Abstract
Mucosa-associated lymphoid tissue (MALT) lymphoma is a relatively common, indolent B-cell lymphoma. MALT lymphoma with large tumor cells (LTCs) is believed to have the potential to transform to aggressive diffuse large B-cell lymphoma (DLBCL) which may have a poor prognosis. C-MYC is a transcription factor. Its translocation and overexpression predicts an inferior prognosis and poor response to therapy in cases of DLBCL. In the current study, C-MYC expression was detected in MALT lymphomas, and its relationship to the occurrence of LTCs, clinicopathological parameters and prognosis was assessed. A total of 69 cases were enrolled in the study, including 42 cases of MALT lymphoma without LTCs, 20 cases of MALT lymphoma with LTCs and 7 cases of DLBCL with a MALT lymphoma component (DLBCL+MALT). Immunohistochemistry and fluorescent in situ hybridization analyses were performed. In total, 15/42 (35.7%) cases were nuclear positive for C-MYC expression in the group without LTCs, whereas 15/20 (75.0%) and 4/7 (57.1%) cases were positive in the group with LTCs and in the group with DLBCL+MALT, respectively (P=0.004). Univariate and multivariate analysis were used to determine the correlations of C-MYC expression and clinicopathological parameters with overall survival (OS). C-MYC expression, Ann Arbor stage, LDH level and IPI were considerably associated with OS according to the univariate analysis. However, only C-MYC expression ≥20% showed a statistical significance in the multivariate analysis (HR=20.604, 95% CI: 1.909-222.412, P=0.013). Therefore, C-MYC overexpression may play an important role in aggressive transformation and is an independent prognostic factor in MALT lymphoma.
Keywords: C-MYC, MALT lymphoma, prognosis, immunohistochemistry, fluorescent in situ hybridization
Introduction
Extranodal marginal zone lymphoma of the mucosa-associated lymphoid tissue is a relatively common extranodal lymphoma, accounting for 7-8% of all B-cell lymphomas [1,2]. MALT is predominantly composed of morphologically heterogeneous small tumor cells admixed with variable numbers of transformed centroblast- and immunoblast-like cells [1]. Although MALT lymphomas have an indolent natural clinical course and are slow to disseminate, a small number of them evolve into aggressive DLBCLs [3-5], which have a poor prognosis. Three different subgroups of MALT were mentioned in the 2008 World Health Organization (WHO) classification of hematopoietic and lymphoid tissues tumors [1]: MALT lymphoma, MALT lymphoma with LTCs and DLBCL+MALT. However, in spite of the distinct histological features of each subgroup described by WHO, no definitive number of transformed large cells was included in order to make a diagnosis of MALT lymphoma with LTCs. Due to lack of quantitative diagnostic criterion, diagnosis of the subtype of MALT lymphoma is sometimes poorly reproducible in daily clinical practice, especially the cases with atypical morphologic features. In addition, differences in prognosis between MALT lymphoma with and MALT lymphoma without LTCs remain controversial [6,7].
Recurrent chromosomal abnormalities, including API2-MALT1, IGH-MALT1, IGH-BCL10 and IGH-FOXP1 translocation, are well known that they seem to be closely related to MALT lymphoma [8-12]. A few previous studies have discussed the current insights into the mechanisms underlying the transition from MALT lymphoma into aggressive DLBCL [4,6,7,13,14], as well as the molecular prognostic signatures of MALT lymphoma. Some studies have reported that FOXP1 may play a role in the aggressive transformation and that overexpression of FOXP1 predicted an inferior prognosis [6,7,15]. In addition, several studies performed global microRNA (miRNA) expression profiling of MALT lymphomas and reported that the MYC and NF-κB pathways were involved in dysregulation of miRNA expression in aggressive disease [16,17] and 20% of MALT lymphoma showed C-MYC overexpression [17], which may be caused by posttranscriptional regulation of miRNAs exerted by regulation of its target FOXP1.
C-MYC is a transcription factor [18]. It has been determined that C-MYC translocation predicts an inferior prognosis and poor response to therapy in cases of DLBCL [19-25]. Few studies found that C-MYC and its target genes were involved in the transformation of follicular lymphoma to DLBCL [26,27]. However, to the best of our knowledge, few studies have been performed [17], which have investigated the role of C-MYC in the transformation and prognostic assessment of MALT lymphoma.
In the current study, the expression of C-MYC was examined in a series of MALT lymphomas. The relationship between C-MYC expression and cellular components was analyzed and the relationship between these effects and prognosis in cases of MALT lymphomas were compared in order to determine a possible role of C-MYC in the prognostic evaluation of MALT lymphoma and/or the transition of MALT lymphoma to aggressive DLBCL.
Materials and methods
Patient selection and clinical information
A total of 69 MALT lymphomas samples including 42 MALT lymphoma without LTCs, 20 MALT lymphoma with LTCs and 7 DLBCL+MALT samples were collected at the Cancer Institute and Hospital, Chinese Academy of Medical Sciences (CICAMS) in Beijing, between May 2004 and January 2013. All samples were formalin-fixed paraffin embedded (FFPE) and reviewed to confirm for the diagnoses based on hematoxylin and eosin (H&E)-stained sections and immunohistochemical staining by two experienced pathologists. Other types of B-cell lymphomas, such as small lymphocytic lymphomas, follicular lymphomas and mantle cell lymphomas, were excluded. The clinicopathological parameters of these patients were recorded, including age at diagnosis, gender, primary site, Ann Arbor stage, the International Prognostic Index (IPI) and follow-up data. This study protocol was approved by the ethics committee of the Cancer Hospital, Chinese Academy of Medical Sciences. And the ethics document number was NCC2013RE-047.
