Introduction
Outside of treatments for melanoma and renal cell, immunotherapy for solid tumors has been a major challenge. However, more recent data with agents targeting negative regulatory molecules on activated T cells, such as cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1), are showing promise not only in the traditionally more immunogenic tumors such as melanoma and renal cell carcinoma but are also showing glimpses of activity in non–small cell lung cancer, as well as anecdotal cases of responses in colorectal cancer, and gastric cancer.1-5 Identification of predictive biomarkers would increase the therapeutic yield of these agents across all disease types. A complementary area of immunotherapy research is that of therapeutic cancer vaccines. Cancer vaccines are not vaccines in the traditional sense in that they aim to treat and not prevent established disease. The most notable success is that of sipuleucel-T, an autologous cellular product immunotherapy, which has shown a survival benefit in the treatment of metastatic castration-resistant prostate cancer.6
Despite successes in other cancers, immunotherapy in pancreatic ductal adenocarcinoma (PDA) remains difficult. However, there are recent signals of activity that suggest there is potential for success. Lessons learned from successes and failures can be used to move immunotherapy forward in PDA. Furthermore, knowledge from preclinical testing in more immune-tolerant models must be integrated into clinical testing. Unlike melanoma, PDA does not have many tumor-infiltrating lymphocytes at baseline to be activated by immune stimulants. Successful immunotherapy strategies most often involve vaccines that efficiently deliver antigen to an antigen-presenting cell (APC) in the context of costimulatory signals or combination strategies that induce tumor-specific T cells and promote their activity by adding agents that either provide costimulation or block negative regulatory signaling. Although preclinical modeling does not always predict success in patients, they can serve as a guide as to what is more likely to work, and data suggest the need for vaccines and combinations that are more potent.
This review focuses on recent completed immunotherapy trials in PDAs and the lessons learned from both failures and successes that may help guide future development in this very exciting field, with the hopes of bringing this treatment modality to patients with PDA.
Recent vaccine studies
GV1001
GV1001 is a peptide vaccine consisting of a 16-amino acid peptide from human telomerase (hTERT), a protein that has been shown to contribute to cancer cell immortalization and carcinogenesis.7 When developing vaccine platforms that target only 1 or 2 tumor-associated antigens (TAA), such as with peptide vaccines, identifying the appropriate TAA is critical for its success. Criteria often used in this process include the following: (1) antigens that are highly expressed; (2) antigens that are important in tumor survival, growth, or metastases; and (3) antigens that have low expression in normal tissue. Additional considerations with single-antigen peptide vaccines are the potential for immune escape and inefficient delivery of antigens and costimulatory signals to APCs. GV1001 is given with granulocyte-macrophage colony-stimulating factor (GM-CSF) to improve its immunogenicity (Fig 1).
Fig. 1.
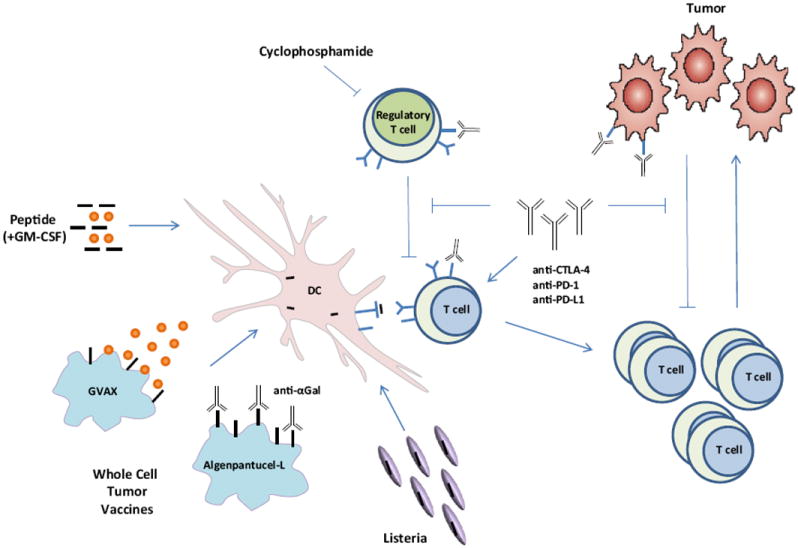
Immunotherapy strategies for pancreatic ductal adenocarcinoma activate antitumor immune responses through multiple mechanisms. Vaccine platforms such as peptide vaccination, whole-cell tumor vaccines, and attenuated Listeria vaccines are currently being pursued in the clinic. These strategies aim to either recruit (through GM-CSF) or target dendritic cells (DC), as well as deliver tumor-associated antigens to be processed and presented by the DCs. Preclinical and clinical studies have demonstrated that combining immune-modulating agents such as cyclophosphamide (CY) and checkpoint blockade inhibitors (α-CTLA-4, α-PD-1, and α-PD-L1) with vaccine strategies can enhance antitumor immune responses as well as block the tolerizing mechanisms that would otherwise inhibit these responses.
