Abstract
Objective: To evaluate the risk factors influencing the recurrence of papillary thyroid carcinoma. Methods: This meta-analysis used MEDLINE (PubMed), EMBASE and CNKI including all cohort studies reporting the risk factors influencing the recurrence after the initial operation on PTC up to February 23, 2014. Software RevMan 5.2 was used for meta-analysis. Results: Thirteen studies with a total of 7048 patients were included in our meta-analysis. Of all variables, gender, extrathyroid extension, LNM, tumor size, distance metastasis, thyroid surgery types and 131-I given or not were significantly correlated with recurrence, While overall recurrence was similar between the group of ≤ 45 years and > 45 years, multifolicality and solitary. However, when stratified the participants by study location (ie, Asian including China, Korea, Japan, Western country including America, France, Italy, Australia), a statistically significant summary odds ratio for age were found in Western country but none in Asian. Conclusion: The risk factors influencing recurrence includes male, extrathyroid extension, LNM, tumor size more than 2 cm, distance metastasis and subtotal thyroidectomy. However, selection of operation mode should be based on not only the recurrence but the comprehensive consideration of the clinical features.
Keywords: Papillary thyroid carcinoma, risk factors, recurrence
Introduction
Papillary thyroid carcinoma (PTC) is the most common endocrine malignancy and the most common type of the thyroid cancer accounting for approximately 85% of all thyroid cancer [1,2]. It has a usually favorable course, with 10-year survival exceeding 90% [3]. However, there are literatures reporting a recurrence rate of 8-23% after the initial operation treatment [4,5]. Although the re-operation to recurrence still has a good prognosis, it wil affect the QOF (quality of life) of patients undoubtedly, therefore reducing the recurrence is necessary.
Various factors such as age, sex, tumor size, extrathyroid extension, and distant metastasis have been mentioned associated with the recurrence of PTC, beside parts of patients can develop local recurrence and may die from it, which indicating the existence of more aggressive variants of the disease. So, the researchers have attempted to classify the disease into low-, moderate-, high-risk group based on these clinicopathological factors after diagnosing well-differentiated thyroid cancer [6,7]. Several predictive scoring systems have been established so far in attempt to evaluate the biological characteristics and prognostic factors for PTC patients more accurate, of which the UICC/AJCC TNM classification is the most commonly and widely used [8].
With an increasing number of new publications on this controversial subject in recent years, we conducted a systematic review and meta-analysis to evaluate the related factors influencing the papillary thyroid carcinoma by current literature.
Methods
Our review protocol has not previously been published or registered. We were specifically focused on the number of patients with positive recurrence at a different stratification of given parameters.
Search strategy
Studies evaluating the factors influencing the recurrence of patients with PTC were retrieved from the Medline (PubMed), EMBASE and CNKI on February 23, 2014. We used the following free-text search terms in “All fields”: “papillary thyroid carcinoma” and “recurrence”. There was no language restriction and no methodological filters.
Study inclusion/exclusion criteria
All titles identified by the search strategy were independently screened by two authors (Guo Kai, Wang Zhuoying). Search results were compared, and disagreements were resolved by consensus. Abstracts of potentially relevant titles were then reviewed for eligibility, and full-length articles were selected for closer examination if there was a specific description on patients with PTC. The criteria for eligibility were as follows. First, any prospective or retrospective studies on patients with PTC only were included. Studies that analyzed differentiated thyroid carcinoma were considered if results of PTC were separately reported. Second, studies with evaluating the prognostic factors on the recurrence of postoperative PTC patients were included. Third, odds ratios (ORs) in case-control studies or relative risks in cohort studies were reported with the 95% confidence intervals (CIs) (or, if 95% CIs were not reported, the reported data were sufficient to calculate them). Fourth, articles specifically on microcarcinoma and family history thyroid carcinoma were excluded; study which focus on the recurrence of adolescent PTC were not included.
Data extraction and management
Each study was abstracted by the same two independent reviewers who reached a consensus about any discrepancies in the collection process. Authors were contacted when there were any questions about their methodology or results.
All data were extracted onto a standardized form. The primary data extracted from each article included first authorship, country of origin, year of publication, follow-up duration, mean recurrence time, patient demographics, tumor characteristics, number of patients who undergoing recurrence and non-recurrence, surgery method, 131-I therapy, disease-free survival (DFS), and disease-specific survival (DSS).
Assessment of risk of bias in included studies
Risk of bias across studies may be present, particularly with regard to publication bias. As the topic involves surgical procedures and outcomes, it is very likely that smaller studies or those with unfavorable outcomes may not be published in the literature. A funnel plot was created to assess publication bias.
