Abstract
Objective: To explore the relationship between the number of tumor-associated macrophages (TAMs) and proliferative activity of tumor cells and the relationship between two macrophage biomarkers CD68 and CD163 in nasopharyngeal NK/T-cell lymphoma. Methods: Immunohistochemistry was used to reconfirm the diagnosis of nasal NK/T-cell lymphoma and detect the numbers of TAMs and the ki-67 label index of the tumor cells in all 31 cases. In addition, 12 cases of inflammatory cases were collected as controls, for which the immunostaining of CD68 and CD163 were done as well. Then staining results were analyzed with Pearson correlation and t test. Results: The number of TAMs was positively correlated with tumor proliferative activity (P = 0.024) in nasopharyngeal NK/T-cell lymphoma. The expression of CD68 and CD163 was closely related (P = 0.009), and the positive rate of CD68 was generally higher than CD163, however there is no statistical significance. Conclusion: The increase in numbers of TAMs in nasopharyngeal NK/T-cell lymphoma is related to higher proliferative index, indicating the TAMs play an important role in tumor proliferation. Meanwhile both CD68 and CD163 might be the markers for TAMs but CD163 would be the better one.
Keywords: NK/T-cell lymphoma, Ki-67 label index, tumor-associated macrophages (TAMs), CD68, CD163
Introduction
Nasopharyngeal NK/T-cell lymphoma is one of the most commonly malignant lymphoma in Asian countries including China. Clinically, patients with Nasopharyngeal NK/T-cell lymphoma usually have ulcer and perforation on multiple areas such as nasopharynx, nasal septum, soft palate and other areas, which could cause the face damage, resulting in patients’ deaths at last. Histopathologically, obvious hyperplasia of the tumor cells and the corrosion of blood vessels are characteristics of the tumor. Therefore, it is generally accepted that nasopharyngeal NK/T-cell lymphoma is a malignant tumor with a relatively high lethality [1]. Recently, people have paid more attention to tumor microenvironment in tumorigenesis and tumor development [2]. As an important immunocyte, TAMs in tumor diagnosis and treatment is drawing widely attention as well. Abundant research findings showed that at present TAMs are closely associated with tumorigenesis, tumor development and poor prognosis [3,4] in the studies in breast cancer, gastric carcinoma and carcinoma of large intestine [5-7]. However, TAMs in nasopharyngeal NK/T-cell lymphoma and the study on tumor proliferation activity have not been reported so far. Therefore, two TAM markers, i.e. CD68 and CD163, were used for detecting TAMs in nasopharyngeal NK/T-cell lymphoma and the correlation of TAM number with the tumor proliferative activity was further studied. We found that the increase in numbers of TAMs in nasopharyngeal NK/T-cell lymphoma was related to higher proliferative index and it indicated the TAMs play an important role in tumor cell proliferation. To our knowledge, this is first report about TAMs study on nasopharyngeal NK/T-cell lymphoma.
Materials and methods
Case collection
Thirty-one cases of nasopharyngeal NK/T-cell lymphoma were collected from the archives in department of pathology of our hospital from the year 2005 to 2007. There were 23 males and 8 females from the age of 4 to 80 years and the median of the age was 39 years. Meanwhile, 10 cases of reactive lesion were collected from same anatomical locations and same period and the median of the age was 19 years. Among them there were 8 males and 2 females from the age of 4 to 59 years.
Immunohistochemical study
All of the primary antibodies and the EnVision kit were commercially obtained from DAKO (Denmark) including CD3, CD45RO, TIA-1, CD56, CD20, CD79α, CD68, CD163 and Ki67. The staining protocols of antibodies according to the manufacture’s instructions. Among them, CD3, CD45RO, TIA-1 and CD56 were used for recognizing T/NK cells, CD20 and CD79α for B-cells, CD68 and CD163 for macrophages and Ki67 for detecting tumor cell proliferation. Afterwards, we used general type second antibody and conducted DAB chromogenic and hematoxylin stain. All 31 cases of NK/T-cell lymphoma were studied by EnVision immunohistochemistry and 10 cases of reactive proliferative cases were used as the control group. First of all, we reconfirmed the diagnosis of the tumor cases according to WHO criteria of both morphology and immunohistochemistry [8]. Then, tumor-associated macrophages and Ki67 labeling index were independently determined in double blind condition.
