs, laboratory test results, physical examination findings, and ratings on the PDSS. The PDSS, an eight question self-report scale suitable for children and adolescents 11–15 years of age (Drake et al. 2003), was administered to subjects (with parental assistance) at visits 1 (screening), 2 (baseline), and all subsequent scheduled visits through visit 10 (week 8 of treatment). Each item on the PDSS is scored using Likert scale ratings from 0 (never) to 4 (always) for a maximum total score of 32, with higher scores reflecting greater levels of daytime sleepiness.
Data analyses
The analysis population included all subjects who were randomized and had taken one or more doses of the study drug. Mean CPRS–R:S scores and mean change from baseline in CPRS–R:S scores were summarized at each time point (morning, afternoon, and evening), and mean scores across the three time points were calculated by treatment group at visits 7, 8, 9, and 10; if scores at any time point from a given visit were missing, the mean was taken from available time points at that visit. Changes in CPRS–R:S total scores were examined at each time point, and a daily mean score was calculated across all time points combined. Changes in CPRS–R:S subscale scores were only examined across all time points combined (daily mean scores).
PDSS total score and mean change from baseline were summarized at each visit through visit 10, using LOCF. A post-hoc item analysis of the PDSS was conducted to further evaluate the effects of treatment on daytime sleepiness. Response distributions to individual PDSS questions were evaluated using the Cochran–Mantel–Haenszel test. Strengths of association were evaluated between PDSS total score change from baseline to visit 10, LOCF, and weight (Pearson correlation), age (Spearman rank correlation), and sex (Spearman rank correlation). Changes from baseline in CPRS–R:S and PDSS scores were analyzed using analysis of covariance (ANCOVA) models at each applicable visit, using LOCF methodology to account for missing data.
Results
Subject disposition
A total of 340 subjects were enrolled in the study. Seven subjects (six in the GXR a.m. group and one in the placebo group) did not receive study drug for the following reasons: protocol violations, being lost to follow-up, or withdrawal from study. Of 333 subjects included in the analysis population, 107 subjects were in the GXR a.m. group, 114 were in the GXR p.m. group, and 112 were in the placebo group. A total of 247 subjects completed the study through visit 10, with 80 subjects (70.8%) in the GXR a.m. group, 90 (78.9%) in the GXR p.m. group, and 77 (68.1%) in the placebo group. Subject demographic and baseline characteristics were similar among treatment groups (Table 1). The majority of subjects were male (70.6%), white (57.1%), and classified as having combined ADHD subtype (96.1%). The mean (standard deviation [SD]) age was 9.1 (1.77) years and the mean time since diagnosis was 1.7 (2.17) years.
Table 1.
Demographic and Baseline Characteristics
| Characteristic | GXRa.m.(n=107) | GXR p.m.(n=114) | Placebo(n=112) |
|---|---|---|---|
| Mean (SD) age, y | 9.1 (1.77) | 9.3 (1.76) | 8.9 (1.78) |
| Sex, n (%) | |||
| Male | 72 (67.3) | 78 (68.4) | 85 (75.9) |
| Female | 35 (32.7) | 36 (31.6) | 27 (24.1) |
| Race, n (%) | |||
| White | 66 (61.7) | 68 (59.6) | 56 (50.0) |
| Black or African American | 38 (35.5) | 35 (30.7) | 47 (42.0) |
| Asian | 1 (0.9) | 0 | 1 (0.9) |
| American Indian or Alaska Native | 0 | 1 (0.9) | 0 |
| Other | 2 (1.9) | 10 (8.8) | 8 (7.1) |
| Mean (SD) body weight, lb | 77.95 (19.44) | 80.38 (20.91) | 75.79 (17.57) |
| Mean (SD) BMI, kg/m2 | 17.92 (2.42) | 18.25 (2.35) | 17.96 (2.33) |
| ADHD subtype, n (%) | |||
| Predominately inattentivea | 3 (2.8) | 3 (2.6) | 1 (0.9) |
| Predominately hyperactive-impulsive | 3 (2.8) | 2 (1.8) | 1 (0.9) |
| Combined subtype | 101 (94.4) | 109 (95.6) | 110 (98.2) |
| Mean (SD) time since ADHD diagnosis, y | 1.5 (2.12) | 2.0 (2.24) | 1.6 (2.13) |
Predominately inattentive subtype was exclusionary.