IHC analysis
Immunohistochemical staining was performed on 4-μm-thick FFPE tissue sections. Briefly, the slides were deparaffinized and antigen retrieval was performed for 1.5 min in 1 mM EDTA (pH 8.0) (Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, China) using a pressure cooker. The slides were then incubated for 1 h in C-MYC rabbit monoclonal anti-human antibody (catalog #1472-1, Epitomics, Inc., Burlingame, CA, USA) plus SignalStain® antibody diluent, at a concentration of 1:100 (Cell Signaling Technology, Danvers, MA, USA). Slides were then incubated in universal secondary antibody (DAKO) for 15 min. Diaminobenzidine (DAB) was the chromogen used and slides were counterstained with hematoxylin before mounting.
The staining pattern for C-MYC was nuclear staining. The expression level of C-MYC was evaluated independently, by 2 ‘masked’ pathologists, for the proportion of positively staining tumor cells in, regardless of the staining intensity [7,17], and recorded in 10% increments. Grades the percentage of positive tumor cells as follows: <10%, 10%, 20%, 30%, 40%, 50% and >50% [28]. Grades <10% were defined as “negative expression” [17,29] and ≥10% were defined as “positive expression”. The diagnostic accordance rate between the two pathologists was 84.06% (58/69). Cases with different diagnoses were discussed by the two pathologists until agreement was reached.
FISH analysis
FISH was done on 3-μm-thick FFPE tumor tissue samples, using a break-apart probe specific to the C-MYC locus (Vysis LSI C-MYC Dual Color, Break Apart Rearrangement Probe; Abbott Molecular, Abbott Park, IL, USA), according to the manufacturer’s instructions. FISH signals were scored with a Zeiss AxioImager M2 epifluorescence microscope (Carl Zeiss, Oberkochen, Germany) equipped with ×100 oil immersion objectives and 4’, 6’-diamidino-2-phenylindole (DAPI)/Spectrum Green/Orange single and triple band pass filters. Tumor cells, which had nuclei with one or more FISH signals of each color, were enumerated. A positive cell was defined as one in which the nucleus had split signals (three or more signal diameters apart) [17,22,23].
Statistical analysis
Clinical pathological characteristics of different groups were compared using the Fisher’s exact test or chi-square test. The correlations between the various variables and overall survival (OS) were estimated using the Kaplan-Meier analysis and the survival distributions were compared using the log-rank test. Cox regression analysis was performed to determine independent prognostic factors. All statistical analyses were two-sided, and P values less than 0.05 were considered statistically significant. Data were analyzed using the SPSS 16.0 statistical software program (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
The demographic and clinical characteristics of all patients enrolled in this study were listed in Table 1. Patients ranged in age from 16 to 80 years (median 60), 23 to 77 years (median 52) and 36 to 78 years (median 59) in the MALT lymphoma without LTCs, MALT lymphoma with LTCs and DLBCL+MALT groups, respectively. There was a significant difference in the primary site between the MALT lymphoma without LTCs and the MALT lymphoma with LTCs groups (P=0.027). In the MALT lymphoma without LTCs group, 31/42 (73.8%) cases occurred primarily in the gastrointestinal tract, whereas in the MALT lymphoma with LTCs group only 9/20 (45%) cases had gastrointestinal tract involvement. No significant differences were found between the two groups with respect to age, gender, Ann Arbor stage and IPI (P>0.05).
Table 1.
Demographic, clinical characteristics and C-MYC expression of all patients
| Characteristics | MALT lymphoma without LTCs | MALT lymphoma with LTCs | DLBCL+MALT | P value |
|---|---|---|---|---|
| Total | 42 | 20 | 7 | |
| Age (years) | >0.05 | |||
| Mean age | 57 | 53 | 60 | |
| Median age | 60 | 52 | 59 | |
| Gender | 0.123 | |||
| Male | 24 | 6 | 4 | |
| Female | 18 | 14 | 3 | |
| Primary Site | 0.081 (0.027 a) | |||
| GI tract | 31 | 9 | 4 | |
| Stomach | 24 | 6 | 3 | |
| Intestine | 7 | 3 | 1 | |
| Non-GI tract | 11 | 11 | 3 | |
| Lung | 8 | 1 | 0 | |
| Oral cavity | 1 | 3 | 0 | |
| Thyroid | 0 | 4 | 1 | |
| Breast | 0 | 1 | 1 | |
| Tonsil | 0 | 2 | 0 | |
| Ocular adnexa | 1 | 0 | 0 | |
| Salivary gland | 1 | 0 | 1 | |
| Ann Arbor stageb | 0.083 | |||
| I | 26 | 7 | 3 | |
| II | 6 | 6 | 3 | |
| III | 1 | 0 | 1 | |
| IV | 3 | 4 | 0 | |
| IPIb | 0.281 | |||
| Low (0, 1) | 31 | 11 | 5 | |
| Intermediate (2) | 3 | 3 | 2 | |
| High (3, 4) | 2 | 3 | 0 | |
| LDHb | 0.515 | |||
| Normal | 30 | 12 | 5 | |
| High | 6 | 5 | 2 | |
| C-MYC expression | 0.004 a | |||
| Negative (<10%) | 27 | 5 | 3 | |
| Positive (≥10%) | 15 | 15 | 4 |
Significant differences are highlighted in bold.