CD68-, CD163- and Ki67-positive cells were counted in 5 randomly selected high power fields out of necrosis with Image Pro-Plus 5.1 software according to the literatures and the percentages were calculated [9].
Statistics
Pearson’s correlation analysis was used to study the relationships between CD68 and Ki67, CD163 and Ki67, CD68 and CD163 with SPSS13.0 software, P < 0.05 is considered significant.
Results
Immunophenotypic analysis in nasopharyngeal NK/T-cell lymphoma
Tumor cells in the 31 cases all expressed hyalomitome type CD3 (Figure 1) and CD45RO with different levels. Tumor cells expressed CD56 in 24 cases (Figure 2); and 25 cases expressed TIA-1. In the 7 cases of CD56 negative, 4 among them didn’t express TIA-1 but they had explicit expression of hyalomitome type CD3 and CD45RO. Furthermore, in 3 cases of CD56 single negative and 2cases of TIA-1 single negative, the tumor cells also expressed hyalomitome type CD3 and CD45RO. Only in a few cases could we see diffuse distribution of CD79α positive cells (Table 1). Based on the information mentioned above, 31 cases of NK/T-cell lymphoma were all confirmed by the return visits.
Figure 1.
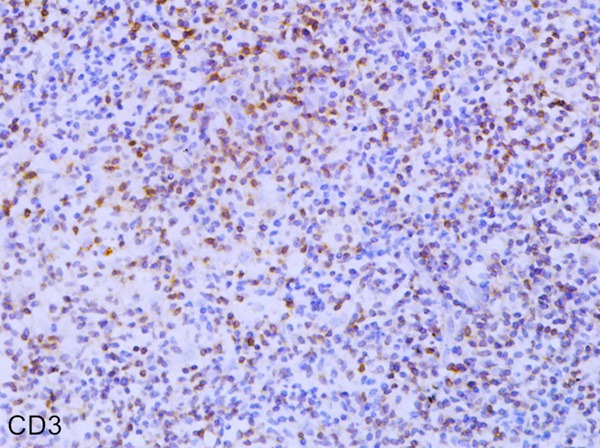
Immunohistochemical staining of CD3 in Nasopharyngeal NK/T cell lymphoma (EnVision × 400).
Figure 2.
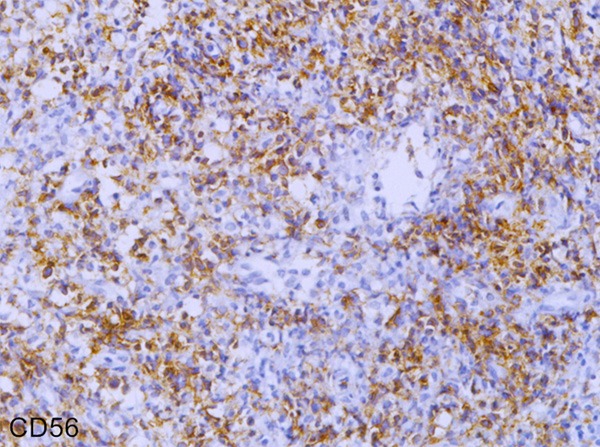
Immunohistochemical staining of CD56 in Nasopharyngeal NK/T cell lymphoma (EnVision × 400).
Table 1.
