Abstract
Objective: This study was to clone, identify and analyze the characteristics of egG1Y162 gene from Echinococcus granulosus. Methods: Genomic DNA and total RNAs were extracted from four different developmental stages of protoscolex, germinal layer, adult and egg of Echinococcus granulosus, respectively. Fluorescent quantitative PCR was used for analyzing the expression of egG1Y162 gene. Prokaryotic expression plasmid of pET41a-EgG1Y162 was constructed to express recombinant His-EgG1Y162 antigen. Western blot analysis was performed to detect antigenicity of EgG1Y162 antigen. Gene sequence, amino acid alignment and phylogenetic tree of EgG1Y162 were analyzed by BLAST, online Spidey and MEGA4 software, respectively. Results: EgG1Y162 gene was expressed in four developmental stages of Echinococcus granulosus. And, egG1Y162 gene expression was the highest in the adult stage, with the relative value of 19.526, significantly higher than other three stages. Additionally, Western blot analysis revealed that EgG1Y162 recombinant protein had good reaction with serum samples from Echinococcus granulosus infected human and dog. Moreover, EgG1Y162 antigen was phylogenetically closest to EmY162 antigen, with the similarity over 90%. Conclusion: Our study identified EgG1Y162 antigen in Echinococcus granulosus for the first time. EgG1Y162 antigen had a high similarity with EmY162 antigen, with the genetic differences mainly existing in the intron region. And, EgG1Y162 recombinant protein showed good antigenicity.
Keywords: EgG1Y162 antigen, phylogenetic tree, fluorescent quantitative PCR, prokaryotic expression
Introduction
Echinococcus disease, also called hydatid disease, is a severe zoonotic parasitic disease mainly caused by Echinococcus, and has a worldwide distribution [1-3]. Currently, Echinococcus disease is mainly treated with surgery and drugs. However, the disease recurrence rate is relatively high after surgery treatment and the damage on human body caused by surgery is also relatively severe. Meanwhile, treatment with drugs has some side effects, which cannot be tolerated by some patients. In addition, some patients may also develop resistance to chemotherapy. Therefore, early diagnosis and immune protection of hydatid disease have become very important for the treatment of hydatid disease [4].
Studies in Echinococcus multilocularis found that there were some secreted proteins with membrane surface anchoring functions, which could be used as candidate proteins for vaccine production. These candidate vaccine proteins usually have an amino-terminal hydrophobic signal peptide and a carboxyl-terminal hydrophobic transmembrane region, and are involved in the interactions between parasites and hosts [5,6]. These transmembrane proteins could help parasites penetrate the cell membrane of host cells to infect the host and regulate the host immune response. Therefore, these secreted proteins may be used for vaccine production or in diagnosis of Echinococcus disease and they were named as EMY162 antigen [5,6]. In addition, it was found that EMY162 recombinant antigens could induce a 74.3% immune protective effect in mouse. Meanwhile, the positive rate of EMY162 recombinant antigens in the serum of patients with alveolar echinococcosis was significantly higher than that of EM95. The expression of emY162 gene was found in all the four developmental stages of Echinococcus multilocularis (protoscolex, metacestode, larvae, adults). Western blot analysis in serum of Echinococcus multilocularis infected dog showed that the EMY162 antigen could produce a strong IgG immune response. It has been confirmed that EMY162 is a secretory protein, which can stimulate the immune system of the dog to produce effective immune responses. Therefore, EMY162 antigen is considered as an ideal candidate for vaccine development.
However, whether there are similar antigens of EMY162 in Echinococcus granulosus and whether they can be used as ideal protective antigens have been not reported. In this study, we cloned EgG1Y162 antigen, similar antigen of EMY162, from Echinococcus granulosus for the first time. And EgG1Y162 antigen exists in stages of adult, germinal layer, protoscolex and egg of Echinococcus granulosus. Furthermore, we constructed a prokaryotic expression plasmid of pET-41a/EgG1Y162 and induced EgG1Y162 expression in vitro. The antigenicity of EgG1Y162 was detected. Gene sequence, amino acid alignment and phylogenetic tree of EgG1Y162 were also analyzed. Our results provide experimental data for further study of EgG1Y162’s role in immune protection and for production of recombinant vaccines against Echinococcus granulosus infection.