The Ann Arbor Stage, IPI and LDH level of 9 patients were not known.
Morphology and immunohistochemistry
The H&E-stained sections were reviewed. 42 cases of MALT lymphoma without LTCs predominantly showed small B cells, including centrocyte-like cells, monocytoid cells and small lymphocyte-like cells. 20 cases of MALT lymphoma with LTCs displayed a variable number of transformed centroblast- or immunoblast-like large cells, which were scattered among the small tumor cells (Figure 1A and 1B). The DLBCL+MALT cases presented as solid or sheet-like proliferations of large tumor cells in part of the area of each of the 7 samples.
Figure 1.
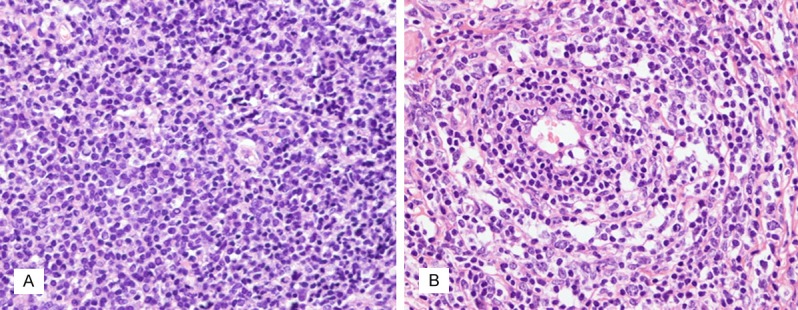
A. MALT lymphoma without large tumor cells (×400). B. MALT lymphoma with large tumor cells showing centroblast- or immunoblast-like large cells scattered among the small tumor cells (×400).
The expression of C-MYC was examined in all 69 cases (Table 1). C-MYC positive cells were predominantly large tumor cells with strong to moderate nuclear staining, which were prominent among a large amount of inflammatory cells. In addition to the large tumor cells, it is worthwhile to note that some of the small tumor cells also displayed variable intensities of C-MYC expression (Figure 2A).
Figure 2.
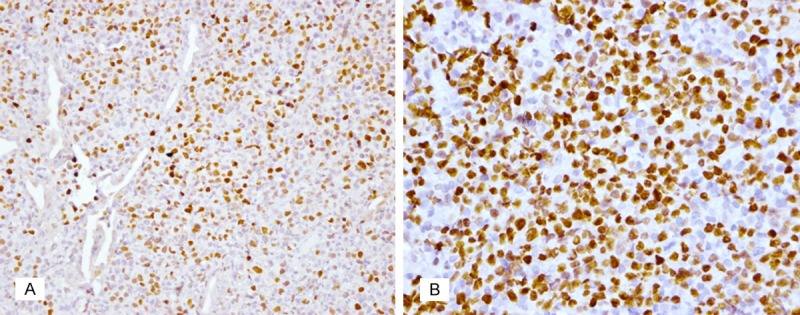
A. Staining shows that both large and small tumor cells were positive for C-MYC in MALT lymphoma (×400). B. In one of the DLBCL+MALT cases, 80% of tumor cells show strong nuclear staining for C-MYC (×200).
In the group without LTCs, 15/42 (35.7%) cases appeared to be nuclear positive for C-MYC vs. 15/20 (75.0%) cases in the group with LTCs (Figure 3). Thus, the expression of C-MYC was significantly higher in the MALT lymphoma with LTCs group compared to the group without LTCs (P=0.004). The percentage of samples with C-MYC expression was also higher in the DLBCL+MALT group compared to the MALT lymphoma without LTCs group (4/7, 57.1%). In fact, in one positive case, 80% of tumor cells exhibited nuclear staining in a partial area of the tumor (Figure 2B).
Figure 3.
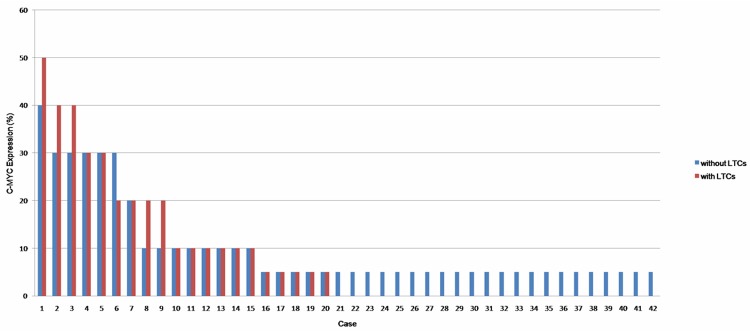
It shows the C-MYC expression of each case in all 62 MALT lymphomas.
