Abstract
Background and aim: Ventricular assist devices (VAD) have become an established therapy for patients with end-stage heart failure. The two main reasons for this development are the shortage of appropriate donor organs and the increasing number of patients waiting for heart transplantation (HTX). Furthermore, the enormous advances in the technical equipment and the rising clinical experience have improved the implantation technique, the durability and the long-term patient outcomes. Methods: We reviewed all cases of left ventricular assist device (LVAD) implantation at our Erlangen Heart Center during January 2000-July 2013. The main aim of this study was to analyze the underlying pathology from the cardiac apex removed during the implantation. From all patients, we created a follow-up, analyzed the pathological features with the clinical diagnoses and described the overall outcome. Results: VAD implantation was performed in 266 cases at our center in the last 13 years (2.2% of the total of 12254 cardiac surgical operations in that period). From these patients, 223 underwent LVAD or biventricular (BVAD) implantation; the remaining received a right (RVAD) implantation. The most frequent underlying clinical diagnoses were dilated (n = 84, 37.7%, DCM) or ischemic (n = 61, 27.4%, ICM) cardiomyopathy. The pathological findings in the apex biopsy were generally non-specific and showed variable interstitial myocardial fibrosis with evidence of fibre loss, fatty degeneration and variable irregular atrophy of muscle fibres, consistent with dilated and ischemic cardiomyopathies as the most frequent causes of heart failure in these patients. Only a few cases showed other specific features such as myocarditis and AL-amyloidosis. Conclusions: Pathological findings in cardiac apex removed during LVAD implantation are rather non-specific and they generally reflect the late stage or consequences of chronic myocardial damage in cases of dilated or ischemic cardiomyopathies. Variable patchy chronic inflammatory changes may be observed in cardiomyopathies as a non-specific reaction caused by myocardial fiber damage and should not lead to misinterpretation as evidence of myocarditis or revision of original diagnosis.
Keywords: Left ventricular assist devices (LVAD), apex pathology, dilatative cardiomyopathy, ischemic cardiomyopathy, myocarditis
Introduction
A variety of acute and chronic myocardial diseases are well known to persist causing chronic heart disease that may ultimately result in end-stage heart failure. While heart transplantation (HTX) represents the ultimate option for cure of these patients who developed end-stage heart disease, left ventricular assist devices (LVAD) have emerged as an established therapy for patients with end-stage heart failure [1,2]. The two main reasons for this development are the shortage of appropriate donor organs on one hand and the increasing number of patients waiting for heart transplantation (HTX) on the other hand [3,4]. Furthermore, the enormous advances in the technical equipment and the rising clinical experience have improved the implantation technique, the durability and the long-term patient outcomes [5,6].
During implementation of LVAD, the apex of the heart is usually removed because the inflow cannula would insert in the ventricle through the apex. The blood flows through this cannula into the LVAD and from there through an outflow cannula to the patient’s aorta, i.e. the left ventricle relieved by this pump system. Although histopathological evaluation of cardiac apex in these circumstances might represent a useful adjunct for further assessment of prognosis and planning of treatment strategies for patients on an individual base, there are only very limited data on this aspect in the clinical and pathological cardiology literature [7,8]. To our knowledge, only two studies have analysed this in more details [9,10].
The aim of the current study was to evaluate and review the histopathological findings in cardiac apices removed during LVAD implantation in a series of 266 patients treated at our center during the last 13 years to correlate histopathological findings with clinical diagnoses.
Patients and methods
All patients who underwent VAD implantation from January 2000 to July 2013 at the Center for Cardiac Surgery, University Hospital of Erlangen, Germany, have been included in this retrospective analysis. These were 266 cases (2.2%) of all 12254 consecutive open heart procedures performed during the same period at our department (Figure 1). From these terminal heart failure patients 223 underwent LVAD or BVAD (biventricular) implantation, the remaining 43 patients’ received RVAD (right) implantation. From all LVAD and BVAD patients, we created a follow-up, reviewed and analyzed the pathological findings and correlated them with the clinical diagnoses and described the overall outcome.
Figure 1.