Materials and methods
Reagents
Echinococcus granulosus was extracted from abdominal cavity of mice infected with Echinococcus multilocularis and mice were provided by the Experimental Animal Center of Xinjiang Medical University. PUCmT vector, pET-41a vector, PCR kit, T4 DNA ligase, DL2000 DNA markers were all purchased from TaKaRa (TaKaRa Biotechnology (Dalian) Co., Ltd., Dalian, China). E. coli DH5 was preserved in our research group. DNA extraction kit was purchased from Tiangen (Tiangen Biotech Co., Ltd., Beijing, China). X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactoside) and other biological reagents were purchased from Sangon (Sangon Biotech Co., Ltd., Shanghai, China). Reverse transcription kit and Bradford protein quantification kit were purchased from Invitrogen (Invitrogen Co., Carlsbad, California, USA).
By utilizing DNAman software, the upstream and downstream primers were designed according to the reported sequence of emY162 cDNA [7]. Primers were synthesized by Sangon (Sangon Biotech Co., Ltd., Shanghai, China). The sequences of the primers were shown as follows. Upstream primer: 5’-CCGAATTCATGGTACTTCGATTCTGT-3’, downstream primer: 5’-CCAAGCTTAGTAAGTAATAGGAGCCCA-3’. The restriction sites of EcoR I and Hind III were respectively added to the primers (indicated by underlined italics).
Fluorescent quantitative RT-PCR
Total RNAs were extracted from four different developmental stages of protoscolex, germinal layer, adults and eggs of Echinococcus granulosus, and reverse-transcribed into cDNAs. Then these cDNAs of Echinococcus granulosus were used as templates to conduct fluorescent quantitative RT-PCR. Based on the known copy numbers and the standard curve of standard samples, the mean values of copy numbers (SQ mean) and CT values of sample cDNAs of Echinococcus granulosus were calculated. Housekeeping gene egAct II was used as an internal control. The expression of egG1Y162 in each developmental stage of Echinococcus granulosus was then compared.
Construction of pET41a/EgG1Y162 recombinant plasmid
After amplication by PCR, EgG1Y162 gene was first cloned into pUCm-T vector by utilizing TA cloning. Then colonies formed by pUCm-T/EgG1Y162 were sequenced and correct EgG1Y162 fragments were collected by enzyme digestion. Prokaryotic expression plasmid of pET41a-EgG1Y162 was then constructed with these EgG1Y162 fragments and sequenced again.
Expression and purification of His-EgG1Y162 protein
Expression of pET-41a/EgG1Y162 plasmid was induced by IPTG (final concentration of 0.5 mmol/L) for 0 h, 2 h, 4 h, 6 h, respectively, at 30°C. The cells were collected at 4°C by centrifugation of 12000 r/min for 10 min. Then cells were lysed by ultrasound and His-EgG1Y162 proteins were extracted by centrifugation. His column was used for purification and a step-by-step-elution was conducted by using different concentrations of elution buffer (0, 100, 200, 300, 500 mmol/L of imidazole, pH 7.4). SDS-PAGE (12%) was used for testing the purity of the eluate. The eluate with highest purity and concentration of proteins was collected and was dialyzed in PBS buffer (pH = 8.0) for 24 h. The PBS buffer was changed three times during the dialysis and the proteins were collected after dialysis. The dialyzed proteins were then concentrated by using concentration column. The concentrated proteins were quantified by using Bradford protein quantification kit. The purified proteins were then frozen at -80°C for further use.
Western blot analysis
The purified His-EgG1Y162 protein was separated on 12% SDS-PAGE. Then proteins were transferred onto nitrocellulose membrane. After blocking with non-fat milk, the membrane was incubated with serum samples from normal or Echinococcosis granulosus infected human and dog (1:3000 dilution) at 4°C overnight. After washing, the membrane was then incubated with secondary antibodies of anti-human/anti-dog serum (1:3000 dilution) at room temperature for 1 h. Finally, the membrane was developed by enhanced chemiluminescence plus reagent.
Phylogenetic analysis of EgG1Y162 antigen
DNAman software and GeneBank sequence alignment function (BLAST) were used for sequence analysis (http://www.ncbi.nlm.nih.gov/). Online Spidey (http://www.ncbi.nih.gov/spidey) was used for analyzing the construction of EgG1Y162 cDNA. MEGA4 software was used for analyzing the evolutionary relationship of EgG1Y162 antigen.
Statistical analysis
Data were analyzed using the SPSS 19.0 software. One-Way ANOVA analysis was used to compare differences among different groups. P < 0.05 was considered statistically significant.