Statistical analysis
The effect measures of interest were odds ratios for case-control studies and the corresponding 95% confidence intervals which were examined for the outcomes including patient demographic and tumor characteristic. All the individual outcomes were integrated with the meta-analysis software Review Manager Software 5.2 (Cochrane Collaborative, Oxford, United Kingdom). Publication bias was estimated by using funnel plots and Begg’s rank regression test through the software STATA 12.0 (Stata Corporation, College Station, TX, USA) [9]. P value less than 0.1 was considered to indicate statistically significant publication bias. Statistical heterogeneity among studies was evaluated by random-effects model using the χ2 test, P values, and I2 statistics [10]. A P value less than 0.05 was considered statistically significant.
Main results
Study selection
Search results were reviewed in four stages. The first stage included a duplicate review, which result in a reduction from 1171 to 1036. The second stage included a title review, which resulted in a reduction from 1036 to 107 articles. The third stage included an abstract review, which resulted in a reduction from 107 to 57 articles. The final stage included an assessment of the full article for preselected inclusion/exclusion criteria that yielded 13 full-text articles for which we could extract our metameters (14-31). Any disagreements at this stage were resolved by consensus. Figure 1 shows the flowchart of studies retrieved and excluded.
Figure 1.
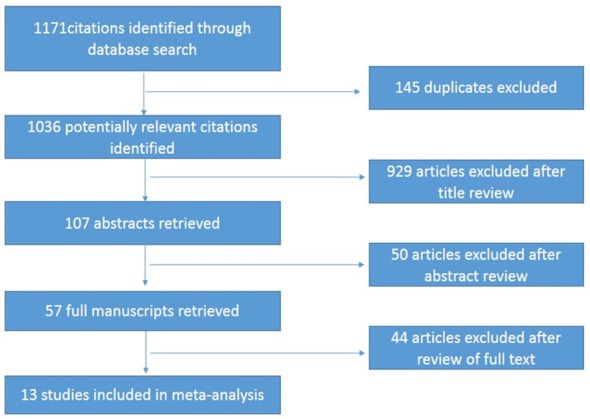
Flow chart for articles identified and included in the meta-analysis.
Study characteristics
Table 1 shows a comparison of the baseline characteristics between the 13 eligible studies. The 13 studies in the meta-analysis were published between 2004 and 2014. Three of these studies were from America, two from Europe, one from Australia, and seven from Asia (four from Korea, two from China, one from Japan). Each study had between 117 and 2095 patients.
Table 1.
Characteristics of studies included in the meta-analysis
| Reference | Country | Study of year | Rec/Total patients (%) | Follow-up durations | Mean Rec time | DFS | DSS |
|---|---|---|---|---|---|---|---|
| Albuja-Cruz [11] | America | 2012 | 9/117 (8%) | 25 (1-256) M | 35 m | ---- | ---- |
| Baek [12] | Korea | 2010 | 33/189 (17.5%) | 81 (48-386) M | — | LNM (+) 77.8% | ---- |
| LNM (-) 57.9% | |||||||
| (P < 0.05) | |||||||
| Grogan [13] | America | 2013 | 75/269 (28%) | 11-27 y | 8.1 y | 10-year 81.0% | 10-year 94.8% |
| 20-year 75.1% | 20-year 92.6% | ||||||
| 30-year 73.2% | 30-year 92.2% | ||||||
| 40-year 72.1% | 40-year 91.1% | ||||||
| Kim 2012 [14] | Korea | 2012 | 36/416 (8.7%) | 120.79 M | 87 m | 10-year 90.4% | 10-year 91.6% |
| 100% (one) | |||||||
| 83.1% (multiple) | |||||||
| Kim 2013 [15] | Korea | 2013 | 138/2095 (6.6%) | 84 (1-188) M | 43 (11-130) m | ---- | ---- |
| Kruijiff [16] | Australia | 2014 | 94/1183 (7.9%) | — | 31 (6-407) m | 5-year 95% | 10-year 98.9% |
| 10-year 92% | |||||||
| Leboulleux [17] | France | 2005 | 8/148 (7%) | 8 (0.1-6 y) | 4.7 (1-7) y | 5-year 96% | 10-year 99% |
| 10-year 91% | |||||||
| Ma [18] | China | 2013 | 18/206 (8.7%) | 4.1 (3-5) y | 30.4 (3-42) m | ---- | ---- |
| Ryu [19] | Korea | 2014 | 17/295 (5.8%) | 78 (63-137) M | — | 5-year 94.6% | 10-year 100% |
| 10-year 92.8% | |||||||
| LNR ≤ 0.65 98.6% | |||||||
| LNR > 0.65 | |||||||
| 75.4% | |||||||
| (P < 0.001) | |||||||
| Shah [20] | America | 2012 | 27/444 (6.1%) | 27.5 M | 21 m | 10-year 93.9% | 10-year 100% |
| Tanaka [21] | Japan | 2004 | 39/386 (10.1%) | 125 m | — | 20-year 87% | 10-year 97.8% |
| 20-year 96% | |||||||
| Toniato [22] | Italy | 2008 | 79/950 (7.8%) | 7.8 (2-17) y | — | ---- | 10-year 91.38% |
| 15-year 88.69% | |||||||
| Zhao [23] | China | 2013 | 109/459 (23.7%) | > 5 y | 40 (1.5-108) m | ---- | ---- |
Rec, Recurrence; DFS, Disease-Free Survival; DSS, Disease-Specific Survival; LNM, Lymph Node Metastasis; LNR, Lymph Node Ratio.