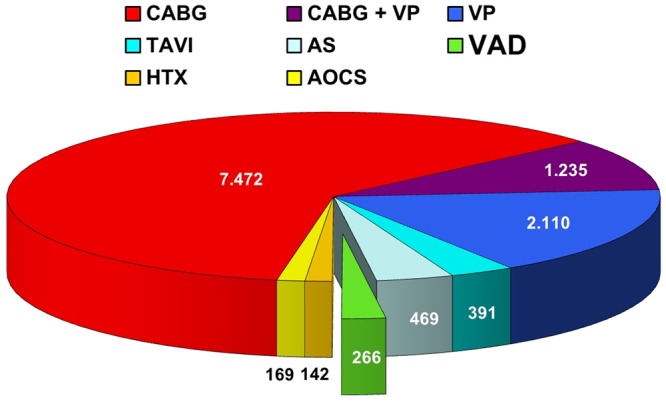
Cardiac Surgery at the University of Erlangen between January 2000 and July 2013. CABG = Coronary Artery Bypass Grafting; VP = Valve Procedures; TAVI = Transcatheter Aortic Valve Implantation; AS = Aortic Surgery; VAD = Ventricular Assist Device; HTX = Heart Transplantation; AOCS = Any Other Cardiac Surgery.
There are many different LVAD types used worldwide. Here in Erlangen we implanted 8 different LVAD during the above mentioned study period (Figure 2). The most important differentiation is the localization of the LVAD, i.e. either extracorporeal or intracorporeal in the pericardium. EXCOR® and MEDOS® are extracorporeal assist devices, whereas the remaining LVAD’s are intracorporeal systems (INCOR®, HeartWare®, Ventracor®, Total Artificial Heart® (TAH), Deltastream® and Novacor®).
Figure 2.
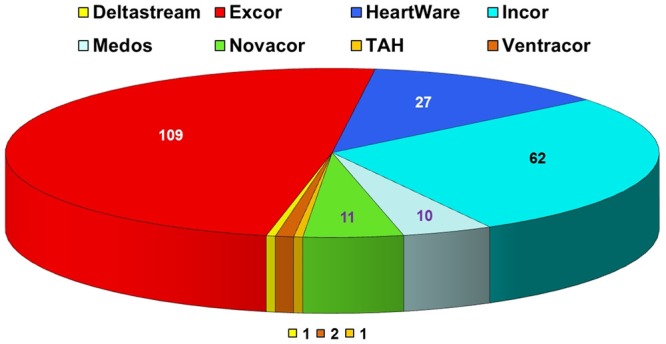
Distribution of the 223 implanted Left Ventricular Assist Devices (LVAD) at he University of Erlangen between January 2000 and July 2013. TAH = Total Artificial Heart.
The surgical technique for the implantation of the different LVAD types is similar and varies only in few particular features among the different companies. Figure 3 shows a typical implantation procedure. First, the apex of the impaired left ventricle will be removed and send to the pathology for further histological investigations (Figure 3A). Then, the inflow cannula would insert through this hole into the left ventricle and fixed via a felt ring suture (Figure 3B). Afterwards, the inflow cannula will be connected with the LVAD, and then connected with the outflow cannula, which inserts into the ascending aorta via a small felt ring suture (Figure 3C).
Figure 3.
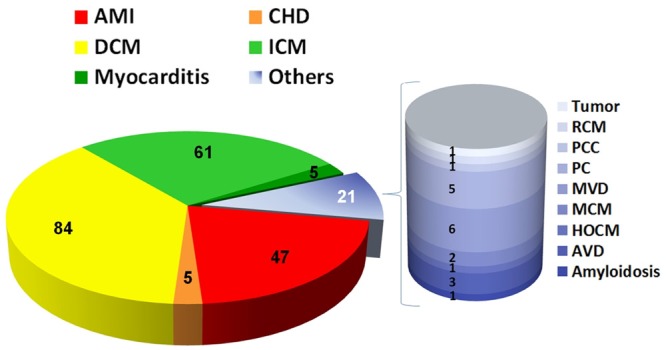
Distribution of the underlying reasons for the LVAD implantation. AMI = Acute Myocardial infarction; AVD = Aortic Valve Disease; CHD = Congenital Heart Defect; DCM = Dilative Cardiomyopathy; HOCM = Hypertrophic Obstructive Cardiomyopathy; ICM = Ischemic Cardiomyopathy; MCM = Metabolic Cardiomyopathy; MVD = Mitral Valve Disease; PC = Post-Surgery-Cardiomyopathy; PCC = Post-Chemotherapy Cardiomyopathy; RCM = Restrictive Cardiomyopathy.