Results
Expression of egG1Y162 gene is different in four developmental stages of protoscolex, germinal layer, adult and egg of Echinococcus granulosus
To determine the expression level of egG1Y162 gene in different developmental stages of Echinococcus granulosus, fluorescent quantitative RT-PCR was performed. Total RNAs were extracted from tissues of different developmental stages of Echinococcus granulosus. Housekeeping gene egAct II was used as an internal control. When using the total RNAs of four developmental stages as templates in fluorescent quantitative RT-PCR, the amplification products of egG1Y162 cDNA were approximately 350 bp. The relative expression levels and copy numbers of egG1Y162 gene were shown in Figure 1A and Table 1. The expression levels of egG1Y162 gene were different in four developmental stages of adult, germinal layer, protoscolex and egg. And, the expression level of egG1Y162 was the highest in adult stage of Echinococcus granulosus, with relative value of 19.526, followed by the germinal layer stage, with relative value of 5.122, and protoscolex stage, with the relative value of 5.083. The expression level of egG1Y162 gene was the lowest in egg stage, with relative value of 1.6588. Statistically, the difference between adult stage and other three stages was significant (P < 0.01). Thus, the four developmental stages of Echinococcus granulosus had different expression levels of egG1Y162 gene and the adult stage had the highest level.
Figure 1.
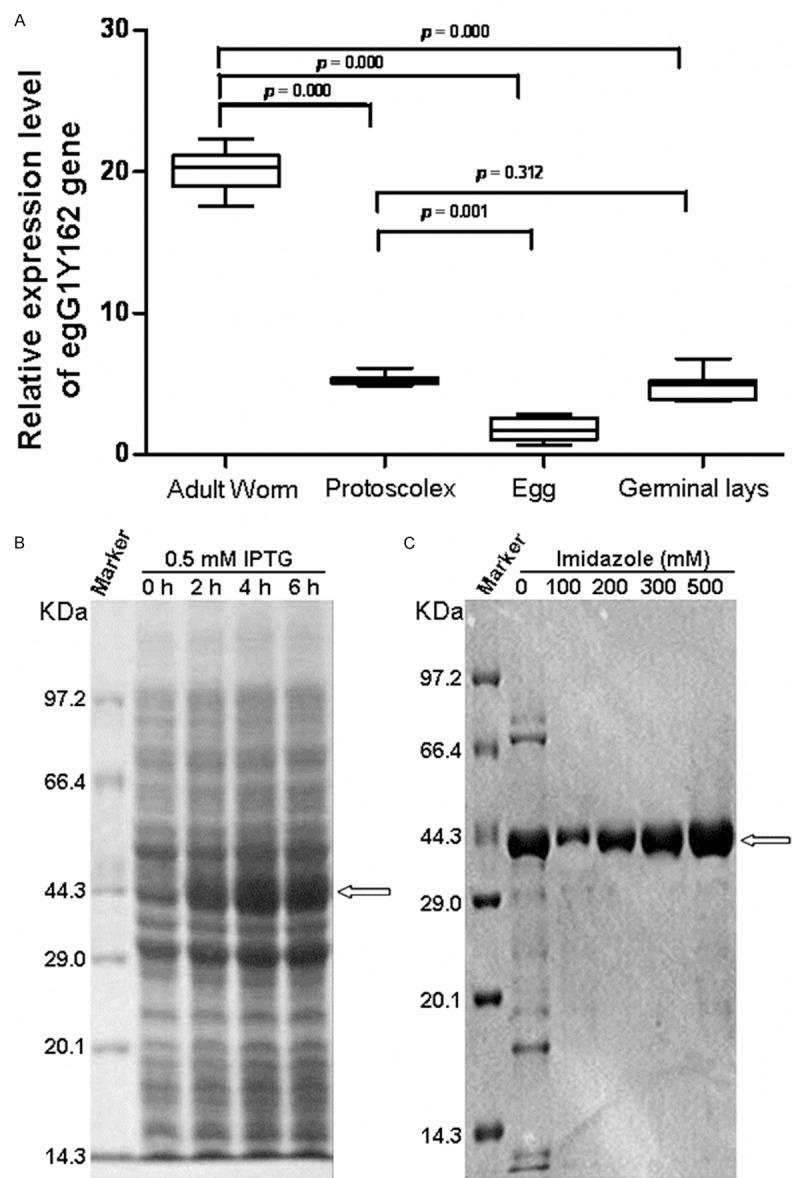
Analysis of EgG1Y162 gene and protein expression. A. Relative expression level of egG1Y162 gene in developmental stages of Echinococcus granulosus. Expression of egG1Y162 gene was analyzed by fluorescent quantitative RT-PCR. Housekeeping gene egAct II was used as an internal control. B. Results of EgG1Y162 recombinant protein expression by SDS-PAGE electrophoresis analysis. Expression of pET-41a/EgG1Y162 plasmid was induced by IPTG (final concentration of 0.5 mmol/L) for 0 h, 2 h, 4 h, 6 h, respectively, at 30°C. C. Results of purified EgG1Y162 recombinant protein by SDS-PAGE electrophoresis analysis. EgG1Y162 recombinant protein was eluted with imidazole at concentrations of 0, 100, 200, 300, 500 mmol/L (pH 7.4).