There were no significant differences between the groups when comparing the relationship between the expression levels of C-MYC and any of the clinicopathological features, including age, gender, primary site, Ann Arbor stage, IPI and LDH level.
FISH analysis
None of the 62 cases of MALT lymphoma had C-MYC translocation. C-MYC translocation was found in the case of DLBCL+MALT that had positive C-MYC expression in 80% of tumor cells (Figure 4).
Figure 4.
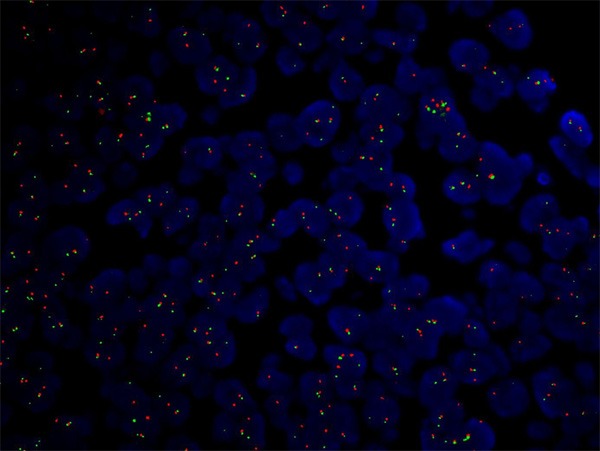
C-MYC translocation in a DLBCL+MALT case as shown by split fluorescent in situ hybridization (FISH) signals.
Prognostic analysis
Among the 62 MALT lymphoma cases, follow-up data was available for 49 patients (79%). Of these, follow- up was done in 87.8% (43/49) of patients for at least 3 years and in 38.8% (19/49) of patients for at least 5 years. A total of 9 patients died, 6 from lymphoma progression or recurrence; 5 of these occurred within 3 years of diagnosis. Lymphoma recurrence and dissemination occurred in 3 of the 40 living patients more than 3 years after the initial diagnosis. The mean OS time was 43.2 months (range of 1 to 84 months) and the overall 3-year and 5-year survival rates were 89.31% and 85.42%, respectively. According to the Kaplan-Meier analysis shown in Figure 5, OS was significantly associated with C-MYC expression, Ann Arbor stage, LDH level and IPI (Table 2), whereas age, gender, primary site and cellular composition were not associated with OS. The correlation between OS and C-MYC expression was even more obvious when looking at C-MYC expression values of ≥20%. However, only C-MYC expressions of ≥20% were found to be significant in the multivariate analysis (multivariate HR=20.604, 95% CI: 1.909-222.412, P=0.013) (Table 3).
Figure 5.
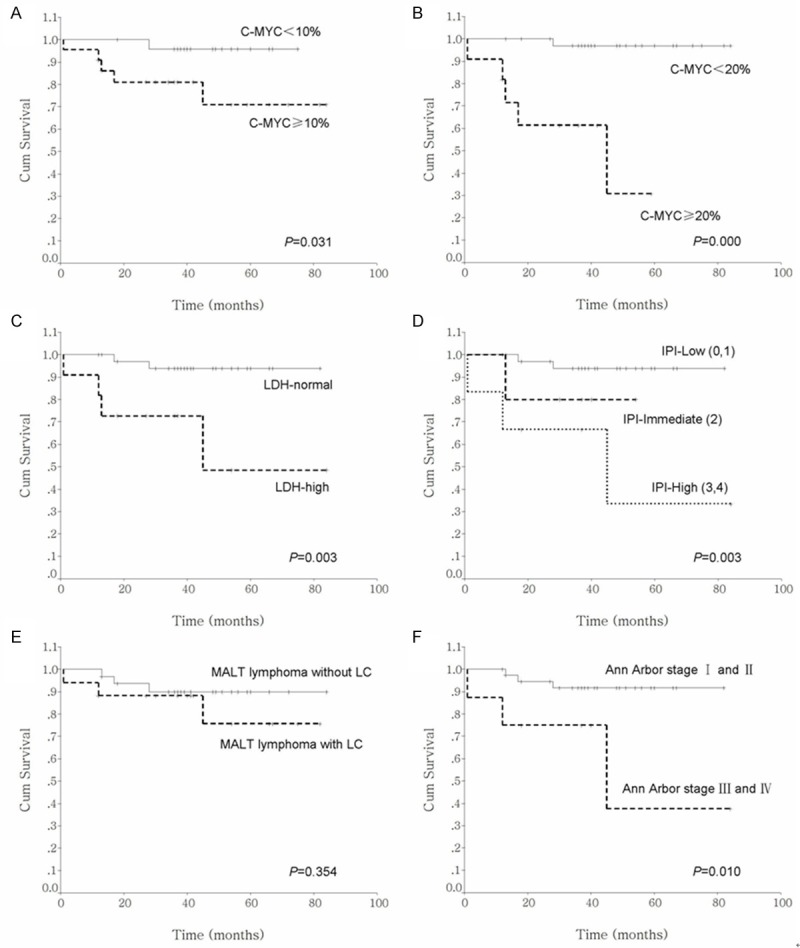
Kaplan-Meier survival curves with respect to C-MYC expression (A and B), LDH level (C), IPI (D), cellular composition (E) and Ann Arbor stage (F).
Table 2.
