Abstract
The prognostic value of histological types in gastric cancer is not well defined. This study aims to clarify the clinicopathologic features of various WHO histological types and their prognostic significance in advanced gastric cancer (AGC). We retrospectively reviewed 741 patients with gastric cancer in our hospital from 1997 to 2007. The AGC (741 cases) were divided into five histological types: well-differentiated carcinoma (WD), moderately differentiated carcinoma (MD), poorly differentiated carcinoma (PD), mucinous carcinoma (MC), and signet ring cell carcinoma (SRC). The various AGC histological types presented significant differences in their clinical and tumor features. The five-year survival rates of patients with WD, MD, PD, MC, and SRC were 87.1%, 57.1%, 50.6%, 62.7%, and 43.4%, respectively (P=0.012). Multivariate analysis showed that cell differentiation, age, depth of invasion, and lymph node metastasis were independent prognostic factors in AGC, whereas MC and SRC were not. Cell differentiation is related to tumor aggression or patient stage. Advanced stage SRC carcinoma had more aggressive features and worse prognosis than the other types. MC carcinoma survival is correlated with the stage at diagnosis. The degree of cell differentiation is an important predictor of survival in AGC.
Keywords: Advanced gastric cancer, histologic type, prognosis, cell differentiation
Introduction
Although the incidence rate of gastric cancer (GC) or stomach adenocarcinoma has steadily declined in the past decades, this global health problem persists primarily because most patients present with the disease in the advanced stage. Thus, clarifying the predictors of survival in advanced gastric cancer (AGC) is very important to establish a therapeutic strategy. The histological type in WHO classification [1] is an important clinicopathologic parameter of GC. This parameter has been recognized as one of the resection criteria for Endoscopic Submucosal Dissection (ESD) in patients with early GC (EGC) in Japan [2]. However, the influence of WHO histological type on GC prognosis is uncertain. In particular, the prognostic value of histological type in AGC has been rarely reported. Saito et al. reported [3] that histology types (differentiated or undifferentiated) are strong indicators of poor prognosis in node-negative AGC patients, whereas others [4] believed that histologic type has no prognostic value in AGC patients. This paper describes the differences in biological behavior. The prognostic value in AGC with various WHO histological types is evaluated, which may provide insight into the nature and clinical features of GC.
Methods
Patient enrollment and classification
During the period between 1997 and 2007, 741 patients with primary gastric adenocarcinoma underwent curative gastrectomy at the Department of Surgical Gastroenterology, Affi-liated Tumor Hospital of Harbin Medical University, Harbin, China. EGC is defined as GC confined to the mucosa or submucosa, regardless of the presence or absence of lymph node metastasis. AGC is defined as GC extending into or beyond the muscularis propria. Of the patients identified, (11.9%) patients had EGC, and (88.1%) patients had AGC. The AGC patients consisted of 563 males and 178 females with a mean age of 57 years (between 24 and 83 years). We analyzed the clinico-pathologic features of AGC with various WHO histological types and their prognostic value. This retrospective study was approved by the Ethics Committee of Harbin Medical University, and all patients signed an informed consent before the study. None of the patients received pre-operative chemotherapy or radiotherapy. The tumors were histologically classified according to WHO classification [1]. In cases with two or more histological types, the histological type was defined by the predominant tumor type. All of the tumor specimens were examined by two independent experienced pathologists. According to this criterion, all AGCs were classified into five histological types, namely, well-differentiated (WD) carcinoma, moderately differentiated (MD) carcinoma, poorly differentiated (PD) carcinoma, mucinous carcinoma (MC) and signet ring cell (SRC) carcinoma. The associated clinicopathological data were obtained from the patient’s operation and pathological reports. Data included gender (male or female), age (mean±SD), tumor size (mean±SD), primary tumor site (upper, middle, lower, or entire), gross appearance (Borrmann I+II, III+IV, X), total gastrectomy (yes or no), depth of tumor invasion (T1: tumor has invaded the mucosa or submucosa layer; T2: tumor has invaded the muscular layer or the sub-serosa; or T3: tumor has invaded the serosa or penetrating serosa; or T4: tumor invaded adjacent organs), 7th American Joint Committee on Cancer lymph node status (N0, N1, N2, N3a, or N3b), and TNM stage (I, II, III, or IV).
Follow-up and statistical analysis
Follow-ups were conducted until the death of the patient or the cut-off date of December 7, 2011. For surviving patients, data were censored at the date of the last contact. Only the patients who died of GC were regarded as tumor-related death cases. Chi-squared test was used for analyzing associations between categorical variables. Independent samples t-test was used to compare the means of the five groups. Survival data were estimated using the Kaplan-Meier method, and the log-rank test was used to compare differences in survival rates among various histologic types. Cox stepwise proportional hazards test was used to carry out multivariate analysis of prognostic factors. The criterion for statistical significance was P < 0.05. All data analyses were performed using SPSS (SPSS Inc., Chicago, IL, USA) for Windows Version 17.0 software.