ADHD, attention-deficit/hyperactivity disorder; BMI, body mass index; GXR, guanfacine extended release; SD, standard deviation.
Reprinted from Journal of the American Academy of Child & Adolescent Psychiatry 52(9), Jeffrey H. Newcorn, Mark A. Stein, Ann C. Childress, Sharon Youcha, Carla White, Gail Enright, and Jonathan Rubin, Randomized, double-blind trial of guanfacine extended release in children with attention-deficit/hyperactivity disorder: Morning or evening administration, 921–930, ©2013, with permission from Elsevier.
Optimized dose
More than half of the subjects (54.3%) reached an optimal dose at 3 or 4 mg. The mean (SD) optimal dose was similar between the GXR a.m. (2.9 [0.92] mg) and GXR p.m. (3.0 [0.98] mg) groups. The mean weight-adjusted optimal dose was also similar in both active treatment groups (GXR a.m., 0.083 mg/kg; GXR p.m., 0.085 mg/kg), with the majority of subjects achieving optimal doses in the 0.05–0.08 mg/kg or 0.09–0.12 mg/kg weight-adjusted dose ranges.
CPRS–R:S total scores by time of assessment
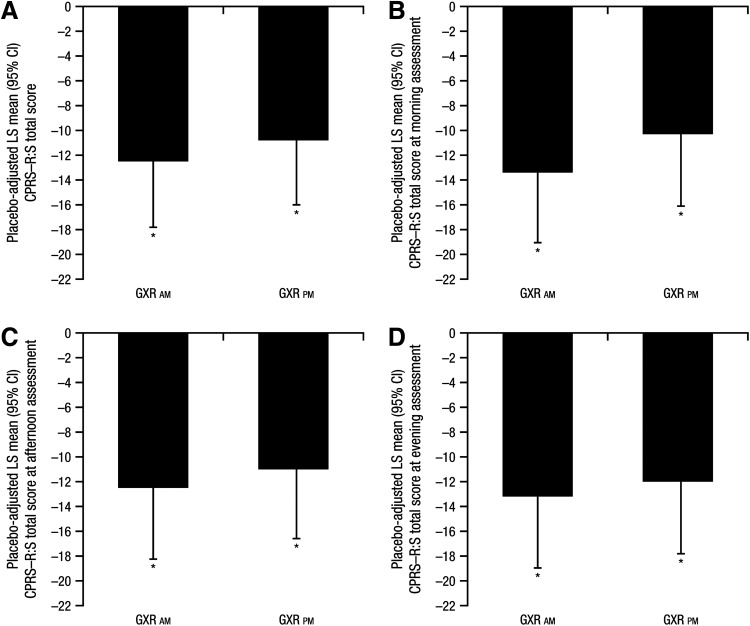
At baseline, daily mean (SD) CPRS–R:S total scores were similar among treatment groups (47.0 [18.88] for the GXR a.m. group, 48.0 [15.63] for the GXR p.m. group, and 49.6 [17.51] for the placebo group); baseline CPRS−R:S total scores were also similar across treatment groups at each of the three daily assessments (morning, afternoon, and evening). At visit 10, LOCF, subjects receiving GXR had a significantly greater improvement from baseline in the daily mean CPRS–R:S total score than did those receiving placebo, regardless of the time of GXR administration (p<0.001 for GXR a.m. and GXR p.m.; Fig. 1A). Effect sizes were similar for both morning and evening GXR administration (0.71 and 0.62, respectively). Subjects on GXR also showed significant improvement compared with the placebo group starting at visit 7 (i.e., the first CPRS–R:S assessment postbaseline, 5 weeks on treatment or LOCF; p<0.001 for GXR a.m. and GXR p.m.).