Immunohistochemistry of nasopharyngeal NK/T cell lymphoma
| Num | Gender | Age | CD3 | CD45RO | CD56 | TIA-1 | CD20 | CD79α | CD68 | CD163 | Ki67 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | male | 17 | + | + | -- | - | - | + | 0.094 | 0.087 | 0.53 |
| 2 | female | 30 | + | + | + | - | - | - | 0.141 | 0.092 | 0.5 |
| 3 | male | 30 | + | + | + | + | - | + | 0.087 | 0.085 | 0.4 |
| 4 | male | 32 | + | + | - | + | - | - | 0.054 | 0.067 | 0.15 |
| 5 | male | 40 | + | + | + | + | - | - | 0.088 | 0.041 | 0.04 |
| 6 | female | 35 | + | + | + | + | - | - | 0.125 | 0.104 | 0.54 |
| 7 | male | 71 | + | + | + | + | - | - | 0.049 | 0.070 | 0.28 |
| 8 | male | 44 | + | + | + | + | + | - | 0.064 | 0.035 | 0.2 |
| 9 | male | 40 | + | + | - | + | - | - | 0.135 | 0.082 | 0.57 |
| 10 | male | 70 | + | + | + | + | - | - | 0.079 | 0.040 | 0.52 |
| 11 | male | 18 | + | + | + | + | - | - | 0.102 | 0.073 | 0.16 |
| 12 | male | 43 | + | + | + | + | - | - | 0.073 | 0.058 | 0.42 |
| 13 | male | 46 | + | + | + | + | - | - | 0.066 | 0.040 | 0.38 |
| 14 | male | 32 | + | + | - | - | - | - | 0.041 | 0.087 | 0.39 |
| 15 | female | 40 | + | + | - | - | - | - | 0.078 | 0.058 | 0.53 |
| 16 | male | 58 | + | + | + | + | - | - | 0.063 | 0.038 | 0.38 |
| 17 | male | 33 | + | + | + | + | - | - | 0.103 | 0.088 | 0.15 |
| 18 | female | 27 | + | + | + | + | - | - | 0.080 | 0.037 | 0.27 |
| 19 | male | 4 | + | + | - | - | - | - | 0.079 | 0.041 | 0.17 |
| 20 | female | 41 | + | + | + | + | - | - | 0.176 | 0.094 | 0.59 |
| 21 | male | 33 | + | + | + | + | - | - | 0.096 | 0.050 | 0.57 |
| 22 | male | 51 | + | + | + | + | - | - | 0.093 | 0.066 | 0.3 |
| 23 | male | 18 | + | + | + | + | - | - | 0.065 | 0.050 | 0.56 |
| 24 | female | 80 | + | + | + | + | - | - | 0.123 | 0.092 | 0.48 |
| 25 | female | 39 | + | + | + | - | - | - | 0.062 | 0.106 | 0.46 |
| 26 | male | 53 | + | + | + | + | - | - | 0.094 | 0.059 | 0.19 |
| 27 | male | 37 | + | + | + | + | - | - | 0.041 | 0.074 | 0.3 |
| 28 | male | 55 | + | + | + | + | - | - | 0.060 | 0.050 | 0.16 |
| 29 | female | 76 | + | + | - | + | - | - | 0.100 | 0.082 | 0.56 |
| 30 | male | 30 | + | + | + | + | - | - | 0.064 | 0.056 | 0.33 |
| 31 | female | 30 | + | + | + | + | - | - | 0.064 | 0.058 | 0.32 |
Ki67 labeling index was positively correlated with TAMs numbers
All the cases of nasopharyngeal NK/T-cell malignant lymphoma were positive for Ki67 (Figure 3), CD68 (Figure 4) and CD163 (Figure 5). However, CD163 is able to more clearly outline the cell contour. Ki67 labeling index and the numbers of both CD68- and CD163-positive cells were listed in Table 1. In inflammatory control group, the mean of both CD68 and CD163-positive cells was 0.057 ± 0.006 and 0.045 ± 0.004 respectively. The statistic analysis indicated that the mean of both CD68 and CD163-positive cells in the cancer group was significantly higher than that of the inflammatory group respectively (t = 3.019, P = 0.004; t = 3.410, P = 0.001). Meanwhile, statistics also indicated that there was a significant positive correlation between Ki67 labeling index and the average numbers of both CD68 (Figure 6) and CD163 (Figure 7) positive cells (P = 0.024; P = 0.031). Furthermore, there is a significant positive correlation between CD68 and CD163 staining (Figure 8).
Figure 3.
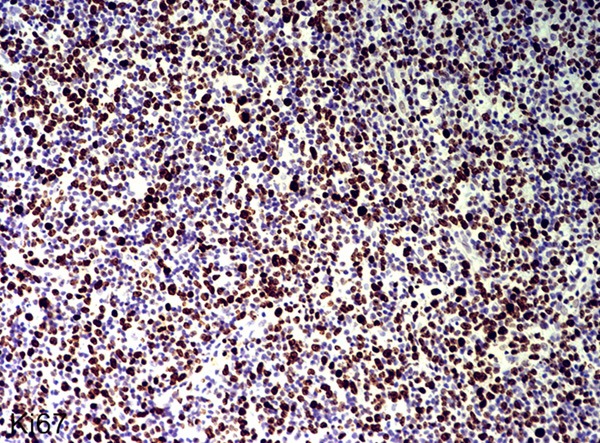
Immunohistochemical staining of Ki67 in nasopharyngeal NK/T cell lymphoma (EnVision × 400).
