Abstract
Insulin-like growth factor binding protein-1 (IGFBP-1) plays an important role in the development and progression of cancer. However, the expression of IGFBP-1 remains equivocal, and little is known about its clinicopathological significance and prognostic value in hepatocellular carcinoma (HCC). In this study, we evaluated the expression of IGFBP-1 in 90 paired HCC tissues and adjacent non-cancerous liver tissues and analyzed its clinical and prognostic significance. The results showed that IGFBP-1 was detected in cytoplasm as well as cell nucleus, and down-regulated in HCC tissues compared to the adjacent non-cancerous liver tissues. The decreased expression of IGFBP-1 was correlated with tumor differentiation, liver cirrhosis, microvascular invasion or metastasis, TNM stage and poor survival. Moreover, low levels of IGFBP-1 may be an independent prognostic indicator for the survival of patients with HCC. We also evaluated its function by adding recombinant IGFBP-1 to the cultured HCC cell lines HepG2 and MHCC97-H. The result of the invasion chamber assay showed that IGFBP-1 could inhibit the invasion of HepG2 and MHCC97-H. MMP-9 secretion by these cells was significantly decreased when the cells were treated with IGFBP-1. Our results suggest that IGFBP-1 inhibits the invasion and metastasis of HCC cells and that IGFBP-1 may be useful as a valuable marker for the prognosis of patients with HCC.
Keywords: IGFBP-1, hepatocellular carcinoma, poor prognosis, invasion, MMP-9, tumor suppressor gene
Introduction
Hepatocellular carcinoma (HCC) has become the second leading cause of cancer-related deaths, and the incidence continues to rise worldwide [1,2]. Globally, approximately 750,000 new cases of liver cancer are reported each year. Population-based studies show that the incidence rate continues to parallel the death rate, which indicates that most individuals who develop HCC die from this disease [1]. Despite the advances in the surveillance of high-risk patients, surgical intervention for patients with early-stage disease and chemotherapy for advanced patients, the overall outcome of patients with HCC remains poor. It is therefore necessary to find more effective treatment strategies and to further investigate the detailed mechanisms of this deadly disease.
Insulin-like growth factor binding protein-1 (IGFBP-1) is one of the six soluble binding proteins which can affect the bioactivities of insulin-like growth factors (IGFs) through binding IGFs with high affinity. IGFBP-1, as well as IGFBP-3, -4 and -6 usually impair the access of IGFs to the IGF-receptor (IGF-R), and therefore, they diminish the effects if IGFs on target cells, including cancer cells [3]. Other IGFBPs such as IGFBP-2 and -5 seem to promote the bioavailability of IGF ligands [3]. IGFBP-1, which is produced primarily by hepatocytes and is secreted into the serum, is also synthesized by the kidney and ovarian granulosa cells and, in pregnant women, by decidualized uterine endometrium [4]. Serum IGFBP-1 modulates cell growth, differentiation and metabolism in an IGF-dependent manner. In addition, IGFBP-1 functions in IGF-independent effects on proliferation, migration and apoptosis of different cell types through its interaction with cell surface molecules [5]. It is therefore conceivable that the biological activity of IGFBP-1 is more complicated than we previously presumed.
Recently, the IGF axis has emerged as a significant pathway in the development and progression of HCC and as a hopeful therapeutic target [6]. Due to their central role in the regulation of bio-available IGFs, the IGFBPs have also emerged as potential mediators of liver cancer. Although many studies have documented the role of IGFBP-1 in liver regeneration [7,8], nonalcoholic steatohepatitis (NASH) [9], liver cirrhosis [10] and stress-related events [11-15], the expression of this binding protein in HCC remains elusive and even controversial. For example, Kondoh et al. [16] reported that IGFBP-1 mRNA was elevated in 4 HCC samples compared with non-cancerous normal tissue samples. Additionally, cultured human hepatoma cells were shown to over-express IGFBP-1 protein upon the administration of cytokines or oxidative stress [14]. However, Gong et al. reported that IGFBP-1 mRNA expression was uniformly and significantly downregulated in patients with HCC compared to adjacent cirrhotic tissues as well as to normal liver tissues [17]. As a significant plasma biomarker, the serum level of IGFBP-1 in patients with HCC has also been explored. Hwang et al. [10] found a significantly higher level of serum IGFBP-1 in patients with HCC than in patients with cirrhosis and in normal controls. However, studies that used an IGF signaling antibody array also showed no differences in the level of IGFBP-1 protein in HCC tissues compared to adjacent unaffected tissues [18].
By up-regulating IGFBP-1 expression in transgenic mice, Lu et al. [19] observed an inhibitory effect of IGFBP-1 on hepatic preneoplasia possibly through a decrease in the mitogenic activity of IGF-1 and/or IGF-2. This supports the concept that IGFBP-1 inhibits the carcinogenesis of HCC. Recently, in a phase II study of cixutumumab, a monoclonal antibody that targets IGF1-R in advanced HCC, researchers found that elevated plasma IGFBP-1 was correlated with improved progression-free survival (PFS) and overall survival (OS) in 24 patients [20]. However, no direct evidence has demonstrated the clinicopathological significance and prognostic value of IGFBP-1.
