Abstract
Advanced abdominal pregnancy is rare. The low incidence, high misdiagnosis rate, and lack of specific clinical signs and symptoms explain the fact that there are no standard diagnostic and treatment options available for advanced abdominal pregnancy. We managed a case of abdominal pregnancy in a woman who was pregnant for the first time. This case was further complicated by a concurrent singleton intrauterine pregnancy; the twin pregnancy was not detected until 20 weeks of pregnancy. The case was confirmed at 26 weeks gestational age using MRI to be an abdominal combined with intrauterine pregnancy. The pregnancy was terminated by cesarean section at 33 + 5 weeks gestation. We collected the relevant data of the case while reviewing the advanced abdominal pregnancy-related English literature in the Pubmed, Proquest, and OVID databases. We compared and analyzed the pregnancy history, gestational age when the diagnosis was confirmed, the placental colonization position, the course of treatment and surgical processes, related concurrency rate, post-operative drug treatment programs, and follow-up results with the expectation to provide guidance for other physicians who might encounter similar cases.
Keywords: Advanced abdominal pregnancy, obstetricians, clinical
Introduction
Advanced abdominal pregnancy is rare and often misdiagnosed [1]. Currently, among third trimester pregnancies, secondary abdominal pregnancies are relatively common. Many patients with advanced abdominal pregnancies have undergone uterine surgeries or dilation and curettage, a history of tubal or uterine horn pregnancies, or a history of artificial insemination [2,3]. In vitro fertilization (IVF)-induced consecutive abdominal pregnancies has occasionally been reported [4]. Some scholars believe that there is a correlation between cocaine use and abdominal pregnancy; however, because the results are based on a 55-case abdominal pregnancy study, the credibility of this connection is limited [5]. The clinical manifestation of advanced abdominal pregnancy is most often hemorrhage, which is caused by rupture of the gestational sac or the absence of labor at term. Advanced abdominal pregnancy can also be discovered in the process of elective cesarean section [6,7]. While infrequent, there are reports of fetal survival from advanced abdominal pregnancies [8-10]. Only two cases of an abdominal pregnancy combined with an intrauterine pregnancy has been reported [11,12]. In one of the cases, the patient underwent in vitro IVF and embryo transfer. Three months before the embryo transfer, the patient had a hysteroscopy. Neither of the fetuses survived. Primary abdominal pregnancy or primary abdominal and intrauterine pregnancies without a history of uterine surgery or assisted reproduction are very rare [13].
There is no standard diagnosis and treatment procedure for abdominal pregnancy. Standardization of the treatment principles for advanced abdominal pregnancy, peri-operative treatment options, and post-operative management measures would improve newborn survival, reduce complications, and mortality.
In addition, uterine malformations cause uterine horn rupture, a gestational sac located outside the uterus, and a placenta inside the uterine cavity are not classified as abdominal pregnancies because the pathogenesis involves rupture of the uterine horn.
Case report
A 30-year-old female conceived spontaneously and had no vaginal bleeding during early pregnancy. She was diagnosed at 12 weeks gestation with a singleton intrauterine pregnancy using B-ultrasound. During a routine obstetric ultrasound examination at 20 weeks of pregnancy, it was shown that she has twins and a chorionic membrane was not identified. At 26 weeks of pregnancy, it was suspected that there was an intrauterine pregnancy combined with an abdominal pregnancy based on sonographic findings. Intrauterine fetal development was consistent with the number of weeks of pregnancy, while abdominal pregnancy fetal growth was slightly behind (similar to 24 weeks of pregnancy; Figure 1). The patient was diagnosed with an abdominal pregnancy and intrauterine pregnancy by MRI. The abdominal pregnancy placenta was attached to the lower end of the uterine wall (Figure 2). The patient denied a history of assisted reproduction and uterine surgery. She also denied a history of pelvic inflammatory disease (PID), endometriosis, cigarette use, and alcohol consumption.
Figure 1.
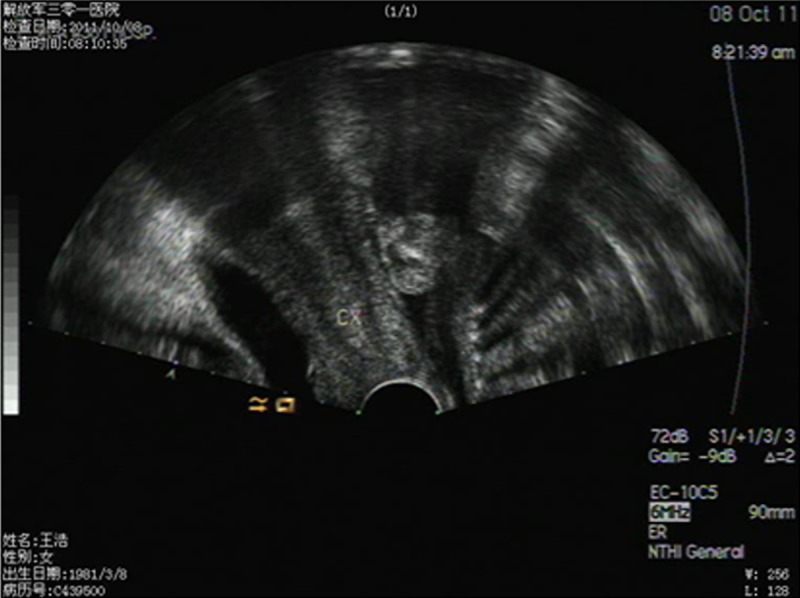
Transvaginal ultrasound results of a 26-week pregnancy (longitudinal). The abdominal pregnancy fetus is posterior to the cervix. The surface did not reach the muscular layer of the uterus.
Figure 2.