Figure 4.
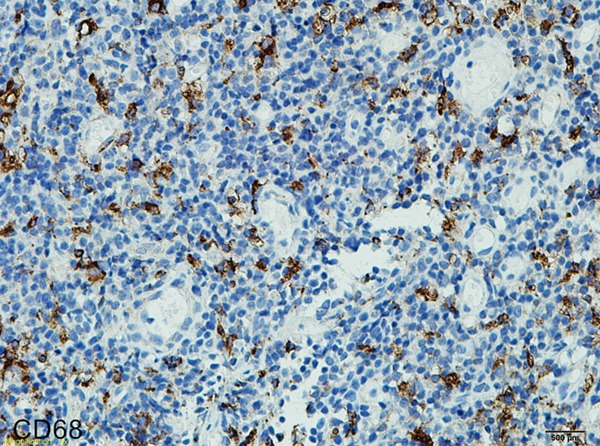
Immunohistochemical staining of CD68 in nasopharyngeal NK/T cell lymphoma (EnVision × 400).
Figure 5.
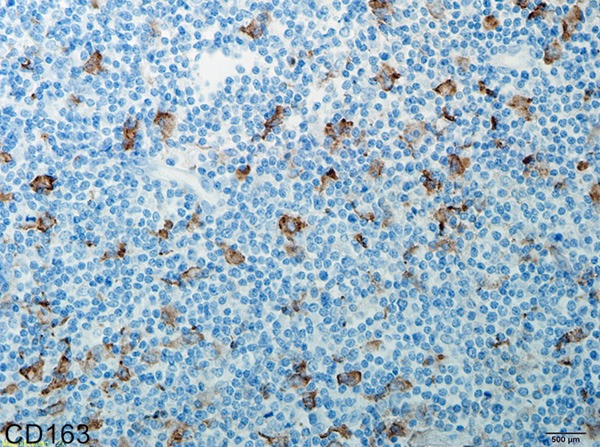
Immunohistochemical staining of CD163 in nasopharyngeal NK/T cell lymphoma (EnVision × 400).
Figure 6.
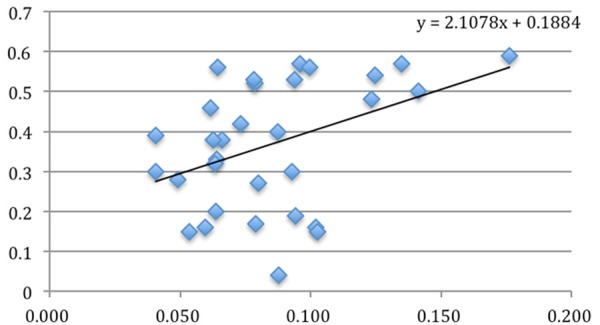
The relationship between CD68 positivity and Ki67 labeling index (P = 0.024).
Figure 7.
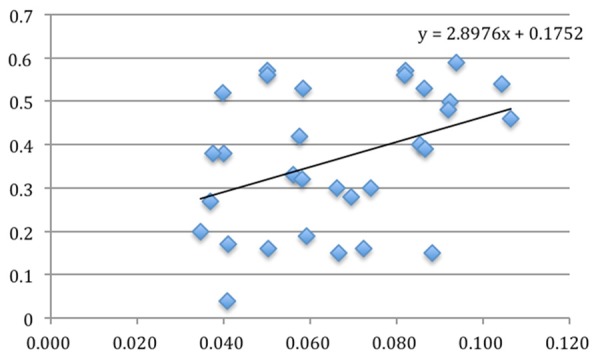
The relationship between CD163 positivity and Ki67 labeling index (P = 0.024).
Figure 8.
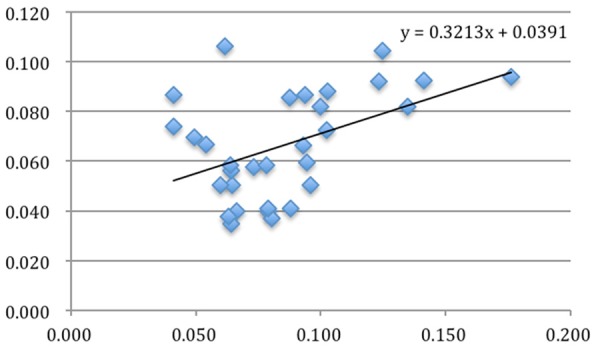
The relationship between CD68 positivity and CD613 positivity (P = 0.009).