Variables associated with C-MYC expression, tumor cellular composition, primary site, clinicopathological parameters and OS by univariate analysis in 62 MALT lymphomas
| Variables | N | Log-rank test | P value |
|---|---|---|---|
| C-MYC expression ≥10% | 30 | 4.64 | 0.031 a |
| C-MYC expression ≥20% | 16 | 19.31 | 0.000 a |
| Gender | 2.12 | 0.145 | |
| Male | 30 | ||
| Female | 32 | ||
| Primary site | 0.02 | 0.902 | |
| GI tract | 40 | ||
| Non-GI tract | 22 | ||
| IPIb | 11.62 | 0.003 a | |
| Low (0, 1) | 42 | ||
| Intermediate (2) | 6 | ||
| High (3, 4) | 5 | ||
| LDHb | 8.78 | 0.003 a | |
| Normal | 42 | ||
| High | 11 | ||
| Ann Arbor stageb | 6.59 | 0.010 a | |
| I-II | 45 | ||
| III-IV | 8 | ||
| Cellular composition | 0.86 | 0.355 | |
| Without LTCs | 42 | ||
| With LTCs | 20 |
Significant differences are highlighted in bold.
The Ann Arbor Stage, IPI and LDH level of 9 patients were not known.
Table 3.
Correlation between C-MYC expression, clinicopathological parameters and OS by multivariate analysis in 62 MALT lymphomas
| Variables | Hazard ratio | 95% CI | P value | |
|---|---|---|---|---|
| (A) | C-MYC ≥10% | 4.351 | 0.468-40.431 | 0.196 |
| IPI | 1.824 | 0.138-24.169 | 0.648 | |
| LDH | 2.594 | 0.129-52.266 | 0.534 | |
| Ann Arbor stage | 0.968 | 0.033-28.073 | 0.985 | |
| (B) | C-MYC ≥20% | 20.604 | 1.909-222.412 | 0.013 a |
| IPI | 0.820 | 0.037-18.389 | 0.900 | |
| LDH | 7.169 | 0.244-210.341 | 0.253 | |
| Ann Arbor stage | 0.799 | 0.012-52.410 | 0.916 |
Significant differences are highlighted in bold.
One of the 7 DLBCL+MALT patients died approximately one year after diagnosis and one progressed to DLBCL after seven months.
Discussion
Although MALT lymphomas are generally considered to be indolent diseases, they can transform to DLBCL on rare occasions [1]. For this reason, patients diagnosed with MALT lymphoma with LTCs are usually considered to be at risk for developing DLBCL and should be treated with an aggressive therapy regimen similar to DLBCL [30-32], which are differ from the patients of MALT lymphoma without LTCs. However, the diagnostic criteria for MALT lymphomas with LTCs are not clear. Even the 2008 WHO classification of hematopoietic and lymphoid tissues tumors did not specify the number of the scattered large tumor cells necessary to make a definitive diagnosis of MALT lymphomas with LTCs. To some extent, the diagnosis of MALT lymphoma with LTCs may be subjective in clinical practice, especially for the cases lack of atypical morphological features. In addition, the differences in prognoses between MALT lymphoma patients with LTCs and without LTCs remain controversial [6,7,32,33]. In the current study, there was no significant difference in the prognosis between these two groups (P=0.355). We presumed that the inconsistent results of these studies may be attributed to the lack of definitive diagnostic criteria, leading to imprecise classification of the cases, as well as the relatively low number of the samples in the study. Therefore, objective molecular markers for assessing the prognosis in cases of MALT lymphoma are necessary.
Previous reports have shown that C-MYC deregulation in lymphoma was typically associated with aggressive clinical behavior [20,34] and that the overexpression of C-MYC or C-MYC translocation in DLBCL was predictive of inferior prognosis, poor response to therapy, and was an independent predictor of outcome [19,21,35-37]. In the current study, the expression of C-MYC in MALT lymphoma was determined and the role of C-MYC in the transformation of large tumor cells was investigated. A total of 30 out of 62 MALT lymphoma cases had C-MYC expression and the C-MYC positive cells were predominantly large tumor cells. C-MYC staining may help to identify large tumor cells in lymphomas with obviously inflammatory background. In the current study, the expression level of C-MYC in the MALT lymphoma with LTCs group was higher than in the group without LTCs (75.0% vs. 35.7%, P=0.004). In the DLBCL+MALT group, 4/7 (57.1%) cases were positive for C-MYC. From this, it could be perceived that the positive rate of C-MYC expression increased as well as the increase in large tumor cells. Therefore, we speculate that C-MYC might play a role in the transformation of large tumor cells in MALT lymphoma. Furthermore, it is worthwhile to note that some small tumor cells were also positive for C-MYC, which is similar to Jiang’s research [7]. We hypothesized that the C-MYC positive small tumor cells may precursors of the large tumor cells and that the two different sizes of C-MYC expressing tumor cells may be derived from the same clone.
Only one DLBCL+MALT case displayed C-MYC translocation, in which 80% of tumor cells showed nuclear expression of C-MYC. In all other cases, nuclear staining was present in less than 50% of tumors and no C-MYC aberrations were found. The concordance between C-MYC expression and C-MYC translocation was consistent with previous studies [24,38,39]. The cases of MALT lymphoma with LTCs were found to be significantly more likely to occur outside the gastrointestinal tract compared with cases of MALT lymphoma without LTCs. There was no significant difference between C-MYC expression and different clinicopathological parameters, including different primary sites, IPI, Ann Arbor stage and LDH level.