Our previous studies demonstrated that the down-regulation of MMP-9 could suppress the invasion capability of HCC cells in HCC [21]. Leu JI et al. reported that IGFBP-1 may inhibit the expression of MMP-9 [22]. Whether IGFBP-1 participates in the invasion of HCC cells via the regulation of MMP-9 remains unknown. In the present study, we used immunohistochemistry (IHC) to examine the expression of IGFBP-1 in 90 HCC samples and in adjacent non-tumor tissues. We also analyzed the clinical and prognostic significance of IGFBP-1. Moreover, we assayed its function in the HCC cell lines, HepG2 and MHCC97-H in vitro.
Materials and methods
Patients and tissue specimens
Fifteen adult patients with hepatic haemangioma and a total of 90 adult patients with HCC who underwent curative resection at the Department of Hepatobiliary Surgery, Xijing Hospital, Fourth Military Medical University (Xi’an, China) between 2006 and 2010 were included in this study. All enrolled patients with HCC met the diagnostic criteria of the American Association for the Study of Liver Diseases. None of these patients had received chemotherapy, ethanol injections, radiofrequency ablation, or transarterial chemoembolization prior to surgical resection. Several related clinical and pathological characteristics (including age, gender, tumor size, tumor differentiation, hepatitis B virus infection, liver cirrhosis, portal vein invasion, lymph node metastasis, and TNM stage) were collected for further analysis. The study protocol was approved by the Ethics Committee of Xijing Hospital, and written informed consent was obtained from each patient or from his/her legal guardians.
Construction of tissue microarrays and immunohistochemistry
The total of 15 paraffin-embedded normal liver tissues, 90 HCC samples and their corresponding adjacent liver tissues were used for the construction of a tissue microarray (Shanghai Outdo Biochip Co., LTD. Shanghai, China). The tissue microarray was used to detect IGFBP-1 expression by IHC. Briefly, the slide was routinely deparaffinized and hydrated in consecutive changes of xylene and ethanol, then heated to a boil in 10 mM sodium citrate antigen retrieval buffer (pH 6.0) and maintained at a sub-boiling temperature for 10 minutes. After the inactivation of endogenous peroxidase by incubating the section in 3% hydrogen peroxide for 10 min, 5% normal goat serum was used to block the section for 1 h at room temperature. The tissue section was incubated at 4°C overnight with an IGFBP-1 polyclonal antibody (Abcam USA, ab111203) at a 1:1000 dilution. After washing with PBS, the slide was incubated with a horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody (ZSGB-BIO, Beijing, China) for 30 minutes at room temperature. Then, the slide was washed and treated with 3, 3’-diamino-benzedine (DAB) for approximately 5 minutes. Hematoxylin was used to counterstain the section. Finally, the slide was consecutively dehydrated in ethanol and xylene, mounted with a coverslip and examined by microscopy. As a negative control, PBS was used in place of the primary IGFBP-1 antibody under the same experimental conditions.
The IHC results were analyzed by two independent experienced pathologists who were not informed of the distribution of the tissue microarray and who were not given the clinical information of the patients. The evaluation of IGFBP-1 staining was performed according to a previously described method [23]. The percentage of positive cells (staining area) was scored as follows: 0, <5%; 1, 5-25%; 2, 25-50%; 3, >50%. The staining intensity was scored as: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. The IGFBP-1 immunostaining score was calculated by multiplying the staining area score by the staining intensity score, and thus ranged from 0 to 9. High IGFBP-1 expression was defined as a total score of ≥4, while low IGFBP-1 expression level was defined as a total score of <4.
Follow-up study
The hepatectomy surgeries were performed between August 2006 and September 2010. The patients were followed-up for 3-7 years. All the follow-up data were summarized at the end of September 2013, and it was found that the patients had a median survival time of 14 months (range 1-80 months). Patients were reexamined every 4-5 months after hepatic resection. All follow-up examinations were performed by full-time staff who were unaware of the study. Overall survival (OS) was considered the primary endpoint and was calculated from the date of hepatic resection to the date of death or the last follow-up.
Cell culture
The human liver non-tumor cell line HL-7702 (obtained from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences), the HCC cell lines HuH-7, HepG2, and SMMC-7721 (obtained from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences) and MHCC97-H (obtained from the Liver Cancer Institute of Fudan University), were cultured at 37°C in 5% CO2 in DMEM supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA).
Invasion assays
Cell invasion was analyzed in Matrigel-coated transwell cell culture chambers (8 μm pore size) (Millipore). Briefly, IGFBP-1-treated cells and non-treated controls (5 × 104 cells/well) were serum-starved for 24 h and plated in the upper insert of a 24-well chamber in a serum-free medium. After 24 h incubation, non-invasive cells on the upper surface of the filters were removed gently by a cotton-tip swab, and the invading cells present on the lower membrane surface were fixed with methanol for 5 mins, stained with 0.1% crystal violet for 30 mins, and then photographed under an inverted microscope (OLYMPUS, Tokyo, Japan). The invading cells were counted at × 200 magnification from 5 random fields per filter. With respect to the treatment of the cells with human recombinant IGFBP-1, the cells were pretreated for 2-4 h, and the treatment continued during the invasion experiment. Each experiment was performed in triplicate wells and was repeated at least three times.