Results
Clinic-pathological findings
Clinicopathologic features of the patients with various histologic types in AGC are presented in Table 1. Patients with WD and MD carcinomas were older than those with PD carcinoma, and patients in the MC and SRC groups had younger mean ages at diagnosis (53.0 and 52.9 years, respectively, P < 0.001). With respect to tumor size, all the histologic types in AGC had a higher proportion of larger size tumors. Table 1 shows that the proportion of larger tumors in PD were higher than those in MD and WD carcinoma (60.1% vs. 56.9% and 50.0%), but they were not statistically significant. The mean tumor size in SRC was larger than those in non-SRC carcinoma (6.52 vs. 5.28, P=0.03, data not shown). In terms of gross appearance, SRC patients had higher proportion of Borrmann III+IV than the other histological types (SRC: 85.4% vs. WD: 57.5% MD: 77.3%, PD: 75.1% and MD: 54.4%). By contrast, the proportion of Borrmann III+IV in MC carcinoma was equal to that in WD carcinoma. The proportion of Borrmann III+IV type increased with poorer cell differentiation, and the incidence of Borrmann I+II type decreased accordingly. The tumors were most commonly located in the lower third of stomach. With the worsening of cell differentiation, an increased percentage was found located in the entire stomach and the middle of the stomach. The SRC type was more likely to be located in the middle of the stomach (20.5% vs. 4.2%, 11.5%, 14.6%) and the entire stomach (15.9% vs. 0%, 2.3%, 2.4%) compared with the WD, MD, and PD types, respectively. Moreover, the percentage of patients who underwent total gastrectomy with worse cell differentiation and patients with SRC carcinoma significantly increased (P < 0.01).
Table 1.
Clinico-pathological characteristics of various histology types in patients with advanced gastric cancer
| Variable | WDa (n=24) | MDb (n=218) | PDc (n=414) | MCd (n=41) | SRCe (n=44) | P value |
|---|---|---|---|---|---|---|
| Gender | 0.272 | |||||
| Male | 17 (70.8%) | 177 (81.2%) | 305 (73.7%) | 32 (78.0%) | 32 (72.7%) | |
| Female | 7 (29.2%) | 41 (18.82%) | 109 (26.3%) | 9 (22.0%) | 12 (27.3%) | |
| Age (mean±SDf) | 55.6±11.3 | 61.1±9.71 | 55.7±11.35 | 53.0±11.52 | 52.9±13.91 | 0.000 |
| Tumor size (cm) | 4.15±1.67 | 5.27±5.34 | 5.29±2.55 | 5.88±3.05 | 6.52±3.67 | 0.09 |
| Borrmann | 0.005 | |||||
| I+II | 10 (45.5%) | 53 (24.9%) | 93 (22.7%) | 17 (42.5%) | 6 (14.6%) | |
| III+IV | 11 (54.5%) | 160 (75.1%) | 316 (77.3%) | 23 (57.5%) | 35 (85.4%) | |
| Unknow | 2 (8.3%) | 5 (2.3%) | 5 (1.2%) | 1 (2.4%) | 3 (6.8%) | |
| Location | 0.000 | |||||
| Upper | 5 (20.8%) | 24 (11.0%) | 55 (13.3%) | 6 (14.6%) | 2 (4.5%) | |
| Middle | 1 (4.2%) | 25 (11.5%) | 80 (19.3%) | 6 (14.6%) | 9 (20.5%) | |
| Lower | 18 (75.0%) | 164 (75.2%) | 239 (57.7%) | 28 (68.3%) | 26 (59.1%) | |
| Entire | 0 (0%) | 5 (2.3%) | 40 (9.7%) | 1 (2.4%) | 7 (15.9%) | |
| Total gastrectomy | 0.001 | |||||
| Yes | 2 (8.3%) | 28 (12.5%) | 103 (24.9%) | 7 (17.1%) | 14 (31.8%) | |
| No | 22 (91.7%) | 196 (87.5%) | 311 (75.1%) | 34 (82.9%) | 30 (68.2%) | |
| Stage T* | 0.006 | |||||
| T2 | 14 (58.3%) | 70 (32.1%) | 100 (24.2%) | 10 (24.4%) | 18 (40.9%) | |
| T3 | 9 (37.5%) | 125 (57.3%) | 268 (64.7%) | 29 (70.7%) | 23 (52.3%) | |