Study heterogeneity
The studies ranged in heterogeneity with I2 statistics of 80, 53, 57, 54, 65, 74, 86, 86, 84 and 56 for age, gender, multifocality, extrathyroid extension, LNM, tumor size, thyroid surgery, distance metastasis and 131-I therapy, respectively. This ranges from moderate to high range of heterogeneity between studies.
Results of the search
The risk estimates of PTC for recurrence including age, gender, multifocality, extrathyroid extension, LNM, tumor size, distance metastasis and 131-I therapy in cohort studies and summary estimates are shown in Figures 2, 3, 4, 5, 6, 7, 8, 9 and 10. Overall, we observed statistically significant associations between gender (OR 1.53, CI [1.28, 1.84]), extrathyroid extension (OR 2.83, CI [2.32, 3.44]), LNM (OR 3.24, CI [2.61, 4.02]), tumor size (OR 2.69, CI [2.06, 3.50]), thyroid surgery types (OR 2.38, CI [1.81, 3.12]), distance metastasis (OR 11.96 [8.43, 16.97]) and 131-I therapy (OR 1.34, CI [1.01, 1.78]) and the risk of recurrence respectively. However, age of patients (OR 0.83, CI [0.69, 1.01]) and multifocality lesions (OR 1.15, CI [0.96, 1.39]) were not statistically significantly associated with recurrence.
Figure 2.
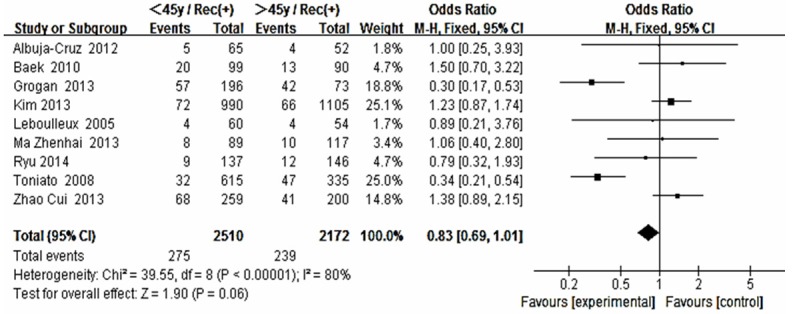
Forest plot for recurrence according to age.
Figure 3.
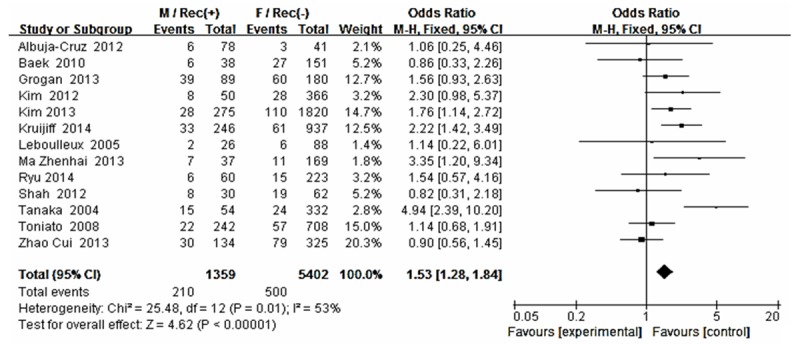
Forest plot for recurrence according to gender.
Figure 4.
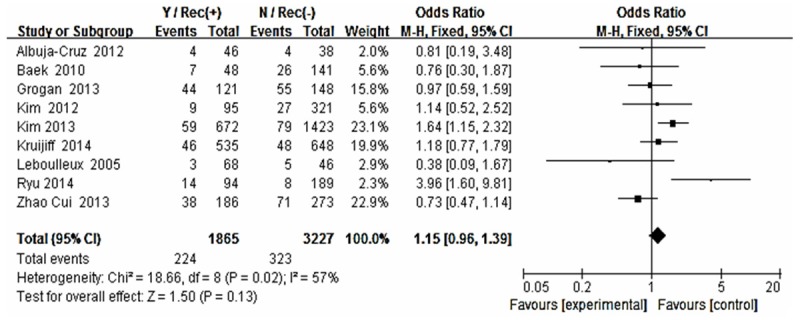
Forest plot for recurrence according to multifocality.
Figure 5.