Patient characteristics are summarized in Table 1. Notably, the majority of the 223 recovered patients were men (n = 191; 85.7%), whereas there was no gender specific significance concerning age, body mass index, EuroScore, creatinine or bilirubin values (Table 1). Furthermore, there were no significant differences between the underlying causes for terminal heart failure concerning the patient characteristics.
Table 1.
Patients characteristics
| Patient Characteristics | AMI (n = 47) | CHD (n = 5) | DCM (n = 84) | ICM (n = 61) | Myocarditis (n = 5) | Others (n = 21) |
|---|---|---|---|---|---|---|
| male/female | 39/8 | 3/2 | 70/14 | 56/5 | 2/3 | 12/9 |
| Age male (mean ± SD) | 59.9 ± 11.0 | 8.9 ± 14.6 | 52.9 ± 12.5 | 59.9 ± 8.7 | 7.7 ± 5.0 | 48.7 ± 16.3 |
| Age female (mean ± SD) | 55.6 ± 12.1 | 7.5 ± 3.2 | 31.4 ± 29.0 | 57.3 ± 18.1 | 41.2 ± 11.6 | 48.2 ± 21.5 |
| Body Mass Index (BMI) male (mean ± SD) | 27.6 ± 3.4 | 19.0 ± n.a. | 28.3 ± 5.6 | 27.6 ± 4.0 | 27.0 ± n.a. | 24.3 ± 3.6 |
| Body Mass Index (BMI) female (mean ± SD) | 28.8 ± 5.3 | 18.0 ± n.a. | 22.6 ± 4.9 | 26.8 ± 6.8 | 27.0 ± 3.6 | 24.2 ± 6.2 |
| EuroScore additive male (mean ± SD) | 28.8 ± 5.3 | 6.7 ± 2.9 | 9.1 ± 3.4 | 7.5 ± 3.2 | 5.0 ± 0.0 | 8.8 ± 3.4 |
| EuroScore additive female (mean ± SD) | 11.0 ± 3.9 | 4.5 ± 2.1 | 6.7 ± 2.5 | 12.6 ± 3.2 | 7.7 ± 1.5 | 9.4 ± 2.6 |
| Creatinine [mg/dl] male (mean ± SD) | 1.4 ± 0.7 | 0.6 ± 0.6 | 1.8 ± 0.9 | 1.3 ± 0.4 | 2.1 ± 2.4 | 1.2 ± 0.3 |
| Creatinine [mg/dl] female (mean ± SD) | 1.3 ± 0.9 | 0.6 ± n.a. | 0.9 ± 0.5 | 1.2 ± 0.4 | 1.7 ± 0.8 | 1.4 ± 0.8 |
| Bilirubin [mg/dl] male (mean ± SD) | 0.9 ± 0.4 | 0.6 ± n.a. | 2.7 ± 1.7 | 1.3 ± 0.8 | n.a. | 1.6 ± 1.3 |
| Bilirubin [mg/dl] female (mean ± SD) | 1.4 ± 1.4 | n.a. | 1.8 ± 1.8 | 1.2 ± 1.1 | 0.7 ± 0.4 | 1.9 ± 2.8 |
AMI = Acute Myocardial infarction; CHD = Congenital Heart Defect; DCM = Dilative Cardiomyopathy; ICM = Ischemic Cardiomyopathy.
All VAD-patients were routinely followed up at the heart failure and transplantation outpatient clinic, University Hospital Erlangen. After initial hospital stay for the implantation, patients were seen on a routine protocol for VAD justification and coagulation controls or in cases with clinically suspected VAD dysfunction. To these routine investigations belongs a transthoracic echocardiogram (TTE), a conventional chest x-ray in two planes and a complete blood screening as well as readout of the stored data of the VAD.