FIG. 1.
Placebo-adjusted least squares (LS) mean (95% CI) change from baseline in Conners' Parent Rating Scale–Revised: Short Form (CPRS–R:S) total scores at visit 10, last observation carried forward (LOCF): (A) across all three time points; (B) at morning assessment; (C) at afternoon assessment; and (D) at evening assessment. *p<0.001. GXR, guanfacine extended release. LS means and p values are based on type III sum of squares from an analysis of covariance (ANCOVA) model for the change from baseline, with treatment group as a fixed effect and baseline value as a covariate.
At each of the morning, afternoon, and evening CPRS–R:S assessments at visit 10, LOCF, subjects receiving GXR showed significantly greater improvements from baseline than did those receiving placebo, regardless of the time of GXR administration (p<0.001 for morning, afternoon, and evening CPRS–R:S assessments for GXR a.m. and GXR p.m.; Fig. 1B–D). At each of the three daily CPRS–R:S assessments, significant improvement from baseline in the total score was evident in subjects receiving GXR compared with those receiving placebo, starting at the first visit at which a postbaseline measurement was obtained (visit 7 [5 weeks on treatment or LOCF]; p≤0.002 for morning, afternoon, and evening assessments).
CPRS–R:S subscale scores
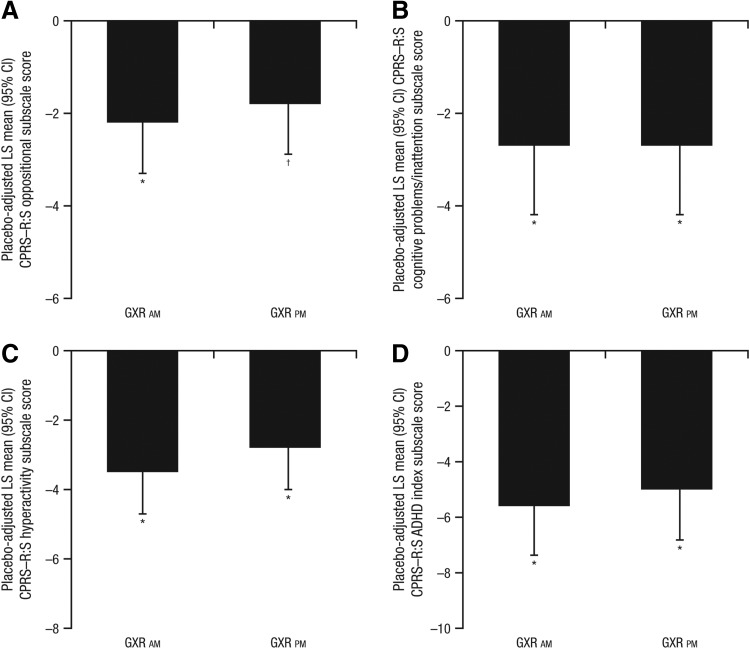
At baseline, all mean CPRS–R:S subscale scores were similar among treatment groups. At visit 10, LOCF, subjects receiving GXR showed significantly greater improvements from baseline in each subscale score (oppositional, cognitive problems/inattention, hyperactivity, and ADHD index) than did those receiving placebo, regardless of time of administration (p<0.003 across all subscales for GXR a.m. and GXR p.m.; Fig. 2). Effect sizes were similar within each subscale across the different times of GXR administration (effect size range, 0.55–0.85 for GXR a.m. and 0.47–0.68 for GXR p.m.). Subjects on GXR also showed significant improvement on each subscale compared with those receiving placebo starting at visit 7 (5 weeks on treatment or LOCF); p<0.008 across all subscales for GXR a.m. and GXR p.m. vs. placebo.
FIG. 2.