Western blot analysis
Equal amounts of protein were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 1% BSA for 2 h at room temperature and incubated with primary MMP-9 antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and with primary β-actin antibody (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA, US) at 4°C overnight, followed by horseradish peroxidase (HRP)-conjugated goat anti-mouse (1:10000; Santa Cruz Biotechnology) or goat anti-rabbit (1:10000; Santa Cruz Biotechnology) secondary antibody, respectively. Protein expression levels were quantified using the software Quantity One to detect the intensity of the protein bands.
Real-time PCR analysis for mRNA expression
Total RNA from different cells was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Real-time quantitative PCR was conducted in an Mx4000 Multiplex QPCR System (Stratagene, La Jolla, CA) using 2X SYBR-Green PCR Master mix (Applied Biosystems) and Premix Ex Taq reagent (TaKaRa). The cycling program was as follows: 94°C for 5 min and 40 cycles of 94°C for 15 s, 56°C for 20 s, 72°C for 20 s, and a final step of 10 min at 72°C. The primers used in the PCR reaction were: IGFBP-1, forward primer (5’-AGGCGGA CATTCTGGAAATG-3’) and reverse primer (5’-TCGTTCA TGCACTCGCTGA-3’); β-actin, forward primer (5’-ACCGCAT CAACAGCAGCATT-3’) and reverse primer (5’-AGGCTTT GCTGTGCTTCAGGT-3’). Data were analyzed according to the comparative Ct method and were normalized by β-actin expression in each sample (relative quantity, 2-ΔΔCt).
Statistical analysis
Statistical analyses were conducted with SPSS statistical software (version 13.0). The χ2 test was used to analyze the IGFBP-1 expression levels in different groups and its relationship with the clinicopathological characteristics of the patients. Survival curves were calculated by the Kaplan-Meier method and were analyzed by the Log-rank test. The Cox proportional hazards model was used to estimate the significance of various variables for survival in the multivariate analysis. P<0.05 was considered statistically significant.
Results
Expression of IGFBP-1 in HCC by IHC
To explore the IGFBP-1 protein level in HCC tissues, IHC was performed to assess its expression in a tissue microarray of 15 normal liver tissues, 90 paired HCC and adjacent non-cancerous liver tissue samples. IGFBP-1 was expressed mainly in the cytoplasm as well as in the nuclear of liver cells and liver cancer cells, but not in inflammatory cells or stromal cells. High expression of IGFBP-1 was observed in 100% (15/15) of the normal liver tissues and in 96.7% (87/90) of the adjacent non-cancerous liver tissues. While 36.7% (33/90) of the HCC samples demonstrated low IGFBP-1 expression (Figure 1). As shown in Table 1, the difference in IGFBP-1 expression that was observed between the HCC samples and the adjacent non-cancerous liver tissue was statistically significant (P<0.05).
Figure 1.
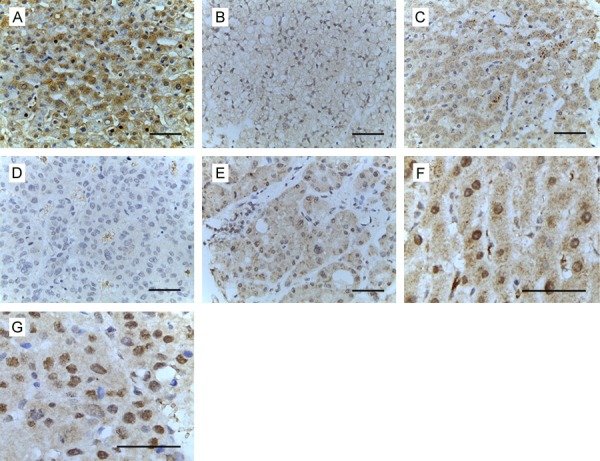
Expression analysis of IGFBP-1 in HCC by immunohistochemistry (A-G). (A) Expression of IGFBP-1 in normal liver. (B) Low expression of IGFBP-1 in adjacent non-tumorous tissue. (C) High expression of IGFBP-1 in adjacent non-tumorous tissue. (D) Low expression of IGFBP-1 in HCC. (E) High expression of IGFBP-1 in HCC. (F) Nuclear expression of IGFBP-1 in adjacent non-tumorous tissue. (G) Nuclear expression of IGFBP-1 in HCC. Bar, 50 μm.
Table 1.
Expression of IGFBP-1 in HCC by IHC
| IGFBP-1 expression | HCC tissues (n = 90) | Adjacent liver tissues (n = 90) | P value |
|---|---|---|---|
| High expression | 57 | 86 | <0.001 |
| Low expression | 33 | 4 |
Correlation between IGFBP-1 expression and the clinicopathological features
To further evaluate the clinical relevance of the decreased expression of IGFBP-1 in the HCC samples, we compared the IGFBP-1 expression with the clinicopathological features of the patients. We found that decreased expression of IGFBP-1 was correlated with tumor differentiation, liver cirrhosis, microvascular invasion or metastasis, and TNM stage, but there was no significance with respect to gender, age, tumor number, tumor size, HBV infection and serum AFP level (Table 2).