The first randomized phase III trial testing this agent was performed in patients with unresectable and metastatic PDA and compared PrimoVax (GV1001 + GM-CSF) administered sequentially with gemcitabine against gemcitabine alone. This trial was halted in 2008 after the enrollment of 365 of 520 patients owing to a lack of improvement in survival (median overall survival (OS) 5.9 vs 7.3 months).8 More recently, data from a second GV1001 phase III trial (TeloVac) in unresectable and metastatic PDA were presented. This trial integrated peptide vaccination with either subsequent or concurrent chemotherapy (gemcitabine and capecitabine) vs chemotherapy alone. There were no significant survival differences observed between the 2 peptide-containing chemotherapy combinations vs the chemotherapy alone arm (median OS 6.94 and 8.36 vs 7.89 months, respectively).9 These arms were selected despite a lack of prior clinical evidence that concurrent administration of vaccine with potentially immunosuppressive chemotherapy was beneficial. Furthermore, patients in the sequential arm received only 2 months of chemotherapy before being taken off an active therapy that has a historical median progression-free survival of 4.3 months.10 Despite the disappointing phase III results, the investigators have identified biomarkers that may predict response to this vaccine and propose that new research may show benefit in a subgroup of patients.11
Algenpantucel-L
To avoid the difficulty of picking the optimal tumor antigen to target for immunotherapy, whole-cell vaccines offer a solution in that they express an array of antigens. One such whole-cell vaccine platform under investigation is algenpantucel-L (NewLink Genetics Corporation). This vaccine is made of 2 human PDA cell lines (HAPa-1 and HAPa-2) that were genetically modified to express α (1,3)-galactosyl epitopes (αGAL). The strategy behind this platform is to induce complement and antibody-dependent cell-mediated hyperacute rejection of the vaccine through anti-αGal antibodies that are generated owing to the presence of bacterial flora in the intestinal tract.12,13 In doing so, the hope is that this proinflammatory process would stimulate the processing and presentation of TAAs expressed by the vaccine and activate host B and T cells (Fig 1).
A phase II non-randomized clinical trial investigating the incorporation of algenpantucel-L immunotherapy to adjuvant chemotherapy and chemoradiation in patients with resected PDA was recently completed. Hardacre et al12 reported an enhancement in the 12-month disease-free survival over the historical control (62% vs 50%). Although the median OS of 24.1 months does not definitely prove that there is a benefit to adding algenpantucel-L immunotherapy to adjuvant therapy, there are some intriguing findings that have led to further development of this platform in randomized phase III clinical trials.14 Interestingly, the patients who received a higher dose of vaccine in the study (300 vs 100 million cells/dose) had an increase in 12-month disease-free survival (81% vs 51%; p = 0.02) and 12-month OS (96% vs 79%; p = 0.053). Additionally, patients in this trial had a higher percentage of lymph node positivity (stage IIb) in comparison with the RTOG-9704 trial (81% vs 68%). Retrospective analysis using the Brennan nomogram would have predicted the 12-month OS for a similar patient population to be 63%, although the actual reported percentage was 86%.12 Finally, patients with a ≥25% enhancement in the production of antimesothelin antibodies in comparison with baseline significantly correlated with enhanced median OS (42 vs 20 months). Elevation of multiple antibody responses (antimesothelin, anti-CEA, and anti-αGal) also correlated with a median OS that has not been reached as of 36 months.15 These antibody responses can potentially serve as immunologic biomarkers that may aid in the determination of subsequent treatment decisions, which should be more definitively defined in a phase III trial. Based on these data, algenpantucel-L administration is currently being evaluated in 2 phase III trials, one in resected PDA (NCT01072981) and another in borderline resectable or locally advanced PDA (NCT01836432).