Results
General patient’s characteristics, clinical diagnoses and types of LVAD
The distribution of underlying reasons for terminal heart failure and subsequent LVAD implantation is demonstrated in Figure 4. Assist devices were performed in most of the cases due to dilated (n = 84, 37.7%, DCM) and ischemic (n = 61, 27.4%, ICM) cardiomyopathy. Further underlying causes for LVAD were acute myocardial infarction (n = 47, 21.1%, AMI), congenital heart defect (n = 5, 2.2%, CHD), myocarditis (n = 5, 2.2%) and other cardiac diseases (n = 21, 9.4%, others). The latter 21 cases are further detailed in Figure 4: amyloidosis (n = 1, 0.4%), aortic valve disease (n = 3, 1.3%), hypertrophic obstructive cardiomyopathy (n = 1, 0.4%), metabolic cardiomyopathy (n = 2, 0.9%), mitral valve disease (n = 6, 2.7%), post-surgery-cardiomyopathy (n = 5, 2.2%), post-chemotherapy cardiomyopathy (n = 1, 0.4%), restrictive cardiomyopathy (n = 1, 0.4%) and tumor (n = 1, 0.4%).
Figure 4.
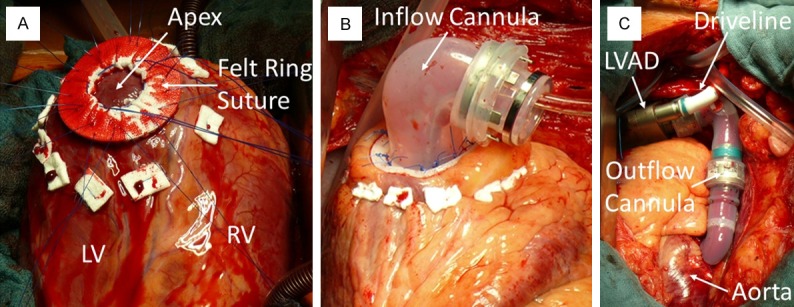
A: Intraoperative photograph showing the apex of the left ventricle, usually removed for the inflow cannula and then send for further histopathological examination. B: The inflow cannula was inserted into the left ventricle. C: This cannula was connected with the LVAD, then connected with the outflow cannula and inserted into the ascending aorta, finally.
Patient follow-up data is summarized in Table 2. There were no gender specific significant difference concerning the LVAD duration, the 30 days lethality, the one year lethality, the percentage of heart transplantations and the on-going LVAD percentage. Notably, the LVAD duration varied greatly between 0.5 and 1304 days, i.e. less than one day and more than 3 and half years. Additionally, there were no statistically significant differences between the follow-up characteristics and the underlying causes for the LVAD implantation (Table 2).
Table 2.
Follow-up during LVAD assistance Patients characteristics
| Follow-up | AMI (n = 47) | CHD (n = 5) | DCM (n = 84) | ICM (n = 61) | Myocarditis (n = 5) | Others (n = 21) |
|---|---|---|---|---|---|---|
| male/female | 39/8 | 3/2 | 70/14 | 56/5 | 2/3 | 12 / 9 |
| LVAD duration (days) male [mean (range)] | 160.6 (0.5-848) | 34.0 (8-84) | 193.0 (0.5-925) | 206.8 (0.5-1304) | 104.5 (85-124) | 125.2 (2-590) |
| LVAD duration (days) female [mean (range)] | 94.5 (1-252) | 15.0 (4-26) | 389.4 (3-1012) | 60.8 (2-258) | 134.3 (7-385) | 6.4 (0.5-20) |
| 30 days Lethality male (n/% of subgroup) | 21/53.8 | 2/66.7 | 25/35.7 | 24/42.9 | 0/0 | 9/75.0 |
| 30 days Lethality female (n/% of subgroup) | 3/37.5 | 2/100 | 3/21.4 | 3/60.0 | 2/66.7 | 9/100 |
| 1 year Lethality male (n/% of subgroup) | 26/66.7 | 3/100 | 38/54.3 | 32/57.1 | 0/0 | 9/75.0 |
| 1 year Lethality female (n/% of subgroup) | 6/75.0 | 2/100 | 4/28.6 | 4/80.0 | 2/66.7 | 9/100 |
| Heart transplantation male (n/% of subgroup) | 4/10.3 | 0/0 | 18/25.7 | 12/21.4 | 0/0 | 1/8.3 |
| Heart transplantation female (n/% of subgroup) | 0/0 | 0/0 | 3/21.4 | 0/0 | 1/33.3 | 0/0 |
| On-going LVAD male (n/% of subgroup) | 5/12.8 | 0/0 | 6/8.6 | 8/14.3 | 0/0 | 1/8.3 |
| On-going LVAD female (n/% of subgroup) | 1/12.5 | 0/0 | 4/28.6 | 1/20.0 | 0/0 | 0/0 |
AMI = Acute Myocardial infarction; CHD = Congenital Heart Defect; DCM = Dilative Cardiomyopathy; ICM = Ischemic Cardiomyopathy.