| T4 | 1 (4.2%) | 23 (10.6%) | 46 (11.1%) | 2 (4.9%) | 3 (6.8%) | |
| Stage N+ | 0.001 | |||||
| N0 | 16 (66.7%) | 97 (44.5%) | 132 (31.88%) | 18 (43.9%) | 19 (43.2%) | |
| N1 | 3 (12.5%) | 60 (27.5%) | 89 (21.5%) | 10 (24.4%) | 9 (20.5%) | |
| N2 | 5 (20.8%) | 36 (16.5%) | 98 (23.7%) | 6 (14.6%) | 10 (22.7%) | |
| N3a | 0 (0%) | 23 (10.6%) | 76 (18.4%) | 7 (17.1%) | 6 (13.6%) | |
| N3b | 0 (0%) | 2 (0.9%) | 19 (4.6%) | 0 (0%) | 0 (0%) | |
| Stage TNM | 0.000 | |||||
| I | 14 (58.3%) | 39 (17.9%) | 59 (14.3%) | 4 (9.8%) | 8 (18.2%) | |
| II | 6 (25.0%) | 73 (33.5%) | 96 (23.2%) | 20 (48.8%) | 16 (36.4%) | |
| III | 4 (16.7%) | 97 (44.5%) | 246 (59.4%) | 17 (41.5%) | 20 (45.5%) | |
| IV | 0 (0%) | 9 (4.1%) | 13 (3.1%) | 0 (0%) | 0 (0%) |
T2 tumor has invaded muscular layer or the subserosa; T3: tumor has invaded serosa or penetrating serosa; T4: invading adjacent organs;
N0 no regional lymph node metastasis; N1 1-2 regional lymph node metastasis; N2 3-6 regional lymph node metastasis; and N3a 7-15 regional lymph node metastasis; N3b ≥ 15 regional lymph node metastasis.
WD, well differentiated carcinoma;
MD, moderately differentiated carcinoma;
PD, poorly differentiated carcinoma;
MC, mucinous carcinoma;
SRC, signet ring cell carcinoma.
SD, standard deviation.
Moreover, cell differentiation was significantly related to invasion depth, lymph node metastasis number, and cancer stage. PD carcinoma increased with the depth of tumor invasion in terms of layers, and the reverse was true with WD carcinoma. A depth of tumor invasion greater than T2 was observed more frequently in patients with PD carcinoma than those with the WD and MD types (T3: 37.5%, 57.3%, 64.7%; T4: 4.2%, 10.6%, 11.1%, Table 1, P=0.006), whereas the incidence of WD carcinoma was higher than MD and PD carcinoma at stage T2 (58.3% vs. 32.1% vs. 24.2%, respectively). Moreover, more lymph node metastases greater than N2 were found in PD carcinoma than in MD and WD carcinomas. As shown in Table 1, WD carcinoma had a higher proportion of patients with stage N0 than the MD and PD carcinoma type (66.7% vs. 44.5% and 31.88%, respectively), whereas PD had a larger proportion of N2, N3a, and N3b compared with MD and WD carcinoma (N2: 23.7% vs. 16.5%, 20.80%; N3a: 18.4% vs. 10.6%, 0%; N3b: 4.6% vs. 0.9%, 0%). MC and SRC carcinoma were more likely to be observed at stage T3 (70.7% and 52.3%, respectively). Finally, the degree of cell differentiation had an inverse relationship with respect to TNM stage. WD carcinoma had a higher proportion of patients at stage I than MD and PD carcinoma (58.3% vs. 14.9% and 14.3%), whereas PD carcinoma had a larger proportion of stage III than MD and WD carcinoma (59.4% vs. 44.5% and 16.7%). With respect to the MC type, tumors presented with more stage N0 or N1 and stage T3, and MC type outcomes were more likely to be at stage II, which was higher than in other histologic types (48.8% vs. 25%, 33.5%, 23.2%, and 22.7%). In addition, SRC carcinoma was more likely to present in stage T3 and III, which was similar to the PD type. Figures 1, 2 and 3 demonstrate the relationship between cell differentiation (WD, MD, and PD) and stage (T, N, and TNM, respectively).
Figure 1.