Placebo-adjusted least squares (LS) mean (95% CI) change from baseline in Conners' Parent Rating Scale–Revised: Short Form (CPRS–R:S) subscale scores at visit 10, last observation carried forward (LOCF): (A) oppositional; (B) cognitive problems/inattention; (C) hyperactivity; and (D) attention-deficit/hyperactivity disorder (ADHD) index. *p<0.001; †p<0.01. GXR, guanfacine extended release. LS means and p values are based on type III sum of squares from an analysis of covariance (ANCOVA) model for the change from baseline, with treatment group as a fixed effect and baseline value as a covariate.
Safety
Treatment-emergent adverse events (TEAEs) from this study have been previously discussed in detail (Newcorn et al. 2013). In brief, TEAEs occurred in 81.4% of subjects receiving GXR (79.4% for GXR a.m. and 83.3% for GXR p.m.) compared with 57.1% of subjects receiving placebo. The most frequently reported AEs (reported in >10% of subjects) in the GXR groups were somnolence, headache, sedation, upper abdominal pain, and fatigue. The majority of AEs were mild or moderate, and 7.2% of those on GXR (eight subjects each in the a.m. and p.m. groups) discontinued the study because of AEs. Three subjects reported serious AEs (SAEs): one subject in the GXR a.m. group (syncope) and two subjects in the GXR p.m. group (syncope and self-injurious ideation/suicidal ideation). All SAEs were determined by the investigators to be of mild/moderate intensity and to be related to study drug; all resolved by each subject's final study visit.
The impact of GXR lowering blood pressure and pulse was consistent with the known safety profile of GXR. For the GXR a.m. and p.m. groups, respectively, mean decreases in supine pulse rate were −3.7 and −3.8 bpm; decreases in systolic blood pressure were −1.6 and −2.1 mm Hg; and decreases in diastolic blood pressure were −0.8 and −2.1 mm Hg. At visit 10, LOCF, subjects receiving GXR demonstrated a mean decrease from baseline in supine pulse rate, systolic blood pressure, and diastolic blood pressure (−3.8 bpm, −1.9 mm Hg, and −1.5 mm Hg) compared with subjects receiving placebo (+1.0 bpm, −0.5 mm Hg, and −0.3 mm Hg).
PDSS scores
At baseline, mean (SD) PDSS total scores were similar among treatment groups: 13.9 (5.73) for the GXR a.m. group, 14.9 (5.93) for the GXR p.m. group, and 14.8 (5.75) for the placebo group. By comparison, the normative sample of 450 students from 6th to 8th grades had a mean (SD) PDSS total score of 15.3 (6.2) (Drake et al. 2003), indicating that sleep was not especially disrupted in this population. No significant correlations were found between change from baseline to visit 10, LOCF, in PDSS total scores by treatment group and age, weight, or gender (p≥0.260 among groups for all coefficients). In addition, there were no consistent effects of treatment on PDSS total scores based on whether subjects experienced a sedative TEAE, including somnolence, sedation, and hypersomnia.
Discussion
There is increasing awareness that children with ADHD can experience symptoms throughout the day: In school, at home, and during evening activities (Pelham et al. 2001). Therefore, children with ADHD may have a need for long-lasting therapeutic options for the treatment of ADHD symptoms. The current analysis demonstrates that morning or evening administration of once-daily GXR reduces ADHD-related symptoms compared with placebo, as measured by a modified parent/guardian-rated CPRS–R:S. Importantly, symptom reductions were observed at three intervals during the day, at morning, afternoon, and evening CPRS–R:S assessments, regardless of morning or evening GXR administration, supporting once-daily dosing of GXR.