Histopathological findings
The pathological findings in the apex biopsy were generally non-specific. Variable interstitial myocardial fibrosis, either with reticular interstitial patterns or with prominent subendocardial and perivascular accentuation was seen in a majority of cases, irrespective of the underlying cause of cardiomyopathy (ischemic or dilated). A frequent finding common to most cases was the presence of variable fibre loss, occasionally associated with fatty degeneration and variable usually patchy mononuclear inflammatory infiltrates (Figure 5A-C). The cardiomyocytes showed prominent irregular hypertrophy of muscle fibres, consistent with late features of dilated and ischemic cardiomyopathies as the most frequent causes of heart failure in these patients. Only a few cases showed other specific features such as myocarditis and ALamyloidosis (Figure 6A-C). Mural thrombi were also seen in several cases representing secondary events. Cases with ischemic cardiomyopathy featured significant narrowing and transmural fibrosis of the apex thus reflecting aneurysmal dilatation of the apex as a consequence of old infarction scars.
Figure 5.
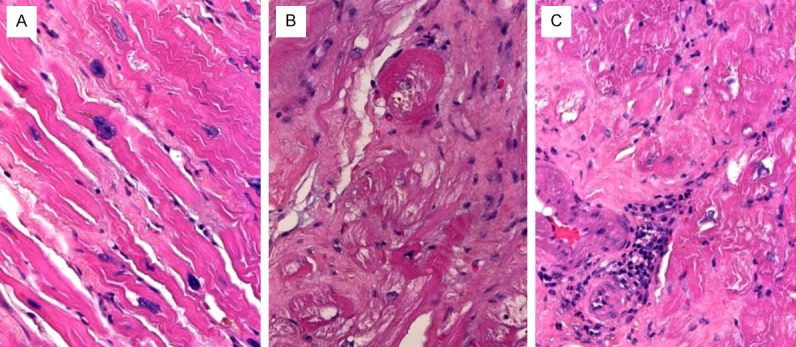
Example of apex pathology in dilated (A) and ischemic (B, C) cardiomyopathy showed variable interstitial fibrosis with occasional reactive mononuclear cell aggregates (C) and myofibre hypertrophy.
Figure 6.
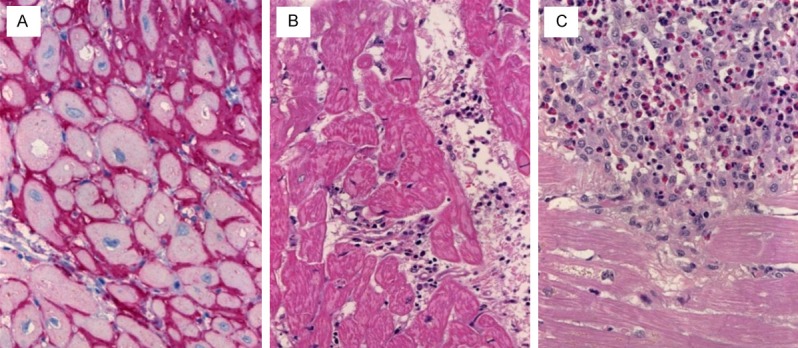
A: Case of cardiac failure due to AL-amyloidosis (immunostaining for light chain). B: A case of myocarditis showed increased mononuclear inflammatory cells between muscle fibres. C: severe eosinophilic myocarditis.