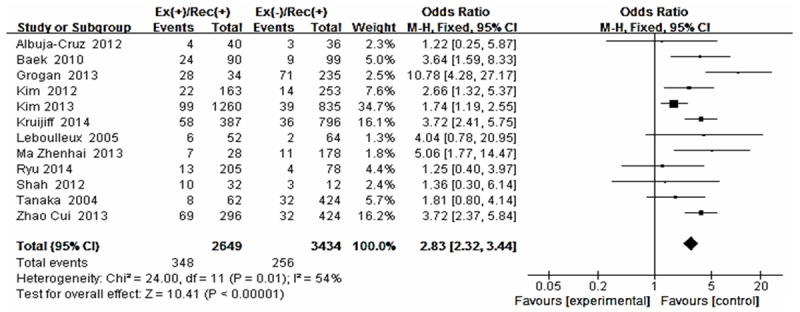
Forest plot for recurrence according to extrathyroid extension.
Figure 6.
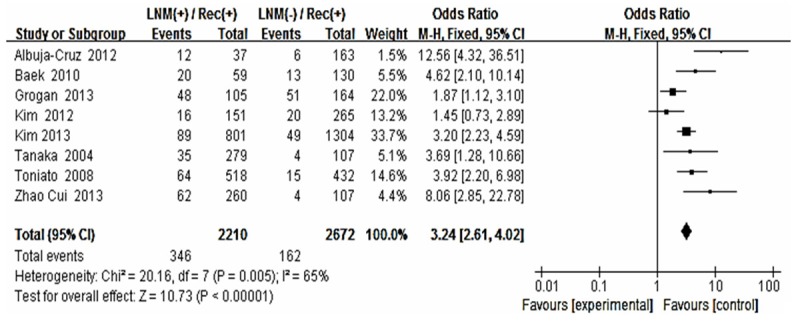
Forest plot for recurrence according to LNM.
Figure 7.
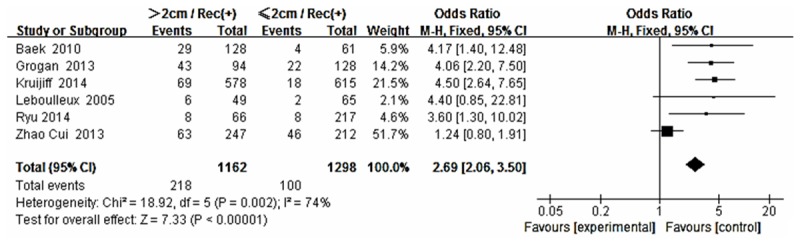
Forest plot for recurrence according to tumor size.
Figure 8.
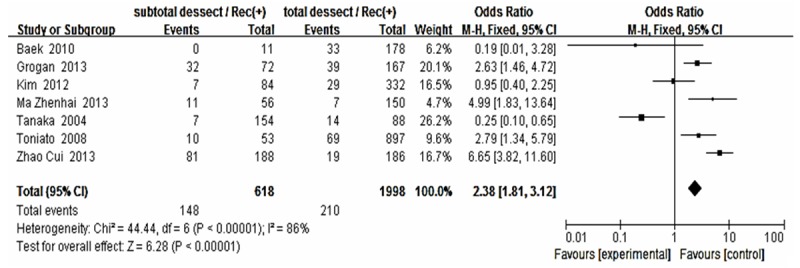
Forest plot for recurrence according to thyroid surgery method.
Figure 9.
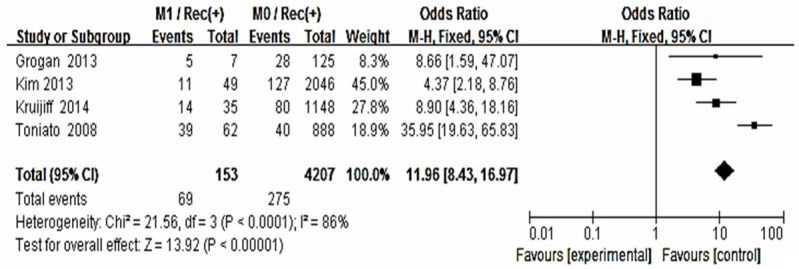
Forest plot for recurrence according to distance metastasis.
Figure 10.
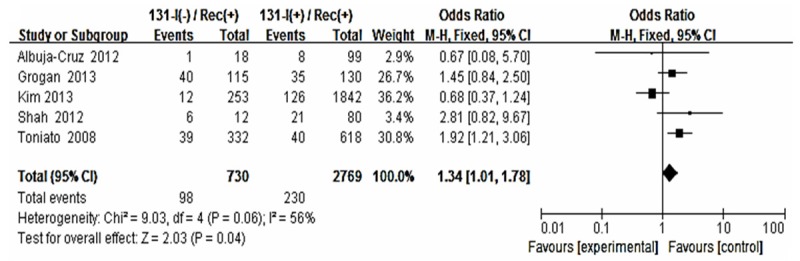
Forest plot for recurrence according to 131-I therapy.
The statistically significant summary odds ratio for gender, multifocality, extrathyroid extension, LNM, tumor size, thyroid surgery, distance metastasis and 131-I therapy were essentially unchanged when we stratified the participants by study location (i.e., Asian including China, Korea, Japan, Western country including America, France, Italy, Australia). However, a statistically significant summary odds ratio for age was found in group B (OR 0.37, CI [0.26, 0.51] I2 = 27%) but none of that in group A (OR 1.25, CI [0.98, 1.58] I2 = 0%) (Figures 11, 12).