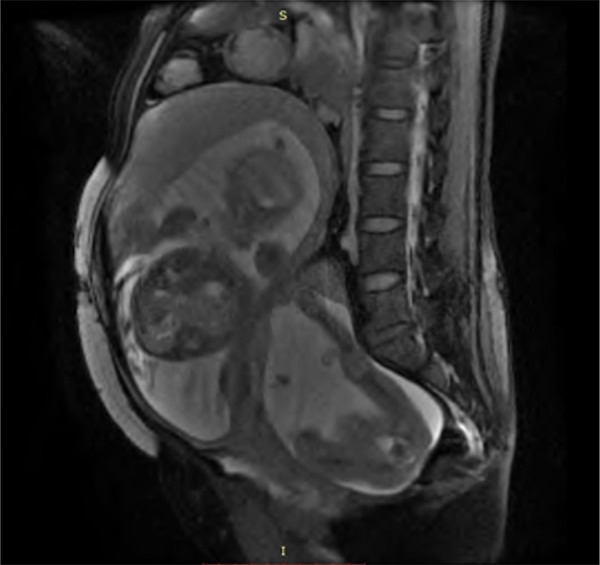
Pre-operative MRI results. Pre-operative magnetic resonance imaging indicated a breech fetus in the uterus. Posterior to the uterus, an abdominal pregnancy with a single breech fetus can be seen. The abdominal pregnancy placenta is located inferior to the uterine wall.
This woman was admitted to the hospital at 26 weeks gestation. We restricted the movement of the patient after admission, adjusted the diet with high fiber foods to prevent constipation and resulting abdominal pressure, and required the patient to wear anti-thrombosis stockings to prevent blood clots. Weekly monitoring of blood pressure, hemogram panels, urine testing, and biochemical indicators were carried out. After communicating with the patient and her family, to the decision was made to continue the pregnancy. At 28 weeks gestation, the development of the fetus outside of the uterus was 2 weeks behind that of the fetus in the uterine cavity with no other obvious abnormalities, based on an ultrasonographic examination. This observation was followed by weekly monitoring of fetal development. During treatment, the patient had no complaints of discomfort. The ultrasound results suggested that starting from 28 weeks of pregnancy, fetal growth differences between the 2 fetuses gradually increased. At 32 weeks of pregnancy, an ultrasonographic examination indicated that the abdominal pregnancy fetus was suspected to have cardiac malformations. At 33 + 5 weeks of pregnancy, an ultrasonographic examination showed that fetal diastolic blood flow to the abdominal fetus had disappeared and the development was 5 weeks behind. Thus, an emergent abdominal delivery was arranged.
General anesthesia was used. After the laparotomy incision was made, the intrauterine fetus (in the breech position) and the placenta were removed first. The newborn was given an Apgar score of 9 at 1 minute and 10 at 5 minutes, and weighed 1990 g without deformities. After closing the uterine incision, the abdomen was explored to expose the abdominal gestational sac behind the uterus (Figure 3). The gestational sac surface was adhesed to the omentum, and was separated and ligated, followed by incision of the sac wall along an avascular zone. With gradual expansion of the incision, it was noted that the amniotic fluid was stained (third degree) and the fetus sat with a single hip inside the sac. The newborn was taken out of the sac carefully and given an Apgar score of 0 at 1 minute and 0 at 5 minutes, and weighed 1600 g. Exploration of the abdomen revealed that the placenta was posterior to the uterine wall, covering the upper anterior sigmoid colon and the rectum, and the uterorectal fossa could not be exposed. Because the placental attachment had no active bleeding, the umbilical cord was cut close to the base, excluding the placenta. The uterine horn and fallopian tube were not defective or damaged. There were two indwelling abdominal drainage tubes; one of the tubes was placed in the abdominal gestational sac and the other was placed in the pelvic cavity. The abdomen was then closed layer-by-layer. The surgical blood loss was approximately 600 ml and no blood transfusion was indicated. The patient was transferred back to the maternity ward. After 5 days, no bloody drainage outflow was observed and the drainage tubes were removed.
Figure 3.
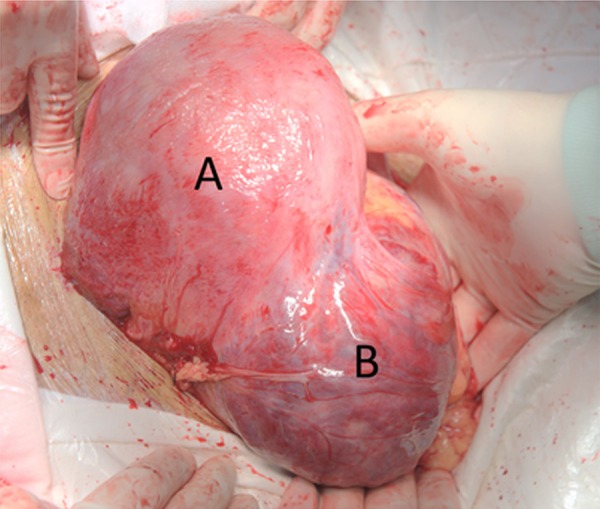
Intra-operative photograph. A is the uterine pregnancy; the anterior incision was completely sutured. B is the abdominal pregnancy sac with an intact surface and visibly abundant blood vessels.
The patient was given cefazolin (2 g bid) to prevent infection. She was given mifepristone (50 mg bid) the next day and the β-HCG level dropped from 19033 mIU/mL to 16078 mIU/mL 12 days later. An ultrasonographic examination showed an abundance of abdominal placental blood flow signals (Figure 4). After 12 days, the oral mifepristone was discontinued and methotrexate (75 mg intramuscular) was administered. When the patient was discharged 2 days later, the β-HCG level had dropped to 4411.5 mIU/mL. Fifty days after the surgery, an ultrasonographic examination suggested that the blood flow in the abdominal pregnancy placental had decreased significantly (Figure 5). Three months after surgery, the β-HCG dropped to the normal range. The patient was followed up once a year after surgery. During the follow-up, the patient had no complaints of discomfort and reported no impact on her sexual life. No intestinal obstruction symptoms developed and elevation of the serum β-HCG was not observed. A MRI 1 and 2 years later suggested that the abdominal pregnancy placenta was still in the same site and there were no blood flow signals (Figures 6, 7).