Discussion
In this study, tumor-associated macrophages were detected by using two markers of macrophage, i.e., CD68 and CD163 in 31 cases of nasopharyngeal NK/T-cell lymphoma tissues. Meanwhile, Ki67 labeling index was also determined in double blind condition. Furthermore, the relationships between Ki67 labeling index and the number of both CD68- and CD163- positive cells as well as between CD68-positive and of CD163-positive cell numbers were analyzed. We found for the first time that both CD68- and CD163-positive cells were significantly correlated with Ki67 labeling index in this cancer. Meanwhile, a significant correlation between CD68- and CD163-positive cell numbers was also found. The findings above indicated that both CD68 and CD163 can recognize macrophages in nasopharyngeal NK/T-cell lymphoma tissues and these macrophages can promote lymphoma cell proliferation.
Tumor microenvironment is composed of three components: tumor cells, non-transformed cells and extra cellular matrix (ECM) [10]. It is found that non-transformed cells and ECM can provide living signals and condition to tumor cells by secreting growth factors, angiogenesis factors, and adhesion molecule [11-13] etc. So, it is believed that tumor microenvironment plays an important role in tumor cell hyperplasia, vascularization, infestation and metastasis [14]. The non-transformed cells include stroma cells, T cells, mononuclear macrophage even dendritic cells. These cells can form a specific network by connecting with cytokine and chemotactic factors to promote tumor formation and hyperplasia through cell-to-cell interaction. Among them, TAMs play a significant role.
In different microenvironments, macrophage can be induced into different subgroups with different molecular and functional characteristics. At present, macrophages are divided into two categories, that is, classically activated macrophage, M1 macrophages, and alternatively activated macrophage, M2 macrophages [15]. M1 cells can secrete NO, diverse cytokines, iNOS etc and act as inducer cells and effector cells to participate in killing pathogen and tumor cells in Th1 immune response [16]. On the contrary, M2 macrophages have the tendency to promote tumor to grow. M2 cells can promote vascularization and formation of lymphatic vessels; destroy base membrane and plerosis damnification [17]. Therefore, we need TAM subtype-specific markers to recognize them in situ tissue.
In this study, we indeed found that CD68-positive macrophages are not the same as CD163-positive macrophages. First, the cellular morphology of CD68-positive cells was clearly different from that of 163-positive cells and the boundary of the later cells was much clear. Second, the number of CD68-positive cells was more than that of CD163-positive cells in nasopharyngeal NK/T-cell lymphoma tissues, although the difference was not significant. (Figure 9). Furthermore, compared with the control group of nasopharyngeal reactive lesions, the number of CD 163-positive macrophages was significantly increased (CD163, P = 0.001; CD 68, P = 0.004). So, coupled with recent results in literatures, it is believed that CD68 is a general marker of macrophages and CD163 is more specific marker of M2 cells [18,19]. Although we found that there was a significant correlation between the numbers of CD68- and CD 163-positive macrophages, in our opinion, CD163 would be better and suitable for TAMs recognition. Although the results of us were similar with Harris JA et al. [20], they believed that CD68 and CD163 uncorrelated was inconsistent with the results of our study. Because CD68 marked the M1 and M2 cells in TAM, which CD163 labeled M2 only. Sowe had reason to believe that there was a correlation between them.
Figure 9.
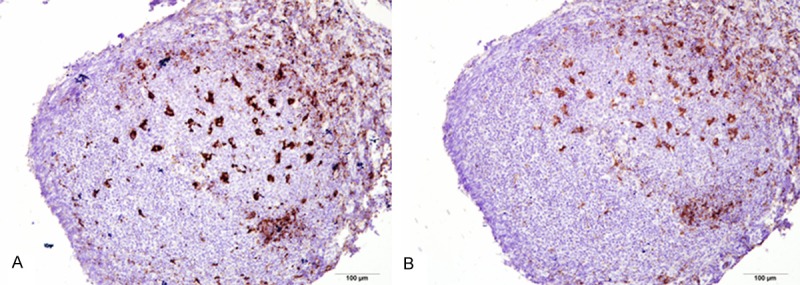
The positive rate of CD68 (A) and CD163 (B) serial sections under the same vision in the germinal center. CD68-positive rate was significantly higher than the CD163. (EnVision × 400).