Figure 11.
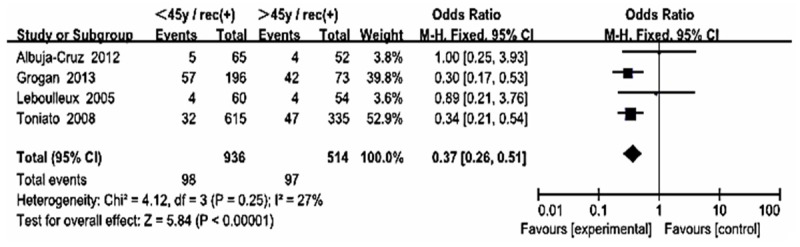
Forest plot for recurrence according to age in Western countries.
Figure 12.
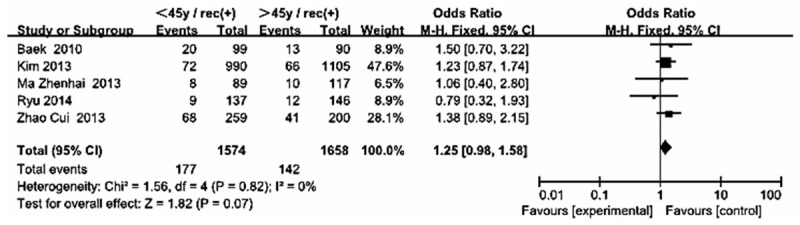
Forest plot for recurrence according to age in Asian.
We also collected the 5-, 10-, 15-, 20-year DFS and DSS. The data show, no matter how DFS changed, 10-year DSS were more than 90% in all cohort studies. Furthermore, we knew exactly that LNM will affect recurrence but not reduce the survival rate (Table 1).
Publication bias
Begg’s funnel plot and Egger’s test were performed to access the publication bias of literatures. The sharps of the funel plots did not reveal any evidence of obvious asymmetry (Figure 13). Then, the Egger’s test was used to provide statistical evidence of funnel plot symmetry. The results still did not suggest any evidence of publication bias (P = 0.903).
Figure 13.
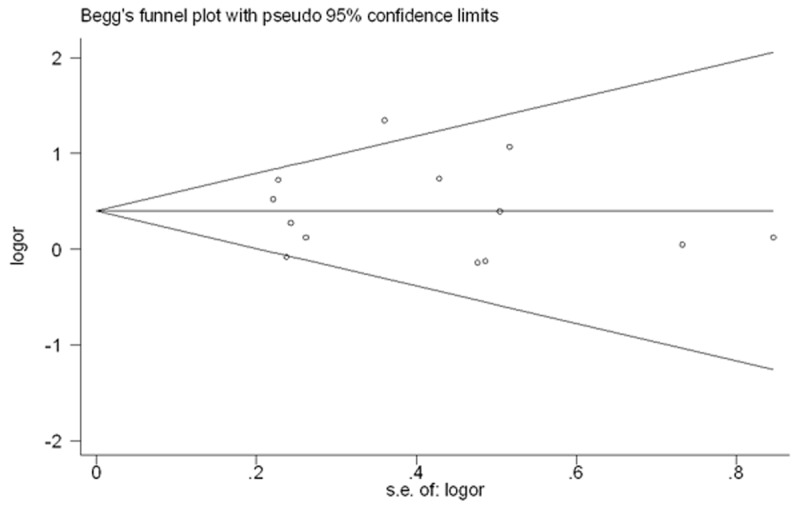
Begg’s funnel plot for publication bias test. P > |z| = 0.903. We didn’t detect publication bias of the included articles.
Discussion
Many factors may affect the thyroid cancer recurrence, yet there still existed some controversy so far. Although some authors believed patients with postoperative recurrence rate may not affect the overall survival, but the combat bring by the reoperation to the patient was certainly and they undoubtable consume considerable medical resources in the follow-up period. So the prevalence of recurrence disease in PTC patients should not be negligible, to this purpose, recurrence-related variables involved in PTC patients’ outcome assumes a great importance.
This study was the first systematic review and meta-analysis of the literature on exploring the factors for patients with recurrence after initial operation treatment. Included in our analysis were 13 unique studies from 2005 to 2014 with 7048 patients. Data shows that gender, extrathyroid extension, LNM, size, and distance metastasis were each associated with a statistically significantly increased risk of recurrence.