GVAX
Another whole-cell vaccine approach is that of GVAX. This platform uses 2 irradiated cancer cell lines (PANC 6.03 and PANC 10.05) that were engineered to express GM-CSF. GM-CSF has been shown to recruit and activate APCs, such as dendritic cells (DC), which are central to activation of antitumor immune responses (Fig 1). Several phase I and II studies have been reported in both the adjuvant and previously treated metastatic setting. In summary, these trials demonstrate postvaccination induction of CD8+ T cells to multiple mesothelin-specific epitopes, which correlate with improved survival.16-18 Mesothelin is a TAA that is overexpressed in most PDAs and is thought to be involved in cell adhesion, and therefore play a role in metastases.19 Interestingly, the maintenance of enhanced mesothelin-specific CD8+ T-cell responses and an increase in the number of epitopes against which patients’ lymphocytes responded throughout treatment also correlated with survival, thus demonstrating the importance of an expanded, and therefore more diverse and complete repertoire.18,20 In a proof of concept neoadjuvant study, GVAX, preceded by a low dose of cyclophosphamide (CY) to target suppressive regulatory T cells, induced immune cells and tertiary lymphoid aggregates in resected surgical specimens only 2 weeks after vaccination.21 Ongoing and recently completed studies with GVAX, described later in the section immune combinatorial strategies, are building on the platform to be used in combination strategies to improve on its potency in a variety of settings, including neoadjuvant therapy, adjuvant therapy, and metastatic disease.
CRS-207
CRS-207 (Lm ΔactA/ΔinlB-mesothelin) is a live-attenuated Listeria monocytogenes (Lm) modified to express mesothelin. Lm is an obligate intracellular organism that targets DCs and monocytes and delivers antigen into both the MHC class I and II antigen-processing pathway resulting in activation of both CD4+ and CD8+ T cells (Fig 1). Lm, as an infectious agent, is also unique in that it provides its own danger signals and provides stimulation through toll-like receptors and other pathogen-recognition receptors. The systemic response to Lm infusion results in proinflammatory cytokine release, which also promotes antitumor immunity. Furthermore, Lm activates innate immunity and has antitumor effects even without the integration of a tumor antigen. Unlike viral vectors, repeated vaccinations do not result in neutralization. A phase 1 study of ANZ-100 (Lm ΔactA/ΔinlB), which is the platform strain without encoded antigen, showed natural killer cell activation at all dose levels tested.22 A subsequent phase 1 study of CRS-207 in patients with treatment-refractory cancers known to express mesothelin demonstrated induction of mesothelin-specific T-cell responses and a dose-dependent ability to induce proinflammatory cytokines. The 7 patients with PDA in this study lived a median of 7 months.22 CRS-207 is currently being studied in pleural mesothelioma and in combination strategies in PDA. One limitation in the use of CRS-207 alone is that it only targets 1 antigen. However, unlike peptide vaccines, full-length antigens as well as multiple antigens can be integrated in the Lm construct. Multiantigen (polyvalent) strains are being developed for a variety of disease types. Furthermore, other mechanisms of action of Lm add to its immunostimulatory effects. In fact, preclinical models support the use of Lm as a boosting vaccine to a variety of vaccine constructs including GVAX, DC, DNA, protein, adenovirus, and vaccinia-based vaccines (Dirk Brockstedt, personal communication). Interestingly, 3 of the 7 patients with PDA from the phase 1 study lived ≥ 15 months and all had prior GVAX.22 A GVAX prime and CRS-207 boost study has been completed (immune combinatorial strategies).