Table 1.
Expression of EgG1Y162 gene in four developmental stages of protoscolex, germinal layer, adult and egg of Echinococcus granulosus
| Gene copy numbers | Adult worm | Protoscolex | Egg | Germinal lay |
|---|---|---|---|---|
| EgG1Y162 | 6.89×105 | 4.44×104 | 1.23×105 | 1.77×105 |
| egAct II | 3.53×104 | 8.73×103 | 7.45×103 | 3.46×103 |
| Relative level of EgG1Y162 | 19.526* | 5.083 | 1.6588 | 5.122 |
Ρ < 0.01, compared with adult worm stage.
Housekeeping gene egAct II was used as an internal control.
His-EgG1Y162 protein is successfully expressed and purified
To express egGY162 gene in vitro, the prokaryotic expression plasmid pET41a-EgG1Y162 was constructed. Before construction of pET41a-EgG1Y162 plasmid, egG1Y162 gene was cloned and sequenced. When using genome DNA was as an amplification template and egG1Y162 specific primers in PCR amplification, a fragment of about 1600 bp was obtained. The recombinant pET41a-EgG1Y162 prokaryotic expression plasmid was constructed and induced for expression by IPTG. The molecular weight of His tag of pET41a plasmid is about 32.29 KDa. Thus it is speculated that the molecular weight of the recombinant protein His-EgG1Y162 was approximately 44 KDa. As shown in Figure 1B, there was an obvious band at 44 KDa after IPTG induction, suggesting that the recombinant protein His-EgG1Y162 was successfully induced. And the protein amount was gradually increased with prolonged induction. This indicated that the expression of EgG1Y162 recombinant proteins was highly effective. His-EgG1Y162 protein was purified by His column and eluted with different concentrations of imidazole. As shown in Figure 1C, the purified protein was with good purity and the protein level was the highest when eluting with 500 mM imidazole. These results showed that His-EgG1Y162 protein was effectively expressed and purified in vitro.
His-EgG1Y162 protein has good antigenicity
To analyze the antigenicity of His-EgG1Y162 protein, Western Blot analysis was conducted. The first antibodies were serum samples from normal or Echinococcosis granulosus infected human and dog and the secondary antibodies were anti-human/anti-dog serum. Representative Western Blot results were shown in Figure 2. Figure 2A showed the reaction of His-EgG1Y162 protein with dog serum samples. The serum of 6 dogs infected with Echinococcus all showed positive reaction, whereas the serum of 6 dogs uninfected with Echinococcus showed negative reaction. This result suggests that His-EgG1Y162 protein was identified by serum samples from Echinococcosis granulosus infected dog but not by normal dog serum samples. And the specific band of His-EgG1Y162 was detected at 44.0 KDa.
Figure 2.
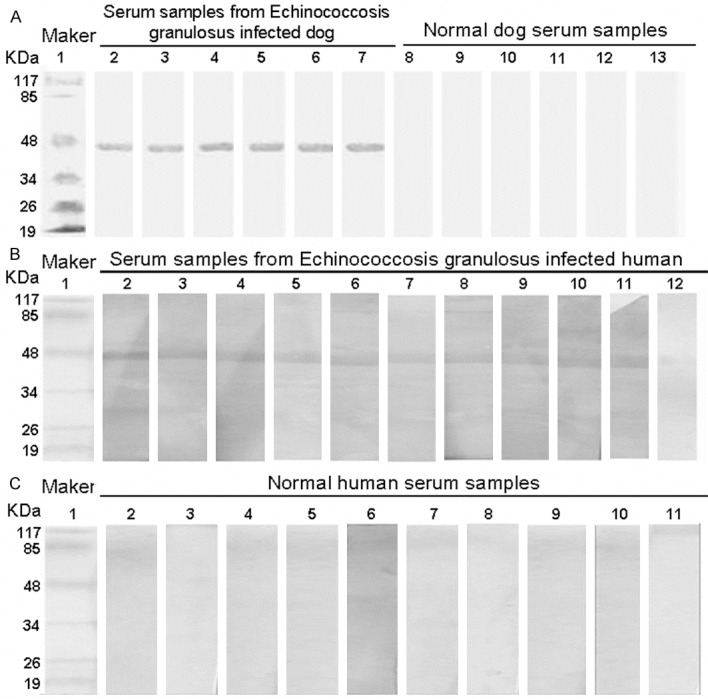
Antigenicity analysis of EgG1Y162 protein. Antigenicity of EgG1Y162 protein was analyzed by Western Blot analysis. Serum samples from Echinococcus infected human/dog and from normal human/dog were used. A. Representative Western Blot results of dog serum blotting with EgG1Y162 protein. Lane 1: Protein molecular weight marker; Lane 2-7: serum samples from Echinococcus infected dog; Lane 8-13: serum samples from normal dog. B. Representative Western Blot results of Echinococcus infected human serum blotting with EgG1Y162 protein. Lane 1: protein molecular weight marker; Lane 2-12: serum samples from Echinococcus infected human. C. Representative Western Blot results of normal human serum blotting with EgG1Y162 protein. Lane 1: protein molecular weight marker; Lane 2-11: serum samples of normal human.