In the present study, C-MYC expression level was significantly associated with OS according to univariate analysis of prognostic factors. OS was inferior in cases with C-MYC expression in ≥10% of tumor cells compared to those with C-MYC expression in <10% of tumor cells (P=0.031). When the cut off value for C-MYC expression was set at 20%, the difference in OS between the two groups became more remarkable (P=0.000). In addition, we found that Ann Arbor stage III-IV, higher IPI score and elevated LDH level also appeared to have a significantly adverse impact on OS. However, when the variables that were significant according to the univariate analysis were analyzed using a multivariate analysis, only C-MYC expression ≥20% was statistically significant (multiva riate HR=20.604, 95% CI: 1.909-222.412, P=0.013). Therefore, an inferior prognosis may be indicated when ≥20% of tumor cells are positive for C-MYC in MALT lymphoma.
In conclusion, C-MYC staining may be contributed to recognize the large tumor cells, especially in an obviously inflammatory background. The positive rate of C-MYC expression was higher in the group of MALT lymphoma with LTCs and in the group of DLBCL+MALT than that in the group of MALT lymphoma without LTCs, which suggested that C-MYC would play an important role in aggressive transformation in MALT lymphoma. And according to the Cox regression analysis, C-MYC overexpression appeared to be an objective independent prognostic factor in MALT lymphoma.
However, it should be noted that the cohort of patients with outcome data was small in the current study and the results need to be further validated in larger patient cohorts.
Acknowledgements
This work was supported by Capital Medical Development Research Fund (2009-2008) and Beijing Hope Run special fund (LC2013B40).
Disclosure of conflict of interest
None.
References
- 1.Swerdlow SH. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) In: Isaacson PG, editor. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. Lyon: IARC; 2008. pp. 314–319. [Google Scholar]
- 2.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 3.Huang HC, Cheng AL, Lin CW, Kuo SH. Primary central nervous system diffuse large B cell lymphoma transformed from orbital mucosa-associated lymphoid tissue lymphoma: complete response to combined intrathecal and systemic rituximab. Ann Hematol. 2013;92:989–992. doi: 10.1007/s00277-012-1651-7. [DOI] [PubMed] [Google Scholar]
- 4.Flossbach L, Holzmann K, Mattfeldt T, Buck M, Lanz K, Held M, Moller P, Barth TF. High-resolution genomic profiling reveals clonal evolution and competition in gastrointestinal marginal zone B-cell lymphoma and its large cell variant. Int J Cancer. 2013;132:E116–127. doi: 10.1002/ijc.27774. [DOI] [PubMed] [Google Scholar]
- 5.Iwano M, Watanabe N, Matsushima Y, Seno H, Oki K, Sakurai T, Inagaki H, Okazaki K, Chiba T. Rapid development of diffuse large B-Cell lymphoma after successful eradication of Helicobacter pylori for gastric MALT lymphoma. Am J Gastroenterol. 2006;101:2878–2883. doi: 10.1111/j.1572-0241.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 6.Sagaert X, de Paepe P, Libbrecht L, Vanhentenrijk V, Verhoef G, Thomas J, Wlodarska I, De Wolf-Peeters C. Forkhead box protein P1 expression in mucosa-associated lymphoid tissue lymphomas predicts poor prognosis and transformation to diffuse large B-cell lymphoma. J. Clin. Oncol. 2006;24:2490–2497. doi: 10.1200/JCO.2006.05.6150. [DOI] [PubMed] [Google Scholar]
- 7.Jiang W, Li L, Tang Y, Zhang WY, Liu WP, Li GD. Expression of FOXP1 in mucosa-associated lymphoid tissue lymphoma suggests a large tumor cell transformation and predicts a poorer prognosis in the positive thyroid patients. Med Oncol. 2012;29:3352–3359. doi: 10.1007/s12032-012-0288-7. [DOI] [PubMed] [Google Scholar]
- 8.Du MQ. MALT lymphoma: many roads lead to nuclear factor-kappab activation. Histopathology. 2011;58:26–38. doi: 10.1111/j.1365-2559.2010.03699.x. [DOI] [PubMed] [Google Scholar]
- 9.Hamoudi RA, Appert A, Ye H, Ruskone-Fourmestraux A, Streubel B, Chott A, Raderer M, Gong L, Wlodarska I, De Wolf-Peeters C, MacLennan KA, de Leval L, Isaacson PG, Du MQ. Differential expression of NF-kappaB target genes in MALT lymphoma with and without chromosome translocation: insights into molecular mechanism. Leukemia. 2010;24:1487–1497. doi: 10.1038/leu.2010.118. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa M, Seto M, Hosokawa Y. Molecular pathogenesis of MALT lymphoma: two signaling pathways underlying the antiapoptotic effect of API2-MALT1 fusion protein. Leukemia. 2006;20:929–936. doi: 10.1038/sj.leu.2404192. [DOI] [PubMed] [Google Scholar]