Table 2.
Correlation between IGFBP-1 expression and the clinicopathological features of the patients
| Clinicopathological Variables | IGFBP1 expression | P value | |
|---|---|---|---|
|
| |||
| High expression | Low expression | ||
| Gender | |||
| Male | 48 | 29 | >0.05 |
| Female | 9 | 4 | |
| Age | |||
| <45 | 25 | 12 | >0.05 |
| ≥45 | 32 | 21 | |
| Tumor number | |||
| Single | 49 | 23 | >0.05 |
| Multiple | 8 | 10 | |
| Maximal tumor size | |||
| ≤5 cm | 27 | 11 | >0.05 |
| >5 cm | 30 | 22 | |
| Tumor differentiation | |||
| I-II | 39 | 15 | <0.05 |
| III-IV | 18 | 18 | |
| Liver cirrhosis | |||
| No | 30 | 25 | <0.05 |
| Yes | 27 | 8 | |
| Serum AFP | |||
| ≤400 (ng/ml) | 16 | 8 | >0.05 |
| >400 (ng/ml) | 41 | 25 | |
| HBsAg status | >0.05 | ||
| Negative | 8 | 5 | |
| Positive | 49 | 28 | |
| Microvascular invasion or metastasis | |||
| No | 53 | 24 | <0.05 |
| Yes | 4 | 9 | |
| TNM stage | |||
| I-II | 36 | 6 | <0.001 |
| III | 21 | 27 | |
Survival analysis
We used Kaplan-Meier survival curves to evaluate the over-all survival rates of the patients with HCC according to their levels of IGFBP-1 expression. As shown in Figure 2, patients in the low IGFBP-1 group showed reduced overall survival rates compared with patients in the high IGFBP-1 group (Log rank = 6.072, P = 0.014).
Figure 2.
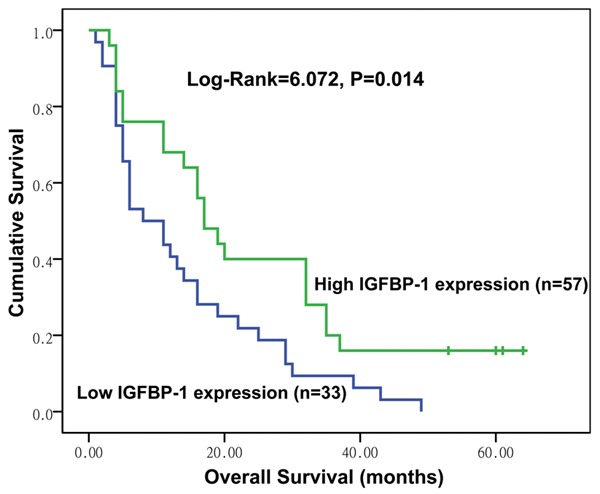
Kaplan-Meier survival analysis of overall survival in patients with HCC according to IGFBP-1 expression; the log-rank test was used to calculate P values.
Low expression of IGFBP-1 is an independent factor that predicts poor prognosis in patients with HCC
According to univariate Cox regression analyses, tumor size, microvascular invasion, TNM stage and IGFBP-1 expression were correlated with the overall survival rates of the patients with HCC. Furthermore, to determine the potential role of IGFBP-1 expression as an independent prognostic indicator in the prediction of the outcomes of patients with HCC, multivariate Cox regression analyses were performed. In this analysis, microvascular invasion, TNM stage and IGFBP-1 expression were recognized as independent prognostic indicators of the overall survival of the patients (Table 3).
Table 3.
Low expression of IGFBP-1 is an independent factor that predicts a poor prognosis of patients with HCC
| Clinicopathological characteristics | Univariate analyses | Multivariate analyses | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| RR | 95% CI | P value | RR | 95% CI | P value | |
| Gender | 0.662 | 0.226 - 1.940 | 0.452 | |||
| Age | 1.051 | 0.433 - 2.548 | 0.913 | |||
| Tumor size | 1.552 | 1.085 - 2.592 | 0.026* | 1.386 | 0.863 - 2.273 | 0.076 |
| Tumor nodules | 0.969 | 0.485 - 1.936 | 0.929 | |||
| Tumor differentiation | 1.030 | 0.587 - 1.810 | 0.917 | |||
| Liver cirrhosis | 0.876 | 0.433 - 1.771 | 0.712 | |||
| Microvascular invasion | 0.248 | 0.114 - 0.538 | <0.001* | 0.252 | 0.122 - 0.521 | <0.001* |
| HBsAg | 0.834 | 0.616 - 1.130 | 0.241 | |||
| AFP | 0.765 | 0.559 - 1.048 | 0.095 | |||
| TNM stage | 0.561 | 0.353 - 0.890 | 0.014* | 0.782 | 0.394 - 0.931 | 0.037* |
| IGFBP-1 expression level | 2.383 | 1.203 - 4.719 | 0.013* | 2.596 | 1.476 - 4.566 | 0.001* |
Statistically significant (P<0.05).