Recent immune checkpoint blockade studies
Ipilimumab
Tolerizing mechanisms have proven to be a major hurdle in the activation of tumor-specific immune responses against PDA. Regulatory (CD4+CD25+) T cells and negative costimulatory pathways in particular play a crucial role in tumor immune evasion (Fig 1). Enhancement of regulatory T-cell numbers23-25 and expression of negative costimulatory molecules26 are frequently observed in patients with cancer and mouse models. CTLA-4 is a negative costimulatory molecule known to downregulate T-cell responses when engaged by its ligands B7-1 and B7-2. CTLA-4 has also been shown to be expressed on regulatory T cells.26,27 It has also been recently demonstrated that anti-CTLA-4 not only blocks engagement of CTLA-4 but also eliminates or depletes regulatory T cells, which would otherwise contribute to the suppression of the antitumor immune response.28 Ipilimumab (IPI), an anti-CTLA-4 antibody, prolongs survival in patients with metastatic melanoma. A phase II trial was conducted in locally advanced and metastatic PDA using IPI as a monotherapy at a dose of 3 mg/kg. Using RECIST criteria, there were no responders on this clinical trial; however, 1 patient did exhibit a transient delayed response with CA 19-9 tumor marker decline.29 This response highlights the possibility that immunotherapy could work in PDA if predictive biomarkers were discovered or combination strategies could increase the potency. A GVAX + IPI combination study has been completed and reported in the section immune combinatorial strategies.
BMS-936559
BMS-936559 is a high-affinity, fully human, PD-ligand-1 (PD-L1)–specific, IgG4 monoclonal antibody that inhibits the binding of PD-L1 to both PD-1 and CD80. Similar to CTLA-4, PD-1 and its ligand PDL-1 and 2 are responsible for downregulating immune responses that are unwanted or no longer needed. Binding of either PD-L1 or PD-L2 to PD-1 inhibits T-cell activation triggered through the T-cell receptor.30,31 Unlike the ligand for CTLA-4, PD-L1 is selectively expressed on many tumor types, including PDA, and stroma cells found in the tumor microenvironment.32,33 Use of inhibitory antibodies directed at this pathway has been shown to break immune tolerance and potentiate antitumor immune responses.34,35 Promising clinical data have been shown in studies of both anti-PD-12,4,36 and anti-PD-L13 antibodies. In the BMS-936559 study, 14 patients with PDA were treated on this trial, but none of these patients demonstrated a clinical response.3 Based on the clinical experience of the anti-PD-L1 antibody and IPI as monotherapy, these agents would most likely better succeed in a combinatorial approach for PDA.
Recent immune combinatorial studies
GVAX + CRS-207
Based on the previously mentioned preclinical studies and the anecdotal prolonged survival of patients with PDA from the CRS-207 phase 1 study,22 enrollment in a follow-up heterologous prime-boost vaccination study has been completed. This phase 2 study is a randomized trial in patients with metastatic PDA who had received or refused prior therapy. Patients on this trial were randomized to either 2 doses of CY/GVAX followed by 4 boosting doses of CRS-207 doses (Arm A) or 6 doses of CY/GVAX (Arm B). This study would evaluate the safety, immune response, and overall survival of these treatments. Results from this trial are expected to be released this year.
GVAX + ipilimumab
Preclinical models support the synergy of GVAX in combination with anti-CTLA-4 antibodies.27 A clinical trial evaluating either IPI administered alone (Arm 1) or in combination with GVAX (Arm 2) in patients with previously treated, locally advanced or metastatic PDA has been completed. Overall, 15 patients were treated on each arm. Among them, 2 patients in Arm 1 showed evidence of stable disease (7 and 22 weeks) but none demonstrated CA19-9 biochemical responses. In contrast, 3 patients in Arm 2 had evidence of prolonged disease stabilization (31, 71, and 81 weeks) and 7 patients experienced CA19-9 declines. In 2 of these patients, disease stabilization occurred after an initial period of progression. Mesothelin-specific responses were observed in both arms of the study; however, an increase in the diversity of that response was more prominent in the combinatorial arm. This diversification of mesothelin-specific responses correlated with OS (15.7 vs 4.1 months). Even though the sample size was small in this study (30 patients) and not powered for a direct comparison, the immune and clinical responses put in perspective the survival benefit of the combinatorial therapy vs the monotherapy where the 12-month OS was 27 vs 7% and the median OS was 5.7 vs 3.6 months (HR = 0.51; p = 0.072).37 These data support that even in the advanced disease setting, the immunologic responses correlated with clinical responses. The next GVAX + IPI study will randomize patients who have stable disease after 8-12 doses of FOLFIRINOX(5-fluorouracil, irinotecan, oxaliplatin, leucovorin) to treatment with GVAX + IPI or to continue chemotherapy. Integrating immunotherapy after upfront chemotherapy has several advantages. Chemotherapy can predispose cancer cells to cell death mediated by immune cells. Furthermore, chemotherapy is currently the best therapy to stabilize patients with PDA. Immunotherapy can take weeks to months to be effective and is unlikely to work in patients who cannot initially be stabilized. GVAX + IPI showed promise in heavily pretreated patients and giving it immediately after front-line chemotherapy is likely to improve activity.