- 11.Sagaert X, De Wolf-Peeters C, Noels H, Baens M. The pathogenesis of MALT lymphomas: where do we stand. Leukemia. 2007;21:389–396. doi: 10.1038/sj.leu.2404517. [DOI] [PubMed] [Google Scholar]
- 12.Streubel B, Lamprecht A, Dierlamm J, Cerroni L, Stolte M, Ott G, Raderer M, Chott A. T(14;18)(q32;q21) involving IGH and MALT1 is a frequent chromosomal aberration in MALT lymphoma. Blood. 2003;101:2335–2339. doi: 10.1182/blood-2002-09-2963. [DOI] [PubMed] [Google Scholar]
- 13.Deutsch AJ, Steinbauer E, Hofmann NA, Strunk D, Gerlza T, Beham-Schmid C, Schaider H, Neumeister P. Chemokine receptors in gastric MALT lymphoma: loss of CXCR4 and upregulation of CXCR7 is associated with progression to diffuse large B-cell lymphoma. Mod Pathol. 2013;26:182–194. doi: 10.1038/modpathol.2012.134. [DOI] [PubMed] [Google Scholar]
- 14.Kondo T, Oka T, Sato H, Shinnou Y, Washio K, Takano M, Morito T, Takata K, Ohara N, Ouchida M, Shimizu K, Yoshino T. Accumulation of aberrant CpG hypermethylation by Helicobacter pylori infection promotes development and progression of gastric MALT lymphoma. Int J Oncol. 2009;35:547–557. doi: 10.3892/ijo_00000366. [DOI] [PubMed] [Google Scholar]
- 15.He M, Gao L, Zhang S, Tao L, Wang J, Yang J, Zhu M. Prognostic significance of miR-34a and its target proteins of FOXP1, p53, and BCL2 in gastric MALT lymphoma and DLBCL. Gastric Cancer. 2013 doi: 10.1007/s10120-013-0313-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Hother C, Rasmussen PK, Joshi T, Reker D, Ralfkiaer U, Workman CT, Heegaard S, Ralfkiaer E, Gronbaek K. MicroRNA profiling in ocular adnexal lymphoma: a role for MYC and NFKB1 mediated dysregulation of microRNA expression in aggressive disease. Invest Ophthalmol Vis Sci. 2013;54:5169–5175. doi: 10.1167/iovs.13-12272. [DOI] [PubMed] [Google Scholar]
- 17.Craig VJ, Cogliatti SB, Imig J, Renner C, Neuenschwander S, Rehrauer H, Schlapbach R, Dirnhofer S, Tzankov A, Muller A. Myc-mediated repression of microRNA-34a promotes high-grade transformation of B-cell lymphoma by dysregulation of FoxP1. Blood. 2011;117:6227–6236. doi: 10.1182/blood-2010-10-312231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang WT, Lu N, Guo L. [Significance and application of c-myc in diffuse large B-cell lymphoma] . Zhonghua Bing Li Xue Za Zhi. 2013;42:638–640. [PubMed] [Google Scholar]
- 19.Nitsu N, Okamoto M, Miura I, Hirano M. Clinical significance of 8q24/c-MYC translocation in diffuse large B-cell lymphoma. Cancer Sci. 2009;100:233–237. doi: 10.1111/j.1349-7006.2008.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slack GW, Gascoyne RD. MYC and aggressive B-cell lymphomas. Adv Anat Pathol. 2011;18:219–228. doi: 10.1097/PAP.0b013e3182169948. [DOI] [PubMed] [Google Scholar]
- 21.Barrans S, Crouch S, Smith A, Turner K, Owen R, Patmore R, Roman E, Jack A. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J. Clin. Oncol. 2010;28:3360–3365. doi: 10.1200/JCO.2009.26.3947. [DOI] [PubMed] [Google Scholar]
- 22.Savage KJ, Johnson NA, Ben-Neriah S, Connors JM, Sehn LH, Farinha P, Horsman DE, Gascoyne RD. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009;114:3533–3537. doi: 10.1182/blood-2009-05-220095. [DOI] [PubMed] [Google Scholar]
- 23.Kluk MJ, Chapuy B, Sinha P, Roy A, Dal Cin P, Neuberg DS, Monti S, Pinkus GS, Shipp MA, Rodig SJ. Immunohistochemical detection of MYC-driven diffuse large B-cell lymphomas. PLoS One. 2012;7:e33813. doi: 10.1371/journal.pone.0033813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tapia G, Lopez R, Munoz-Marmol AM, Mate JL, Sanz C, Marginet R, Navarro JT, Ribera JM, Ariza A. Immunohistochemical detection of MYC protein correlates with MYC gene status in aggressive B cell lymphomas. Histopathology. 2011;59:672–678. doi: 10.1111/j.1365-2559.2011.03978.x. [DOI] [PubMed] [Google Scholar]