IGFBP-1 mRNA expression in HCC cell lines
We evaluated the expression of IGFBP-1 in a human liver non-tumor cell line (HL-7702) and in HCC cell lines (HuH-7, HepG2, SMMC-7721, MHCC97-H) (Figure 3). The invasion ability of the above cells was gradually increased. This result showed that the expression of IGFBP-1 decreases gradually in the above cell lines, which suggests that IGFBP-1 may participate in the cellular invasion process of HCC cells.
Figure 3.
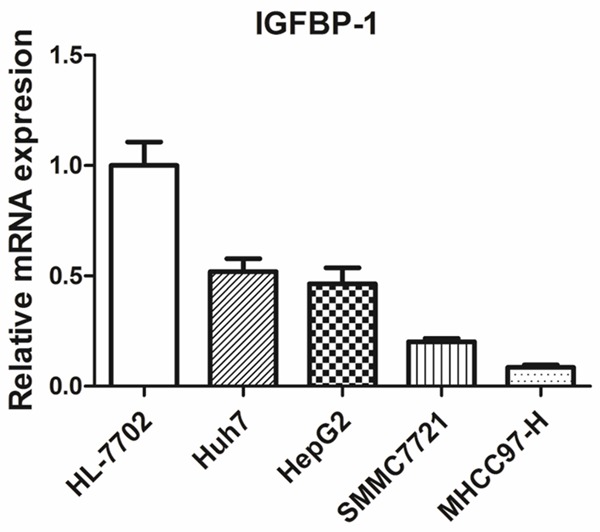
Expression analysis of IGFBP-1 mRNA in HCC cells by RT-PCR.
IGFBP-1 could suppress HCC cells invasion
We then analyzed the effect of IGFBP-1 on the invasion capability of HepG2 and MHCC97-H cells using a transwell invasion assay. As shown in Figure 4, the invasion capability of HepG2 and MHCC97-H cells was significantly impaired after IGFBP-1 treatment in a dose-dependent manner. Therefore, we concluded that IGFBP-1 may participate in the cellular invasion process of hepatic cancer cells.
Figure 4.
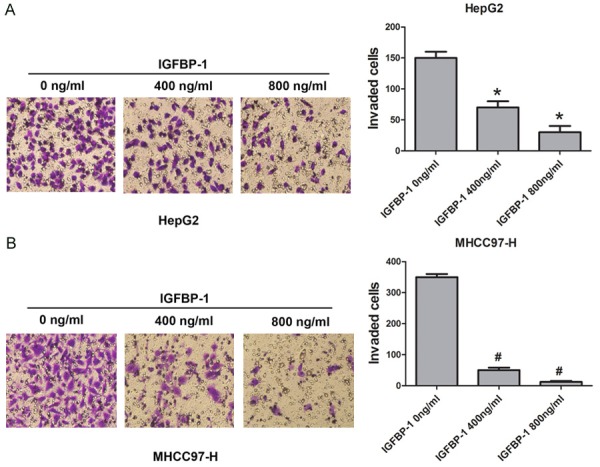
IGFBP-1 inhibits the invasion ability of HCC cells (A and B). HepG2 and MHCC97-H cells were treated with IGFBP-1 at different doses (400 and 800 ng/ml) to evaluate the invasion capacities of the cells. Non-treated cells served as controls. *P<0.05 compared to HepG2 cells non-treated with IGFBP-1, #P<0.05 compared to MHCC97-H cells non-treated with IGFBP-1.
Effects on MMP-9 expression of HepG2 and MHCC97-H cells after treatment with human recombinant IGFBP-1
MMPs have been recognized as the predominant molecules in the cellular invasion process [21]. We employed a Western blot assay to detect the change in protein expression of MMP-9 (an important member of the MMP family) after treatment with IGFBP-1. Western blot was used to analyze the protein expression as shown in Figure 5. IGFBP-1 was able to effectively inhibit the expression of MMP-9 protein in a dose-dependent manner in HepG2 and MHCC97-H cells. The results indicated that IGFBP-1 may regulate MMP-9 in HCC cells.
Figure 5.
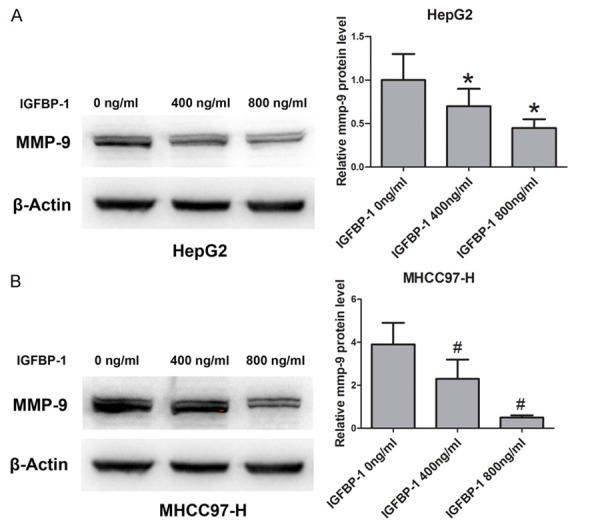
IGFBP-1 inhibits the expression of MMP-9 in HCC cells (A and B). HepG2 and MHCC97-H cells were treated with IGFBP-1 at different doses (400 and 800 ng/ml) to evaluate the protein level of MMP-9. Non-treated cells served as controls. *P<0.05 compared to HepG2 cells non-treated with IGFBP-1, #P<0.05 compared to MHCC97-H cells non-treated with IGFBP-1.