Conclusion and future directions
The use of immunotherapy for treatment of PDA is promising. However, the immune-tolerant environment created by PDA continues to be a major hurdle. This is evident in clinical trials evaluating immunotherapies as single agents for PDA. We have shown that therapeutic vaccines have the ability to activate antitumor immune responses; however, these strategies need to be combined with immune-modulating agents (such as IPI, anti-PD-1, and anti-PD-L1), chemotherapies, or radiation depending on the patient disease status to yield better results in PDA. There is also a great deal of need to optimize vectors, antigens, and patient selection. Additionally, more preclinical and early-phase clinical trials need to be done to determine if and which chemotherapies would complement immunotherapies and determine how to optimally sequence the administration of immunotherapy with chemotherapy and radiation. These investigations would lead to greater success in phase III trials.
References
- 1.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [Epub 2010/06/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106 in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–75. doi: 10.1200/JCO.2009.26.7609. [Epub 2010/06/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694. [Epub 2012/06/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [Epub 2012/06/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabernero J, Powderly JD, Hamid O, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic CRC, gastric cancer (GC), SCCHN, or other tumors. ASCO Meeting Abstracts; 2013. p. 3622. [Google Scholar]
- 6.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–22. doi: 10.1056/NEJMoa1001294. [Epub 2010/09/08] [DOI] [PubMed] [Google Scholar]
- 7.Bernhardt SL, Gjertsen MK, Trachsel S, et al. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: a dose escalating phase I/II study. Br J Cancer. 2006;95(11):1474–82. doi: 10.1038/sj.bjc.6603437. [Epub 2006/10/25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buanes T, Maurel J, Liauw W, et al. Randomized phase III study of gemcitabine (G) versus GV1001 in sequential combination with G in patients with unresectable and metastatic pancreatic cancer (PC). ASCO Meeting Abstracts; 2009. p. 4601. [Google Scholar]
- 9.Middleton GW, Valle JW, Wadsley J, et al. A phase III randomized trial of chemoimmunotherapy comprising gemcitabine and capecitabine with or without telomerase vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer. ASCO Meeting Abstracts; 2013. p. LBA4004. [Google Scholar]
- 10.Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25(16):2212–17. doi: 10.1200/JCO.2006.09.0886. [Epub 2007/06/01] [DOI] [PubMed] [Google Scholar]
- 11.Press release. Available from: http://www.businesswire.com/news/home/20130603005934/en/Final-Results-Phase-III-TeloVac-Trial-Pancreatic
- 12.Hardacre JM, Mulcahy M, Small W, et al. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: a phase 2 study. J Gastrointest Surg. 2013;17(1):94–100. doi: 10.1007/s11605-012-2064-6. [discussion p-1. Epub 2012/12/12] [DOI] [PubMed] [Google Scholar]
- 13.Galili U. Anti-Gal: an abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology. 2013 doi: 10.1111/imm.12110. [Epub 2013/04/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardacre JM, Mulcahy MF, Small W, et al. Addition of algenpantucel-L immunotherapy to standard of care (SOC) adjuvant therapy for pancreatic cancer. ASCO Meeting Abstracts; 2012. p. 4049. [DOI] [PubMed] [Google Scholar]
- 15.Rossi GR, Hardacre JM, Mulcahy MF, et al. Effect of algenpantucel-L immunotherapy for pancreatic cancer on anti-mesothelin antibody (Ab) titers and correlation with improved overall survival. ASCO Meeting Abstracts; 2013. p. 3007. [Google Scholar]
- 16.Jaffee EM, Hruban RH, Biedrzycki B, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19(1):145–56. doi: 10.1200/JCO.2001.19.1.145. [Epub 2001/01/03] [DOI] [PubMed] [Google Scholar]