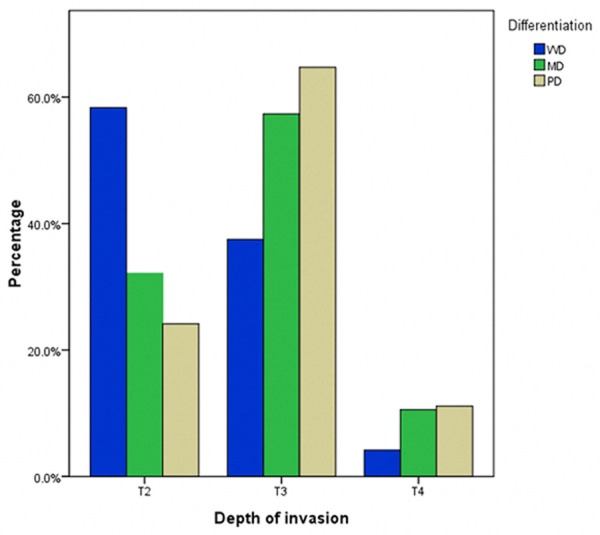
Relationship between cell differentiation (WD, MD, and PD) and stage T. WD: well-differentiated carcinoma; MD: moderately differentiated carcinoma; PD: poorly differentiated carcinoma.
Figure 2.
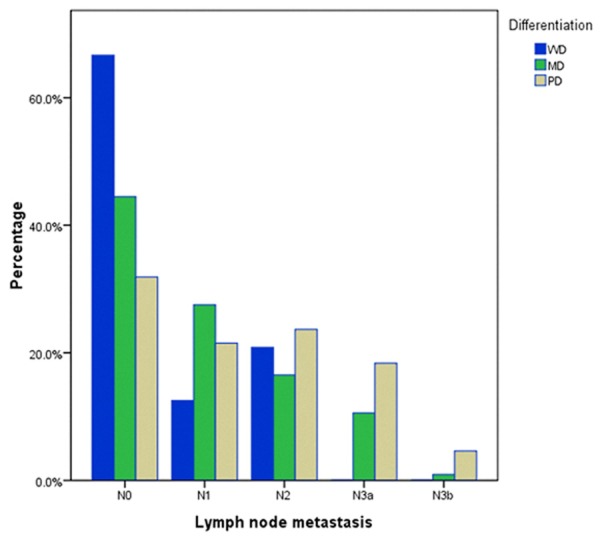
Relationship between cell differentiation (WD, MD, and PD) and stage N. WD: well-differentiated carcinoma; MD: moderately differentiated carcinoma; PD: poorly differentiated carcinoma.
Figure 3.
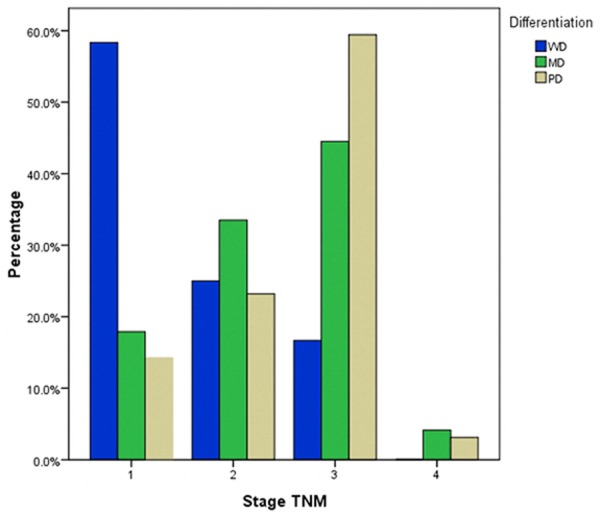
Relationship between cell differentiation (WD, MD, and PD) and stage TNM. WD: well-differentiated carcinoma; MD: moderately differentiated carcinoma; PD: poorly differentiated carcinoma.
Survival and histologic type
To clarify the influence of histological type on GC as a whole, we first performed multivariate Cox stepwise proportional hazard test for overall survival in patients according to T stage (EGC vs. AGC, Table 2). Histologic type was not an independent prognostic factor in EGC, and lymph node metastasis was the only independent prognostic factor. However, in AGC, histologic type was an independent prognostic factor. Thus, we further analyzed the prognostic value of histologic type in AGC. The five-year survival rate of AGC patients with WD, MD, PD, MC, and SRC were 87.1%, 57.1%, 50.6%, 62.7%, and 43.4%, respectively (P=0.012, Figure 4). This result demonstrated the significant correlation between cell differentiation degree and patient survival; the survival of WD carcinoma patients was significantly higher than those with MD and PD carcinomas. The survival of SRC patients was lower than those of other histologic types. MC survival was between that of WD and MD. Multivariate analysis showed that histologic type, age, tumor size, lymph node metastasis, and invasion depth were independent prognostic factors in AGC (Table 2). Given that the survival curves included crosses, we plotted Cox survival curves including sub-variables for histologic type by adjusting for age, tumor size, and other parameters. The Cox survival curves (Figure 5) demonstrated that the differences in survival rates were similar to the results of univariate analysis. Multivariate analysis with sub-variables for histologic types showed that the histologic types WD, MD, and PD were independent prognostic factors, whereas MD and SRC were not independent prognostic factors in AGC (Table 3).