Present study found for the first time that TAMs number was closely related to cell proliferation of nasal NK/T-cell lymphomas. Unfortunately, however, we didn’t clearly understand how TAMs promote tumor cell proliferation. But some literatures have reported that TAMs can interact with tumor cells through activation of cellular factors such as TNF-α, IL-2 by autocrine and/or paracrine secretion in the inflammation circumstances [21]. TAMs can also regulate NF-kB pathway to release large amount of cytokines (EGFR, GM-CSF, TGF-β etc.) and to promote the hyperplasia and growth of a tumor, induce vascularization and formation of lymphatic vessels, destroy base membrane and encourage tumorigenesis and development [22-25]. Among them, study showed ligands of EGFR family play important roles in tumorigenesis. The members of this family can form homologous or allogenetic dimeride on the cell surface which can mediate transference of cell multiplication signals. Another piece of literature reported that TAMs can release G O/G 1 switch gene 2 and accelerate cell growth cycle to promote hyperplasia of tumor cells [26]. To our knowledge, at present there are no any reports for a direct demonstrating a link between TAMs and tumor cell proliferation. In spite of Ishiih et al. described monocytes may be contribute to lymphoma progression in nasal NK/T-cell lymphomas [27], but the CD14-positive monocytes was the precursor cell what was different from the macrophages we reported.
In brief, increase number of TAMs was originally found in 31 cases of nasal NK/T-cell lymphoma tissues in this study and it indicated that TAMs could promote the cancer cell proliferation. Meanwhile, as M2 is much more important for cancer cell behavior, it suggested that CD163, a recommended marker of M2, was preferentially used in TAMs recognition. Furthermore, the mechanism of how TAMs promote NK/T-cell lymphoma cell proliferation needs to be directly investigated.
Disclosure of conflict of interest
None.
References
- 1.Zhou DM, Chen G, Zheng XW, Li C, He YZ. Clinicopathologic analysis of 7 cases of primary cutaneous NK/T cell lymphoma, nasal type. Zhonghua Bing Li Xue Za Zhi. 2011;40:772–773. [PubMed] [Google Scholar]
- 2.Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment - tumorigenesis and therapy. Nat Rev Cancer. 2005;5:867–875. doi: 10.1038/nrc1735. [DOI] [PubMed] [Google Scholar]
- 3.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 5.Erreni M, Mantovani A, Allavena P. Tumor-associated Macrophages (TAM) and Inflammation in Colorectal Cancer. Cancer Microenviron. 2011;4:141–154. doi: 10.1007/s12307-010-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Okumura H, Matsumoto M, Miyazono F, Hokita S, Aikou T. Tumor-associated macrophage (TAM) infiltration in gastric cancer. Anticancer Res. 2003;23:4079–4083. [PubMed] [Google Scholar]
- 7.Jonjic N, Valkovic T, Lucin K, Iternicka Z, Krstulja M, Mustac E, Dobi-Babic R, Sasso F, Melato M. Comparison of microvessel density with tumor associated macrophages in invasive breast carcinoma. Anticancer Res. 1998;18:3767–3770. [PubMed] [Google Scholar]
- 8.Pathology and Genetics: Tumours of Haematopoietic and Lymphoid Tissues (World Health Organization Classification of Tumours) Intl Agency for Research on Cancer. 2003 Jul 7; Elaine Jaffe NCI, Bethesda, MD 20892-1500, USA. Nancy Lee Harris, Massachusetts General Hospital, Boston, MA, USA. Harald Stein, Universitatsklinikum Benjamin Franklin, 12200 Berlin, Germany. [Google Scholar]