UICC/AJCC TNM classification is the most commonly and widely accepted scoring system for thyroid cancer [7,24]. The system included in the risk stratification of age, tumor size, LNM, extra-thyroid extension, distance metastasis and pathological subset of PTC. Our results were much similar to that except age (0.83 [0.69, 1.01]). Interestingly, in our meta-analysis, we found that the OR for age in Western country (OR 0.37, CI [0.26, 0.51]) was statistically significance, but not in the Asian patients (OR 1.25, CI [0.98, 1.58]). This result showed that the treatment stratification for patients ≥ 45 years may be not suitable for Asians. It’s not exactly clear what has caused this ethnic difference. Of course, although we got the significant difference, only 5 and 4 articles were included in each group. We look forward to the future large sample statistical studies to confirm this finding.
There existed significant relationship between LNM and postoperative recurrence in PTC patients. Cases with preoperative LNM, no matter proven by clinical radiological evidence or occult LNM, were attended to be recurrence and relapse [25]. The sensitivity to detect these positive lymph nodes in the central compartment by means of ultrasound was low [26,27]. So performing a prophylactic CLND at the first operation may harvest advantages including the opportunity for better staging and avoiding the higher risk of permanent hypoparathyroidism and RLN injury caused by reoperation in the central compartment. Our data show significant reduced DFS in patients with LNM at initial presentation. However, the node dissection and the node metastases were correlated with disease recurrence but not with survival rate (DSS) (Table 1).
For surgery types, Mazzaferri et al. found recurrence rate of partial thyroidectomy was almost 2 times higher than the total/subtotal thyroidectomy [28]. In 2007, Bilimoria et al. examined 52173 adults, of which 43227 (82.9%) underwent total thyroidectomy, and 8,946 (17.1%) underwent lobectomy. For tumors that were 1 cm or larger, lobectomy resulted in higher risk of recurrence and death. But they did not observe the same results in the patients with tumors smaller than 1 cm [29]. This was consistent with our result (OR 2.38, CI [1.81, 3.12]). Our result showed the 131-I therapy can significantly decreased the recurrence (OR 1.34, CI [1.01, 1.78]). While other articles think RAI given to those with cervical metastases should be consider all risk factors such as older age, tumor type, and quality and quantity of LN individually [30].
This meta-analysis not related to pathological subtype and coexistent HT (Hashimoto’s thyroiditis). Research suggests that, thyroid cancer pathological types were independent risk factors of recurrence after operation, can directly affect the prognosis [31]. Differentiated thyroid cancer has a low recurrence rate than other types of thyroid cancer. In differentiated thyroid carcinoma, follicular variant recurrence rate was relatively high [32]. Wittle et al. suggest that, Follicular thyroid cancer has a recurrence rate as high as 43.5%, and more than half of the recurrence will occur within 3 years [33]. The data from Ganly et al. show that though the subtype of PTC did not influence neck RFS, patients with TCV (tall-cell variant of PTC) and PTC TCF (PTC with tall-cell features) had a poorer distant RFS in comparison to those with classical PTC (P = 0.03) [34,35]. HT was closely related with PTC; beside, the PTC patients coexistent with HT demonstrated a better prognosis. Scholars think that may be cytokines secreted by infiltrated lymphocytes inhibit tumor growth and metastasis [36].
Despite above important positive findings, we remained cautious in our conclusions, as there were numbers of potential limitations. Whenever performing a meta-analysis to estimate an outcome or effect from a group of similar studies, the measure of heterogeneity informs whether the combined estimate was meaningful. First, the initial operation performed by different doctors, even according to the standard mode, operation quality may influence the post-operation recurrence. Secondly, we only counted the number of patients with or without recurrence, didn’t distinguish the site of recurrence, ipsilateral or bilateral, synchronous or metachronous, one or more time/site recurrence. Third, since only two articles related to the pathological subtypes, unfortunately, this meta-analysis did not include the subtype into the study, but some researchers suggested that the follicular variant of papillary carcinoma had higher recurrence rate.
Conclusions
Male patients, extrathyroid extension, LNM (lymph node metastasis), distant metastasis, tumor size greater than 2 cm, subtotal thyroidectomy and without postoperative 131-I treatment are the PTC recurrence risk factors. For the treatment strategy in these patients, we believe that proper alternative of operation method is necessary, taking corresponding measures with close follow-up should be suggested and popularized because early detection lead to effective surgery salvage. Although the patients with risk factors may develop recurrence someday, they can be surgical remission and not appear to reduce DSS (Disease-specific Survival).
Acknowledgements
Supported by Science & Technology Commission of Shanghai (No. 124119a0203).
Disclosure of conflict of interest
None.