Figure 4.
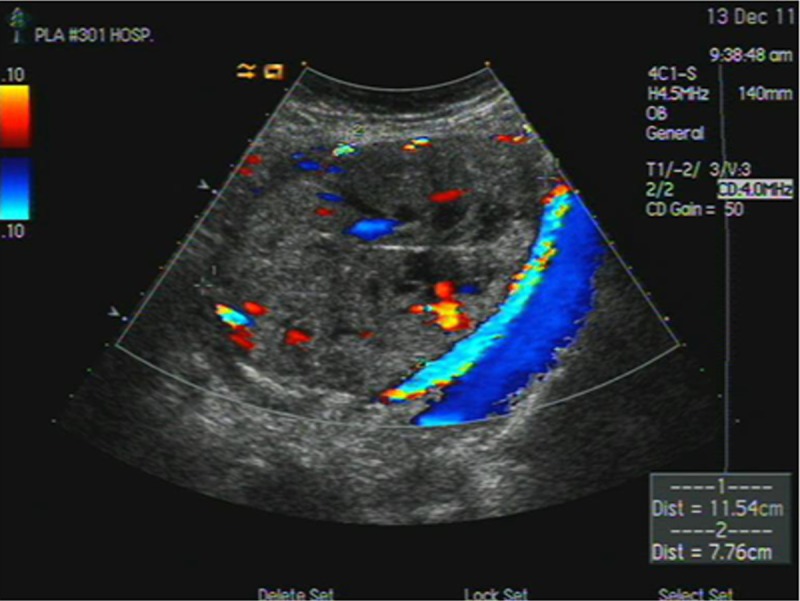
Fourteen days after surgery, a post-operative ultrasound indicated rich blood flow signals of the residual abdominal pregnancy placenta.
Figure 5.
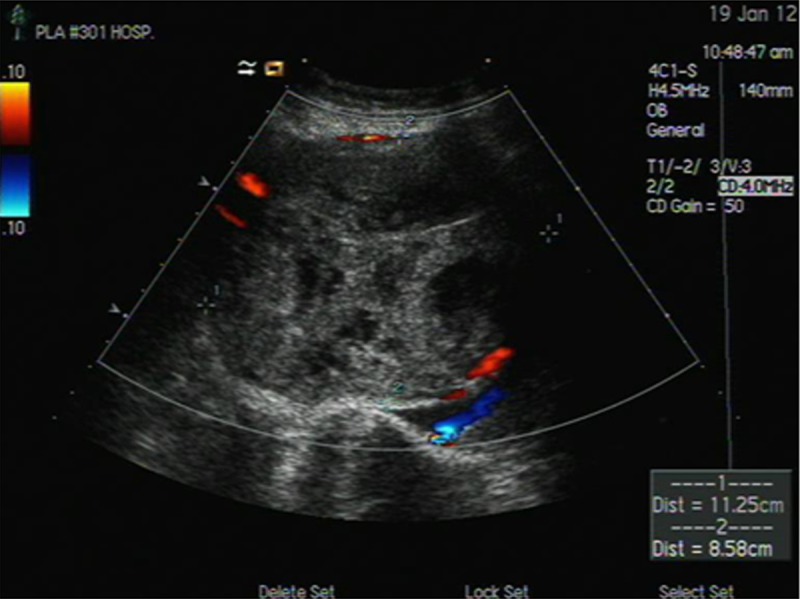
After 50 days of surgery, the ultrasound results suggested a small amount of placental blood flow signals at the edge of the abdominal pregnancy residual placenta.
Figure 6.
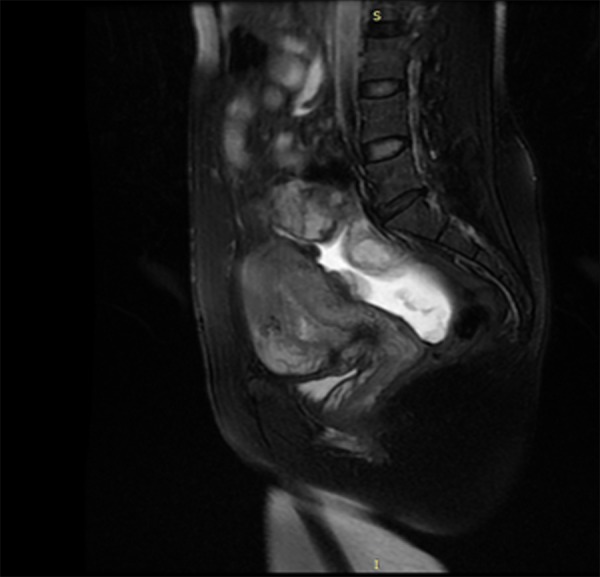
MRI results of the follow-up after 1 year. Placenta located at the uterorectal fossa did not significantly shrink in size. The border with the anterior uterus was not clear. No obvious bowel oppression symptoms were noted.
Figure 7.