To further verify this, the antigenicity of EgG1Y162 was also analyzed with the serum of patients with hydatid disease. Serum samples from 11 patients infected with Echinococcosis granulosus and from 10 normal persons were used. Consistently, the specific positive band of His-EgG1Y162 was only found at 44.0 KDa. There were 10 positive reactions between His-EgG1Y162 protein and serum samples of patients with hydatid disease (Figure 2B, samples 1-11), while no positive reaction occurred between EgG1Y162 and serum of normal humans (Figure 2C). These results indicated that EgG1Y162 recombinant protein had good antigenicity.
Analysis of gene sequence, amino acid sequence and homology of EgG1Y162
To analyze the evolutionary relationship of EgG1Y162 with other antigens, the gene sequence, amino acid sequence and phylogenetic tree of EgG1Y162 were analyzed and compared. The sequencing results analyzed by DNAman software showed that the length of egG1Y162 gene was 1,648 bp, while the length of cDNA was 459 bp. Through homology comparisons with gene sequences (GenBank sequence number AB303297) and cDNA sequences (GenBank sequence No. AB303298) of emY162, egG1Y162 gene was identified as a new gene of Echinococcus granulosus. The sequences of egG1Y162 and cDNA have been registered in GenBank, with the registration numbers of AB458258 and AB45825, respectively. The online Spidey analysis showed the sequence of egG1Y162 gene was composed of three exons and two introns (Figure 3). According to the gene sequence of emY162 in GenBank, there were three nucleotides in the transcription initiation region. Thus, in EmY162, the sequences of exon regions were 4-73, 1067-1384 and 1581-1651. Online Blast analysis found that there were 20 different nucleotide sites between the nucleotide sequence of EgG1Y162 and EmY162. The similarity in cDNA sequence between EgG1Y162 and emY162 was 95%. Meanwhile the similarity in amino acid sequence between EgG1Y162 and EG95 was 30.61% and was 33.58% between EgG1Y162 and EM95 (Figure 4). Then we did homology analysis among EgG1Y162 antigen, other antigens of Echinococcus granulosus and emY162 antigen of Echinococcus multilocularis by using MEGA4 software. We found that EgG1Y162 and EmY162 antigen had the closest homology (Figure 5). Therefore, EgG1Y162 antigen was genetically conserved.
Figure 3.
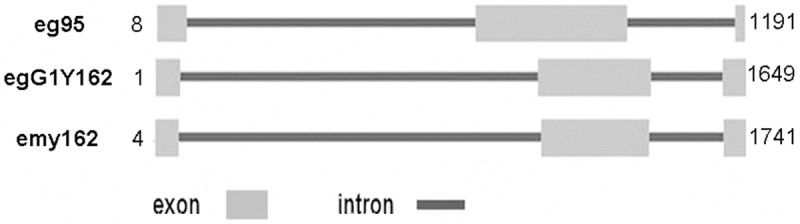
Analysis of gene structure. Gene constructions of eg95, egG1Y162 and emy162 were analyzed by online Spidey (http://www.ncbi.nih.gov/spidey). Gray rectangles represent exons and dark grey bold lines represent introns.
Figure 4.

Amino acid sequence alignment of EgG1Y162, EMY162, EG95 and EM95. BLAST analysis was performed for amino acid sequence alignment. Strictly conserved residues are highlighted in bright yellow and partially conserved residues are depicted in bright green.
Figure 5.