Table 2.
Multivariate Cox Stepwise Proportional Hazard Model for overall survival in 100 EGC and 741 AGC patients with gastrectomy
| Stage | Variable | χ 2 | P | Hazard ratio (95% CI) |
|---|---|---|---|---|
| EGC | Lymph node metastasis | 6.236 | 0.013 | 7.138 (1.526, 33.378) |
| AGC | Age | 12.542 | < 0.001 | 1.019 (1.009, 1.030) |
| Tumor size | 10.909 | 0.001 | 1.552 (1.196, 2.014) | |
| Lymph node metastasis | 55.555 | < 0.001 | 1.498 (1.347, 1.6663) | |
| Depth of invasion (T2-T4) | 17.750 | < 0.001 | 1.570 (1.273, 1.936) | |
| Histology | 4.820 | 0.028 | 1.177 (1.018, 1.361) |
EGC: early gastric cancer. AGC: advanced gastric cancer.
Figure 4.
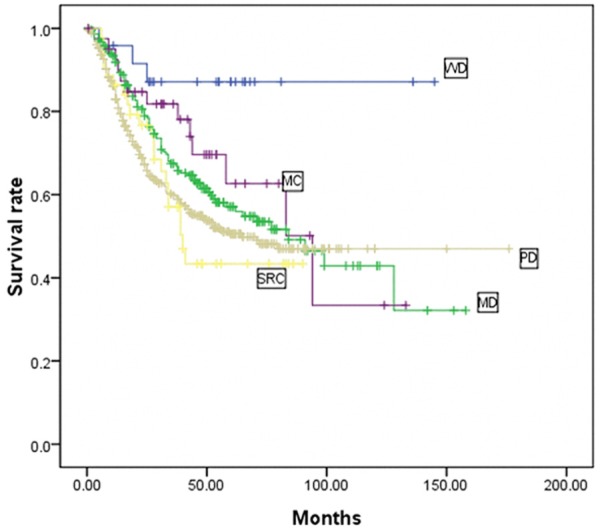
Kaplan-Meier survival curves of AGC patients according to various histological types (n=741). In AGC, the five-year survival rate of patients with WD, MD, PD, MC, and SRC were 87.1%, 57.1%, 50.6%, 62.7%, and 43.4%, respectively (P=0.012). WD: well-differentiated carcinoma; MD: moderately differentiated carcinoma; PD: poorly differentiated carcinoma; MC: mucinous carcinoma; SRC: signet ring cell carcinoma.
Figure 5.
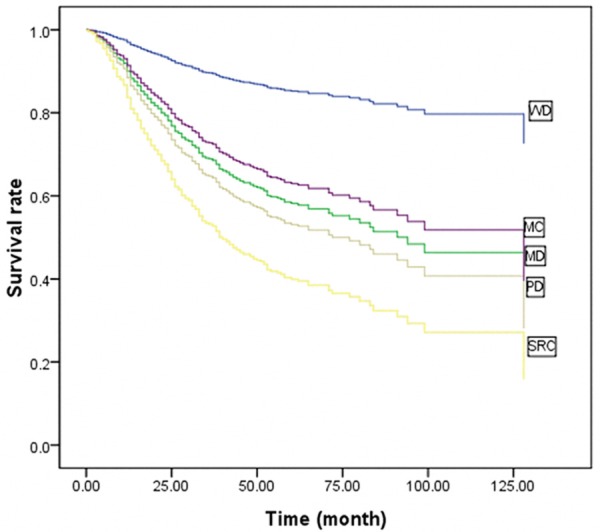
Cox survival curves of AGC patients according to various histological types (n=741). WD: well-differentiated carcinoma; MD: moderately differentiated carcinoma; PD: poorly differentiated carcinoma; MC: mucinous carcinoma; SRC: signet ring cell carcinoma.
Table 3.