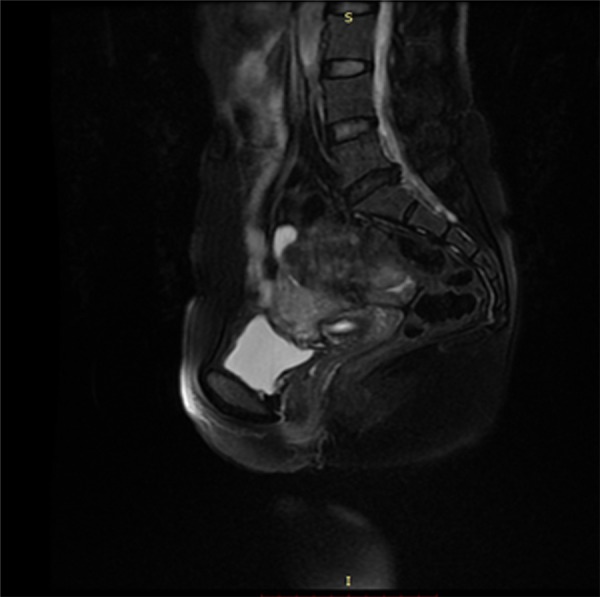
MRI results of the follow-up after 2 years. After 2 years, a MRI still indicated that the placenta was at the uterorectal fossa and was not significantly reduced in size. The border with the anterior uterus was not clear. No obvious bowel oppression symptoms were noted.
Characteristics and diagnostic key points of advanced abdominal pregnancy
An abdominal pregnancy is a special type of ectopic pregnancy, accounting for approximately 1% of the total number of ectopic pregnancies. Abdominal pregnancy is easily missed and mostly diagnosed after substantial emergency bleeding, which is caused by an insecure abdominal pregnancy placenta, a weak gestational sac, and the lack of protection of the myometrium [14]. The etiology of this disease is unknown and early detection is difficult. There are no widely accepted diagnostic criteria for abdominal pregnancies and the current diagnostic criteria for primary abdominal pregnancy are based on 1942 Studdiford standards. Abdominal pregnancy often leads to early spontaneous abortion, causing abdominal bleeding. In rare cases, the pregnancy can develop to late stages.
For advanced abdominal pregnancy, placental location tends to be relatively stable. The majority of the placentas are located near the uterine wall and the placenta has a relatively abundant blood supply to maintain fetal development [15]. There are different degrees of fetal growth retardation with advanced abdominal pregnancies, but no increase in the fetal malformation rate has been reported with advanced abdominal pregnancies.
Abdominal pregnancy can be easily missed or misdiagnosed. The diagnostic value of ultrasonography alone is limited. When the intestines are close to the abdominal pregnancy, ultrasound diagnosis is of lesser value [9,16,17]. With nine cases of abdominal pregnancy, Lockhat et al. [18] confirmed the value of MRI in the diagnosis of abdominal pregnancy. MRI can be used to diagnose an abdominal pregnancy, and more importantly, MRI can help locate and identify the relationship between the placenta and the adjacent organs and tissues. The location of the placental site can help decide whether or not to continue the pregnancy, and help develop a relatively safe and reasonable treatment option and surgical planning. This patient was misdiagnosed with a singleton pregnancy before the twin pregnancy was diagnosed at 20 weeks of pregnancy. Because careful analysis of the reasons for misdiagnosis of twin pregnancies was not performed, the abdominal pregnancy was not discovered. At 26 weeks of pregnancy, however, the patient was suspected to have an intrauterine pregnancy combined with an abdominal pregnancy. The relatively low sensitivity and specificity of ultrasound diagnosis was the main reason for the late diagnosis in the current case. At 26 weeks of pregnancy, the patient was diagnosed by MRI with an abdominal pregnancy and an intrauterine pregnancy. The abdominal pregnancy placenta, which was located below the uterine wall, can maintain fetal development in an abdominal pregnancy. Gestational fetal reduction at this stage is associated with a risk for intra-abdominal infection, bleeding, and intestinal perforation, thus endangering the safety of the fetus in utero. When considering the gestational age with the development of the twin gestation, and after thorough communication with the patient and her family, the decision was made to continue the tocolytic therapy.
Treatment principles for advanced abdominal pregnancy
With the early detection of an abdominal pregnancy, artificial termination of pregnancy is safe. Depending on the number of weeks of pregnancy and the physical condition of the gravida, different pregnancy termination options, including laparoscopic or open surgery, arterial embolization, and intracapsular injection of potassium chloride into the abdominal pregnancy sac are available; however, different complications occur to various degrees, with bleeding being the more common problem.
Whether or not fetal survival is the goal in an abdominal pregnancy, open surgery is the main means of treatment for advanced abdominal pregnancies. Previous experience suggests that uterine artery embolization, injection of potassium chloride into the abdominal pregnancy sac, and other conservative treatments can lead to a higher incidence of infection and late bleeding during the fetus ossification process because the dead fetus cannot be absorbed completely. There are also reports of intestinal fistulas after fetal ossification [19]. Thus, for the fetus in an advanced abdominal pregnancy, if the development is acceptable, expectant treatment can be adopted to ensure a live birth [20]. In the case herein, because the diagnosis of twin pregnancy was established late, irrespective of the method of treatment for abdominal pregnancy that was utilized, the survival for the fetus in the uterine cavity was at risk. Because both fetuses developed reasonably well and the patient had no complaints of discomfort, upon full disclosure to the patient, expectant management was chosen.
Timing of pregnancy termination
This case was a twin pregnancy with the abdominal pressure higher than in a singleton abdominal pregnancy. We initially planned termination at 32-34 weeks of pregnancy. There were several considerations. First, a fetus after 34 weeks of gestation has a high survival rate. Of note, we were concerned that with the increase in the number of weeks of pregnancy, especially after 34 weeks gestation, the size of the pregnancy sac increases rapidly and the risk of abdominal pregnancy sac rupture was significantly higher. Based on a literature review, abdominal discomfort or abdominal pain may not be a harbinger of gestational sac rupture sensitivity, and MRI is still the most reliable method to evaluate the integrity of the gestational sac. Therefore, the original plan was after 34 weeks of pregnancy, MRI would be performed weekly to assess the integrity of the abdominal pregnancy sac. To our surprise, at 33 + 5 weeks gestation, we found that the diastolic flow of the fetal abdominal pregnancy disappeared based on an ultrasonographic examination, and the decision was made to proceed with an emergent abdominal delivery.