Several other studies have used the CPRS–R:S as an outcome measure. Clinical studies with other nonstimulant therapies such as atomoxetine and clonidine have also demonstrated improvements in CPRS–R:S scores (Michelson et al. 2001, 2002; Spencer et al. 2002). These studies presumably administered unmodified versions of the CPRS–R:S, with baseline and endpoint assessments at least 1 month apart. The current study examined a modified CPRS–R:S at three time points across the day, as was done previously with the stimulant lisdexamfetamine dimesylate (Lopez et al. 2008; VYVANSE 2013). Results not only demonstrated that once-daily GXR is effective in reducing the CPRS–R:S total score, but these improvements were observed consistently throughout the day. However, as this study used a modified version of the CPRS–R:S, direct comparisons cannot be made with the previous studies of nonstimulants.
In addition to improvements in the CPRS–R:S total score, morning or evening administration of GXR was more effective than placebo across all symptom subscales (oppositional, cognitive problems/inattention, hyperactivity, and ADHD index). Effect sizes for morning or evening administration of GXR were similar across all subscales; the overall improvement in CPRS–R:S total score was not predominantly driven by any particular subscale. Importantly, GXR showed improvements in the oppositional symptomatology as assessed by the oppositional subscale of the CPRS, symptomatology that is not assessed by the ADHD-RS-IV.
Exploratory secondary safety analyses were conducted using the PDSS to evaluate the effects of GXR (morning or evening administration) on overall daytime sleepiness in children with ADHD, as nonstimulant agents including GXR have been associated with somnolence, sedation, and hypersomnia (Jain et al. 2011; Kollins et al. 2011). Baseline PDSS total scores across treatment groups were similar to the normative sample, and there were no consistent effects of GXR treatment (morning or evening administration) on PDSS total scores based on whether subjects experienced a sedative TEAE. This result is noteworthy, given the sedative side effects typically associated with α agonists, and the fact that sedation was a frequently occurring AE in this study, as previously indicated.
There are several limitations to the methodology of this study that should be considered. First, as previously mentioned, the CPRS–R:S was modified to evaluate ADHD-related symptoms at several time points throughout the day, rather than completing the measure while considering symptoms during the previous month (as it is validated for use) (Conners 1997), which may limit the interpretation of these results. Second, although the study drug was administered at two different time points (morning and evening), this study was not powered to formally assess differences between the a.m. and p.m. cohorts. Last, the PDSS is a subjective assessment and has not demonstrated correlation with objective measures of sleep (e.g., polysomnography) (Patil 2010). As a self-report measure, the PDSS results may have been skewed by inherent rater bias; there appears to be a trend toward inaccuracy for self-appraisal by children and adults with ADHD, although controversy exists regarding this topic (Knouse et al. 2005; Owens et al. 2007; Rizzo et al. 2010; Manor et al. 2012). In addition, although parental assistance was permitted, it is important to note that the PDSS was developed for children and adolescents 11–15 years of age (Drake et al. 2003), but was administered in a younger population (6–12 years) in this study. Furthermore, it is possible that the PDSS scores did not correlate with sedative TEAE incidence because the event may have resolved prior to being captured by the PDSS. For these reasons, the PDSS, as employed in this study, may not have been the most appropriate measure to examine daytime sleepiness.
Conclusions
Once-daily GXR monotherapy has demonstrated efficacy in reducing ADHD symptoms in children, as measured previously by the clinician-rated ADHD-RS-IV, and in the current study by using a modified parent/guardian-rated CPRS–R:S. Once-daily GXR was effective in reducing ADHD symptoms consistently throughout the day as assessed by the CPRS–R:S, regardless of whether the medication was administered in the morning or evening. These improvements were observed in oppositional symptoms and consistently across all other subscales of the CPRS–R:S. Furthermore, the long-lasting effects of GXR support once-daily dosing.
Clinical Significance
These data suggest that once-daily GXR effectively reduces ADHD symptoms and oppositional symptoms in children with ADHD throughout the day. GXR demonstrated similar efficacy whether administered in the morning or evening, consistent with its long half-life and extended time to maximum blood concentrations, thus providing the convenience of either morning or evening administration, as preferred by clinicians and/or patients.