- 25.Tzankov A, Xu-Monette ZY, Gerhard M, Visco C, Dirnhofer S, Gisin N, Dybkaer K, Orazi A, Bhagat G, Richards KL, Hsi ED, Choi WW, van Krieken JH, Ponzoni M, Ferreri AJ, Ye Q, Winter JN, Farnen JP, Piris MA, Moller MB, You MJ, McDonnell T, Medeiros LJ, Young KH. Rearrangements of MYC gene facilitate risk stratification in diffuse large B-cell lymphoma patients treated with rituximab-CHOP. Mod Pathol. 2014;27:958–971. doi: 10.1038/modpathol.2013.214. [DOI] [PubMed] [Google Scholar]
- 26.Gentles AJ, Alizadeh AA, Lee SI, Myklebust JH, Shachaf CM, Shahbaba B, Levy R, Koller D, Plevritis SK. A pluripotency signature predicts histologic transformation and influences survival in follicular lymphoma patients. Blood. 2009;114:3158–3166. doi: 10.1182/blood-2009-02-202465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu X, Zhang L, Wang Y, Zhang Q, Zhang L, Sun B, Zhang Y. Double-hit and triple-hit lymphomas arising from follicular lymphoma following acquisition of MYC: report of two cases and literature review. Int J Clin Exp Pathol. 2013;6:788–794. [PMC free article] [PubMed] [Google Scholar]
- 28.Green TM, Young KH, Visco C, Xu-Monette ZY, Orazi A, Go RS, Nielsen O, Gadeberg OV, Mourits-Andersen T, Frederiksen M, Pedersen LM, Moller MB. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J. Clin. Oncol. 2012;30:3460–3467. doi: 10.1200/JCO.2011.41.4342. [DOI] [PubMed] [Google Scholar]
- 29.Gupta M, Maurer MJ, Wellik LE, Law ME, Han JJ, Ozsan N, Micallef IN, Dogan A, Witzig TE. Expression of Myc, but not pSTAT3, is an adverse prognostic factor for diffuse large B-cell lymphoma treated with epratuzumab/R-CHOP. Blood. 2012;120:4400–4406. doi: 10.1182/blood-2012-05-428466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aviles A, Neri N, Nambo MJ, Huerta-Guzman J, Cleto S. Surgery and chemotherapy versus chemotherapy as treatment of high-grade MALT gastric lymphoma. Med Oncol. 2006;23:295–300. doi: 10.1385/MO:23:2:295. [DOI] [PubMed] [Google Scholar]
- 31.Montalban C, Norman F. Treatment of gastric mucosa-associated lymphoid tissue lymphoma: Helicobacter pylori eradication and beyond. Expert Rev Anticancer Ther. 2006;6:361–371. doi: 10.1586/14737140.6.3.361. [DOI] [PubMed] [Google Scholar]
- 32.Skacel M, Ross CW, Hsi ED. A reassessment of primary thyroid lymphoma: high-grade MALT-type lymphoma as a distinct subtype of diffuse large B-cell lymphoma. Histopathology. 2000;37:10–18. doi: 10.1046/j.1365-2559.2000.00941.x. [DOI] [PubMed] [Google Scholar]
- 33.Ang MK, Hee SW, Quek R, Yap SP, Loong S, Tan L, Tao M, Lim ST. Presence of a high-grade component in gastric mucosa-associated lymphoid tissue (MALT) lymphoma is not associated with an adverse prognosis. Ann Hematol. 2009;88:417–424. doi: 10.1007/s00277-008-0604-7. [DOI] [PubMed] [Google Scholar]
- 34.Zhao XF, Hassan A, Perry A, Ning Y, Stass SA, Dehner LP. C-MYC rearrangements are frequent in aggressive mature B-Cell lymphoma with atypical morphology. Int J Clin Exp Pathol. 2008;1:65–74. [PMC free article] [PubMed] [Google Scholar]
- 35.Savage KJ, Johnson NA, Ben-Neriah S, Connors JM, Sehn LH, Farinha P, Horsman DE, Gascoyne RD. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009;114:3533–3537. doi: 10.1182/blood-2009-05-220095. [DOI] [PubMed] [Google Scholar]
- 36.Zhou K, Xu D, Cao Y, Wang J, Yang Y, Huang M. C-MYC aberrations as prognostic factors in diffuse large B-cell lymphoma: a meta-analysis of epidemiological studies. PLoS One. 2014;9:e95020. doi: 10.1371/journal.pone.0095020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valera A, Lopez-Guillermo A, Cardesa-Salzmann T, Climent F, Gonzalez-Barca E, Mercadal S, Espinosa I, Novelli S, Briones J, Mate JL, Salamero O, Sancho JM, Arenillas L, Serrano S, Erill N, Martinez D, Castillo P, Rovira J, Martinez A, Campo E, Colomo L. MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Haematologica. 2013;98:1554–1562. doi: 10.3324/haematol.2013.086173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green TM, Nielsen O, de Stricker K, Xu-Monette ZY, Young KH, Moller MB. High levels of nuclear MYC protein predict the presence of MYC rearrangement in diffuse large B-cell lymphoma. Am J Surg Pathol. 2012;36:612–619. doi: 10.1097/PAS.0b013e318244e2ba. [DOI] [PubMed] [Google Scholar]
- 39.Ruzinova MB, Caron T, Rodig SJ. Altered subcellular localization of c-Myc protein identifies aggressive B-cell lymphomas harboring a c-MYC translocation. Am J Surg Pathol. 2010;34:882–891. doi: 10.1097/PAS.0b013e3181db83af. [DOI] [PubMed] [Google Scholar]