Surgical principles for advanced abdominal pregnancy
The primary goal of surgery is to save the fetus. The secondary goal is to properly treat the abdominal pregnancy placenta.
There are no conclusive treatment procedures for placentas in advanced abdominal pregnancies. When the placenta is located in a blood vessel-rich area, such as an advanced ovarian pregnancy, forcible removal of the placenta surgically can cause serious bleeding [10]. Tshivhula et al. [21] reported an abdominal pregnancy diagnosed at 29 weeks gestation in which conservative treatment was carried out until week 32, followed by elective laparotomy through which the abdominal fetus was removed and the placenta was manually removed. Because the placenta was near the uterus and close to the broad ligament and right peritoneum and some parts of the colon, there was significant blood loss (2 L) during surgery [21]. Miguel Echenique-Elizondo et al. [22] reported a case of placental invasion of the omentum, mesentery, colon, small intestine, and left ureter and iliac vessels (mostly the iliac vein). During the surgical removal of the placenta, 8 units of whole blood and 6 units of freshly frozen plasma was used for infusion. Thus, for abdominal pregnancy patients in whom the placental colonization site is relatively stable, intra-operative exclusion of the placenta is safer. We determined that the placenta was attached to the lower portion of the uterine wall pre-operatively by MRI. During the surgery, we selected the uterine avascular zone to perform the abdominal gestational sac incision, and after removal of the abdominal pregnancy and the incision of the fetal umbilical cord, no placental separation was observed. Further investigation revealed widespread attachment of the abdominal pregnancy placenta to the lower portion of the uterine wall, the anterior aspect of the sigmoid colon, and the uterine rectal fossa, with no active bleeding. We made the decision to keep the pelvic ectopic placenta in situ, and inserted abdominal drainage tubes. After surgery, we used mifepristone to induce placental degeneration, followed by intramuscular methotrexate (75 mg) 12 days later. No pelvic infection occurred during the treatment period. Three weeks after surgery, the serum HCG returned to normal and serial ultrasound examinations showed no placental blood flow.
Literature review
We used “advanced abdominal pregnancy or late trimester and pregnancy or ectopic pregnancy” and “advanced extra-uterine pregnancy” as keywords to search the English literature in the Medline database from 1989 to March 2014, and all English literature in the Proquest and OVID databases from 1980 to December 2013. There were 47 papers meeting the study requirements, which are summarized below.
Clinical characteristics
The etiology of primary abdominal pregnancies is unknown. Many patients with secondary abdominal pregnancies have a history of uterine surgery or are IVF patients [23]. It is difficult to detect an abdominal pregnancy early; the diagnosis is usually based on abdominal pain or emergency bleeding caused by abdominal pregnancy rupture [24,25]. Abdominal pregnancies often result in early abortion; a few cases can develop to advanced pregnancy and the use of MRI diagnosis is more reliable than ultrasound [26].
Clinical manifestations
There are no specific early symptoms or signs for abdominal pregnancy; bleeding is the main complication. It is believed that in advanced abdominal pregnancy, bleeding is not only related to the gestational sac, but also to the site of colonization. Based on the available literature, abdominal pregnancy sac colonization on the omentum and liver and spleen surface are high bleeding risk factors, and colonization in the uterus or other parts of the uterus surface is associated with a relatively low risk of bleeding. When placental colonization is on the uterine wall, the likelihood of abdominal pregnancy fetal growth retardation is lower.
Treatment of abdominal pregnancy
For advanced abdominal pregnancies diagnosed based on acute blood loss, surgery should aim to completely stop the bleeding and remove the abdominal pregnancy fetus. Combined with the existing literature, for abdominal pregnancy cases diagnosed in the mid-trimester, we believe that an individualized treatment approach should be adopted based on the site of placental colonization. This is especially important for gravidas with an intrauterine pregnancy who also have an abdominal pregnancy. In addition to protecting pregnant women, it is also necessary to take into account the safety of the two fetuses. It should be noted that abdominal pain is not a harbinger of gestational sac rupture. For cases in which the abdominal pregnancy is diagnosed during an ongoing pregnancy, MRIs must be periodically performed to assess the integrity of the gestational sac and timely termination of pregnancy is performed when necessary.
Expectant treatment is suitable for abdominal pregnancies for which placenta colonization is in the uterus or at the uterine wall because these sites can provide a relatively stable blood supply and the probability of acute bleeding is low. Periodic review of obstetric ultrasound and monitoring of fetal development is necessary. At the beginning of 32 weeks of pregnancy, it is recommended to have a weekly MRI to evaluate the integrity of the gestational sac to detect any early signs of abdominal gestational sac rupture [21].
When colonization is outside the uterus, such as the omentum, bowel, ovarian ligament, and liver surface, more life- threatening complications occur in gravidas during the second trimester. Active treatment of such cases is recommended, and there are several treatment methods available, as below.
Vascular embolization
Because the arterial supply to the placenta is not well-defined and may involve the surrounding blood supply to vital organs, this method tends to yield poor results. In addition, after arterial embolization, fetal bone ossification-associated intestinal perforation and other complications are not uncommon. Therefore, vascular embolization is not preferred.
Gestational sac injection or umbilical intravenous injection of potassium chloride
This technique is suitable for early pregnancies. For advanced abdominal pregnancy, due to the risk of bleeding, infection, fetal ossification-associated secondary damage and the subsequent second surgery, this method is not recommended.
Laparotomy
This approach is applicable to patients at various stages of pregnancy. A thorough evaluation before surgery is required and MRI is used to determine the site of abdominal pregnancy colonization before surgery.