Multivariate Cox Stepwise Proportional Hazard Model for overall survival in patients by sub-variable of various histologic types
| Histology | χ 2 | P | Hazard ratio (95% CI) |
|---|---|---|---|
| Well differentiated | 9.148 | 0.027 | |
| Moderately differentiated | 5.527 | 0.019 | 0.174 (0.04, 0.748) |
| Poorly differentiated | 4.346 | 0.037 | 0.590 (0.36, 0.969) |
| Mucinous cell | 2.428 | 0.119 | 0.689 (0.432, 1.101) |
| Signet ring cell | 3.645 | 0.056 | 0.504 (0.250, 1.018) |
Discussion
We analyzed the clinical-pathological features and the prognosis with various histologic types in advanced stage tumors. Results showed that the various histologic types of GC possess different clinical and tumor characteristics. With respect to cell differentiation, it was closely related to the infiltration of the gastric wall and lymph node metastases, that is, tumor malignancy increased as cell differentiation worsened (Figures 1 and 2). The PD and SRC tumors were associated with more aggressive tumor features, whereas WD, MD and MC carcinoma were associated with less aggressive features. Moreover, univariate survival analysis showed that histologic type, age, invasion depth, and lymph node metastasis were significant prognostic factors. Multivariate analysis confirmed that cell differentiation, age, invasion depth, and lymph node metastasis were independent prognostic factors (Table 2). Survival was poor in SRC patients than that in other histologic types, whereas that of MC was between the values for WD and MD (Figure 5). The MC and SRC types alone were not independent prognostic factors in AGC (Table 3).
Histological type is an important tumor clinical parameter. In thyroid carcinoma, histologic type is an important factor for evaluating the patient’s prognosis [5]. However, the prognostic value of histologic type in GC has not been defined. Histological type has been classified differently by various researchers. Sugano et al. [6] classified gastric carcinoma into two major categories: differentiated and undifferentiated types. The former responds to the intestinal-type carcinoma in Lauren’s classification [7] and to the expanding type according to Ming’s classification [8], whereas the latter responds to diffuse carcinoma in Lauren’s classification and infiltrative type according to Ming’s classification. Both Lauren’s and Ming’s classifications have been reported to possess prognostic value in GC [9]. Research on the prognosis of WHO histologic classification is rare. Saito et al. [3] and Baiocchi et al. [4] demonstrated that histologic type is an independent prognostic indicator in node-negative AGC patients, whereas others [11] believed that histologic type do not have prognostic value in AGC patients. The current study was designed to identify the prognostic value of various WHO histologic types in AGC.
The majority of GC patients were presented in the advanced stage. In this paper, the proportion of advanced stage tumors was 88.1%, which is significantly higher than that of early stage tumors (11.9%). Our results demonstrated a significant correlation between the degree of cell differentiation and aggression of tumors or stages of patients. With respect to Borrmann type, the proportion of Borrmann III+IV type increased with poorer cell differentiation, and the incidence of Borrmann I+II type decreased accordingly. PD tumors were more frequently located in the middle of the stomach (19.3% vs. 4.2% and 11.5%) and the entire stomach (9.7% vs. 0% and 2.3%) compared with WD and MD tumors, which led to a higher percentage of total gastrectomy in these patients. Moreover, cell differentiation had an inverse relationship with respect to stages T, N, and TNM. With the deterioration of cell differentiation, the tumors were more likely to present with deeper layer invasion, higher incidence of lymph node metastasis, and more advanced stage diseases (Figures 1, 2 and 3). The survival rates of patients also correlated with degree of cell differentiation and ranged from 87.1% in WD cancers to 57.1% and 50.6% in patients with MD and PD cancers (Figure 4). Moreover, multivariate survival analysis confirmed that histological type (WD, MD, and PD) accompanied by age, tumor size, invasion depth, and lymph node metastasis are independent prognostic factors in AGC. These results are similar to those in previous studies. Adachi et al. [11] believed that histological type (WD vs. PD) is one of the independent prognostic factors in GC patients. Zheng et al. [12] showed that WHO classification is an independent prognostic factor for gastric carcinoma patients (P < 0.05). The current study demonstrated that histologic type (WD, MD, and PD) is a significant prognostic factor only in AGC, whereas in EGC, histologic type has no prognostic value. Cell differentiation may be an important predictor of survival in AGC.
Compared with AGC, patient survival in EGC is less influenced by histological type. The multivariate analysis showed that lymph node metastasis is the only independent prognostic factor in EGC (Table 2), which agreed with other reports [13,14]. In previous reports, lymph node metastases were confirmed to be independent prognostic indicators. This finding indicates that lymph node metastases are more likely important than histologic type in predicting prognosis in EGC. Lymph node resection is imperative in these patients. Studies [15] have shown that histologic type is associated with incidence of lymph node metastasis in EGC. Thus, histologic type is recognized as one of the resection criteria for ESD or endoscopic mucosal resection (EMR) in patients with EGC in Japan.