Discussion
IGFBP-1, one of the binding proteins with high affinity to IGF ligands, is a relatively tissue-specific molecule mainly produced by the liver [24] that can negatively regulate the activation of IGF-R. Because the IGF signaling pathway plays an important role in the development and progression of HCC [6], it is believed that IGFBP-1 may function as a tumor suppressor gene by blunting the IGF axis.
The complexity of IGFBP-1 has also been demonstrated in malignancies. IGFBP-1 promotes apoptosis of cancer cells under some conditions but not under others [25,26]. Furthermore, IGFBP-1 expression has been documented to be both positively and negatively correlated with cancer risk [25,27]. However, there is limited evidence from the literature concerning the role of IGFBP-1 in HCC. According to the existing data, one study showed that IGFBP-1 was elevated in HCC tissues [16], while the other demonstrated a decreased IGFBP-1 expression level in HCC [17]. No differences in IGFBP-1 expression between HCC and adjacent unaffected tissues have been reported [18]. In addition, paradoxical data have been observed in terms of the serum level of IGFBP-1 in patients with HCC [10,20]. While a shortage of these types of studies should be noted, a large number of samples are also lacking, and the method of detection varies among studies. Thus, the expression pattern of IGFBP-1 in HCC remains unclear.
Herein, we have utilized 15 normal liver samples, 90 HCC samples and corresponding adjacent non-cancerous liver tissues to explore the expression profile of IGFBP-1 by IHC. Our data demonstrated that IGFBP-1 protein expression was decreased in HCC tissues compared with adjacent non-tumor liver tissues and normal liver. The IGFBP-1 protein is thought to be expressed mainly in the cytoplasm and from there, is secreted into the serum. Our IHC results, however, strikingly found some HCC samples and adjacent liver samples, but not normal liver tissues, were delivering an evident nuclear stain of IGFBP-1. Actually, the same expression features have been reported in IGFBP-3 in vitro studies [28]. The detailed mechanisms and the biological effects of nuclear translocated IGFBP-1, therefore, deserve our prospective investigation. Furthermore, clinicopathological analyses have shown that the decreased IGFBP-1 level was significantly correlated with tumor differentiation, liver cirrhosis, microvascular invasion or metastasis and TNM stage. Moreover, a worse prognosis was observed in patients with low IGFBP-1 expression compared with those with high IGFBP-1 expression. Univariate and multivariate analyses also showed that low expression of IGFBP-1 was an independent predictor of the overall survival of the patients. These findings were in agreement with the previous reports that demonstrated that IGFBP-1 mRNA was decreased in HCC [17] and that transgenic overexpression of IGFBP-1 in mice could inhibit the development of HCC [19]. Similarly, the prognostic value of serum IGFBP-1 in cases of advanced HCC was verified in a recent preliminary study [20]. Thus, it can be presumed that IGFBP-1 is negatively correlated with HCC and might function as a tumor suppressor gene.
However, some in vitro studies have shown that IGFBP-1 is more than just a binding protein that modulates the affinity of IGFs to IGF-R. It has been demonstrated that IGFBP-1 contains an Arg-Gly-Asp (RGD) sequence that is capable of recognizing and binding to certain integrins on the cell surface, and thus they may function in an IGF-independent manner [29,30]. The binding of the IGFBP-1 RGD sequence to a recognition site on the α5β1 integrin could, as reported previously, enhance the migration ability of several cell types [30-33]. This may be accomplished through direct activation of focal adhesion kinase (FAK) and by stimulation of the mitogen-activated protein kinase (MAPK) pathway [32,33].
In our study, we first evaluated the expression of IGFBP-1 in the human liver non-tumor cell line HL-7702, HCC cell lines HuH-7, HepG2, SMMC-7721 and MHCC97-H which had been showed a gradually increased invasion capability. The result showed that the expression of IGFBP1 declines gradually in the above cell lines. This suggested that IGFBP-1 may participate in the cellular invasion process of HCC cells. We then examined the effects of IGFBP-1 on the invasion capability of HepG2 and MHCC97-H cells using a transwell invasion assay. Compared to controls, HepG2 and MHCC97-H cells treated with IGFBP-1 showed a significant decrease in the number of invasive cells. This strongly indicates that human recombinant IGFBP-1 suppresses the invasion capabilities of HCC cell lines. As MMPs have been recognized as the predominant molecules in the cellular invasion process [21], we employed a Western blot assay to detect the expression of MMP-9 in MHCC97-H and HepG2 cells treated with IGFBP-1. The results showed a significant decrease in the expression of MMPs in these two cell lines, which indicates that IGFBP-1 may regulate MMP-9 expression in HCC cells.