References
- 1.Khan A, Nose V. In: Endocrine pathology: differential diagnosis and molecular advances. 2nd edition. Lloyd RV, editor. New York: Springer; 2010. pp. 181–236. [Google Scholar]
- 2.Ito Y, Kihara M, Takamura Y, Kobayashi K, Miya A, Hirokawa M, Miyauchi A. Prognosis and prognostic factors of papillary thyroid carcinoma in patients under 20 years. Endocr J. 2012;59:539–45. doi: 10.1507/endocrj.ej12-0086. [DOI] [PubMed] [Google Scholar]
- 3.Malterling RR, Andersson RE, Falkmer S, Falkmer U, Niléhn E, Järhult J. Differentiated thyroid cancer in a Swedish county--long-term results and quality of life. Acta Oncol. 2010;49:454–9. doi: 10.3109/02841860903544600. [DOI] [PubMed] [Google Scholar]
- 4.Popadich A, Levin O, Lee JC, Smooke-Praw S, Ro K, Fazel M, Arora A, Tolley NS, Palazzo F, Learoyd DL, Sidhu S, Delbridge L, Sywak M, Yeh MW. A multicenter cohort study of total thyroidectomy and routine central lymph node dissection for cN0 papillary thyroid cancer. Surgery. 2011;150:1048–57. doi: 10.1016/j.surg.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Hartl DM, Mamelle E, Borget I, Leboulleux S, Mirghani H, Schlumberger M. Influence of prophylactic neck dissection on rate of retreatment for papillary thyroid carcinoma. World J Surg. 2013;37:1951–8. doi: 10.1007/s00268-013-2089-3. [DOI] [PubMed] [Google Scholar]
- 6.Pacini F, Schlumberger M, Dralle H, Ilisea R, Smith Y, Viersinga V. [European consensus on the management of patients with differentiated carcinoma of the thyroid from follicular epithelium] . Vestn Khir Im I I Grek. 2008;167:52–6. [PubMed] [Google Scholar]
- 7.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 8.Ito Y, Miyauchi A, Jikuzono T, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Ichihara K, Kuma K. Risk factors contributing to a poor prognosis of papillary thyroid carcinoma: validity of UICC/AJCC TNM classification and stage grouping. World J Surg. 2007;31:838–48. doi: 10.1007/s00268-006-0455-0. [DOI] [PubMed] [Google Scholar]
- 9.Mueller KF, Meerpohl JJ, Briel M, Antes G, von Elm E, Lang B, Gloy V, Motschall E, Schwarzer G, Bassler D. Detecting, quantifying and adjusting for publication bias in meta-analyses: protocol of a systematic review on methods. Syst Rev. 2013;2:60. doi: 10.1186/2046-4053-2-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowden J, Tierney JF, Copas AJ, Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol. 2011;11:41. doi: 10.1186/1471-2288-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albuja-Cruz MB, Thorson CM, Allan BJ, Lew JI, Rodgers SE. Number of lymph nodes removed during modified radical neck dissection for papillary thyroid cancer does not influence lateral neck recurrence. Surgery. 2012;152:1177–1183. doi: 10.1016/j.surg.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Baek S, Jung KY, Kang SM, Kwon SY, Woo JS, Cho SH, Chung EJ. Clinical risk factors associated with cervical lymph node recurrence in papillary thyroid carcinoma. Thyroid. 2010;20:147–152. doi: 10.1089/thy.2008.0243. [DOI] [PubMed] [Google Scholar]
- 13.Grogan RH, Kaplan SP, Cao H, Weiss RE, Degroot LJ, Simon CA, Embia OM, Angelos P, Kaplan EL, Schechter RB. A study of recurrence and death from papillary thyroid cancer with 27 years of median follow-up. Surgery. 2013;154:1436–1447. doi: 10.1016/j.surg.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Kim KM, Park JB, Bae KS, Kang SJ. Analysis of prognostic factors in patients with multiple recurrences of papillary thyroid carcinoma. Surg Oncol. 2012;21:185–190. doi: 10.1016/j.suronc.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Sohn SY, Jang HW, Kim SW, Chung JH. Multifocality, But Not Bilaterality, Is a predictor of disease recurrence/persistence of papillary thyroid carcinoma. World J Surg. 2013;37:376–384. doi: 10.1007/s00268-012-1835-2. [DOI] [PubMed] [Google Scholar]
- 16.Kruijff S, Petersen JF, Chen P, Aniss AM, Clifton-Bligh RJ, Sidhu SB, Delbridge LW, Gill AJ, Learoyd D, Sywak MS. Patterns of structural recurrence in papillary thyroid cancer. World J Surg. 2014;38:653–659. doi: 10.1007/s00268-013-2286-0. [DOI] [PubMed] [Google Scholar]
- 17.Leboulleux S, Rubino C, Baudin E, Caillou B, Hartl DM, Bidart JM, Travagli JP, Schlumberger M. Prognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid capsule at initial diagnosis. J Clin Endocrinol Metab. 2005;90:5723–5729. doi: 10.1210/jc.2005-0285. [DOI] [PubMed] [Google Scholar]
- 18.Ma Z. Analysis of factors influencing the postoperative recurrence of thyroid papillary carcinoma. Chinese Journal of Bases and Clinics in General Surgery. 2013;12:1410–1413. [Google Scholar]