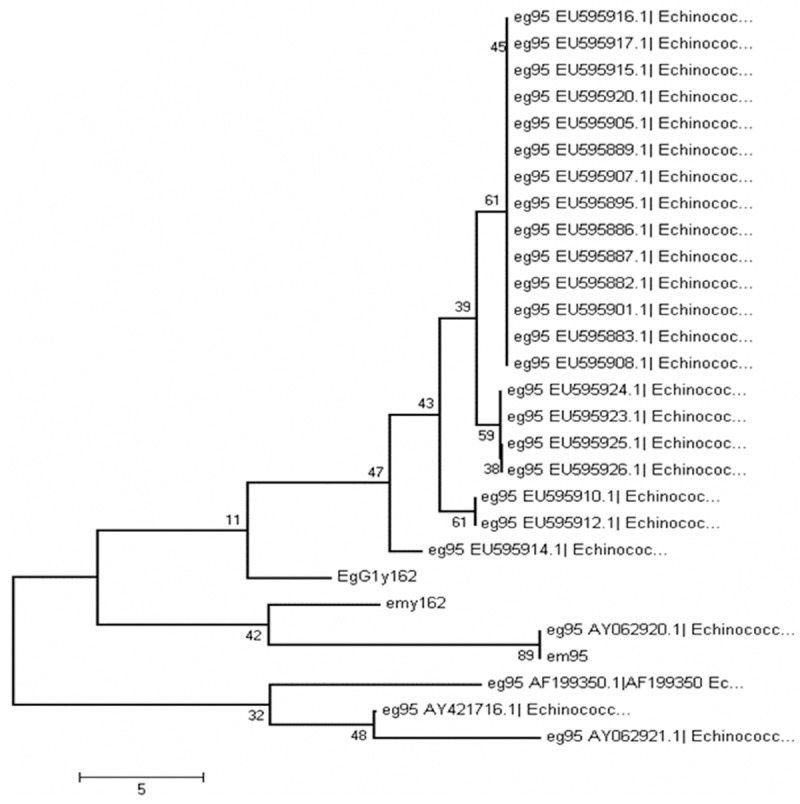
Phylogenetic analysis of EgG1Y162 protein. MEGA4 software was used for analyzing the evolutionary relationship of EgG1Y162 protein. And the phylogenetic tree of EgG1Y162 protein was depicted. Numbers indicate the phylogenetic relationship.
Discussion
The hydatid disease is prevalent in the Middle East, Southern Europe, Latin America, Asia, Australia and Africa [8-11]. In China, hydatid disease is mainly distributed in provinces of Xinjiang, Qinghai, Gansu, Ningxia, Tibet, and Sichuan [12-15]. With the increasing breeding of pets (dogs) and economic animals (foxes), hydatid disease is spreading and patients with hydatid disease have been found in many provinces (areas) of China. Treatment with genetically engineered vaccines and DNA vaccines is one of the most effective ways to completely control the epidemic of hydatid disease [16-18]. Gauci et. al. [19] first reported the Em95 antigen gene in 2002 and found that it could induce protective immune response of the host. Now, Em95 and Eg95 antigen have been used as the first candidate vaccines to prevent the intermediate host again Echinococcus granulosus infection. Although the development of recombinant vaccines currently has achieved some progress [7,20-26], the desired effects of prevention and protection have not been achieved. Thus more effective and protective antigens still need to be developed.
This study was the first report to identify a new antigen named EgG1Y162 in Echinococcus granulosus. Through DNA sequence analysis and homology analysis, we found that the sequencing difference between egG1Y162 and emY162 gene mainly existed in the intron region, which maintained the conservation of the coding sequence and stability of protein antigens. EgG1Y162 gene was present in four developmental stages of Echinococcus granulosus. Through phylogenetic analysis, it was found that EgG1Y162 protein was the closest to EmY162 protein. Thus, this data indicate that EgG1Y162 may be a protective antigen of the final host and that EgG1Y162 antigen may be useful for the development of vaccines against Echinococcus granulosus. Furthermore, our data provide experimental evidence for the further study of the role of EgG1Y162 in protecting the intermediate host and the final host against Echinococcus infection. The expression of egG1Y162 gene in different developmental stages of protoscolex, germinal layer, adult and eggs of Echinococcus granulosus was analyzed by fluorescent quantitative PCR. The results showed that egG1Y162 gene had a high level of expression in the stages of germinal layer and adult, especially in the adult stage. These results indicate that the expression of EgG1Y162 antigen was significantly difference in four developmental stages of Echinococcus granulosus and this difference may be related to the function of EgG1Y162 antigen at different stages. EgG1Y162 antigen was further expressed in vitro. Through blotting EgG1Y162 antigen with dog serum, it was found that the serum of Echinococcus granulosus infected dogs had a specific reaction with EgG1Y162 recombinant protein at 44 KDa, while the normal dog serum showed negative reaction. This result indicates that EgG1Y162 was highly specific and sensitive.