Collectively, our study is the first to explore the expression pattern of IGFBP-1 in HCC at the protein level and evaluate its prognostic value in patients with HCC. We have found that IGFBP-1 protein is decreased in HCC tissues compared with adjacent unaffected liver tissue and that decreased expression of IGFBP-1 in HCC tissues is associated with a poor prognosis. We also found that IGFBP-1 could inhibit HCC invasion via the down-regulation of MMP expression. Our study proved that IGFBP-1 is a tumor suppressor gene that can be used as a prognostic marker for HCC.
Acknowledgements
This work was supported by grants from the Major Program of the National Natural Science Foundation of China (Grants No. 81030010/H0318).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 4.Lee PD, Giudice LC, Conover CA, Powell DR. Insulin-like growth factor binding protein-1: recent findings and new directions. Proc Soc Exp Biol Med. 1997;216:319–357. doi: 10.3181/00379727-216-44182. [DOI] [PubMed] [Google Scholar]
- 5.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 6.Wu J, Zhu AX. Targeting insulin-like growth factor axis in hepatocellular carcinoma. J Hematol Oncol. 2011;4:30. doi: 10.1186/1756-8722-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohn KL, Melby AE, Tewari DS, Laz TM, Taub R. The gene encoding rat insulinlike growth factor-binding protein 1 is rapidly and highly induced in regenerating liver. Mol Cell Biol. 1991;11:1393–1401. doi: 10.1128/mcb.11.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J, Greenbaum L, Haber BA, Nagle D, Lee V, Miles V, Mohn KL, Bucan M, Taub R. Structure and localization of the IGFBP-1 gene and its expression during liver regeneration. Hepatology. 1994;19:656–665. doi: 10.1002/hep.1840190317. [DOI] [PubMed] [Google Scholar]
- 9.Younossi ZM, Gorreta F, Ong JP, Schlauch K, Del Giacco L, Elariny H, Van Meter A, Younoszai A, Goodman Z, Baranova A, Christensen A, Grant G, Chandhoke V. Hepatic gene expression in patients with obesity-related non-alcoholic steatohepatitis. Liver Int. 2005;25:760–771. doi: 10.1111/j.1478-3231.2005.01117.x. [DOI] [PubMed] [Google Scholar]
- 10.Hwang DL, Huang SP, Lan WS, Lee PD. Elevated insulin, proinsulin and insulin-like growth factor-binding protein-1 in liver disease. Growth Horm IGF Res. 2003;13:316–321. doi: 10.1016/s1096-6374(03)00042-x. [DOI] [PubMed] [Google Scholar]
- 11.Leu JI, George DL. Hepatic IGFBP1 is a prosurvival factor that binds to BAK, protects the liver from apoptosis, and antagonizes the proapoptotic actions of p53 at mitochondria. Genes Dev. 2007;21:3095–3109. doi: 10.1101/gad.1567107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magne L, Blanc E, Marchand A, Fafournoux P, Barouki R, Rouach H, Garlatti M. Stabilization of IGFBP-1 mRNA by ethanol in hepatoma cells involves the JNK pathway. J Hepatol. 2007;47:691–698. doi: 10.1016/j.jhep.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Jousse C, Bruhat A, Ferrara M, Fafournoux P. Physiological concentration of amino acids regulates insulin-like-growth-factor-binding protein 1 expression. Biochem J. 1998;334:147–153. doi: 10.1042/bj3340147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang CH, Nystrom GJ, Frost RA. Regulation of IGF binding protein-1 in hep G2 cells by cytokines and reactive oxygen species. Am J Physiol. 1999;276:719–727. doi: 10.1152/ajpgi.1999.276.3.G719. [DOI] [PubMed] [Google Scholar]
- 15.Tazuke SI, Mazure NM, Sugawara J, Carland G, Faessen GH, Suen LF, Irwin JC, Powell DR, Giaccia AJ, Giudice LC. Hypoxia stimulates insulin-like growth factor binding protein 1 (IGFBP-1) gene expression in HepG2 cells: a possible model for IGFBP-1 expression in fetal hypoxia. Proc Natl Acad Sci U S A. 1998;95:10188–10193. doi: 10.1073/pnas.95.17.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kondoh N, Wakatsuki T, Ryo A, Hada A, Aihara T, Horiuchi S, Goseki N, Matsubara O, Takenaka K, Shichita M, Tanaka K, Shuda M, Yamamoto M. Identification and characterization of genes associated with human hepatocellular carcinogenesis. Cancer Res. 1999;59:4990–4996. [PubMed] [Google Scholar]