Discussion
In this study, we reviewed our experience with cardiac diseases underlying the indications for VAD implantation and also reviewed pathological findings reported in surgical biopsies of the cardiac apex removed during VAD implantation in patients receiving either left (LVAD) or biventricular (BVAD) devices. To our knowledge, data on the pathology of cardiac apex are sparse with only two previous studies dealing with this aspect in more details [9,10]. In most cases, studies with left ventricular mechanical support described the properties and complications of such assist devises either as bridge to transplantation, as definitive therapy or as a bridge to recovery [11-16].
Our results showed generally non-specific histopathological findings in the apex biopsy. Indeed, this is not unexpected as histopathological findings in dilated and ischemic cardiomyopathies as the most frequent indications for LVAD implantation are generally non-specific. Furthermore, the chronic nature of end-stage cardiac disease and the lengthy period prior to implantation significantly impact the histopathological findings, i.e. secondary and nonspecific histopathological features common to a variety of diseases having in common chronic myocardial fibre damage are seen in a majority of cases. Thus, histological picture is dominated by chronic fibre damage or fibre loss, associated with secondary compensatory myocardial hypertrophy which is usually disproportional and very irregular in distribution. These changes are accompanied by varying degree of interstitial fibrosis with frequent subendocardial and perivascular accentuation, all are common sequelae of chronic myocardial damage.
Cases with specific etiology were rather uncommon and constituted 21 cases only (9.4%). In some of these cases, the specific etiology can still be recognized in the apex biopsy such as in cases of amyloidosis. However, some cases with viral myocarditis may not feature specific findings based on the duration between the disease activity that caused cardiac failure and the time of implantation. Diagnosis in such cases was mainly clinical and/or on the basis of myocardial biopsy during the active stage of the disease. On the other hand, several cases of cardiomyopathies with extensive interstitial fibrosis showed reactive patchy mononuclear inflammatory infiltrates, probably as a consequence of active fibre loss due to ischemic or other pathogenetic mechanisms associated with the underlying cardiomyopathies. These findings might on occasion be mistaken for active myocarditis thus leading to the erroneous revision of the initial diagnosis. This finding should therefore be considered in all cases with end-stage heart disease, particularly in cases of ischemic cardiomyopathies. Correlation with clinical finings is thus mandatory to avoid erroneous revised diagnosis of an inflammatory disease.
Recent studies pointed out the remodeling capacity of cardiomyocytes of chronically failing hearts in response to VAD implantation [17,18]. At a translational level, these studies have compared the pre- and post-VAD cardiomyocytes with regard to functional contractility and gene expression profiling.
In conclusion, once being considered of no specific pathological interest, cardiac apices removed during LVAD transplantation would be of particular interest for future studies on the different molecular aspects comparing functional status and properties of end-stage failing and regenerating heart muscle fibres. Accordingly, it would be mandatory to cryopreserve parts of the apex biopsy for future molecular and translational studies.
Acknowledgements
We thank all colleagues at our heart failure and transplantation ambulance, University Hospital Erlangen, for their untiring assistance and supervision of all VAD patients and all heart transplant recipients. We acknowledge support by Deutsche Forschungsgemeinschaft and Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) within the funding program Open Access Publishing.
Disclosure of conflict of interest
None.