Acknowledgments
Under author direction, Melissa Brunckhorst of MedErgy provided writing assistance for this publication. Editorial assistance in formatting, proofreading, copy editing, and fact checking was also provided by MedErgy.
Disclosures
Joel Young serves on advisory boards for Eli Lilly and Company, Shionogi Inc., and Shire; speakers bureaus for Bristol-Myers Squibb, Eli Lilly and Company, Forest Laboratories, Shionogi Inc., and Shire; and receives grant/research support from Cyberonics, Eli Lilly and Company, Forest Laboratories, Otsuka, Pfizer, Shire, and Takeda. Thomas Rugino has been a consultant to and/or speaker for Bristol-Myers Squibb, NEBA Health, and Shire. Children's Specialized Hospital has received research funding from Bristol-Myers Squibb, Eli Lilly and Company, Forest Laboratories Shire, and Supernus Pharmaceuticals. Ryan Dammerman was an employee of Shire, which funded this study, at the time of manuscript development, and previously held stock/options in Shire. Andrew Lyne is an employee of Shire, which funded this study, and owns stock/options in Shire. Jeffrey Newcorn has received research support from Shire. He has served as an advisor and/or consultant for Alcobra, BioBehavioral Diagnostics, Enzymotec, GencoSciences, Neos Therapeutics, Shire, and Sunovion.
References
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- Banaschewski T, Roessner V, Dittmann RW, Santosh PJ, Rothenberger A: Non-stimulant medications in the treatment of ADHD. Eur Child Adolesc Psychiatry 13Suppl 1:I102–I116, 2004 [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV: Attention-deficit hyperactivity disorder. Lancet 366:237–248, 2005 [DOI] [PubMed] [Google Scholar]
- Biederman J, Melmed RD, Patel A, McBurnett K, Konow J, Lyne A, Scherer N: A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics 121:e73–e84, 2008 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention: Increasing prevalence of parent-reported attention-deficit/hyperactivity disorder among children—United States, 2003 and 2007. MMWR Morb Mortal Wkly Rep 59:1439–1443, 2010 [PubMed] [Google Scholar]
- Coghill DR, Banaschewski T, Lecendreux M, Zuddas A, Dittmann RW, Otero IH, Civil R, Bloomfield R, Squires LA: Efficacy of lisdexamfetamine dimesylate throughout the day in children and adolescents with attention-deficit/hyperactivity disorder: Results from a randomized, controlled trial. Eur Child Adolesc Psychiatry, 23:61–68, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK: Conners Rating Scales-Revised. Toronto: Multi-Health Systems, Inc.; 1997 [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN: The revised Conners' Parent Rating Scale (CPRS-R): Factor structure, reliability, and criterion validity. J Abnorm Child Psychol 26:257–268, 1998 [DOI] [PubMed] [Google Scholar]
- Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Pietro B: The pediatric daytime sleepiness scale (PDSS): Sleep habits and school outcomes in middle-school children. Sleep 26:455–458, 2003 [PubMed] [Google Scholar]
- Faraone SV, Pucci M, Coghill D: Pharmacotherapy for attention-deficit-hyperactivity disorder. EUR Psychiatry Rev 2:42–52, 2009 [Google Scholar]
- INTUNIV (guanfacine) extended-release tablets [package insert]. Wayne, PA: Shire Pharmaceuticals LLC; 2011 [Google Scholar]
- Jain R, Segal S, Kollins SH, Khayrallah M: Clonidine extended-release tablets for pediatric patients with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 50:171–179, 2011 [DOI] [PubMed] [Google Scholar]
- Knouse LE, Bagwell CL, Barkley RA, Murphy KR: Accuracy of self-evaluation in adults with ADHD: Evidence from a driving study. J Atten Disord 8:221–234, 2005 [DOI] [PubMed] [Google Scholar]