- 17.Gong Y, Cui L, Minuk GY. The expression of insulin-like growth factor binding proteins in human hepatocellular carcinoma. Mol Cell Biochem. 2000;207:101–104. doi: 10.1023/a:1007010818094. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Q, Mao YQ, Jiang WD, Chen YR, Huang RY, Zhou XB, Wang YF, Shi Z, Wang ZS, Huang RP. Development of IGF signaling antibody arrays for the identification of hepatocellular carcinoma biomarkers. PLoS One. 2012;7:e46851. doi: 10.1371/journal.pone.0046851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu S, Archer MC. Insulin-like growth factor binding protein-1 over-expression in transgenic mice inhibits hepatic preneoplasia. Mol Carcinog. 2003;36:142–146. doi: 10.1002/mc.10105. [DOI] [PubMed] [Google Scholar]
- 20.Abou-Alfa GK, Capanu M, O’Reilly EM, Ma J, Chou JF, Gansukh B, Shia J, Kalin M, Katz S, Abad L, Reidy-Lagunes DL, Kelsen DP, Chen HX, Saltz LB. A phase II study of cixutumumab (IMC-A12, NSC742460) in advanced hepatocellular carcinoma. J Hepatol. 2014;60:319–324. doi: 10.1016/j.jhep.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou L, Wang DS, Li QJ, Sun W, Zhang Y, Dou KF. Downregulation of the Notch signaling pathway inhibits hepatocellular carcinoma cell invasion by inactivation of matrix metalloproteinase-2 and -9 and vascular endothelial growth factor. Oncol Rep. 2012;28:874–882. doi: 10.3892/or.2012.1880. [DOI] [PubMed] [Google Scholar]
- 22.Leu JI, Crissey MA, Taub R. Massive hepatic apoptosis associated with TGF-beta1 activation after Fas ligand treatment of IGF binding protein-1-deficient mice. J Clin Invest. 2003;111:129–139. doi: 10.1172/JCI16712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue YZ, Sheng YY, Liu ZL, Wei ZQ, Cao HY, Wu YM, Lu YF, Yu LH, Li JP, Li ZS. Expression of NEDD9 in pancreatic ductal adenocarcinoma and its clinical significance. Tumour Biol. 2013;34:895–899. doi: 10.1007/s13277-012-0624-8. [DOI] [PubMed] [Google Scholar]
- 24.Lewitt MS, Saunders H, Phyual JL, Baxter RC. Regulation of insulin-like growth factor-binding protein-1 in rat serum. Diabetes. 1994;43:232–239. doi: 10.2337/diab.43.2.232. [DOI] [PubMed] [Google Scholar]
- 25.Perks CM, Newcomb PV, Norman MR, Holly JM. Effect of insulin-like growth factor binding protein-1 on integrin signalling and the induction of apoptosis in human breast cancer cells. J Mol Endocrinol. 1999;22:141–150. doi: 10.1677/jme.0.0220141. [DOI] [PubMed] [Google Scholar]
- 26.Perks CM, Bowen S, Gill ZP, Newcomb PV, Holly JM. Differential IGF-independent effects of insulin-like growth factor binding proteins (1-6) on apoptosis of breast epithelial cells. J Cell Biochem. 1999;75:652–664. doi: 10.1002/(sici)1097-4644(19991215)75:4<652::aid-jcb11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 27.Kaaks R, Toniolo P, Akhmedkhanov A, Lukanova A, Biessy C, Dechaud H, Rinaldi S, Zeleniuch-Jacquotte A, Shore RE, Riboli E. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst. 2000;92:1592–1600. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- 28.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 29.Brewer MT, Stetler GL, Squires CH, Thompson RC, Busby WH, Clemmons DR. Cloning, characterization, and expression of a human insulin-like growth factor binding protein. Biochem Biophys Res Commun. 1988;152:1289–1297. doi: 10.1016/s0006-291x(88)80425-x. [DOI] [PubMed] [Google Scholar]
- 30.Jones JI, Gockerman A, Busby WJ, Wright G, Clemmons DR. Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the alpha 5 beta 1 integrin by means of its Arg-Gly-Asp sequence. Proc Natl Acad Sci U S A. 1993;90:10553–10557. doi: 10.1073/pnas.90.22.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gockerman A, Prevette T, Jones JI, Clemmons DR. Insulin-like growth factor (IGF)-binding proteins inhibit the smooth muscle cell migration responses to IGF-I and IGF-II. Endocrinology. 1995;136:4168–4173. doi: 10.1210/endo.136.10.7545099. [DOI] [PubMed] [Google Scholar]
- 32.Chesik D, De Keyser J, Bron R, Fuhler GM. Insulin-like growth factor binding protein-1 activates integrin-mediated intracellular signaling and migration in oligodendrocytes. J Neurochem. 2010;113:1319–1330. doi: 10.1111/j.1471-4159.2010.06703.x. [DOI] [PubMed] [Google Scholar]
- 33.Gleeson LM, Chakraborty C, Mckinnon T, Lala PK. Insulin-like growth factor-binding protein 1 stimulates human trophoblast migration by signaling through alpha 5 beta 1 integrin via mitogen-activated protein Kinase pathway. J Clin Endocrinol Metab. 2001;86:2484–2493. doi: 10.1210/jcem.86.6.7532. [DOI] [PubMed] [Google Scholar]