- 19.Ryu IS, Song CI, Choi SH, Roh JL, Nam SY, Kim SY. Lymph node ratio of the central compartment is a significant predictor for locoregional recurrence after prophylactic central neck dissection in patients with thyroid papillary carcinoma. Ann Surg Oncol. 2014;21:277–283. doi: 10.1245/s10434-013-3258-1. [DOI] [PubMed] [Google Scholar]
- 20.Shah PK, Shah KK, Karakousis GC, Reinke CE, Kelz RR, Fraker DL. Regional recurrence after lymphadenectomy for clinically evident lymph node metastases from papillary thyroid cancer: a cohort study. Ann Surg Oncol. 2012;19:1453–1459. doi: 10.1245/s10434-011-1890-1. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka K, Sonoo H, Hirono M, Ohkubo S, Nomura T, Ikeda M, Nakajima K, Kurebayashi J. Retrospective analysis of predictive factors for recurrence after curatively resected papillary thyroid carcinoma. Surg Today. 2005;35:714–719. doi: 10.1007/s00595-005-3021-8. [DOI] [PubMed] [Google Scholar]
- 22.Toniato A, Boschin I, Casara D, Mazzarotto R, Rubello D, Pelizzo M. Papillary thyroid carcinoma: factors influencing recurrence and survival. Ann Surg Oncol. 2008;15:1518–1522. doi: 10.1245/s10434-008-9859-4. [DOI] [PubMed] [Google Scholar]
- 23.Zhao C, Yun XW, Gao J, Gao M. Clinical analysis of 109 cases of recurrent papillary thyroid carcinoma. Tumor. 2013;33:728–733. [Google Scholar]
- 24.Ya M. [Interpretation of the management guidelines for patients with thyroid nodules and differentiated thyroid cancer (2012 Chinese edition)] . Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;27:917–20. [PubMed] [Google Scholar]
- 25.de Meer SG, Dauwan M, de Keizer B, Valk GD, Borel Rinkes IH, Vriens MR. Not the number but the location of lymph nodes matters for recurrence rate and disease-free survival in patients with differentiated thyroid cancer. World J Surg. 2012;36:1262–7. doi: 10.1007/s00268-012-1427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartl DM, Leboulleux S, Al Ghuzlan A, Baudin E, Chami L, Schlumberger M, Travagli JP. Optimization of staging of the neck with prophylactic central and lateral neck dissection for papillary thyroid carcinoma. Ann Surg. 2012;255:777–83. doi: 10.1097/SLA.0b013e31824b7b68. [DOI] [PubMed] [Google Scholar]
- 27.Choi JS, Kim J, Kwak JY, Kim MJ, Chang HS, Kim EK. Preoperative staging of papillary thyroid carcinoma: comparison of ultrasound imaging and CT. AJR Am J Roentgenol. 2009;193:871–8. doi: 10.2214/AJR.09.2386. [DOI] [PubMed] [Google Scholar]
- 28.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–28. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 29.Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS, Sturgeon C. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246:375–84. doi: 10.1097/SLA.0b013e31814697d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Gonzalez R, Bologna-Molina R, Carreon-Burciaga RG, Gómezpalacio-Gastelum M, Molina-Frechero N, Salazar-Rodríguez S. Papillary thyroid carcinoma: differential diagnosis and prognostic values of its different variants: review of the literature. ISRN Oncol. 2011;2011:915–925. doi: 10.5402/2011/915925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Singh B, Tallini G, Carlson DL, Katabi N, Shaha A, Tuttle RM, Ghossein RA. Follicular variant of papillary thyroid carcinoma: a clinicopathologic study of a problematic entity. Cancer. 2006;107:1255–64. doi: 10.1002/cncr.22138. [DOI] [PubMed] [Google Scholar]
- 33.Witte J, Goretzki PE, Dieken J, Simon D, Röher HD. Importance of lymph node metastases in follicular thyroid cancer. World J Surg. 2002;26:1017–22. doi: 10.1007/s00268-002-6668-y. [DOI] [PubMed] [Google Scholar]
- 34.Chrisoulidou A, Boudina M, Tzemailas A, Doumala E, Iliadou PK, Patakiouta F, Pazaitou-Panayiotou K. Histological subtype is the most important determinant of survival in metastatic papillary thyroid cancer. Thyroid Res. 2011;4:12. doi: 10.1186/1756-6614-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganly I, Ibrahimpasic T, Rivera M, Nixon I, Palmer F, Patel SG, Tuttle RM, Shah JP, Ghossein R. Prognostic implications of papillary thyroid carcinoma with tall-cell features. Thyroid. 2014;24:662–670. doi: 10.1089/thy.2013.0503. [DOI] [PubMed] [Google Scholar]
- 36.Mazokopakis EE, Tzortzinis AA, Dalieraki-Ott EI, Tsartsalis AN, Syros PK, Karefilakis CM, Papadomanolaki MG, Starakis IK. Coexistence of Hashimoto’s thyroiditis with papillary thyroid carcinoma. A retrospective study. Hormones (Athens) 2010;9:312–7. doi: 10.14310/horm.2002.1282. [DOI] [PubMed] [Google Scholar]