Neonatal development
The current literature does not support a correlation between abdominal pregnancy and birth defects; however, because most cases of abdominal pregnancy do not achieve full term, and because the blood supply to the placental colonization site is poorer than that of a normal intrauterine pregnancy, the S/D ratio increases in the umbilical cord and neonatal growth retardation is relatively common. In our case, a similar phenomenon was observed [27,28].
Treatment of residual placenta and long-term outcome
Placenta treatment should be individualized according to the colonization site. There are reported cases of colonization at important perivascular sites, such as the iliac vessels and pelvic ligament, where even when the residual placenta has no blood flow and the β-HCG has decreased to normal, late post-operative bleeding still occurs. This may be caused by the relatively large size of the residual placenta and the adjacent blood vessels being torn during activities. We believe that for placenta colonization at the vessel-rich and mobility-poor regions (pelvic ligaments, iliac vessel region, the hepatic portal, or spleen), surgery must be gentle and meticulous to avoid causing placental separation. Aseptic procedures must be strictly followed with adequate drainage, otherwise the incidence of secondary pelvic abscesses will be high [29,30]. When the patient’s condition stabilizes and placental blood flow ceases (approximately 3 months after surgery) a second surgical procedure to remove the placenta can be performed. Otherwise, there may be a post-operative risk of excess residual fluid, bleeding, or infection [2,31-33]. The placenta colonized at other parts does not need to be treated [34,35]. For the cases of abdominal pregnancy diagnosed after fetal death, arterial embolization may be performed before surgery to reduce blood loss [36].
There is no standardized post-operative medication guide. We believe that early post-operative drug therapy for the residual placenta should not be abandoned and a potent short-term drug-induced placental necrosis is not appropriate because the latter may increase the risk of placental separation and postpartum hemorrhage. Instead, relatively mild drugs should be given. When the conditions stabilize and the placenta begins fibrosis, potent drugs should be used to promote placental necrosis, which can reduce late bleeding risks. Mifepristone is effective in inducing degradation of the placenta. We administered oral mifepristone post-operatively and did not find placental separation bleeding; however, the effect on β-HCG reduction is poor. Although methotrexate can quickly reduce the HCG level, use of methotrexate directly after surgery can lead to rapid placental lobular necrosis and likely cause intra-abdominal bleeding. Thus, we only used a single dose of methotrexate (75 mg intramuscular) 12 days after surgery. During treatment, no infection, bleeding, and other complications were observed. The results are similar to the results reported by Cetinkaya [34]. Valenzano et al. reported a more rapid reduction of the serum β-HCG level [37]. In some cases, methotrexate is administered twice daily (10 mg) to achieve a gradual decrease in the β-HCG level. There also are reports of not using any drugs after surgery. Rather, the serum β-HCG level is monitored until it decreases to the normal range, but > 5 weeks is typically required [38,39].
Our patient was followed for 2 years. The patient had no complaints of discomfort. Ossified placentas do not affect the patient’s daily life and sexual activities. A gynecologic examination showed that the residual placenta was still palpable within the uterorectal fossa; however, an ultrasound blood flow signal was not found. Review of the MRIs after 1 and 2 years indicated that the pelvic mass echo had increased, the ossified placenta did not shrink significantly, and no blood flow signals were detected. The serum β-HCG level was undetectable. Our long-term case follow-up continues. In similar cases in the literature, absorption of the residual placenta was not satisfactory either.
Conclusions
Abdominal pregnancies are rare. Cases of advanced intrauterine pregnancies with abdominal pregnancies are even rarer. The diagnostic criteria for abdominal pregnancy, treatment methods, treatment timing, peri-operative considerations, and post-operative follow-up deserve our attention. Clinicians need to be aware of how to improve the rate of early diagnosis and reduce the risks and complications in patients. With the current development of myomectomy and other types of surgeries, cases of desired fertility after surgery have gradually increased and women undergoing IVF treatment are also increasing. Consequently, the occurrence of abdominal pregnancy has shown a gradually increasing trend.
Currently, the most accepted method of diagnosing an abdominal pregnancy is MRI, while ultrasound is suitable for screening. A MRI cannot only diagnose an abdominal pregnancy, but also locate the position of the placenta, which will significantly contribute to the development of treatment principles and a surgical treatment plan [40]. The treatment timing of abdominal pregnancy needs to be individualized according to the location of the placenta. In cases with a relatively stable placental colonization site, expectant treatment is a conservative choice. For abdominal pregnancies with placental colonization involving non- stable parts, an early termination of pregnancy is recommended. We believe that if the abdominal pregnancy placenta colonization involves the uterine wall and MRI does not indicate a threatened rupture or defects of the gestational sac, pregnancy can continue under strict monitoring. Starting from week 32 of gestation, MRI should be used to assess the integrity of the gestational sac every week and early termination of pregnancy should be performed when necessary. Because the abdominal pregnancy sac wall will thicken, the likelihood of continued pregnancy to 34 weeks is high. For treatment of post-operative residual placenta in the pelvis, the consensus is to avoid the use of potent drugs to induce placental necrosis. Relatively mild or slow- acting drugs can be given early on, which is followed by a potent drug-induced necrosis of the placenta or the continued use of small doses of potent drugs to cause placental necrosis, e.g., methotrexate (50 mg intramuscular injection once a week for a total of 4 doses; [41]. For placentas colonized at the major blood vessels (pelvic ligaments and iliac vessel region), we recommend that when placental blood flow ceases 3 months after surgery, a second surgery is performed to remove the residua. Otherwise, there may be late post-operative bleeding and risks of other complications.
Current data has shown that the placenta remaining in the pelvis cannot be self-absorbed. No cases of residual placenta growth or malignancy have been reported. When patients achieve pregnancy again, the residual placenta did not show any reaction to estrogen and progesterone stimulation [41]. The regular follow-up of our patient at 29 months showed no apparent abnormalities.