- 17.Laheru D, Lutz E, Burke J, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14(5):1455–63. doi: 10.1158/1078-0432.CCR-07-0371. [Epub 2008/03/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutz E, Yeo CJ, Lillemoe KD, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253(2):328–35. doi: 10.1097/SLA.0b013e3181fd271c. [Epub 2011/01/11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Argani P, Iacobuzio-Donahue C, Ryu B, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7(12):3862–8. [Epub 2001/12/26] [PubMed] [Google Scholar]
- 20.Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200(3):297–306. doi: 10.1084/jem.20031435. [Epub 2004/08/04] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng L, Edil B, Nguyen T, et al. Novel tertiary lymphoid aggregates induced in pancreatic adenocarcinoma by an allogeneic GM-CSF secreting pancreatic tumor vaccine as a neoadjuvant treatment. ASCO Gastrointestinal Cancers Abstracts. 2010;157 [Google Scholar]
- 22.Le DT, Brockstedt DG, Nir-Paz R, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res. 2012;18(3):858–68. doi: 10.1158/1078-0432.CCR-11-2121. [Epub 2011/12/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ino Y, Yamazaki-Itoh R, Shimada K, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108(4):914–23. doi: 10.1038/bjc.2013.32. [Epub 2013/02/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169(5):2756–61. doi: 10.4049/jimmunol.169.5.2756. [Epub 2002/08/24] [DOI] [PubMed] [Google Scholar]
- 25.Hiraoka N, Onozato K, Kosuge T, et al. Prevalence of FOXP3 + regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12(18):5423–34. doi: 10.1158/1078-0432.CCR-06-0369. [Epub 2006/09/27] [DOI] [PubMed] [Google Scholar]
- 26.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–42. doi: 10.1038/nri3405. [Epub 2013/03/09] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immunol. 2013;13(5) [Epub 2013/02/08] [PMC free article] [PubMed] [Google Scholar]
- 28.Selby MJ, Engelhardt JJ, Quigley M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013 doi: 10.1158/2326-6066.CIR-13-0013. [Epub 2013/04/07] [DOI] [PubMed] [Google Scholar]
- 29.Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33(8):828–33. doi: 10.1097/CJI.0b013e3181eec14c. [Epub 2010/09/16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027. doi: 10.1084/jem.192.7.1027. [Epub 2000/10/04] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–8. doi: 10.1038/85330. [Epub 2001/02/27] [DOI] [PubMed] [Google Scholar]
- 32.Bigelow E, Bever KM, Xu H, et al. Immunohistochemical staining of B7-H1 (PD-L1) on paraffin-embedded slides of pancreatic adenocarcinoma tissue. J Vis Exp. 2013;71 doi: 10.3791/4059. [Epub 2013/01/19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuang DM, Zhao Q, Peng C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206(6):1327–37. doi: 10.1084/jem.20082173. [Epub 2009/05/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Currie AJ, Prosser A, McDonnell A, et al. Dual control of antitumor CD8 T cells through the programmed death-1/ programmed death-ligand 1 pathway and immunosuppressive CD4 T cells: regulation and counterregulation. J Immunol. 2009;183(12):7898–908. doi: 10.4049/jimmunol.0901060. [Epub 2009/12/17] [DOI] [PubMed] [Google Scholar]
- 35.Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19:1):2293–7. doi: 10.1073/pnas.192461099. [Epub 2002/09/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipson EJ, Sharfman WH, Drake CG, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19(2):462–8. doi: 10.1158/1078-0432.CCR-12-2625. [Epub 2012/11/22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le DT, Lutz E, Uram JN, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. [10.1097/ CJI.0b013e31829fb7a2];J Immunother. 2013 36(7):382–9. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]