A number of studies have indicated that patients with MC tumors had lower survival than those with non-MC tumors [12,16]. However, we did not obtain the same results. In the present study, the survival of patients with MC carcinoma was better than those with MD, PD, and SRC carcinomas. This result agrees with the reports of Adachi [17], in which the five-year survival rate after curative resection was the same for advanced MC carcinoma and advanced non-MC carcinoma (58% vs. 56%). In this paper, the better prognosis of the MC type can be attributed to the lower proportion of Borrmann III+IV and the higher proportion of patients in stage II than other histologic types. In other studies, the differences in MC carcinoma survival might be due to the differences in the enrollment of patients. Previous studies included all the patients with MC carcinoma (curative and non-curative gastrectomy). In the present study only the patients with curative gastrectomy were included. Kunisaki et al. [18] reported that no significant difference was found in survival between MC and non-MC gastric carcinoma for advanced stage gastric carcinoma patients who underwent curative resection, which agrees with our study. In previous studies, patients with low survival in MC carcinoma were considered due for diagnosis with advanced stage and were included with non-curative gastrectomy. By contrast, the patients included in the present study were those with curative gastrectomy only who were diagnosed at an earlier stage, which led to a better prognosis. Thus, we believe that the prognosis of MC carcinoma correlates with the stage at diagnosis, not the MC type itself. Improving the early diagnosis is the key to improve survival in patients with MC carcinoma.
Our study also showed that the five-year survival rate of SRC was the lowest among the other histologic types in AGC (Figure 4). However, SRC alone was not an independent prognostic factor in multivariate analysis (Table 3), which agreed with previous reports [19,20]. Li et al. reported [19] that advanced gastric SRC has poorer prognosis than non-SRC. Similarly, Kim et al. [20] demonstrated that no significant difference exists in the five-year survival rates among patients with different histological cell types in EGC. However, in AGC, the prognosis for SRC patients was significantly poorer than that for the other types. Our data indicated that advanced SRC carcinoma had distinct clinico-pathological features characterized by larger tumors, higher incidence of Borrmann III+IV type, and deeper local invasion than in other types. These aggressive features may account for the poorer prognosis in patients with advanced SRC than those with other types. However, findings from previous studies noted that patients with SRC carcinoma had better prognosis compared with the other types [16,21,22], which were mainly caused by the SRC carcinoma that contained a higher proportion of EGC. Moreover, these previous studies considered curative and non-curative gastrectomy, whereas this study included only the curative gastrectomy patients. Therefore, radical surgical procedures should be considered in advanced SRC patients, and SRC carcinoma should be treated separately.
The current study has several limitations because it is an inherent retrospective analysis. Our study lacks data on the receipt of adjuvant chemotherapy because of uncertainty on the extent of systemic adjuvant chemotherapy. Moreover, the regimens for postoperative chemotherapy were varied in GC patients, and we did not consider the impact of adjuvant chemotherapy on patient survival. Studies [23] have shown that differentiation and serosa involvement should be considered to stratify patients with node-negative AGC for adjuvant treatment. In the future, prospective clinical research should be used to assess the survival benefit of chemotherapy in patients with various histologic types in AGC.
In conclusion, this paper showed that various histological types of AGC differed in their clinical and tumor features. Cell differentiation is related to tumor aggression or patient stage. Advanced stage SRC carcinoma had more aggressive features and poorer prognosis than the other types. Patients with advanced stage MC carcinoma have a survival rate between the values of those with WD and MD. MC and SRC carcinoma are not independent prognostic factors in AGC. Degree of cell differentiation, age, invasion depth, and lymph node metastasis are independent prognostic factors in AGC.
Disclosure of conflict of interest
None.
References