Katoh et. al. [27,28] found that EmY162 recombinant antigen could induce a 74.3% immune protection in rats. In addition, the positive rate of EMY162 recombinant antigens in the serum of patients with alveolar echinococcosis was significantly higher than that of Em95. It was speculated that EgG1Y162 antigen could also induce significant host immune protection. This study demonstrated that EgG1Y162 antigen was highly expressed in developmental stages of adult and protoscolex of Echinococcus granulosus. EgG1Y162 antigen may have protective and preventive effects on the intermediate host and the final host.
Structural analysis of protein sequences and Western Blot analysis results showed that EgG1Y162 antigen was highly antigen-specific in the final host, and has certain antigenicity in the intermediate host. Therefore, EgG1Y162 recombinant antigen can be used as a new vaccine candidate, which can reduce the infection risk of human hydatid disease. EgG1Y162 recombinant antigen provides the further experiment basis for studying the immune protective ability of anti-Echinococcus infection of intermediate host and the final host. Furthermore, it provides a new way for the development of new effective vaccines and lays the experimental foundation for prevention of pastoral hydatid tapeworm infection.
Acknowledgements
This study was supported by National Natural Science Foundation (No. 81160378, 81160200, 31000411, 30901374, 31160194 and 81060135) and University Scientific Research Project of Education Department of Xinjiang Autonomous Region (XJEDU2010S25 and XYDXK50780328).
Disclosure of conflict of interest
None.
References
- 1.Eckert J, Deplazes P. Biological, Epidemiological and Clinical Aspects of Echinococcosis, a Zoonosis of Increasing Concern. Clin Microbiol Rev. 2007;17:107–35. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu JH. Border areas hydatid disease causes and prevention of epidemic response. Medical Information. 2007;20:682–3. [Google Scholar]
- 3.Jiang CP. Current epidemic status of echinococcosis in chin. Endemic Diseases Bulletin. 2002;17:77–9. [Google Scholar]
- 4.Wen H, Xu M. PracticaL echinococcosis. 1rd edition. Beijing: Science Publishing House; 2007. pp. 1–19. [Google Scholar]
- 5.Katoh Y, Kouguchi H, Matsumoto J, Goto A, Suzuki T, Oku Y, Yagi K. Characterization of emY162 encoding an immunogenic protein cloned from an adult worm-specific cDNA library of Echinococcus multilocularis. Biochim Biophys Acta. 2007;1780:1–6. doi: 10.1016/j.bbagen.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Kouguchi H, Matsumoto J, Katoh Y, Oku Y, Suzuki T, Yagi K. The vaccination potential of EMY162 antigen against Echinococcus multilocularis infection. Biochim Biophys Acta. 2007;363:915–20. doi: 10.1016/j.bbrc.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Liu X, Zhu Y, Zhou X, Cao C, Hu X, Ma H, Wen H, Ma X, Ding JB. Bioinformatic prediction of epitopes in the Emy162 antigen of Echinococcus multilocularis. Exp Ther Med. 2013;6:335–40. doi: 10.3892/etm.2013.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh BB, Dhand NK, Ghatak S, Gill JP. Economic losses due to cystic echinococcosis in India: Need for urgent action to control the disease. Prev Vet Med. 2014;113:1–12. doi: 10.1016/j.prevetmed.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Tumol’():skaia NI, Zavoĭkin VD, Mazmanian MV, Sergiev VP. Alveolar echinococcosis in European Russia. Med Parazitol (Mosk) 2013:36–7. [PubMed] [Google Scholar]
- 10.Symeonidis N, Pavlidis T, Baltatzis M, Ballas K, Psarras K, Marakis G, Sakantamis A. Complicated liver echinococcosis: 30 years of experience from an endemic area. Scand J Surg. 2013;102:171–7. doi: 10.1177/1457496913491877. [DOI] [PubMed] [Google Scholar]
- 11.Czarkowski MP, Gołab E. Invasive tapeworm infections in Poland in 2011. Przegl Epidemiol. 2013;67:263–6. 365–7. [PubMed] [Google Scholar]
- 12.Zeng SH, Wang SP, Zhang JX. Analyzing the structures and function of SJCWL06 encoding protein of schistosoma japonicum by bioinformatics. Int J Med Parasit Dis. 2006;33:10–4. [Google Scholar]