Disclosure of conflict of interest
None.
References
- 1.Sunday-Adeoye I, Twomey D, Egwuatu EV, Okonta PI. A 30-year review of advanced abdominal pregnancy at the Mater Misericordiae Hospital, Afikpo, southeastern Nigeria (1976-2006) Arch Gynecol Obstet. 2011;283:19–24. doi: 10.1007/s00404-009-1260-4. [DOI] [PubMed] [Google Scholar]
- 2.Roberts RV, Dickinson JE, Leung Y, Charles AK. Advanced abdominal pregnancy: still an occurrence in modern medicine. Aust N Z J Obstet Gynaecol. 2005;45:518–521. doi: 10.1111/j.1479-828X.2005.00489.x. [DOI] [PubMed] [Google Scholar]
- 3.Hyvarinen M, Raudaskoski T, Tekay A, Herva R. [Abdominal pregnancy] . Duodecim. 2009;125:2448–2451. [PubMed] [Google Scholar]
- 4.Moonen-Delarue MW, Haest JW. Ectopic pregnancy three times in line of which two advanced abdominal pregnancies. Eur J Obstet Gynecol Reprod Biol. 1996;66:87–88. doi: 10.1016/0301-2115(96)02388-3. [DOI] [PubMed] [Google Scholar]
- 5.Audain L, Brown WE, Smith DM, Clark JF. Cocaine use as a risk factor for abdominal pregnancy. J Natl Med Assoc. 1998;90:277–283. [PMC free article] [PubMed] [Google Scholar]
- 6.Tungshevinsirikul R, Charutragulchai P, Khunpradit S, Herabutya Y. Advanced abdominal pregnancy: a case report. J Med Assoc Thai. 1990;73(Suppl 1):107–110. [PubMed] [Google Scholar]
- 7.Matovelo D, Ng’walida N. Hemoperitoneum in advanced abdominal pregnancy with a live baby: a case report. BMC Res Notes. 2014;7:106. doi: 10.1186/1756-0500-7-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang J, Jing X, Fan S, Fufan Z, Yiling D, Pixiang P, Xiaomeng X. Primary unruptured full term ovarian pregnancy with live female infant: case report. Arch Gynecol Obstet. 2011;283(Suppl 1):31–33. doi: 10.1007/s00404-010-1755-z. [DOI] [PubMed] [Google Scholar]
- 9.Varma R, Mascarenhas L, James D. Successful outcome of advanced abdominal pregnancy with exclusive omental insertion. Ultrasound Obstet Gynecol. 2003;21:192–194. doi: 10.1002/uog.25. [DOI] [PubMed] [Google Scholar]
- 10.Meseci E, Guzel Y, Zemheri E, Eser SK, Ozkanli S, Kumru P. A 34-week ovarian pregnancy: case report and review of the literature. J Turk Ger Gynecol Assoc. 2013;14:246–249. doi: 10.5152/jtgga.2013.31391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shojai R, Chaumoitre K, Chau C, Panuel M, Boubli L, d’Ercole C. Advanced combined abdominal and intrauterine pregnancy: a case report. Fetal Diagn Ther. 2007;22:128–130. doi: 10.1159/000097111. [DOI] [PubMed] [Google Scholar]
- 12.Das N. Advanced simultaneous intrauterine and abdominal pregnancy. A case report. Br J Obstet Gynaecol. 1975;82:840–842. doi: 10.1111/j.1471-0528.1975.tb00585.x. [DOI] [PubMed] [Google Scholar]
- 13.Mpogoro F, Gumodoka B, Kihunrwa A, Massinde A. Managing a live advanced abdominal twin pregnancy. Ann Med Health Sci Res. 2013;3:113–115. doi: 10.4103/2141-9248.109472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rojansky N, Schenker JG. Heterotopic pregnancy and assisted reproduction--an update. J Assist Reprod Genet. 1996;13:594–601. doi: 10.1007/BF02066615. [DOI] [PubMed] [Google Scholar]
- 15.Dubinsky TJ, Guerra F, Gormaz G, Maklad N. Fetal survival in abdominal pregnancy: a review of 11 cases. J Clin Ultrasound. 1996;24:513–517. doi: 10.1002/(SICI)1097-0096(199611/12)24:9<513::AID-JCU4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 16.Aydogdu M, Heilmann I, Schutte P, Trams G. [Advanced ectopic pregnancy--clinical management] . Zentralbl Gynakol. 2001;123:585–587. doi: 10.1055/s-2001-19091. [DOI] [PubMed] [Google Scholar]
- 17.Blackwelder JT, Varner MW, Brown RC. Sonographic findings in advanced abdominal pregnancy. AJR. 1981;137:1259–1261. doi: 10.2214/ajr.137.6.1259. [DOI] [PubMed] [Google Scholar]
- 18.Lockhat F, Corr P, Ramphal S, Moodley J. The value of magnetic resonance imaging in the diagnosis and management of extra-uterine abdominal pregnancy. Clin Radiol. 2006;61:264–269. doi: 10.1016/j.crad.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Pasternak BM. Uteroenteric fistula due to advanced extrauterine pregnancy. Arch Surg. 1977;112:669. doi: 10.1001/archsurg.1977.01370050129028. [DOI] [PubMed] [Google Scholar]
- 20.Brewster EM Sr, Braithwaite EA, Brewster EM Jr. Advanced abdominal pregnancy: a case report of good maternal and perinatal outcome. West Indian Med J. 2011;60:587–589. [PubMed] [Google Scholar]
- 21.Tshivhula F, Hall DR. Expectant management of an advanced abdominal pregnancy. J Obstet Gynaecol. 2005;25:298. doi: 10.1080/01443610500106819. [DOI] [PubMed] [Google Scholar]