- Kollins SH, Lopez FA, Vince BD, Turnbow JM, Farrand K, Lyne A, Wigal SB, Roth T: Psychomotor functioning and alertness with guanfacine extended release in subjects with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 21:111–120, 2011 [DOI] [PubMed] [Google Scholar]
- Lopez FA, Ginsberg LD, Arnold V: Effect of lisdexamfetamine dimesylate on parent-rated measures in children aged 6 to 12 years with attention-deficit/hyperactivity disorder: A secondary analysis. Postgrad Med 120:89–102, 2008 [DOI] [PubMed] [Google Scholar]
- Manor I, Vurembrandt N, Rozen S, Gevah D, Weizman A, Zalsman G: Low self-awareness of ADHD in adults using a self-report screening questionnaire. Eur Psychiatry 27:314–320, 2012 [DOI] [PubMed] [Google Scholar]
- Michelson D, Allen AJ, Busner J, Casat C, Dunn D, Kratochvil C, Newcorn J, Sallee FR, Sangal RB, Saylor K, West S, Kelsey D, Wernicke J, Trapp NJ, Harder D: Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: A randomized, placebo-controlled study. Am J Psychiatry 159:1896–1901, 2002 [DOI] [PubMed] [Google Scholar]
- Michelson D, Faries D, Wernicke J, Kelsey D, Kendrick K, Sallee FR, Spencer T.: Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: A randomized, placebo-controlled, dose-response study. Pediatrics 108:E83, 2001 [DOI] [PubMed] [Google Scholar]
- Newcorn JH, Stein MA, Childress AC, Youcha S, White C, Enright G, Rubin J: Randomized, double-blind trial of guanfacine extended release in children with attention-deficit/hyperactivity disorder: Morning or evening administration. J Am Acad Child Adolesc Psychiatry 52:921–930, 2013 [DOI] [PubMed] [Google Scholar]
- Owens JS, Goldfine ME, Evangelista NM, Hoza B, Kaiser NM: A critical review of self-perceptions and the positive illusory bias in children with ADHD. Clin Child Fam Psychol Rev 10:335–351, 2007 [DOI] [PubMed] [Google Scholar]
- Patil SP: What every clinician should know about polysomnography. Respir Care 55:1179–1195, 2010 [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Burrows-Maclean L, Williams A, Fabiano GA, Morrisey SM, Chronis AM, Forehand GL, Nguyen CA, Hoffman MT, Lock TM, Fielbelkorn K, Coles EK, Panahon CJ, Steiner RL, Meichenbaum DL, Onyango AN, Morse GD.: Once-a-day Concerta methylphenidate versus three-times-daily methylphenidate in laboratory and natural settings. Pediatrics 107:E105, 2001 [DOI] [PubMed] [Google Scholar]
- Rizzo P, Steinhausen HC, Drechsler R: Self-perception of self-regulatory skills in children with attention-deficit/hyperactivity disorder aged 8–10 years. Atten Defic Hyperact Disord 2:171–183, 2010 [DOI] [PubMed] [Google Scholar]
- Sallee FR, McGough J, Wigal T, Donahue J, Lyne A, Biederman J: Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: A placebo-controlled trial. J Am Acad Child Adolesc Psychiatry 48:155–165, 2009 [DOI] [PubMed] [Google Scholar]
- Spencer T, Heiligenstein JH, Biederman J, Faries DE, Kratochvil CJ, Conners CK, Potter WZ: Results from 2 proof-of-concept, placebo-controlled studies of atomoxetine in children with attention-deficit/hyperactivity disorder. J Clin Psychiatry 63:1140–1147, 2002 [DOI] [PubMed] [Google Scholar]
- VYVANSE (lisdexamfetamine dimesylate) capsules, for oral use, CII [package insert]. Wayne, PA: Shire US Inc.; 2013 [Google Scholar]
- Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, Ganiats TG, Kaplanek B, Meyer B, Perrin J, Pierce K, Reiff M, Stein MT, Visser S: ADHD: Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 128:1007–1022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]