- 9.Paton D, Faragher B, Mustaffa KM, Szestak T, Barrett SD, Craig AG. Automated counting for Plasmodium falciparum cytoadherence experiments. Malar J. 2011;10:91. doi: 10.1186/1475-2875-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swartz MA, Iida N, Roberts EW, Sangaletti S, Wong MH, Yull FE, Coussens LM, Declerck YA. Tumor Microenvironment Complexity: Emerging Roles in Cancer Therapy. Cancer Res. 2012;72:2473–80. doi: 10.1158/0008-5472.CAN-12-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stokes JB, Adair SJ, Slack-Davis JK, Walters DM, Tilghman RW, Hershey ED, Lowrey B, Thomas KS, Bouton AH, Hwang RF, Stelow EB, Parsons JT, Bauer TW. Inhibition of focal adhesion kinase by PF-562,271 inhibits the growth and metastasis of pancreatic cancer concomitant with altering the tumor microenvironment. Mol Cancer Ther. 2011;10:2135–2145. doi: 10.1158/1535-7163.MCT-11-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, Mauerer K, Farina L, Gribben JG. The role of the tumor microenvironment in hematological malignancies and implication for therapy. Front Biosci. 2005;10:1581–1596. doi: 10.2741/1642. [DOI] [PubMed] [Google Scholar]
- 13.Lee HO, Silva AS, Concilio S, Li YS, Slifker M, Gatenby RA, Cheng JD. Evolution of tumor invasiveness: the adaptive tumor microenvironment landscape model. Cancer Res. 2011;71:6327–6337. doi: 10.1158/0008-5472.CAN-11-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarin D. Clinical and Biological Implications of the Tumor Microenvironment. Cancer Microenviron. 2012;5:95–112. doi: 10.1007/s12307-012-0099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantovani A, Germano G, Marchesi F, Locatelli M, Biswas SK. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol. 2011;41:2522–2525. doi: 10.1002/eji.201141894. [DOI] [PubMed] [Google Scholar]
- 16.Schmieder A, Michel J, Schonhaar K, Goerdt S, Schledzewski K. Differentiation and gene expression profile of tumor-associated macrophages. Semin Cancer Biol. 2012;22:289–97. doi: 10.1016/j.semcancer.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Baay M, Brouwer A, Pauwels P, Peeters M, Lardon F. Tumor cells and tumor-associated macrophages: secreted proteins as potential targets for therapy. Clin Dev Immunol. 2011;2011:565187. doi: 10.1155/2011/565187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P. Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur Respir J. 2009;33:118–126. doi: 10.1183/09031936.00065708. [DOI] [PubMed] [Google Scholar]
- 19.Shabo I, Svanvik J. Expression of macrophage antigens by tumor cells. Adv Exp Med Biol. 2011;714:141–150. doi: 10.1007/978-94-007-0782-5_7. [DOI] [PubMed] [Google Scholar]
- 20.Harris JA, Jain S, Ren Q, Zarineh A, Liu C, Ibrahim S. CD163 versus CD68 in tumor associated macrophages of classical hodgkin lymphoma. Diagn Pathol. 2012;7:12. doi: 10.1186/1746-1596-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakagawa J, Saio M, Tamakawa N, Suwa T, Frey AB, Nonaka K, Umemura N, Imai H, Ouyang GF, Ohe N, Yano H, Yoshimura S, Iwama T, Takami T. TNF expressed by tumor-associated macrophages, but not microglia, can eliminate glioma. Int J Oncol. 2007;30:803–811. [PubMed] [Google Scholar]
- 22.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Feng HC, Song YF, Wen YM. [Correlation between expression of vascular endothelial growth factor-C in tumor-associated macrophages and lymphatic metastasis in oral cancer] . Ai Zheng. 2004;23:278–281. [PubMed] [Google Scholar]
- 24.Hemmerlein B, Markus A, Wehner M, Kugler A, Zschunke F, Radzum HJ. Expression of acute and late-stage inflammatory antigens, c-fms, CSF-1, and human monocytic serine esterase 1, in tumor-associated macrophages of renal cell carcinomas. Cancer Immunol Immunother. 2000;49:485–492. doi: 10.1007/s002620000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sica A. Role of tumour-associated macrophages in cancer-related inflammation. Exp Oncol. 2010;32:153–158. [PubMed] [Google Scholar]
- 26.Yuan A, Chen JJ, Yang PC. Pathophysiology of tumor-associated macrophages. Adv Clin Chem. 2008;45:199–223. [PubMed] [Google Scholar]
- 27.Ishii H, Takahara M, Nagato T, Kis LL, Nagy N, Kishibe K, Harabuchi Y, Klein E. Monocytes enhance cell proliferation and LMP1 expression of nasal natural killer/T-cell lymphoma cells by cell contact-dependent interaction through membrane-bound IL-15. Int J Cancer. 2012;130:48–58. doi: 10.1002/ijc.25969. [DOI] [PubMed] [Google Scholar]