- 13.Zhang W, Li J, McManus DP. Coneepts in immunology and diagnosis of hydatid disease. Clin Microbiol Rev. 2003;16:18–36. doi: 10.1128/CMR.16.1.18-36.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eekert J, Sehantz P, Gasser R. Geographie distribution and Prevalenee. In: Eekert J, Gemmell MA, Meslin FX, Pawlowski ZS, editors. WHOI/OIE manual on eehinoeoeeosis in humans and animals: a public health problem of global concern. Paris: World organization for Animal Health; 2001. pp. 100–41. [Google Scholar]
- 15.Eekerta J, Conrathsb F, Taekmannb K. Eehinoeoeeosis: an emerging or re-emerging zoonosis? Int J Parasitol. 2000;30:1283–94. doi: 10.1016/s0020-7519(00)00130-2. [DOI] [PubMed] [Google Scholar]
- 16.Heath DD. The use of a Recombinant Antigen to Immunize Sheep against Echinococcus granulosus. Beijing: Proceedings of the International Congress of Hydatidology; 1993. pp. 10–3. [Google Scholar]
- 17.Lightowlers MW. Vaccination against cestode parasites. Int J Parasitol. 1996;26:819–24. doi: 10.1016/s0020-7519(96)80048-8. [DOI] [PubMed] [Google Scholar]
- 18.Lightowlers MW, Jensen O, Fernandez E, Iriarte JA, Woollard DJ, Gauci CG, Jenkins DJ, Heath DD. Vaccination trials in Australia and Argentina confirm the effectiveness of the Eg95 hydatid vaccine in sheep. Int J Parasitol. 1999;29:531–4. doi: 10.1016/s0020-7519(99)00003-x. [DOI] [PubMed] [Google Scholar]
- 19.Ito A, Sako Y, Yamasaki H, Mamuti W, Nakaya K, Nakao M, Ishikawa Y. Development of Em18-immunoblot and Em18-ELISA for specific diagnosis of alveolar echinococcosis. Acta Trop. 2003;8:173–82. doi: 10.1016/s0001-706x(02)00221-8. [DOI] [PubMed] [Google Scholar]
- 20.Alvite G, Di Pietro SM, Santomé JA, Ehrlich R, Esteves A. Binding properties of Echinococcus granulosus fatty acid binding protein. Biochim Biophys Acta. 2001;15:293–302. doi: 10.1016/s1388-1981(01)00164-0. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Leggatt GR, Kalinna BH, Piva TJ, McManus DP. Cloning and expression of a cDNA encoding a nonintegrin laminin-binding protein from Echinococcus granulosus with localization of the laminin-binding domain. Mol Biochem Parasitol. 1997;87:183–92. doi: 10.1016/s0166-6851(97)00066-2. [DOI] [PubMed] [Google Scholar]
- 22.Virginio VG, Hernández A, Rott MB, Monteiro KM, Zandonai AF, Nieto A, Zaha A, Ferreira HB. A set of recombinant antigens from Echinococcus granulosus with potential for use in the immunodiagnosis of human cystic hydatid disease. Clin Exp Immunol. 2003;13:309–15. doi: 10.1046/j.1365-2249.2003.02123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heath DD, Robinson C, Lightowlers MW. Maternal antibody parameters of cattle and calves receiving EG95 vaccine to protect against Echinococcus granulosus. Vaccine. 2012;30:7321–6. doi: 10.1016/j.vaccine.2012.08.076. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez Rojas CA, Gauci CG, Lightowlers MW. Antigenic differences between the EG95-related proteins from Echinococcus granulosus G1 and G6 genotypes: implications for vaccination. Parasite Immunol. 2013;35:99–102. doi: 10.1111/pim.12009. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez Rojas CA, Gauci CG, Nolan MJ, Harandi MF, Lightowlers MW. Characterization of the eg95 gene family in the G6 genotype of Echinococcus granulosus. Mol Biochem Parasitol. 2012;183:115–21. doi: 10.1016/j.molbiopara.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Chow C, Gauci CG, Vural G, Jenkins DJ, Heath DD, Rosenzvit MC, Harandi MF, Lightowlers MW. Echinococcus granulosus: variability of the host-protective EG95 vaccine antigen in G6 and G7 genotypic variants. Exp Parasitol. 2008;119:499–505. doi: 10.1016/j.exppara.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Lightowlers MW, Gauci CG, Chow C, Drew DR, Gauci SM, Heath DD, Jackson DC, Dadley-Moore DL, Read AJ. Molecular and genetic characterisation of the host-protective oncosphere antigens of taeniid cestode parasites. Int J Parasitol. 2003;3:1207–17. doi: 10.1016/s0020-7519(03)00174-7. [DOI] [PubMed] [Google Scholar]
- 28.Casaravilla C, Malgor R, Rossi A, Sakai H, Nonaka N, Kamiya M, Carmona C. Production and characterization of monoclonal antibodies against excretory/secretory products of adult Echinococcus granulosus, and their application to coproantigen detection. Parasitol Int. 2005;5:43–9. doi: 10.1016/j.parint.2004.08.006. [DOI] [PubMed] [Google Scholar]