- 22.Echenique-Elizondo M, Carbonero K. Fullterm abdominal pregnancy, mother and infant survival. J Am Coll Surg. 2001;192:231. doi: 10.1016/s1072-7515(00)00784-5. [DOI] [PubMed] [Google Scholar]
- 23.Bassil S, Pouly JL, Canis M, Janny L, Vye P, Chapron C, Bruhat MA. Advanced heterotopic pregnancy after in-vitro fertilization and embryo transfer, with survival of both the babies and the mother. Hum Reprod. 1991;6:1008–1010. doi: 10.1093/oxfordjournals.humrep.a137450. [DOI] [PubMed] [Google Scholar]
- 24.Ayinde OA, Aimakhu CO, Adeyanju OA, Omigbodun AO. Abdominal pregnancy at the University College Hospital, Ibadan: a ten-year review. Afr J Reprod Health. 2005;9:123–127. [PubMed] [Google Scholar]
- 25.Zeck W, Kelters I, Winter R, Lang U, Petru E. Lessons learned from four advanced abdominal pregnancies at an East African Health Center. J Perinat Med. 2007;35:278–281. doi: 10.1515/JPM.2007.075. [DOI] [PubMed] [Google Scholar]
- 26.Wagner A, Burchardt AJ. MR imaging in advanced abdominal pregnancy. A case report of fetal death. Acta Radiol. 1995;36:193–195. [PubMed] [Google Scholar]
- 27.Golan A, Sandbank O, Andronikou A, Rubin A. Advanced extra-uterine pregnancy. Acta Obstet Gynecol Scand. 1985;64:21–25. doi: 10.3109/00016348509154682. [DOI] [PubMed] [Google Scholar]
- 28.Radaelli T, Bulfamante G, Cetin I, Marconi AM, Pardi G. Advanced tubal pregnancy associated with severe fetal growth restriction: a case report. J Matern Fetal Neonatal Med. 2003;13:422–425. doi: 10.1080/jmf.13.6.422.425. [DOI] [PubMed] [Google Scholar]
- 29.Rahman MS, Al-Suleiman SA, Rahman J, Al-Sibai MH. Advanced abdominal pregnancy--observations in 10 cases. Obstet Gynecol. 1982;59:366–372. [PubMed] [Google Scholar]
- 30.Yu S, Pennisi JA, Moukhtar M, Friedman EA. Placental abruption in association with advanced abdominal pregnancy. A case report. J Reprod Med. 1995;40:731–735. [PubMed] [Google Scholar]
- 31.Masukume G, Sengurayi E, Muchara A, Mucheni E, Ndebele W, Ngwenya S. Full-term abdominal extrauterine pregnancy complicated by post-operative ascites with successful outcome: a case report. J Med Case Re. 2013;7:10. doi: 10.1186/1752-1947-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spinnato JA, Aksel S, Mendenhall HW. Postpartum polyhydramnios: a unique complication of advanced abdominal pregnancy. Obstet Gynecol. 1987;70:490–492. [PubMed] [Google Scholar]
- 33.Worley KC, Hnat MD, Cunningham FG. Advanced extrauterine pregnancy: diagnostic and therapeutic challenges. Am J Obstet Gynecol. 2008;198:297, e1–7. doi: 10.1016/j.ajog.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 34.Cetinkaya MB, Kokcu A, Alper T. Follow up of the regression of the placenta left in situ in an advanced abdominal pregnancy using the Cavalieri method. J Obstet Gynaecol Res. 2005;31:22–26. doi: 10.1111/j.1447-0756.2005.00236.x. [DOI] [PubMed] [Google Scholar]
- 35.Oneko O, Petru E, Masenga G, Ulrich D, Obure J, Zeck W. Management of the placenta in advanced abdominal pregnancies at an East african tertiary referral center. J Womens Health. 2010;19:1369–1375. doi: 10.1089/jwh.2009.1704. [DOI] [PubMed] [Google Scholar]
- 36.Cardosi RJ, Nackley AC, Londono J, Hoffman MS. Embolization for advanced abdominal pregnancy with a retained placenta: A case report. J Reprod Med. 2002;47:861–863. [PubMed] [Google Scholar]
- 37.Valenzano M, Nicoletti L, Odicino F, Cocuccio S, Lorenzi P, Ragni N. Five-year follow-up of placental involution after abdominal pregnancy. J Clin Ultrasound. 2003;31:39–43. doi: 10.1002/jcu.10124. [DOI] [PubMed] [Google Scholar]
- 38.Steier JA, Sandvei R, Myking OM. Disappearance of human chorionic gonadotropin following removal of the fetus and placenta left in situ in a case of advanced abdominal pregnancy. Acta Obstet Gynecol Scand. 1987;66:729–731. doi: 10.3109/00016348709004153. [DOI] [PubMed] [Google Scholar]
- 39.Gomez E, Vergara L, Weber C, Wong AE, Sepulveda W. Successful expectant management of an abdominal pregnancy diagnosed at 14 weeks. J Matern Fetal Neonatal Med. 2008;21:917–920. doi: 10.1080/14767050802353556. [DOI] [PubMed] [Google Scholar]
- 40.Hall JM, Manning N, Moore NR, Tingey WR, Chamberlain P. Antenatal diagnosis of a late abdominal pregnancy using ultrasound and magnetic resonance imaging: a case report of successful outcome. Ultrasound Obst Gyn. 1996;7:289–292. doi: 10.1046/j.1469-0705.1996.07040289.x. [DOI] [PubMed] [Google Scholar]
- 41.Rahaman J, Berkowitz R, Mitty H, Gaddipati S, Brown B, Nezhat F. Minimally invasive management of an advanced abdominal pregnancy. Obstet Gynecol. 2004;103:1064–1068. doi: 10.1097/01.AOG.0000127946.14387.48. [DOI] [PubMed] [Google Scholar]