References
- 1.Rodriguez LE, Suarez EE, Loebe M, Bruckner BA. Ventricular assist devices (VAD) therapy: new technology, new hope? Methodist Debakey Cardiovasc J. 2013;9:32–7. doi: 10.14797/mdcj-9-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garbade J, Barten MJ, Bittner HB, Mohr FW. Heart transplantation and left ventricular assist device therapy: two comparable options in end-stage heart failure? Clin Cardiol. 2013;36:378–82. doi: 10.1002/clc.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moazami N, Hoercher KJ, Fukamachi K, Kobayashi M, Smedira NG, Massiello A, Horvath DJ. Mechanical circulatory support for heart failure: past, present and a look at the future. Expert Rev Med Devices. 2013;10:55–71. doi: 10.1586/erd.12.69. [DOI] [PubMed] [Google Scholar]
- 4.Moosdorf R. Artificial heart and heart transplantation. Herz. 2012;37:869–74. doi: 10.1007/s00059-012-3702-1. [DOI] [PubMed] [Google Scholar]
- 5.Carrel T, Englberger L, Martinelli MV, Takala J, Boesch C, Sigurdadottir V, Gygax E, Kadner A, Mohacsi P. Continuous flow left ventricular assist devices: a valid option for heart failure patients. Swiss Med Wkly. 2012;142:w13701. doi: 10.4414/smw.2012.13701. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez LE, Suarez EE, Loebe M, Bruckner BA. General surgery considerations in the era of mechanical circulatory assist devices. Surg Clin North Am. 2013;93:1343–57. doi: 10.1016/j.suc.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Drakos SG, Kfoury AG, Selzman CH, Verma DR, Nanas JN, Li DY, Stehlik J. Left ventricular assist device unloading effects on myocardial structure and function: current status of the field and call for action. Curr Opin Cardiol. 2011;26:245–55. doi: 10.1097/HCO.0b013e328345af13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruckner BA, Razeghi P, Stetson S, Thompson L, Lafuente J, Entman M, Loebe M, Noon G, Taegtmeyer H, Frazier OH, Youker K. Degree of cardiac fibrosis and hypertrophy at time of implantation predicts myocardial improvement during left ventricular assist device support. J Heart Lung Transplant. 2004;23:36–42. doi: 10.1016/s1053-2498(03)00103-7. [DOI] [PubMed] [Google Scholar]
- 9.Cazes A, Duong Van Huyen JP, Fornes P, Amrein C, Guillemain R, Grinda JM, Bruneval P. Mechanical ventricular assistance in heart failure: pathology of the cardiac apex removed during device implantation. Cardiovasc Pathol. 2010;19:112–6. doi: 10.1016/j.carpath.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Rose AG, Park SJ. Pathology in patients with ventricular assist devices: a study of 21 autopsies, 24 ventricular apical core biopsies and 24 explanted hearts. Cardiovasc Pathol. 2005;14:19–23. doi: 10.1016/j.carpath.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Sabashnikov A, Mohite PN, Zych B, Garcia D, Popov AF, Weymann A, Patil NP, Hards R, Capoccia M, Wahlers T, De Robertis F, Bahrami T, Amrani M, Banner NR, Simon AR. Outcomes and predictors of early mortality after continuous flow left ventricular assist device implantation as a bridge to transplantation. ASAIO J. 2014 doi: 10.1097/MAT.0000000000000035. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westaby S. Rotary blood pumps as definitive treatment for severe heart failure. Future Cardiol. 2013;9:199–213. doi: 10.2217/fca.12.89. [DOI] [PubMed] [Google Scholar]
- 13.Tsiouris A, Brewer RJ, Borgi J, Nemeh H, Paone G, Morgan JA. Continuous-flow left ventricular assist device implantation as a bridge to transplantation or destination therapy: racial disparities in outcomes. J Heart Lung Transplant. 2013;32:299–304. doi: 10.1016/j.healun.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Cowger J, Romano MA, Stulak J, Pagani FD, Aaronson KD. Left ventricular assist device management in patients chronically supported for advanced heart failure. Curr Opin Cardiol. 2011;26:149–54. doi: 10.1097/HCO.0b013e3283438258. [DOI] [PubMed] [Google Scholar]
- 15.Lahpor JR. State of the art: implantable ventricular assist devices. Curr Opin Organ Transplant. 2009;14:554–9. doi: 10.1097/MOT.0b013e3283303750. [DOI] [PubMed] [Google Scholar]
- 16.Kamdar F, Boyle A, Liao K, Colvin-adams M, Joyce L, John R. Effects of centrifugal, axial, and pulsatile left ventricular assist device support on end-organ function in heart failure patients. J Heart Lung Transplant. 2009;28:352–9. doi: 10.1016/j.healun.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Hall JL, Fermin DR, Birks EJ, Barton PJ, Slaughter M, Eckman P, Baba HA, Wohlschlaeger J, Miller LW. Clinical, molecular, and genomic changes in response to a left ventricular assist device. J Am Coll Cardiol. 2011;57:641–52. doi: 10.1016/j.jacc.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baba HA, Wohlschlaeger J. Morphological and molecular changes of the myocardium after left ventricular mechanical support. Curr Cardiol Rev. 2008;4:157–69. doi: 10.2174/157340308785160606. [DOI] [PMC free article] [PubMed] [Google Scholar]