- 1.Jass JR, Sobin LH, Watanabe H. The World Health Organization’s histologic classification of gastrointestinal tumors. A commentary on the second edition. Cancer. 1990;66:2162–2167. doi: 10.1002/1097-0142(19901115)66:10<2162::aid-cncr2820661020>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 2.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 3.Saito H, Kuroda H, Matsunaga T, Fukuda K, Tatebe S, Tsujitani S, Ikeguchi M. Prognostic indicators in node-negative advanced gastric cancer patients. J Surg Oncol. 2010;101:622–625. doi: 10.1002/jso.21562. [DOI] [PubMed] [Google Scholar]
- 4.Adachi Y, Mori M, Maehara Y, Sugimachi K. Long-term survival after resection for advanced gastric carcinoma. J Clin Gastroenterol. 1995;21:208–210. doi: 10.1097/00004836-199510000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Kerr DJ, Burt AD, Boyle P. Prognostic factors in thyroid tumors. Br J Cancer. 1986;54:475–482. doi: 10.1038/bjc.1986.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugano H, Nakamura K, Kato Y. Pathological studies of human gastric cancer. Acta Pathol Jpn. 1982;32(Suppl 2):329–47. [PubMed] [Google Scholar]
- 7.Laurin P. The two histologic main types of gastric carcinoma:diffuse and so-called intestinal type. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Ming SC. Gastric carcinoma: a pathological classification. Cancer. 1997;39:2475–2485. doi: 10.1002/1097-0142(197706)39:6<2475::aid-cncr2820390626>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 9.Davessar K, Pezzullo JC, Kessimian N, Hale JH, Jauregui HO. Gastric adenocarcinoma: Prognostic significance of several pathologic parameters and histologic classifications. Hum Pathol. 1990;21:325–332. doi: 10.1016/0046-8177(90)90234-v. [DOI] [PubMed] [Google Scholar]
- 10.Baiocchi GL, Tiberio GA, Minicozzi AM, Morgagni P, Marrelli D, Bruno L, Rosa F, Marchet A, Coniglio A, Saragoni L, Veltri M, Pacelli F, Roviello F, Nitti D, Giulini SM, De Manzoni G. A multicentric Western analysis of prognostic factors in advanced, node-negative gastric cancer patients. Ann Surg. 2010;252:70–73. doi: 10.1097/SLA.0b013e3181e4585e. [DOI] [PubMed] [Google Scholar]
- 11.Adachi Y, Yasuda K, Inomata M, Sato K, Shiraishi N, Kitano S. Pathology and prognosis of gastric carcinoma: well versus poorly differentiated type. Cancer. 2000;9:1418–1424. [PubMed] [Google Scholar]
- 12.Zheng HC, Zheng YS, Xia P, Xu XY, Xing YN, Takahashi H, Guan YF, Takano Y. The pathobiological behaviors and prognosis associated with Japanese gastric adenocarcinomas of pure WHO histological subtypes. Histol Histopathol. 2010;25:445–452. doi: 10.14670/HH-25.445. [DOI] [PubMed] [Google Scholar]
- 13.Kikuchi S, Katada N, Sakuramoto S, Kobayashi N, Shimao H, Watanabe M, Hiki Y. Survival after surgical treatment of early gastric cancer: surgical techniques and long term survival. Langenbecks Arch Surg. 2004;389:69–74. doi: 10.1007/s00423-004-0462-2. [DOI] [PubMed] [Google Scholar]
- 14.Itoh H, Oohata Y, Nakamura K, Nagata T, Mibu R, Nakayama F. Complete 10-year postgastrectomy follow-up of early gastric cancer. Am J Surg. 1989;158:14–16. doi: 10.1016/0002-9610(89)90305-x. [DOI] [PubMed] [Google Scholar]
- 15.Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]
- 16.Park JM, Jang YJ, Kim JH, Park SS, Park SH, Kim SJ, Mok YJ, Kim CS. Gastric cancer histology: clinicopathologic characteristics and prognostic value. J Surg Oncol. 2008;98:520–525. doi: 10.1002/jso.21150. [DOI] [PubMed] [Google Scholar]
- 17.Adachi Y, Mori M, Kido A. A clinicopathologic study of mucinous gastric carcinoma. Cancer. 1992;69:866–71. doi: 10.1002/1097-0142(19920215)69:4<866::aid-cncr2820690405>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 18.Kunisaki C, Akiyama H, Nomura M, Matsuda G, Otsuka Y, Ono HA, Shimada H. Clinicopathologic Characteristics and Surgical Outcomes of Mucinous Gastric Carcinoma. Ann Surg Oncol. 2006;13:836–842. doi: 10.1245/ASO.2006.03.077. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Kim S, Lai JF, Hyung WJ, Choi WH, Choi SH, Noh SH. Advanced Gastric Carcinoma with Signet Ring Cell Histology. Oncology. 2007;72:64–68. doi: 10.1159/000111096. [DOI] [PubMed] [Google Scholar]
- 20.Kim JP, Kim SC, Yang HK. Prognostic significance of signet ring cell carcinoma of the stomach. Surg Oncol. 1994;3:221–227. doi: 10.1016/0960-7404(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 21.Maehara Y, Sakaguchi Y, Moriguchi S, Orita H, Korenaga D, Kohnoe S, Sugimachi K. Signet ring cell carcinoma of the stomach. Cancer. 1992;69:1645–1650. doi: 10.1002/1097-0142(19920401)69:7<1645::aid-cncr2820690702>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Zhu G, Zhang H, Gao H, Xue Y. Clinicopathologic features of gastric carcinoma with signet ring cell histology. J Gastrointest Surg. 2010;14:601–606. doi: 10.1007/s11605-009-1127-9. [DOI] [PubMed] [Google Scholar]
- 23.Lee IS, Yook JH, Kim TH, Kim HS, Kim KC, Oh ST, Kim BS. Prognostic factors and recurrence pattern in node-negative advanced gastric cancer. Eur J Surg Oncol. 2013;39:136–40. doi: 10.1016/j.ejso.2012.10.008. [DOI] [PubMed] [Google Scholar]


